Abstract
Summary: Staphylococcus aureus bacteremia (SAB) is an important infection with an incidence rate ranging from 20 to 50 cases/100,000 population per year. Between 10% and 30% of these patients will die from SAB. Comparatively, this accounts for a greater number of deaths than for AIDS, tuberculosis, and viral hepatitis combined. Multiple factors influence outcomes for SAB patients. The most consistent predictor of mortality is age, with older patients being twice as likely to die. Except for the presence of comorbidities, the impacts of other host factors, including gender, ethnicity, socioeconomic status, and immune status, are unclear. Pathogen-host interactions, especially the presence of shock and the source of SAB, are strong predictors of outcomes. Although antibiotic resistance may be associated with increased mortality, questions remain as to whether this reflects pathogen-specific factors or poorer responses to antibiotic therapy, namely, vancomycin. Optimal management relies on starting appropriate antibiotics in a timely fashion, resulting in improved outcomes for certain patient subgroups. The roles of surgery and infectious disease consultations require further study. Although the rate of mortality from SAB is declining, it remains high. Future international collaborative studies are required to tease out the relative contributions of various factors to mortality, which would enable the optimization of SAB management and patient outcomes.
INTRODUCTION
Staphylococcus aureus bacteremia (SAB) is a common and important infection. The exact incidence of SAB is difficult to ascertain, as prospective population-based surveillance studies are infrequently performed. In Scandinavian countries, where data from the nationwide surveillance of SAB are routinely collected, the annual incidence is approximately 26/100,000 population (14, 119, 128). A similar low incidence of 19.7/100,000 population was reported in a Canadian study in 2008 (160), while in countries with a greater burden of methicillin-resistant S. aureus (MRSA), incidence rates are generally higher, between 35 and 39/100,000 population (38, 56, 233). In comparison, even higher rates, approximately 50/100,000 population, are inferred from surveillance data from the United States (143, 201). These large geographical discrepancies probably reflect differences in health care systems, infection control practices, and the completeness of surveillance data.
The incidence of SAB increases with advancing age, with the lowest rates observed in pediatric populations, at approximately 8.4/100,000 population per year (71). Similarly, younger adults have lower incidence rates than older adults (14, 160). Other factors associated with higher incidences include male gender, African American ethnicity, community-onset SAB, and specific patient subgroups that have frequent health care contact, including hemodialysis patients (160, 201, 229). Methicillin-sensitive S. aureus (MSSA) bacteremia (MSSA-B) episodes generally predominate, especially in countries with a low prevalence of MRSA (14, 38). Infecting clonal patterns vary geographically and are in a constant state of flux, as evidenced by the recent phenomenon, especially in the United States, of hospital strain replacement with typical community MRSA clones such as USA300 (229). Nevertheless, although not as common as other infections, such as tuberculosis or chronic hepatitis, SAB remains an important infection.
Mortality
Once established, SAB is not a benign condition, resulting in significant morbidity and mortality, especially in patients in intensive care units (ICUs) (156, 265). SAB mortality rates of between 75% and 83% were observed in the preantibiotic era (182, 196). The introduction of antibiotics in the 1940s and 1950s resulted in better outcomes (320). Subsequently, with a greater understanding of SAB management, improved outcomes have continued to be documented throughout the 20th century, with overall mortality rates declining from 36% and 35% in 1981 to 1985 to 21% and 27% in 1996 to 2004 for hospital- and community-onset SAB episodes, respectively (P < 0.01) (14). However, recent prospective data (2009) of 1,994 SAB episodes suggest that mortality rates may have stabilized, with a 30-day all-cause mortality rate of 20% (296) and an infection-related mortality rate estimated at approximately 13% (164). Despite these improvements, SAB 30-day all-cause mortality results in approximately 2 to 10 deaths annually per 100,000 population (7, 143, 160). Comparatively, the age-adjusted mortality rates are 3, 0.2, and 2.2 per 100,000 population for AIDS, tuberculosis, and viral hepatitis, respectively, while breast and prostate cancers claim 12.5 and 8.6 lives per 100,000 population annually, respectively (21, 145). Despite these similar mortality rates, the impact and significance of SAB in the community remain underestimated.
Multiple factors influence mortality. These factors include host factors, pathogen-host interactions, and pathogen-specific factors. The purpose of this review is to examine factors that influence mortality and investigate the relative impact of each factor on outcomes for patients with SAB.
HOST FACTORS
Age
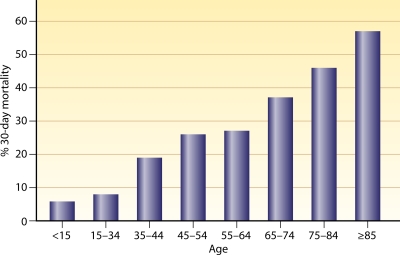
Age is the most consistent and strongest predictor of all-cause and infection-related 30-day mortality, with the majority of SAB cohort studies using multivariate analysis, confirming age as an independent predictor of mortality (Table 1). In one of the largest population-based studies (n = 9,001), where MRSA-B episodes were linked with death certificates to obtain all-cause 30-day mortality rates (155), the mortality rate was found to increase from 6% in young individuals (<15 years old) to 57% in adults older than 85 years of age. This represents an approximately 1.3-fold increase in mortality from SAB for every 10 years of life (Fig. 1). These data are not explained by other problems that come with aging, which include the presence and number of comorbidities, indwelling medical devices, and health care contact (134). Some of the higher death rates may be attributed to differences in SAB-related investigations and management of elderly patients (15). However, in a case-controlled study, age remained a predictor of mortality despite elderly patients (>65 years) having similar baseline characteristics and SAB management (284). Thus, the increased mortality from SAB associated with aging is directly linked to changes within the host as a consequence of the aging process, and age remains a significant confounder when examining other variables that influence outcomes.
Table 1.
Summary of studies that have examined the impact of age on mortality in cases of Staphylococcus aureus bacteremiaa
| Study (outcome of interest) | No. of MSSA and MRSA bacteremic episodes | Overall mortality rate (%) | Age variable; OR (95% CI; P) | Reference |
|---|---|---|---|---|
| Studies that found age to be an independent predictor of mortality (multivariate analysis) | ||||
| Single-center retrospective Taiwan study (1990–2004); 30-day all-cause mortality | 297 MSSA, 851 MRSA | 44.1 | Age >65 yr; 1.85 (1.37–2.50; <0.01) | 308 |
| Single-center retrospective Spanish study (1991–1998); in-hospital infection-related mortality | 683 MSSA, 225 MRSA | 12.1 | Age per 10 yr; 1.17 (1.05–1.31; ND) | 275 |
| Single-center retrospective Canadian study (1991–2005); 30-day mortality | 746 MSSA, 96 MRSA | 24.0 | Age >65 yr; 2.31 (1.43–3.73; <0.01) | 5 |
| Single-center prospective Spanish study (1991–2005); 30-day all-cause mortality | 0 MSSA, 414 MRSA | 28.0 | Age per yr; 1.02 (1.00–1.04; 0.013) | 273 |
| Single-center retrospective Belgian study (1992–1998); 30-day in-hospital mortality | 38 MSSA, 47 MRSA | 37.6 | Age per yr; 1.02 (1.00–1.02; 0.01) | 18 |
| Single-center prospective Danish study (1994–1996); 150-day mortality | 275 MSSA, 3 MRSA | 34 | Age ≥60 yr; 2.4 (1.1–5.3; 0.03) | 127 |
| Single-center prospective U.S. study (1994-1998); 90-day all-cause mortality | 385 SAB | 20.5 | Age >65 yr; 2.39 (1.45–3.95; ND) | 192 |
| Single-center retrospective U.S. study (1995–1999); 30-day all-cause mortality | 204 MSSA, 89 MRSA | 23.2 | Age >65 yr; 2.0 (1.0–3.8; 0.048) | 206 |
| Single-center retrospective Chinese study (2001–2007); 90-day all-cause mortality | 0 MSSA, 115 MRSA | 21.7 | Age per yr; 1.03 (1.01–1.05; 0.019) | 32 |
| Multicenter retrospective U.S. study (1995–2003); 90-day infection-related mortality | 245 MSSA, 193 MRSA | 26.0 | Age >65 yr; 2.3 (1.38–3.8; <0.01) | 266 |
| Single-center retrospective U.S. study (1996–2006); all-cause mortality | 0 MSSA, 489 MRSA | 25.0 | Age per yr; 1.05 (1.03–1.07; <0.001) | 202 |
| Single-center retrospective Taiwan study (1997–2001); 30-day infection-related mortality | 0 MSSA, 162 MRSA | 47.5 | Age ≥60 yr; 2.90 (1.25–6.75; 0.01) | 59 |
| Multicenter retrospective United Kingdom study (1997–2004); 30-day mortality | 229 MSSA, 232 MRSA | 29.0 | Age per decade; 1.79 (1.75–1.82; ND) | 322 |
| Single-center retrospective Australian study (1997–2008); 30-day all-cause mortality | 0 MSSA, 401 MRSA | 28.7 | Age per yr; 1.03 (1.01–1.05; 0.005) | 301 |
| Single-center retrospective Swiss study (1998–2002); in-hospital all-cause mortality | 302 MSSA, 6 MRSA | 19.5 | Age per 10 yr; 1.3 (1.2–1.6; <0.01) | 131 |
| Single-center retrospective Taiwan study (2001–2006); in-hospital infection-related mortality | 0 MSSA, 177 MRSA | 33.3 | Age per yr; 1.04 (1.01–1.08; 0.01) | 167 |
| Single-center retrospective Taiwan study (2001–2007); 90-day all-cause mortality | 0 MSSA, 115 MRSA | 21.7 | Age per yr; 1.01 (1.0–1.02; <0.05) | 33 |
| Single-center retrospective Germany study (2002–2004); in-hospital all-cause mortality | 454 MSSA, 67 MRSA | 21.7 | Age ≥60 yr; 2.4 (1.4–4.2, <0.01) | 237 |
| Single-center retrospective Israeli study (2003–2006); in-hospital infection related-mortality | 0 MSSA, 250 MRSA | 37.2 | Age per yr; 1.02 (1.004–1.04; 0.013) | 185 |
| Single-center retrospective U.S. study (2003–2008); in-hospital mortality | 326 MSSA, 488 MRSA | 13.3 | Age per yr; 1.04 (1.03–1.05; ND) | 257 |
| Single-center prospective U.S. study (2004); in-hospital mortality | 0 MSSA, 132 MRSA | 22.0 | Age >55 yr; 8.09 (2.02-32.5; 0.03) | 261 |
| Multicenter prospective post hoc analysis Asian study (2004–2006); 30-day all-cause mortality | 380 MSSA, 329 MRSA | 24.5 | Age ≥65 yr; 1.69 (1.15–2.48; 0.008) | 133 |
| Multicenter prospective post hoc analysis Asian study (2004–2006); 30-day all-cause mortality | 1,701 MSSA, 2,007 MRSA | 13.4 | Age per 10 yr; 1.19 (1.12–1.27; <0.01) | 134 |
| Single-center retrospective Taiwanese study (2004–2006); 30-day all-cause mortality | 186 MSSA, 30 MRSA | 12.6 | Age per yr; 1.03 (1.01–1.06; <0.01) | 310 |
| Single-center retrospective Singaporean study (2005–2006); in-hospital infection-related mortality | 100 MSSA, 0 MRSA | 23.9 | Age >65 yr; 13.7 (2.75–68.3; <0.01) | 35 |
| Single-center, prospective U.S. study (2005–2006), 30-day all-cause mortality | 90 MSSA, 163 MRSA | Not stated | Age per yr; 1.04 (1.01-1.07) | 77 |
| Multicenter retrospective European study (2007); 30-day all-cause mortality | 257 MSSA, 77 MRSA | 24.0 | Age per yr; 1.06 (1.03–1.10; <0.01) | 6 |
| Multicenter prospective Australian study (2007–2008); 30-day all-cause mortality | 1,415 MSSA, 450 MRSA | 20.6 | Age >70 yr; ND (ND; <0.001) | 296 |
| Multicenter prospective Australian study (2007–2008); 30-day all-cause mortality | 324 MSSA, 199 MRSA | 17.2 | Age per yr; 1.06 (1.04–1.08; <0.01) | 101 |
| Studies where age was not an independent predictor of mortality | ||||
| Single-center prospective Spanish study (1990–1993); infection-related mortality | 100 MSSA, 84 MRSA | 44.0 | Age >65 yr; ND | 244 |
| Single-center retrospective Turkish study (1990–1994); infection-related mortality | 55 MSSA, 46 MRSA | 21.8 | ND | 291 |
| Single-center retrospective Brazilian study (1991–1992); 14-day all-cause mortality | 46 MSSA, 90 MRSA | 39.0 | Age >60 yr; ND | 39 |
| Single-center retrospective French study (1997–1998); infection-related mortality | 69 MSSA, 30 MRSA | 27.3 | Age ≥60 yr; ND | 286 |
| Single-center retrospective U.S. study (1997–2000); in-hospital mortality | 250 MSSA, 96 MRSA | 20.7 | Age >65 yr; ND | 41 |
| Single-center retrospective Korean study (1998–2001); 60-day infection-related mortality | 111 MSSA, 127 MRSA | 37.0 | Age ≥65 yr; 1.88 (0.97–3.62; ND) | 142 |
| Single-center retrospective Korean study (1998–2006); infection-related mortality | 294 MSSA, 0 MRSA | 19.4 | Age ≥65 yr; 1.4 (0.8–2.5; 0.29) | 140 |
| Single-center retrospective Brazilian study (2000–2001); infection-related mortality | 50 MSSA, 61 MRSA | 39.0 | Age per yr; 0.98 (0.96–1.00; 0.07) | 89 |
| Single-center retrospective Taiwanese study (2000–2008); 30-day all-cause mortality | 0 MSSA, 227 MRSA | 44.9 | Age ≥65 yr; 1.93 (0.98–3.78; ND) | 171 |
| Single-center retrospective Belgian study (2002–2004); infection-related in-hospital mortality | 88 MSSA, 66 MRSA | 40.0 | ND | 168 |
| Multicenter retrospective U.S. study (2004–2005); in-hospital mortality | 32 MSSA, 36 MRSA | 19.1 | Age per yr; 1.05 (1.0–1.1; 0.08) | 184 |
| Single-center retrospective Taiwanese study (2006–2008); 30-day all-cause mortality | 0 MSSA, 253 MRSA | 30.5 | ND | 309 |
Studies employed a multivariate logistic regression model.
OR, odds ratio; CI, confidence interval; P, probability; ND, not described.
Fig 1.
Impact of age on overall 30-day mortality from Staphylococcus aureus bacteremia. Percentages of patients who succumbed at 30 days following an episode of Staphylococcus aureus bacteremia are stratified by 10-year age groups. (Adapted from reference 155 with permission of Elsevier.)
Gender
The incidence of SAB is generally higher for males than for females (143, 160). Despite this, several studies have shown an increased mortality rate for females. In several retrospective cohort studies of MRSA-B episodes, female gender was an independent predictor of 30-day all-cause mortality. The odds of death was approximately 2-fold higher than that for males in 116 (odds ratio [OR], 3.88; 95% confidence interval [CI], 1.23 to 12.2; P = 0.02) (261), 510 (OR, 1.74; 95% CI, 1.13 to 2.68; P < 0.001) (220), and 250 (OR, 1.97; 95% CI, 1.06 to 3.64; P = 0.017) (185) MRSA-B episodes. Comparable results were seen in two larger studies of 908 (OR, 1.71; 95% CI, 1.02 to 2.84) and 814 (OR, 1.59; 95% CI, 1.09 to 2.34) SAB episodes from Spain (275) and Canada (5), respectively. Possible reasons for why gender may play a role in outcomes include different health-seeking behaviors adopted by women, the infecting MRSA clone, or hormonal differences (289). Alternatively, age differences may explain some of the observed gender differences, as the age-adjusted 30-day mortality rates were similar for males and females in a large retrospective English cohort study of 9,001 MRSA-B episodes (155).
Regardless, these explanations remain largely speculative, and without further evidence, gender is unlikely to be a major factor in SAB mortality, as the majority of studies (only studies with more than 500 SAB cases have been cited) have not detected any gender difference in regard to outcomes (14, 32, 101, 133, 134, 237, 296).
Ethnicity
In the United States, African American populations have higher rates of SAB (61) and MRSA-B (74, 95, 143) than Caucasian patients. In 2005, in a U.S. population-based surveillance study, predominantly of health care-associated MRSA bacteremic episodes (66%; 5,813/8,792), the annual invasive MRSA infection incidence rate for Caucasians was 27.7/100,000 population (95% CI, 21.9 to 32.4), compared to 66.5/100,000 (95% CI, 43.5 to 63.1) for African Americans (143). Several other studies from New Zealand (109), Australia (96, 289), and the Pacific Islands (98) have documented ethnic differences in SAB incidences, with generally higher incidences in indigenous populations than in nonindigenous populations.
Despite the increased incidences in certain racial groups, the impact of ethnicity on mortality is unclear, primarily because the numbers of deaths are not indicated or are too small to compare (98, 109, 289) and because of multiple confounders associated with indigenous populations (96). These include the prevalence of comorbidities such as diabetes and alcoholism, socioeconomic status (SES), overcrowding, poor infrastructure, limited opportunities, and low income and education (96, 160, 194, 289). There was a suggestion in the literature, however, that mortality may be affected by ethnicity, with higher standardized mortality rates observed for African Americans (10/100,000 per annum) than for Caucasians (5.9/100,000) (143). However, unlike the study by Klevens et al. (143), most studies examining the effect of ethnicity showed no difference in outcomes (96) or lower mortality rates in African Americans than in Caucasian patients (26% and 37%, respectively; P = 0.04) (243). Similarly, a large multicenter observational prospective study in Australia of 1,865 cases observed a significantly lower 30-day mortality rate for indigenous Australians, 5.7% (3/53), than for people of European decent, 22.2% (350/1,575) (P < 0.001) (296). Limitations of these studies include the lack of adjustments for age and comorbidities. Nevertheless, despite these counterintuitive findings, the impact of ethnicity on SAB incidences and mortality remains largely unresolved, with little progress in the understanding of how racial disparities may influence these measures.
Socioeconomic Status
Socioeconomic status (SES) is known to impact a patient's infection risk (10). For SAB, an inverse relationship exists between incidence and SES, with the lowest rates found for the least deprived economic strata than for the most deprived strata (16 and 21.3/100,000, respectively; P < 0.01) (109). In this population-based survey of 779 SAB episodes, SES was measured by using the New Zealand deprivation index, a geographical and census-based measure of income (i.e., place of abode, number of others living in the household, the employment status and qualifications of each person, and access to a telephone and car). Linear regression modeling did not show an effect of the deprivation quintile on mortality rates. Thus, the effect of SES on outcome remains uncertain, as no other SAB study, to our knowledge, has captured these data. Further study is warranted, however, as defining the role of deprivation in SAB outcomes would allow for the better targeting of preventative strategies and government programs.
Immune Status
Immunosuppression can be defined as a congenital or acquired quantitative or qualitative deficiency of phagocytic cells, complement, or humoral or cell-mediated immunity. Acquired immune deficiencies predominate especially in adult populations and remain an important area of research with the continuing expansion of stem cell and solid-organ transplantation and the increasing use of immune modulators in general medical therapeutics. Overall, immunosuppression has been observed infrequently as an independent predictor of mortality in SAB studies (98, 131), with the odds of death being approximately 4-fold higher (OR, 4.1; 95% CI, 1.5 to 11.3; P = 0.007) than for immunocompetent hosts (131). This may reflect the number of heterogeneous disorders that are lumped together, with very few cohort studies examining the impact of specific immune system components on mortality due to SAB and the lack of functional immune data.
Innate immunity.
The innate immunity system forms the initial defense against infections and consists of anatomical barriers, complement, and specific cells. The most common acquired defect of the innate immune system is secondary to chemotherapy-induced neutropenia, especially for the treatment of hematological malignancies. Paradoxically, these patients are significantly less likely to die than nonneutropenic cancer patients (P = 0.01) (305), which may reflect the higher rates of intravenous line-related SAB and low rates of infective endocarditis (IE) in these patients (270). Congenital disorders are rare, with chronic granulomatous disease (CGD) being the most commonly occurring deficiency. This heterogeneous condition is characterized by the inability of cells to phagocytose catalase-positive microorganisms, resulting in an increased risk of recurrent infections, especially SAB (130). However, no comparative data exist to assess the overall impact of CGD on SAB mortality.
Humoral immunity.
The protective role of antibody responses to S. aureus infections remains to be defined. Similarly, the impact of acquired and congenital B-cell dysfunctions on SAB mortality is unknown.
Individuals with prior S. aureus exposure or carriers are known to have lower SAB mortality rates than noncarriers (8% versus 32%; P = 0.006) (316). As antibody levels are shaped during S. aureus colonization prior to infection (146), it is plausible that antibody levels may provide some protection and improve outcomes for these patients. More direct evidence for protective antibody responses was observed in a single-center prospective Swedish study, which detected a significant (P < 0.05) association between 28-day mortality and low levels of antibodies to teichoic acid (relative risk [RR], 4.1; 95% CI, 1.3 to 13.3), lipase (RR, 4.3; 95% CI, 1.3 to 13.1), enterotoxin A (RR, 3.6; 95% CI, 1.1 to 11.9), and scalded skin syndrome toxin (RR, 13.2; 95% CI, 1.7 to 99.7) (118). This study, however, was not limited to bacteremic episodes only and failed to measure other components of the immune system that may impact outcomes.
The failed S. aureus vaccine trials (254, 263) provide further evidence that our understanding of the humoral immune system with respect to outcomes of SAB is unclear. Given the impact of SAB, humoral responses warrant further study, which in turn may enable better vaccination strategies (118, 146, 306).
Cell-mediated immunity.
AIDS secondary to HIV is one of the most common cell-mediated acquired immune deficiency states worldwide (159). The incidence of SAB among HIV-infected individuals remains higher than that among individuals not infected with HIV (25, 124, 138, 159, 260, 295). This risk is directly associated with the degree of immune deficiency, with the relative risk being 31 times higher (incidence rate ratio [IRR], 31.1; 95% CI, 10.3 to 94.0) for patients with CD4 cell counts of <100 cells/μl than for patients with CD4 cell counts of ≥350 cells/μl (159). Despite a higher incidence of SAB among HIV-infected individuals, no study to date has detected an increased mortality rate with SAB in HIV-infected cases (227, 260, 261).
Overall, immune deficiency states seem to have a minimal impact on outcomes for patients with SAB. Paradoxically, there is some evidence that immune suppression may actually be protective, as these dampened responses may lead to reduced inflammation, manifesting as less severe disease. However, as comparative data remain limited, the current data should be interpreted with caution.
Presence of Comorbidities
The presence of one or more comorbidities has been associated, albeit inconsistently, with SAB mortality. These comorbidities include the presence of alcoholism (131), immunosuppression (131), cirrhosis (133, 134, 138, 140), congestive cardiac failure (171), malignancy (33, 77, 133, 134, 140, 308, 309), chronic renal failure requiring hemodialysis (77, 131, 134, 184), and the presence of multiple comorbidities (14).
McCabe and Jackson proposed a system that classifies patients into one of three groups, rapidly fatal, ultimately fatal, and nonfatal, based on the prognosis of the underlying illness (190a). Cohort studies with patients with SAB have found that prognosis as defined by McCabe and Jackson is an independent predictor of mortality (140, 167, 273, 275, 291). Furthermore, an incremental increased risk of death among the three categories has been documented (273, 275), with the odds of death being higher for patients with a rapidly fatal underlying disease (OR, 13.1; 95% CI, 5.7 to 30.9) than for patients with an ultimately fatal underlying disease (OR, 2.02; 95% CI, 1.18 to 3.44) (275). Conversely, patients with nonfatal comorbidities had lower mortality rates (OR, 0.2; 95% CI, 0.1 to 0.4; P < 0.01) (237). Cosgrove and colleagues transformed the McCabe-Jackson categories into a continuous variable by assigning a score to each category (fatal disease, 1; nonfatal disease, 3) (41). In this prospective study of 348 SAB episodes, lower scores on multivariate analysis remained an independent predictor of mortality (OR of 38.5 for each incremental decrease; P < 0.001).
Functional status as a surrogate marker for the underlying disease prognosis has likewise been demonstrated to be an independent predictor of mortality, with bedridden patients (185, 204) and individuals from a long-term care facility (133, 134) having higher death rates. Alternatively, 17 preexisting conditions known to be associated with an increased mortality can be scored by using the Charlson weighted index (CWI) (31). Higher scores are associated with an increased 1-year mortality rate compared to lower scores (e.g., 82% for scores of >5, compared to 12% for scores of 0). Following the publication of the CWI, Lesens et al. were able to validate the utility of this index in a prospective study of 168 patients with SAB (164). Multiple other cohort studies since then have demonstrated that the CWI is an independent predictor of mortality due to SAB (6, 15, 33, 185, 301).
Although several SAB cohort studies have not detected any difference in outcomes for patients with one or more comorbidities (32, 39, 59, 89, 309, 310), this discrepancy probably reflects the small sample size and patient groups selected in these studies. Regardless, overall, the presence of one or more comorbidities generally portends a worse outcome for patients with SAB, and as such, the collection of these data should be encouraged in future SAB studies.
HOST-PATHOGEN INTERACTIONS
Principal Diagnosis and Source of Infection
The overall mortality rate from SAB varies depending on the primary focus of infection, with the highest mortality rates occurring in patients with primary bacteremic pulmonary infections (33, 39, 41, 96, 206, 296, 301) and infective endocarditis (IE) (77, 168, 237, 296, 301). Some of the lowest rates occur in patients with central or peripheral venous catheter-related infections (33) (Table 2).
Table 2.
All-cause mortality for patients with Staphylococcus aureus bacteremia stratified by source of infectiona
| Diagnosis | In-hospital mortality (%) (reference[s]) | 28–30-day mortality (%) (reference[s]) | Description (reference[s]) |
|---|---|---|---|
| Bacteremia without focus | 11–45 (14, 161, 167) | 21.9–47.5 (120, 206, 296, 301) | 14-day mortality rate, 49% (39); independent predictor of mortality relative to other infections in several studies, with an OR range between 3.88 and 12.3 (15, 39, 206, 301) |
| Bone and joint infection | 0–13.9 (14, 19, 76, 80, 167) | 0–29 (120, 296, 301) | 14-day mortality rate, 11.7% (296); mortality dependent on site, with higher rates for vertebral osteomyelitis than for nonvertebral infections; independent predictor of reduced mortality relative to other infections in a single study, with an OR of 0.27 (95% CI, 0.08–0.93; P = 0.038) (133) |
| Prosthetic joint infection | 8.3 (296) | ||
| CNS infection | 25–56 (14, 126, 223, 228) | 10.7 (296) | Independent predictor of mortality relative to other infections in a single study, with an OR of 12.9 (95% CI, 1.1–152.9; P value not stated) (244) |
| Deep abscess | 14.6 (296) | ||
| Intra-abdominal infection | 25 (120) | ||
| Intravenous catheter (type not specified or several types) | 0–21 (14, 54, 113, 167, 169, 205, 235, 313) | 4–32.6 (120, 296, 301) | 14-day mortality rate, 12% (39); device with a metastatic infection associated with higher mortality rates (296); independent predictor of reduced mortality relative to other infections in a single study, with an OR of 0.47 (95% CI, 0.31–0.71; P < 0.01) (33) |
| Central venous catheter | 30 (291) | 20.5 (296) | |
| Hickman catheter | 22 (53) | ||
| Peripheral venous catheter | 16.9 (296) | ||
| Hemodialysis catheter | 6.8 (296) | ||
| Infective endocarditis | 22.4–66 (8, 15, 66, 106, 107, 167, 237, 242, 291) | 25–60 (29, 107, 206, 301) | IVDU-associated IE mortality rate, 11%; non-IVDU IE mortality rate of 21%, vs 29.4% for health care-associated IE (66); infection-related mortality rate, 22% (68); independent predictor of mortality relative to other infections in several studies, with an OR range between 2.8 and 12.1 (77, 168, 237, 296, 301) |
| Left-sided IE | 28.6 (197) | 23.9 (296) | Aortic valve IE was an independent predictor of mortality relative to mitral or right-sided IE, with an OR of 1.91 (95% CI, 1.0–3.66; P = 0.05) (197) |
| Right-sided IE | 5.9 (197) | 11.8 (296) | |
| Prosthetic valve IE | 30.5–50 (66, 242) | ||
| Cardiac device infection | 36.4 (28) | ||
| Pulmonary infection | 41.6–62 (14, 79, 167, 200) | 39–67 (120, 148, 296, 301) | 14-day mortality rate, 65% (39); attributable mortality rate, 46.5% (85); independent predictor of mortality relative to other infections in several studies, with an OR range of between 2.09 and 17.0 (33, 39, 41, 134, 206, 296, 301) |
| Skin and soft tissue infection | 10–18.6 (14, 167) | 14.8–17 (120, 296, 301) | |
| Surgical site infection | 0–23 (14, 291) | ||
| Urinary tract infection | 9.7 (14) | 10 (120) |
For the purpose of this review, no attempt has been made to differentiate between the various study diagnosis definitions, with source of infection, primary focus, or principal diagnosis being used interchangeably.
OR, odds ratio; CI, confidence interval; IVDU, intravenous drug user.
Within a diagnostic category or SAB source, the site and extent of infection influence outcomes, with higher mortality rates for patients with complicated left-sided IE (e.g., the presence of a paravalvular abscess) than for patients with uncomplicated left-sided IE (197). For SAB secondary to medical device infections, the type of device correlates with outcomes, with higher mortality rates associated with a central line (20.5%) than with a peripheral line (16.9%), a hemodialysis catheter (6.8%), or orthopedic implants (8.3%) (296). Patient characteristics further influence these outcomes, with intravenous drug use (IVDU)-associated IE being associated with improved survival compared to non-IVDU-associated IE (P < 0.001) (66). A review of these nuances with respect to presentations and mortality is beyond the scope of this review, as is a review of mortality data for S. aureus compared to other microorganisms.
The link between source and outcome was noted and employed by Soriano and colleagues, who stratified SAB episodes into one of three mortality risk groups by the final source of SAB. An incremental increase in the mortality rate was noted, from 5% to 13% and 30% for “low”-risk (intravenous catheter; urinary tract; ear, nose, and throat; and gynecological foci), “intermediate”-risk (bone and joint, soft tissue, and unknown foci), and “high”-risk (endovascular, lower respiratory tract, intra-abdominal, and central nervous system [CNS] foci) groups, respectively (275). In a subsequent prospective study of 414 MRSA-B episodes, the utility of these categories was confirmed, with 2- and 9-fold-increased odds of death for intermediate-risk (OR, 2.29; 95% CI, 1.21 to 4.31) and high-risk (OR, 9.49; 95% CI, 5.1 to 17.6) SAB diagnoses compared to low-risk SAB sources (273).
At presentation, the extent of S. aureus infection may not be obvious, with approximately one-third of patients developing a metastatic or complicated SAB infection (67, 161, 238). The clinical implication of the above-mentioned association is that for optimal patient outcomes, defining the extent of SAB is critical (40). Accordingly, several groups have proposed criteria based on clinical features and investigation results to recommend a duration of therapy (69, 125, 207). Of these, the criteria developed by Fowler and colleagues are the most well known and classify SAB episodes into one of three groups: complicated, uncomplicated, and simple SAB (69). Similar to the “risk” groups developed by Soriano et al., complicated SAB episodes have worse outcomes than do uncomplicated and simple episodes (24% versus 40% mortality; P < 0.01) (161).
Overall, the source and extent of SAB infection are important and consistent contributors to overall SAB mortality. This association confirms the need to consider SAB as not one single entity but a heterogenous group of infections in future studies. This may enable the refinement of source-specific management factors to be elucidated.
Setting of Bacteremia
The setting of SAB onset has traditionally been divided into two categories, health care associated (formerly nosocomial) and community acquired, when subsequent positive S. aureus blood culture bottles are obtained ≥48 h and within 48 h of hospital admission, respectively (102). With changes in the complexity of modern health care, community-onset infections are now further divided into episodes with health care contact (e.g., health care-associated outpatient) and those without (253). The setting of SAB assisted clinicians in predicting the infecting S. aureus clonal type and, consequently, antibiotic choice. However, with the advent of community-acquired MRSA (CA-MRSA) (defined by the antibiotic resistance pattern and/or staphylococcal cassette chromosome mec [SCCmec] type) strains entering the hospital, causing cross infections and replacing common hospital clones, these definitions are becoming less helpful (229).
Most current cohort studies have not found the setting of SAB to influence outcomes (218, 224, 275, 296, 301), with the exception of two studies, which observed increased 30-day mortality rates for hospital-onset SAB episodes (with one study reporting an OR of 1.75, a 95% CI of 1.05 to 2.92, and a P value of 0.033 [101] and the other reporting an OR of 1.42 and a 95% CI of 1.12 to 1.81 [134]). No typing data were presented in these studies, and therefore, these results should be interpreted with caution, as the setting of bacteremic onset may be a surrogate for a specific S. aureus clone. Regardless, it appears that the setting of SAB has a minimal effect on patient outcomes.
Persistent Bacteremia
Definitions of persistent bacteremia vary between studies and range from 3 days to up to 7 days following appropriate active antibiotic therapy (172, 220, 302). Regardless, persistent bacteremia is a common manifestation of SAB and occurs for between 6% and 38% of infection episodes (65, 93, 139, 210), with a median time to clearance of 7 to 9 days (65, 139, 210). Risk factors include the source of infection (i.e., infective endocarditis or vertebral osteomyelitis) (66, 163), pathogen phenotypes (vancomycin heteroresistance) (302), antibiotic treatment (139, 219), the presence or retention of prosthetic material (139), and the ability to remove foci of infection (e.g., by surgical drainage) (87, 320). MRSA has been associated with a greater likelihood of persistence (median time to clearance of 8 to 9 days) than MSSA (median time to clearance of 3 days) bacteremia (65, 163). This probably reflects suboptimal activity with vancomycin compared to β-lactams in preventing persistence (267). Alternatively, pathogen-specific factors may be responsible for this persistence (259).
Irrespective of the cause, bacteremic persistence probably is a surrogate for complicated SAB (69), as the likelihood of a metastatic infection increases with an increasing duration of bacteremia, to approximately 45% following ≥10 days of SAB (139, 210). These complicated infections in turn lead to poorer outcomes (170, 171). Even in the absence of metastatic complications, persistence per se portends a worse outcome, with infection-related mortality rates being higher for patients with MRSA-B persistence (>3 days) than for nonpersistent episodes (45.2% and 9.4%, respectively; P = 0.002) (323). Similar results were obtained by a larger case-controlled study, where mortality rates for persistent (>7 days) SAB were significantly higher than mortality rates for nonpersistent controls (54.8% and 31.4%, respectively; P < 0.01) (93). Only one cohort study of 177 SAB episodes established persistent bacteremia as being an independent predictor of mortality (OR, 17.5; 95% CI, 1.5 to 212; P = 0.024) (167).
Pathogen factors such as heteroresistance to vancomycin are associated with persistent bacteremia (OR, 2.37; 95% CI, 1.53 to 3.67; P < 0.01) (301). However, heteroresistant vancomycin-intermediate S. aureus (hVISA) bacteremic episodes do not lead to poorer outcomes (302) and may be associated with improved overall survival (301) compared to vancomycin-susceptible S. aureus (VSSA) bacteremic episodes. These complexities require further study, as it seems that not all persistent episodes translate into poorer outcomes. Nevertheless, overall persistent bacteremia generally results in worse outcomes secondary to the causes and complications that persistence usually indicates.
Bacteruria
S. aureus is an uncommon uropathogen in the absence of bladder catheterization, instrumentation, or surgery and thus typically represents hematogenous spread. The presence of concomitant S. aureus bacteruria and bacteremia thus probably represents a higher disease burden or complicated SAB and thus portends a worse outcome. This is supported by a retrospective cohort study of 118 SAB episodes, which detected an increased risk of death for patients with concomitant bacteruria (32%, versus 14% for no S. aureus bacteruria; P = 0.036) (225). In a subsequent case-controlled study of 308 SAB (42% MRSA) episodes, bacteruria (n = 68 episodes) was an independent predictor of SAB in-hospital mortality (OR, 2.87; 95% CI, 1.4 to 5.9; P = 0.004) (36). Similarly, Huggan et al. found that bacteruria was associated with a 2-fold-increased risk of death in SAB episodes (108, 109). Thus, bacteruria is a surrogate for severe disease and, hence, a signal for poorer outcomes.
Shock/Sepsis and Severity of Illness
The presence of sepsis or shock, although definitions vary slightly between studies, is strongly associated with worse outcomes for patients with SAB (Table 3), with mortality rates ranging between 38% and 86% (6, 59). The reason why the study by Holmes et al. did not observe this association is unclear (101), as the larger data set from which the data for this study were drawn found sepsis syndrome to be a strong independent predictor of 30-day all-cause mortality (OR, 4.01; 95% CI, 2.34 to 6.87; P < 0.001) (296). Possible explanations include differences in sepsis definitions and patient populations studied.
Table 3.
Summary of studies that have examined the impact of severity of illness on mortality in Staphylococcus aureus bacteremiaa
| Study and outcome of interest | No. of episodes | % of patients with sepsis/shock | Mortality rate for patients with shock (%) | Variable, OR (95% CI; P) | Reference |
|---|---|---|---|---|---|
| Studies that found severity of illness to be an independent predictor of mortality (multivariate analysis) | |||||
| Single-center retrospective Turkish study (1990–1994); infection-related mortality | 101 SAB | 23.8 | 41.7 | Septic shock, 5.4 (ND; 0.02) | 291 |
| Single-center retrospective Spanish study (1991–1998); in-hospital infection-related mortality | 908 SAB | 9.7 | 55.7 | Shock, 12.6 (7.2–22.2; ND) | 275 |
| Single-center prospective Spanish study (1991–2005); 30-day all-cause mortality | 414 MRSA-B | 20.3 | 63.1 | Shock, 7.38 (4.11–13.3; <0.001) | 273 |
| Single-center retrospective Brazilian study (1991–1992); 14-day all-cause mortality | 134 SAB | 22.1 | 73.3 | Shock, 8.92 (2.9–27.8; ND) | 39 |
| Single-center retrospective Belgian study (1992–1998); 30-day in-hospital mortality | 85 SAB | 62.2 | ND | Hemodynamic instability, 1.76 (1.14–2.71; 0.01); APACHE II per point, 1.04 (1.02–1.06; <0.01) | 18 |
| Single-center prospective Danish study (1994–1996); 150-day mortality | 278 SAB | 25 | 47 | Septic shock, 3.7 (1.5–9.1; 0.004) | 127 |
| Single-center retrospective U.S. study (1995–1999); 30-day all-cause mortality | 293 SAB | 31.4 | 41.3 | Modified APS >60, 15.7 (5.8–49.8; <0.001) | 206 |
| Single-center retrospective U.S. study (1996–2006); all-cause mortality | 489 MRSA-B | Not applicable | Not applicable | SAPS, 1.51 (1.26–1.80; <0.001) | 202 |
| Single-center retrospective Taiwan study (1997–2001); 30-day infection-related mortality | 162 MRSA-B | 13.6 | 86.4 | Septic shock, 9.31 (2.35–36.8; <0.01) | 59 |
| Single-center retrospective Australian study (1997–2008); 30-day all-cause mortality | 401 MRSA-B | 10.5 | 52.4 | APACHE II per point, 1.11 (1.07–1.15; <0.001) | 301 |
| Single-center retrospective Swiss study (1998–2002); in-hospital all-cause mortality | 308 SAB | 10.7 | ND | Septic shock, 14.5 (6.2–61.6; <0.01) | 131 |
| Single-center retrospective Brazilian study (2000–2001); infection-related mortality | 101 SAB | 43.5 | 68.2 | Severe sepsis/shock, 6.68 (3.05–15.4; <0.01) | 89 |
| Single-center retrospective Chinese study (2001–2007); 90-day all-cause mortality | 115 MRSA-B | 11.3 | ND | Septic shock, 7.92 (3.64–17.20; <0.001) | 32 |
| Single-center retrospective Taiwan study (2001–2006); in-hospital infection-related mortality | 177 MRSA-B | Not applicable | Not applicable | Pitt bacteremia score per point, 1.33 (1.04–1.69; 0.024) | 167 |
| Single-center retrospective Taiwan study (2001–2007); 90-day all-cause mortality | 744 SAB | 11.3 | ND | Septic shock, 7.92 (3.64–17.20; <0.001) | 33 |
| Single-center retrospective Belgian study (2002–2004); infection-related in-hospital mortality | 154 SAB | 21.4 | ND | Septic shock, 10.26 (3.65–28.8; <0.001) | 168 |
| Single-center retrospective U.S. study (2003–2008); in-hospital mortality | 814 SAB | Not applicable | Not applicable | SAPS, 1.04 (1.03–1.05; not stated) | 257 |
| Single-center prospective U.S. study (2005–2006), 30-day all-cause mortality | 253 SAB | Not applicable | Not applicable | Illness severity index,b 2.78 (1.94–3.99; <0.001) | 77 |
| Single-center retrospective Taiwanese study (2006–2008); 30-day all-cause mortality | 253 MRSA-B | 41.9 | ND | Septic shock, 8.11 (4.06–16.19; <0.001) | 309 |
| Single-center retrospective Taiwanese study (2004–2006); 30-day all-cause mortality | 215 SAB | 39.9 | ND | Shock, 8.11 (4.06–16.09; <0.001) | 310 |
| Multicenter retrospective European study (2007); 30-day all-cause mortality | 334 SAB | 37.7 | 38.9 | Severe sepsis/shock, 2.68 (1.52–4.75; <0.01) | 6 |
| Multicenter prospective Australian study (2007–2008); 30-day all-cause mortality | 1,865 SAB | 10.8 | 40.3 | Sepsis syndrome, 4.01 (2.40–6.87; <0.001) | 296 |
| Studies where severity of illness was not an independent predictor of mortality (multivariate analysis) | |||||
| Multicenter prospective Australian study (2007–2008); 30-day all-cause mortality | 532 SAB | 12.6 | ND | Sepsis syndrome, not stated | 101 |
Studies employed a multivariate logistic regression model.
OR, odds ratio; CI, confidence interval; P, probability; ND, not described; APACHE, acute physiological and chronic health evaluation; APS, acute physiological score (based on APACHE III); SAPS, simplified acute physiological score.
The illness severity index is a composite scoring system based on the Charlson comorbidity index and a modified acute physiological score.
The use of the acute physiological and chronic health evaluation (APACHE) score (144) for SAB has been met with some criticism (91), as this score was developed and validated for use in intensive care units (ICUs). Nevertheless, the APACHE score (APACHE II or APACHE III), based on the worst parameters within the first 48 h of the positive blood culture, has consistently been established as an independent predictor of mortality, with an incremental increase in mortality rates as scores increase (204, 301, 324). Multiple other scoring systems have been developed, all of which have been shown to be predictors of mortality (167, 202). These scoring systems include the simplified acute physiological score (SAPS), the sequential organ failure assessment score (SOFA), the Pitt bacteremia score, and the multiple organ dysfunction score (MODS).
Single acute organ dysfunction, especially acute renal failure (15, 18, 131); respiratory failure requiring mechanical ventilation (308); altered mental status (82); and hematological dysfunction (33, 134, 310, 312) portend a worse outcome, albeit inconsistently, with SAB. Alternatively, surrogate markers for infection severity such as transit to an ICU (301) or the acquisition of SAB in the ICU (6, 237, 261, 275) have been found to be independent predictors of mortality compared to ward patients. Thus, the severity of SAB, irrespective of the method used to measure this, is one of the more consistent predictors of overall 30-day mortality.
PATHOGEN FACTORS
Methicillin Resistance
Resistance to most β-lactam antibiotics, including the semisynthetic (β-lactamase-resistant) penicillins, such as methicillin and flucloxacillin, is due to the expression of the low-affinity penicillin binding protein PBP2a (92). PBP2a is encoded by the mecA gene and is found on an integrated mobile genetic element called the staphylococcal cassette chromosome mec (SCCmec) element (116, 136, 190, 272).
In many hospital settings over the past 20 years, the incidence of MRSA-B has increased around the world. This increase has not been associated with a corresponding decline in MSSA infections but has added to the overall burden of SAB (21, 322). Numerous studies spanning the last decade have examined the impact of methicillin resistance on mortality in SAB, with conflicting results. Only studies employing multivariate analyses and which were not included in the two meta-analyses mentioned below are summarized in Table 4. Two meta-analyses were performed, by Whitby et al. (317) and Cosgrove et al. (42), in 2001 and 2003, respectively. Although the time periods for study capture were very similar, the numbers of studies included differed greatly due to the fact that Whitby et al. limited their analyses to nosocomial SAB episodes only. Cosgrove et al. examined data from 31 independent studies (1980 to 2000) and showed that mortality rates were significantly higher for MRSA than for MSSA bacteremic episodes (OR, 1.93; 95% CI, 1.54 to 2.42; P < 0.001). Similarly, Whitby et al. detected an increased mortality rate with methicillin resistance (OR, 2.12; 95% CI, 1.76 to 2.57; P < 0.001) from the nine studies included (1978 to 2000). It was suggested that the major weakness of the studies examined in the meta-analyses by Whitby et al. and Cosgrove et al. is that they ignored the length of hospital stay prior to the onset of bacteremia (110, 111), which leads to a time-dependent bias, the significance of which was demonstrated by a study by Wolkewitz et al. (321), as the MRSA mortality rate was similar to that for MSSA episodes following an adjustment for the length of hospital stay. However, these data may themselves be imperfect secondary to the small numbers of MSSA-B (n = 26) and MRSA-B (n = 34) cases detected.
Table 4.
Summary of studies that have examined the impact of methicillin resistance on mortality in Staphylococcus aureus bacteremiaa
| Study | No. of MRSA isolates/no. of MSSA isolates | Mortality rate for MRSA vs MSSA (%) | Mortality risk for MRSA vs MSSA | Adjustment(s) for confounders | Reference |
|---|---|---|---|---|---|
| Meta-analyses | |||||
| Meta-analysis of 9 studies (1990–2000); mortality | 778/1,431 | 29 vs 12 | Pooled RR, 2.03 (95% CI, 1.55–2.65; P < 0.001) | None | 317 |
| Meta-analysis of 31 studies (1980–2000); mortality | 1,360/2,603 | Not stated | Pooled OR, 1.93 (95% CI, 1.54–2.42; P < 0.03) | Varied between studies analyzed | 42 |
| Studies that found MRSA to be an independent predictor of mortality (multivariate analysis) | |||||
| Single-center retrospective Taiwanese study (1990–2004); 30-day mortality | 851/297 | 49.8 vs 27.6 | OR, 1.78 (95% CI, 1.3–2.44; P < 0.001) | None | 308 |
| Single-center retrospective Belgian study (1992–1998); 30-day mortality | 47/38 | 63 vs 18 | HR, 1.93 (95% CI, 1.18–3.18; P < 0.01) | None | 18 |
| Multicenter retrospective U.S. study (1995–2003); 90-day infection-related mortality | 184/235 | 34.2 vs 19.6 | HR, 1.8 (95% CI, 1.2–3.0; P < 0.01) | Patients with pneumonia | 266 |
| Single-center retrospective (2002–2004) and prospective (2005-2007) German study; 90-day all-cause mortality | 67/454 | 42 vs 19 | OR, 2.6 (95% CI, 1.4–4.9; P < 0.01) | None | 237 |
| Single-center retrospective Belgian study (2002–2004); infection-related in-hospital mortality | 66/88 | 52 vs 32 | OR, 3.04 (95% CI, 1.15–8.04; P = 0.021) | None | 168 |
| Multicenter prospective Asian study (2004–2006); 30-day mortality | 329/380 | 33.1 vs 17.1 | OR, 1.69 (95% CI, 1.15–2.49; P < 0.01) | None | 133 |
| Single-center retrospective United Kingdom study (2005–2006); 90-day mortality | 34/26 | 35.3 vs 19.2 | Time-averaged HR of 1.69 (95% CI, 0.72–4) | Age, gender, comorbidities, hospitalization (first admission, time averaged) | 321 |
| Studies that found MRSA not to be an independent predictor of mortality (upon multivariate analysis) | |||||
| Single-center retrospective U.S. study (1991–2000); infection-related mortality | 170/183 | 30.6 vs 15.3 | OR, 1.4 (95% CI, 0.7–3; P = 0.4) | Age, site of infection, APACHE score, ICU at onset, hospital onset, and delayed therapy | 175 |
| Single-center retrospective Canadian study (1991–2005); 30-day mortality | 69/746 | 33 vs 23 | OR, 2.21 (95% CI, 0.99–4.96; P value ND) | Age, gender, comorbidities, residence, absence of treatment, site of infection | 5 |
| Single-center prospective United Kingdom study (1995–2000); infection-related mortality | 382/433 | 29.6 vs 13.6 | OR, 1.72 (95% CI, 0.92–3.2; P = 0.09) | Age, hospital specialty, primary site of infection | 195 |
| Single-center retrospective French study (1997–1998); infection-related mortality | 30/69 | 43.3 vs 20.3 | OR, 2.8 (95% CI, 0.99–7.1; P value ND) | Appropriate treatment within the first 72 h | 286 |
| Single-center prospective U.S. study (1997–2000); in-hospital mortality | 96/252 | 22.9 vs 19.8 | OR, 0.72 (95% CI, 0.39–1.96; P = 0.45) | None | 41 |
| Multicenter retrospective United Kingdom study (1997–2004); 30-day mortality | 227/214 | 34.0 vs 27.0 | OR, 1.49 (95% CI, 0.99–2.26; P value ND) | None | 322 |
| Single-center retrospective Brazilian study (2000–2001); infection-related mortality | 61/50 | 54.9 vs 24.7 | HR, 3.52 (95% CI, 0.96–6.60; P = 0.06) | Adequacy of therapy, gender, age, severity of clinical status and underlying illness | 89 |
| Single-center prospective Taiwanese study (2001–2006); 30-day all-cause mortality | 30/185 | 10.0 vs 13.2 | OR, 0.74 (95% CI, 0.22–2.45; P value ND) | None | 310 |
| Multicenter prospective Asian study (2004–2006); 30-day mortality | 2007/1701 | 16.2 vs 10.8 | Not stated | Age, comorbidities, source of infection, health care onset | 134 |
| Single-center retrospective U.S. study (2004–2008); patients ≥80 yr of age; in-hospital mortality | 46/30 | 34.8 vs 20.0 | OR, 2.1 (95% CI, 0.7–6.3; P = 0.16) | None | 15 |
| Multicenter prospective Australian study (2007–2008); 30-day all-cause mortality | 450/1,415 | 30.0 vs 17.7 | OR, 1.04 (95% CI, 0.58–1.86; P = 0.89) | None | 296 |
Other sources of criticism include the inclusion of studies that failed to adjust for other confounding factors such as comorbidities, age, and severity of illness (224, 276). For example, in a large (n = 815) single-center prospective United Kingdom study (1995 to 2000) by Melzer et al. (195), MRSA-B was not associated with increased mortality after adjustments for the above-mentioned host confounders (OR, 1.72; 95% CI, 0.92 to 3.2; P = 0.09). Conversely, adjusting for these variables may nullify the true impact of resistance (276). Nevertheless, when Cosgrove et al. analyzed only studies that included adjustments for potential confounders (such as age, gender, and severity of illness), the mortality rate remained higher for MRSA-B episodes (OR, 1.88; 95% CI, 1.33 to 2.69; P < 0.001) (42). Similarly, in a cohort study of 753 community-onset SAB episodes (33% MRSA), methicillin resistance remained an independent predictor of mortality when data were stratified by comorbidities or adjusted for confounders in the multivariate logistic regression model (33).
Alternative study designs have attempted to address the significance of methicillin resistance. Harbarth et al. performed a case-controlled study with matching based on previously identified confounders (91), while in a Korean case-controlled study, matching occurred by the use of a propensity score (218). Both studies were unable to detect a difference in mortality for MRSA-B compared to MSSA-B.
Despite these potential weaknesses, the majority of studies summarized in Table 4 support an increased mortality rate associated with MRSA-B (in comparison to MSSA-B). Several explanations for this apparent association have been provided. These include pathogen-specific factors such as SCCmec-associated virulence factors (3), with SCCmec type II being an independent predictor of mortality in a cohort study of 253 SAB (64% MRSA) (77) and 744 SAB (33% MRSA) (33) episodes. Alternatively, differences in empirical prescribing (243) and poor vancomycin efficacy (3, 86, 114) may explain some of these differences. Compared to semisynthetic penicillins, vancomycin has slower bactericidal activity in vitro, especially with high-inoculum infections (158, 252), and variable tissue penetration (2, 88, 157). Finally, MRSA infection may just be a surrogate for host factors such as comorbidities rather than methicillin resistance per se (91). Ultimately, more research is required to clearly define the reasons, which are likely to be multifactorial, for the observed mortality differences.
Vancomycin MIC
The current Infectious Diseases Society of America MRSA-B guidelines continue to recommend vancomycin as the treatment of choice for susceptible MRSA-B isolates (172), with vancomycin-susceptible breakpoints set by the Clinical and Laboratory Standards Institute (CLSI). These breakpoints were lowered in 2006 from an MIC of 4 μg/ml to an MIC of 2 μg/ml following reports of increased mortality associated with vancomycin-intermediate S. aureus (VISA) infections (72, 288).
However, questions remain regarding whether or not these breakpoints should be lowered further, thus limiting the role of vancomycin in the treatment of MRSA-B (47, 198). This controversy is in part maintained by conflicting reports of treatment failure and increased mortality with high- but susceptible-MIC isolates (i.e., an MIC of ≤2 μg/ml). For a more complete examination, including other variables that influence decisions about the use of vancomycin in MRSA-B management, readers are directed to the above-mentioned two publications.
The first study to demonstrate episodes of vancomycin treatment failure with a high MIC (≥1.5 μg/ml by the Etest) was reported in 2006 by Hidayat et al. (97). Subsequently, two other groups observed similar results (173, 181), despite the differing treatment failure definitions and MIC testing methodologies used (Etest and Vitek, respectively).
Although a mortality difference with high-MIC (by Etest) compared to low-MIC episodes was observed in a study by Musta et al., the significance of these results was questioned by the authors themselves, as this association was not upheld on multivariate analysis (202). Soriano et al., in their single-center study of MRSA-B (n = 414), did, however, observe an increased mortality rate with high-MIC (2 μg/ml by Etest) episodes (OR, 6.39; 95% CI, 1.68 to 24.3; P < 0.01) albeit for empirically vancomycin-treated patients only (273). These data were subsequently criticized, as the MIC was an independent predictor of mortality only when shock, which occurred less frequently in high-MIC episodes, was adjusted for (177, 230). However, those authors maintained that shock was a confounding variable rather than an intermediate variable and thus should be corrected for (274). Subsequently, three further studies found a high MIC (≥1.5 μg/ml) to be an independent predictor of mortality (with one study reporting an OR of 2.39, a 95% CI of 1.2 to 4.8, and a P value of 0.014 [312], another study reporting an OR of 6.05, a 95% CI of 2.3 to 15.93, and a P value of 0.001 [285], and a further study reporting an OR of 2.44, a 95% CI of 1.21 to 4.92, and a P value of 0.012 [101]) compared to low-MIC episodes. Of note, a study by Wang et al. was the first to use broth microdilution to determine MICs (312), while a study by Holmes et al. found that the vancomycin MIC also predicted mortality for flucloxacillin-treated MSSA episodes (101).
However, these findings are by no means universal, as no association of mortality with increasing vancomycin MICs was noted in one of the largest retrospective single-center studies of SAB (n = 814) (258). Comparable results were observed by two other retrospective single-center studies (154, 210). The impact of a high MIC is further questioned by the findings of a small study of 49 SAB episodes (MRSA and MSSA), which showed improved survival with high-MIC (≥1.5 μg/ml by the Etest) episodes (232). These studies have likewise come under scrutiny, with contrary findings explained by the inclusion of nontreated patients (258) or vancomycin-treated MSSA episodes in the analysis (232, 258).
Overall, the majority of studies point to a possible increased mortality rate for MRSA-B episodes with high but susceptible vancomycin MICs. As MICs were almost exclusively determined by the Etest, a lowering of the breakpoints is not currently warranted, as the correlation between results of the Etest and the gold standard, broth microdilution (used to set breakpoints), is moderate at best (300). The clinical significance of this association remains unclear, as it may not reflect vancomycin failure per se but pathogen-related characteristics (100), especially in light of recent findings of observed increased mortality rates associated with high-vancomycin-MIC, flucloxacillin-treated MSSA episodes (101). Pending further studies, treatment recommendations continue to support vancomycin use irrespective of the MIC, with changes to therapy guided by the clinical responses (172).
Vancomycin Resistance
Heteroresistant vancomycin-intermediate S. aureus.
Since the first description of hVISA in 1997 by Hiramatsu and colleagues (99), questions remain regarding the best method to detect this phenotype. The current gold standard is the modified population analysis profile-area under the curve (PAP-AUC) method, in which the presence of heteroresistance is confirmed when the ratio of the number of viable colonies plotted against increasing vancomycin concentrations of the test strain to that of the reference strain (Mu3) is ≥0.9. For greater details pertaining to various laboratory detection methods and other factors, including hVISA epidemiology, pathogenesis, and mechanisms of resistance, readers are referred to two recent reviews (104, 302).
Several studies have subsequently examined the impact of hVISA bacteremia compared to vancomycin-susceptible S. aureus (VSSA) episodes. The first study reported was from a single Australian center in 2004, which observed no increased mortality with hVISA (by PAP-AUC) episodes (30). Nevertheless, the rate of mortality of hVISA bacteremia cases was found to be high, at approximately 33%, in a retrospective review of 25 confirmed (by PAP-AUC analysis) hVISA episodes (105). This high mortality rate was confirmed by Maor et al., who detected an in-hospital mortality rate of 75% for 16 confirmed (by the macromethod Etest [MET]) hVISA bacteremic infections from 264 screened MRSA-B episodes (186).
That same group, however, did not detect a 30-day overall mortality difference in their later retrospective comparative study of 250 MRSA-B episodes (51% for hVISA versus 46% for VSSA; P = 0.48) (185). Similar results were seen in a small single-center retrospective review of 56 persistently bacteremic (for more than 7 days) MRSA episodes (63).
In 2009, Musta et al. reported the largest study of hVISA bacteremic episodes (202). In this single-center retrospective study of 489 MRSA-B episodes, 71 (17%) hVISA (confirmed by MET) episodes were detected. No outcome differences were observed between the two groups. Similarly, no increased mortality rate was detected with hVISA (confirmed by PAP-AUC analysis) episodes in a retrospective review of 65 MRSA infective endocarditis patients by the International Collaboration of Endocarditis (ICE) (8).
In contrast, an Australian retrospective study detected a reduced mortality rate with hVISA (confirmed by PAP-AUC analysis) for 401 consecutive MRSA-B episodes over a 12-year period (301). The hVISA phenotype was an independent predictor of reduced mortality (OR, 0.27; 95% CI, 0.09 to 0.83; P = 0.02). Possible explanations for these conflicting findings include the detection of hVISA exclusively in ST239-like MRSA clones and the higher number of hemodialysis patients in the hVISA group.
Overall, hVISA bacteremia is not associated with an increased mortality rate (OR, 1.18; 95% CI, 0.80 to 1.72; P = 0.4) (302) despite hVISA being associated with persistent bacteremia (8, 30, 209, 302), high-bacterial-load infections (e.g., infective endocarditis) (30), and high vancomycin MICs (104, 303), as the likelihood of detection of hVISA increases with rising MICs, with the greatest chance occurring with isolates exhibiting an MIC of 2 μg/ml. The failure to detect a mortality difference may reflect the reduced virulence that was demonstrated in vitro for these isolates (191). Clinical data are lacking but are suggested by the lower rates of shock with infections with high-vancomycin-MIC isolates (154, 273), the lower risk of acquiring hVISA infections than VSSA infections (103), and the lower mortality rates detected in one study (301).
Vancomycin-intermediate S. aureus.
An MRSA isolate is currently defined as being a VISA isolate if the vancomycin MIC is between 4 and 8 μg/ml. These changes occurred in 2006, when the vancomycin breakpoints were lowered from 8 to 16 to 4 to 8 μg/ml for “intermediate” resistance (288). Part of the rationale for doing so was secondary to the findings of a case-controlled study by Fridkin et al. in 2003. In that study, S. aureus isolates with reduced vancomycin susceptibility (RVS) or VISA isolates by the current breakpoints were significantly associated with increased mortality rates compared to vancomycin-susceptible infections (63% versus 12%; OR, 12.7; 95% CI, 3.4 to 48) (72, 73). Despite changes to the breakpoints, VISA remains uncommon (72), and thus, no other large-scale comparative data with respect to VISA outcomes have been reported. Regardless, mortality is generally considered to be increased secondary to vancomycin failure, and as such, alternative antibiotics are recommended for the treatment of VISA infections (172).
Vancomycin-resistant S. aureus.
Vancomycin-resistant S. aureus (VRSA) infections have been limited to a few cases (4, 62, 249, 268). Most have occurred in the United States and have not been associated with bloodstream infections. Thus, the impact of VRSA on mortality in SAB is unknown.
Exotoxins
Secreted toxins (exotoxins) produced by S. aureus contribute to pathogenicity and the ability to colonize the host. These toxins can broadly be categorized as either superantigens (SAgs) or cytotoxic exotoxins. Although there are few studies that have directly examined the impact of exotoxin production on mortality in bacteremic patients, clues to the potential role that exotoxins play in mortality can be garnered from virulence studies and animal models.
Superantigens.
Staphylococcal SAgs include the staphylococcal enterotoxin serotypes (e.g., SEA to SEE, SEG, and SEI), the staphylococcal enterotoxin-like serotypes (e.g., SEl-H and SEl-J to SEI-V), and the distantly related toxic shock syndrome toxin 1 (TSST-1) (formerly serotype SEF) (255). The majority of isolates do not produce SAgs (132), while 90% of isolates that do generally produce more than one enterotoxin (48). Combinations observed include SEA and TSST-1 in 12% of strains, and SEG, SEI, SEN, SEO, and SEM occur together in 100% of strains, as these are carried on the same gene cluster. Conversely, certain toxin combinations remain rare (e.g., SEA and SEB) (123, 132).
SAgs represent a large family of biologically and genetically related toxins that are pyrogenic, cause T-cell proliferation, and induce the release of proinflammatory cytokines (189, 193). Unlike conventional antigens, which stimulate <0.01% of the T-cell population, picomolar concentrations of SAgs are able to stimulate 5 to 30% of the T-cell population (147). The magnitude and speed with which T-cell activation occurs and the accompanying cytokine induction can lead to shock (329).
As shock is a strong independent predictor of mortality in patients with SAB, it is reasonable to assume that isolates expressing these exotoxins would result in greater mortality. However, studies of patients with SAB found similar distributions of enterotoxins (SEA to SEG, SEH, SEI, and TSST-1) in strains isolated from patients who did survive and those who did not survive (48, 55, 241). Furthermore, a prospective study by Desachy et al. found no significant difference (OR, 1.60; 95% CI, 0.34 to 7.59; P = 0.55) between the numbers of enterotoxin-producing strains isolated from surviving (70%; 16/23) and deceased (79%; 11/14) community-onset MSSA-B patients (48). Additional studies using a mouse septic model found no correlation between strains producing SEA, SEB, SEC, SED, or TSST-1 and mouse lethality (287).
Although enterotoxins play an important role in staphylococcal virulence, clinical data do not indicate that the presence of one or more of these factors impacts outcomes.
Cytotoxic exotoxins.
Almost all S. aureus strains produce cytotoxic exotoxins. These toxins are able to disrupt host cells and evade host immune responses. Of the wide variety of staphylococcal cytotoxic exotoxins produced, the most clinically important ones are the hemolysins (alpha-, beta-, gamma-, and delta-hemolysins), which have hemolytic activity toward human erythrocytes and have both dermonecrotic and neurotoxic effects (52).
(i) Alpha-hemolysins.
The presence of alpha-hemolysin, measured by a zone of hemolysis using supplemented nutrient agar, was significantly associated with complicated bacteremia (P = 0.024) but not mortality in a prospective study of 96 SAB infections (118).
(ii) Beta-hemolysins.
A human study by Jin found that bacteremias caused by S. aureus strains with a disrupted beta-hemolysin (hlb) gene (93/161; 58%), due to an insertion of a phage, were associated with a 4.3-fold decrease in mortality rates (129). While this may suggest that beta-hemolysin production increases the risk of mortality, previous mouse and rabbit models of ocular infections and septic arthritis have shown that beta-hemolysin plays a negligible role in S. aureus virulence (44, 213).
(iii) Gamma-hemolysins.
Gamma-hemolysin is a unique three-gene (hlgABC) two-component hemolysin (60, 183). A mouse bacteremic model showed a 20% decrease in mortality, on average, in mice infected with an hlgABC deletion mutant of a USA300 strain, LAC (LAC ΔhlgABC), compared to the wild-type strain at 72 h (183). However, the survival rates after LAC ΔhlgABC infection following phagocytosis did not differ compared to those after infection with the wild-type strain. In addition, the ability of gamma-hemolysin to cause pore formation in human neutrophils was significantly inhibited in the presence of 20 to 50% human serum or plasma. As hlgABC expression is dependent on specific host environments, growth conditions, and/or the disease state, the authors suggested that although gamma-hemolysin may contribute initially to immune evasion or survival in the blood, it plays a minor role in virulence and overall mortality.
(iv) Delta-hemolysin and the accessory gene regulator.
The accessory gene regulator (agr) is a global regulon comprised of two transcripts, RNA II and RNA III. RNA II encodes agrDBCA, which is involved in generating autoinducing peptides (AIPs) for quorum sensing (178). AIPs also induce the expression of the other agr components. RNA III has multiple functions, including coding for delta-hemolysin and controlling the expressions of various exotoxins (e.g., hemolysins, enterotoxins, SEB, SEC, and TSST-1), virulence factors, and housekeeping genes (34). The production of delta-hemolysin can thus be used as a semiquantitative assay for agr function (292).
agr dysfunction has been associated with reduced killing by innate host defense cationic peptides (70, 250) and persistent (duration of ≥7 days) rather than resolving bacteremia (71.4% and 38.9%, respectively; P = 0.057) (70). In addition, antibiotic efficacy, especially vancomycin bactericidal activity, is reduced with agr-dysfunctional isolates (245, 293, 294). All these factors combined probably explain the findings of a recent study by Schweizer et al., who detected an increased 30-day in-hospital mortality rate (RR, 1.52; 95% CI, 1.05 to 2.21; P = 0.03) with agr-dysfunctional SAB (60% MRSA-B) episodes (258). This association was no longer significant following adjustments for age, comorbidities, severity of illness, and methicillin resistance but remained an independent predictor of 30-day mortality in severely ill patients when data were stratified by severity of illness (modified APACHE III score of >28), thus suggesting that agr dysfunction is likely to impact patients who most require an intact immune system and optimal antibiotic efficacy.
Based on the sequence diversity at this locus, 4 agr groups (groups I to IV) have been recognized (179). Several studies have examined the associations between the agr group and mortality, with conflicting results. Increased mortality was noted for agr non-group II (199) and group II (258) isolates, while no mortality difference between agr groups was noted in two studies (49, 120). These discrepancies may be explained in part by the considerable geographical variation in the agr group distribution (37 to 92% for group I, 4 to 50% for group II, 0 to 34% for group III, and 0 to 5% for group IV) among S. aureus isolates obtained from colonized and infected individuals (120, 251, 258, 264, 304).
(v) Panton-Valentine leukocidin.
Like gamma-hemolysin, Panton-Valentine leukocidin (PVL) is a two-component pore-forming toxin (231) with cytolytic activity toward human neutrophils, macrophages, and monocytes (117). The genes encoding PVL, lukS-PV and lukF-PV, are known to be harbored by seven distinct temperate bacteriophages which can be acquired by S. aureus (20). The prevalence of PVL in S. aureus strains varies widely across the world (0 to 50%), and PVL is typically found in community-associated MRSA and MSSA isolates (16, 57, 121, 149, 153).
In vitro studies exposing human neutrophils to the supernatants of isogenic PVL-positive strains and also a PVL-negative strain, grown under conditions that promoted PVL upregulation (i.e., casein-hydrolysate-yeast extract [CCY] medium) (183), have provided conflicting evidence for the role that PVL plays. Neutrophil pore formation was observed in both samples treated with the supernatants from the PVL-positive and PVL-negative strains albeit 25 min later. This suggests that in some strains, there may be functional redundancies that exist, whereby other hemolysins (e.g., alpha-hemolysin) may be involved with disease progression (183). PVL did not affect the survival time (3 to 4 days) in a rabbit bacteremia model (51) despite rabbit neutrophils having similar susceptibilities to the effects of PVL as human neutrophils (217).
The likelihood of developing bacteremia is not affected by the presence of PVL (121, 203), with MRSA-B rates being similar in patients with intranasal MRSA, irrespective of PVL carriage (16). PVL is associated with diseases such as skin and soft tissue infections (22), osteoarticular infections (137), and necrotizing pneumonia (78). With the exception of necrotizing pneumonia (79), PVL has not been found to be associated with increased mortality rates, including SAB (16, 212, 311, 314). Potential reasons for this include the high likelihood of a skin and soft tissue source for SAB (203, 212, 290, 314) and the predominance of PVL-associated disease occurring in younger patients (9, 203). PVL does not seem to augment already-established infections, including bacteremias (153), but is considered important in the early stages of establishing infections (16, 50, 51) or evading an initial innate immune response by lysing neutrophils (22, 51) and is therefore unlikely per se to influence mortality. Nevertheless, the role and contribution of PVL to pathogenesis, morbidity, and mortality remain a controversial area subject to much study.
Overall, the data point to a possible role for various exotoxins in influencing outcomes in SAB. These effects probably result from a complex interplay between toxin expression (concentration and duration) and host immune responses (287), which in turn influence clinical manifestations and subsequent outcomes. However, these complexities are unlikely to be teased apart in the foreseeable future secondary to the multiple confounders (246), and thus, the impact of toxins remains largely speculative based on in vitro and animal data.
S. aureus Clonal Types
The application of various typing methodologies, such as pulsed-field gel electrophoresis (PFGE) and multilocus sequence typing, has shown that S. aureus has a varied clonal population structure. Few studies have examined the impact of different clonal types on mortality in SAB. A single-institution prospective U.S. study of 116 MRSA-B episodes found that USA300 isolates typed by PFGE (34%) were associated with a reduced mortality rate compared to rates associated with USA100 and USA500 isolates upon a univariate analysis only (8%, 29%, and 36%, respectively) (261). The most likely explanation for this finding is that USA300 isolates infected younger patients, with skin and soft tissue infections as the predominant source of SAB. This may change over time as USA300 becomes more established in health care settings (229). A more recent retrospective multicenter U.S. study (2005 to 2008) examined case data for 1,104 typed MRSA-B isolates (138). In contrast to data from the previous study, a multivariate analysis revealed that the USA300 type (37.5% of 1,104 isolates) was an independent predictor of mortality compared to non-USA300 PFGE types after adjustment for age (hazard ratio [HR], 1.63; 95% CI, 1.19 to 2.23; P = 0.002). Although animal data support increased virulence with USA300 strains (166), clinical data remain limited. Clearly, more research is required in this area to determine if various S. aureus clones have a definitive association with increased mortality in SAB.
MANAGEMENT
Antibiotic Choice
The choice of antibiotic is important, especially for the treatment of MSSA-B episodes. Cefazolin, a narrow-spectrum cephalosporin, has been used for the treatment of MSSA-B since the 1970s. The role of this agent was questioned, however, following case reports of treatment failures, suggesting a reduced efficacy compared to the efficacy of antistaphylococcal penicillins (23, 234). Potential explanations for this included an increased susceptibility of cefazolin to the inoculum effect (208) and to staphylococcal β-lactamases (248). No randomized control trial comparing these agents has been performed to definitively answer questions regarding relative efficacies. A single-center cohort study comparing cloxacillin (n = 281) with cefazolin (n = 71) for MSSA-B infections detected no mortality or outcome difference between the two agents (222). Baseline patient characteristics differed significantly between the two groups, and this probably influenced the clinicians' initial antibiotic selections, thus limiting possible conclusions. In a recent propensity-score-matched, case-control study, where the nafcillin supply was interrupted for a 2-year period (i.e., cefazolin period), correction for clinicians' antibiotic choices was able to occur (162). Although no mortality difference or treatment failure was documented at the end of therapy (4 weeks), the low number of episodes (41 in each arm) and the infrequent high-inoculum infections (infective endocarditis, n = 2) likewise limit the conclusions of this study. Thus, questions remain about the comparative efficacies of these agents.
For the treatment of MSSA-B infections, the 30-day mortality rate was higher for patients receiving empirical treatment (i.e., commenced within the first 48 h) with a third-generation cephalosporin (OR, 2.24; 95% CI, 1.23 to 4.08; P = 0.008) or β-lactam–β-lactamase inhibitor combinations (OR, 2.68; 95% CI, 1.23 to 5.85; P = 0.013) than for patients receiving cloxacillin or cefazolin (222). In that same study, no conclusions could be made for definitive MSSA-B therapy (given from days 3 to 9, i.e., the first week after the receipt of blood culture results), as the numbers of patients treated with broad-spectrum β-lactam agents were too small compared to the numbers of patients treated with cloxacillin or cefazolin.
Vancomycin has consistently been associated with increased rates of treatment failure and high mortality rates compared to β-lactams when used for the management of MSSA-B (29, 85, 139, 140, 174, 281). In two cohort studies, vancomycin treatment was significantly associated with infection-related mortality upon a multivariate analysis (with one study reporting an OR of 14.5 and a 95% CI of 1.43 to 146.64 [85] and the other study reporting an OR of 3.3, a 95% CI of 1.2 to 9.5, and a P value of 0.02 [140]). This association remained in the latter study, even after adjusting for other variables, including the likelihood of receiving vancomycin. Further evidence for suboptimal vancomycin treatment responses came from a large prospective cohort study of 1,865 SAB episodes, where glycopeptide use and not antibiotic resistance (i.e., MRSA) was an independent predictor of mortality (OR, 1.64; 95% CI, 1.08 to 2.48; P = 0.02) (296). This difference in outcomes may persist even after replacing vancomycin treatment once susceptibility results become available for patients with endocarditis (174). All groups, including hemodialysis patients, seem equally susceptible to treatment failures with vancomycin (281). Possible reasons for these poor outcomes with vancomycin include slower bactericidal activity, especially in high-inoculum infections (26, 158, 252, 271), and variable tissue penetration (2, 88, 157). Vancomycin is thus considered a suboptimal therapy for MSSA-B compared to semisynthetic penicillins.
For the treatment of MRSA-B, vancomycin remains the treatment of choice, with daptomycin as an alternative, except for patients with left-sided endocarditis (172). This recommendation is based on an open-label, randomized, controlled noninferiority SAB study, where daptomycin was as effective as standard therapy including antistaphylococcal penicillins for MSSA-B (65).
There seems to be little difference between the glycopeptides provided that teicoplanin is dosed appropriately, especially for cases of endocarditis (319). In a recent systematic review and meta-analysis, all-cause mortality rates were similar between teicoplanin and vancomycin treatments, with significantly greater toxicity when vancomycin was prescribed (283).
A direct comparison of treatment outcomes with linezolid compared to vancomycin in SAB is lacking. Although not aimed at investigating the efficacy of linezolid for SAB, three multicenter open-label randomized trials included SAB episodes. For the treatment of suspected S. aureus infections (18% SAB) (278) and Gram-positive infections in pediatric patients (80 SAB episodes) (135), the efficacy of linezolid was comparable to that of vancomycin. In contrast, linezolid was associated with an excess of deaths compared to the number of deaths associated with vancomycin treatment in a trial of catheter-related episodes (142 SAB episodes in the intention-to-treat analysis) (318). Further analyses suggested that these deaths occurred in patients with either Gram-negative pathogens or no pathogens isolated at baseline and not as a result of SAB. In a systematic review and meta-analysis, overall, linezolid was equivalent in efficacy to vancomycin (OR, 0.49 for mortality; 95% CI, 0.49 to 1.58; P = 0.46) for the treatment of SAB (12).
In a randomized controlled trial comparing cotrimoxazole (trimethoprim-sulfamethoxazole) to vancomycin in 101 injection drug users with S. aureus infections (66% SAB), vancomycin was found to be superior with regard to the duration of bacteremia and clinical cure rates (188). As all MRSA infections were cured, the outcome differences were explained by cotrimoxazole treatment failure in MSSA episodes. Although the numbers of patients were small, a recent retrospective case-controlled study observed no mortality or treatment failure difference between patients treated with cotrimoxazole (n = 38) and those treated with vancomycin (n = 76) for MRSA-B (81). These data suggest a possible role for cotrimoxazole in the management of MRSA-B. However, without a randomized controlled trial, these findings remain speculative.
Theoretical considerations suggest a potential role of adjunctive rifampin in the treatment of SAB, as rifampin penetrates biofilms (11, 327) and is rapidly bactericidal (45). Several small clinical studies have been reported, with conflicting results (165, 298, 299). Thus, without a clear benefit (226) and possibly increased hepatic adverse events (236), adjunctive rifampin therapy is generally not recommended (172). The addition of gentamicin has similarly been associated with greater toxicity for no mortality benefit (43, 122) and likewise is no longer recommended (172). With the exception of adjunctive levofloxacin, which did not result in improved outcomes for the treatment of quinolone-sensitive SAB episodes in a randomized trial (247), other combinations remain limited to in vitro data and case reports, with variable successes (211).
For the management of SAB, with the exception of the definitive inferiority of glycopeptides compared to β-lactams for the treatment of MSSA-B, no alternative agent or combination has been shown to result in better patient outcomes than current standard therapy. This may in part reflect antibiotic registration study design, and thus, further research aimed at determining better treatment options is urgently required, especially for MRSA-B episodes.
Empirical Antibiotics/Antibiotic Timing
The harmful effects of delayed appropriate empirical therapy, defined as in vitro antibiotic activity against the infecting pathogen, have been well documented for patients with sepsis (150, 221) and bacteremia (163). Multiple studies have confirmed these observations for the treatment of SAB (MSSA-B or MRSA-B) (139, 176, 256, 275), with a recent meta-analysis observing an overall 2-fold-increased survival benefit (OR, 1.84; 95% CI, 1.25 to 2.71; P < 0.01) with the administration of appropriate empirical therapy for MRSA-B episodes (220).
However, unlike studies of sepsis, no “time response curve” (increasing mortality for every hour of delay in antibiotic administration) has been detected for SAB treatment; rather, time cutoffs have been detected, after which mortality is increased. In a single-center retrospective study of 167 hospital-acquired SAB (67% MRSA) episodes, Lodise at al., using classification regression tree analysis, identified an empirical/delayed antibiotic “breakpoint” of 44.75 h (176). This time cutoff has, however, never been replicated and has ranged between studies from 24 h up to more than 72 h (139, 176, 256, 275). The importance of appropriate empirical antibiotics (within 24 h) remained, even after adjusting for other risk factors of mortality, in a 2010 study by Paul et al. of 510 health care-onset MRSA-B episodes (220). Poorer outcomes have also been demonstrated using a case-controlled study design, with a 2.35-fold increase in mortality in MRSA-B patients whose therapy was delayed by more than 2 days (OR, 2.35; 95% CI, 1.01 to 5.44; P = 0.05) (187).
Provided that the antibiotics prescribed have some activity, even if they are not considered standard therapy, for example, gentamicin alone or in combination with other agents (e.g., ciprofloxacin), mortality may be improved (240). Antibiotic dosing is critical, however, as Shime et al. demonstrated an incremental survival benefit if patients received empirical antibiotics (within 48 h) and attained a vancomycin target of >400 μg · h/ml compared to those who did not achieve appropriate targets (262).
Not all patients may benefit, however, from appropriately timed empirical therapy, with several studies, irrespective of design, being unable to detect a mortality difference (5, 6, 59, 141, 168, 243). An indication as to which patient subgroups would benefit emerged from a study by Lodise et al., who observed that the greatest impact of empirical antibiotics (within 44.75 h) occurred in patients with severe disease (APACHE II score of >15.5) or complicated SAB (176). However, a larger single-center study of 814 SAB episodes detected a reduced mortality rate only for empirically (24 h before to 24 h after the index blood culture) treated patients with the lowest severity-of-illness score (modified APACHE III score) (HR, 2.42; 95% CI, 1.7 to 3.44) (257), whereas a study by Kim et al. noted that empirical antibiotics (within 48 h) resulted in outcome differences only for patients with a noneradicable focus compared to eradicable foci of SAB (142). Conversely, some patients will improve irrespective of inadequate empirical or definitive SAB therapy (6). Theoretically, the greatest benefit is likely to occur when antibiotics are still able to affect the progression of infection (141) and thus impact infection-related mortality (257). This suggests, therefore, that ill but less severely ill patients are most likely to benefit (167).
Potential explanations for these discrepancies include differing empirical therapy or appropriate antibiotic therapy (including vancomycin for MSSA episodes) definitions, patient groups studied, and possible confounders (e.g., initial treatment selection bias). Several studies did not differentiate between empirical and definitive therapies (131, 176, 184), while the need to adjust for confounders, especially shock, remains debated (257, 273). Regardless, earlier appropriate antibiotic prescribing is likely to be of benefit, albeit not for all patients, and is generally recommended by most experts.
Surgery
The impact of surgery on mortality is unclear. Surrogate markers of source control suggest that surgery may improve outcomes. Jensen et al., in a prospective study of 278 SAB cases, demonstrated that eradicable foci were associated with a reduced mortality rate compared to noneradicable foci of infection (13% and 31%, respectively; P = 0.002) (127). Similarly, in a study of 294 MSSA-B episodes, the eradication of infection was an independent predictor of reduced mortality (OR, 0.3; 95% CI, 0.1 to 0.7; P = 0.006) (140). Furthermore, the intervention per se seems to be responsible for better outcomes, as an increased mortality rate was detected for patients with an eradicable focus that was not removed or drained (OR, 4.17; 95% CI, 1.09 to 3.62; P = 0.04) (142). Fowler et al., in a series of 244 infected intravascular device infections, found comparable results, with higher rates of relapse and mortality if the device was not removed (OR, 6.5; 95% CI, 2.1 to 20.2) (69). Only one study to date has found infection-related surgery with respect to SAB to be an independent predictor of reduced mortality (OR, 0.27; 95% CI, 0.09 to 0.83; P = 0.022) (301). This observation is strongly confounded, however, by the patient's ability to undergo surgery or the surgeon's willingness to perform the procedure. As the eradication of infection sources improves outcomes, it would make intuitive sense that surgical source control would have a similar impact.
Unlike uncomplicated SAB diagnoses, there is definitive evidence that surgery improves outcomes compared to medical therapy alone, although this is limited to patients with complicated infective endocarditis. In a multinational propensity-matched prospective cohort study, the adjusted in-hospital mortality rate was lower for patients with paravalvular complications, systemic embolization, and S. aureus native valve IE (152). Other studies have shown improved outcomes with surgery for patients with congestive heart failure and perivalvular complications (1, 307).
For patients with prosthetic joint infections, reduced cure and retention rates are associated with an increased duration of symptoms and suggest that earlier surgical intervention is one of the factors that may result in improved outcomes (328). Besides these data, which provide possible indirect evidence for the efficacy of early intervention, the optimal timing of surgery for the management of SAB is unknown.
Role of Infectious Disease Consultation
Infectious disease (ID) consultation for SAB episodes generally results in a more detailed evaluation, a greater detection of endocarditis or metastatic complications, and improved adherence to treatment guidelines (69, 125). Fowler et al. showed that an acceptance of ID management resulted in significantly fewer relapses, while Jenkins et al. observed a trend toward fewer treatment failures and reduced mortality following the implementation of an ID consultation service for all SAB patients. In two separate studies, of 308 SAB episodes (82% received an ID consult) (131) and 293 SAB episodes (36% received an ID consult) (206), ID consultation was associated with better 30-day mortality rates by a univariate analysis, while in a German study of 521 SAB episodes, ID consultation was an independent predictor of reduced mortality (OR, 0.6; 95% CI, 0.4 to 1.0; P = 0.045) (237). These results were obtained even though sicker patients and patients with more complicated diseases, such as infective endocarditis, were referred. Differences in these results are probably related to study design, patient populations, baseline institutional expertise in SAB management, and adherence to ID advice.
Special Populations
Neonates.
S. aureus is among the most important causes of bacteremia in neonatal intensive care units (NICUs) (115, 279), with specific S. aureus clonal outbreaks (e.g., phage type 86 [112] or USA300 [64]) being documented in newborn nurseries. Similar to adults, SAB is an important cause of both hospital-acquired (325) and community-onset (214, 269, 326) infections in neonates from developing countries.
Data from the U.S. NICU Network (279) and the Danish SAB Registry (n = 300) (58) suggest that overall mortality rates are similar to those for adults (17.2% and 23%, respectively). Identified risk factors for mortality include low birth weight (<1,000 g; P < 0.01) (58) and age at the onset of infection, with early-onset MSSA sepsis (≤48 h of age) being associated with a significantly higher mortality rate than that associated with late-onset MSSA infections (39% versus 7.3%; P < 0.01) (115).
The impact of methicillin resistance is unclear, with MRSA mortality rates being significantly higher than those associated with MSSA episodes (24.6%, versus 9.9% for MSSA; P < 0.001) in an Australian and New Zealand NICU study by Isaacs et al. (115). However, MRSA episodes occurred in infants who were more premature and of lower birth weights. In contrast, at the Duke University Medical Center (37), NICU patient characteristics were similar, with no significant differences in mortality rates (19% for MRSA versus 6% for MSSA infections), length of stay, or later neurodevelopmental impairment between the two groups.
A report of CA-MRSA bacteremia in neonates from Texas (94) noted a high mortality rate of 38%. However, comparative data with other SAB clonal episodes were not documented. In a neonatal intensive care unit study by Kuint et al. (149), of 11 CA-MRSA (pvl-negative), 20 multidrug-resistant MRSA, and 12 MSSA bacteremic episodes, mortality (9%) was not dependent on the S. aureus subtype despite CA-MRSA episodes occurring in younger neonates.
In summary, the data on SAB mortality in neonates are limited. There is a suggestion, however, that patient factors such as the degree of prematurity and birth weight may influence outcomes, whereas the effects of methicillin resistance or S. aureus clonal types cannot be definitively determined.
Children.
Age remains one of the most consistent predictors of mortality, with children generally having lower SAB mortality rates than adults. In one of the largest prospective cohort studies to date that included children, the mortality rate was the lowest for individuals aged between 10 and 19 years (296). Although rates tend to be higher in children aged between 0 and 9 years, this probably reflects mortality in neonates rather than children older than 1 year of age, with mortality being 10- to 17-fold higher in <1-year-olds than in older children (71).
Overall, recent mortality rates vary widely in developing countries, at 35 to 40% in Thailand (0- to 10-year-olds) (212), 25% in Kenya (151), and 15% in South Africa (75), with lower rates documented in Tanzania (7.1%) (17) and Mozambique (6%) (269). These results are difficult to interpret, as they may represent access to and the level of health care as well as the impact of high HIV coinfection rates in these countries. In contrast, overall mortality rates are generally much lower in series from developed countries: 0.7% (infection-related mortality) in Australia (282), 3% in New Zealand (109), 1.5% in Finland (180), and 2% in the United Kingdom (46). In the United States, mortality rates for children with SAB range between 3.2% and 18% (13, 90, 297). The wide range of mortality rates may be explained in part by the associated comorbidities, with a higher 1-year mortality rate (18%) seen in a cohort study including many children with congenital heart disease (297). This is supported by a Danish study of 2,468 pediatric SAB episodes in patients aged between 1 and 20 years, where the presence of comorbidities was an independent risk factor for death (OR, 2.49; 95% CI, 1.71 to 3.62) (71).
The place of SAB onset influences outcomes, with community-onset episodes having a lower mortality rate than health care-acquired episodes (0.6% and 3.9%, respectively) (71), probably secondary to the predominance of skin and soft tissue infections and bone and joint infections in community-onset episodes. Conversely, the presence of comorbidities and, hence, the need for hospitalization may explain the higher mortality rates for children aged between 11 and 20 years with nosocomial SAB episodes. As with adults, the source of infection influences outcomes, with no mortality occurring in oncology patients with line-associated SAB (239, 277). Infective endocarditis, although rare in children, is associated with an increased 1-year mortality rate (40%, versus 12% for children with no IE) (297). Both IE (patients 0 to 10 years old) and pulmonary infections (patients <1 and 11 to 20 years old) were independent predictors of mortality (71). Although small case series have documented high mortality rates associated with pvl-positive and CA-MRSA infections (27, 83, 84), a lack of comparative data and the small sample size limit drawing conclusions about the impact of these factors on outcomes.
The role of antibiotic resistance is likewise unclear. Burke et al., in a retrospective cohort study of 164 U.S. children (24), reported a difference in mortality rates for MRSA-B compared to MSSA-B (10% versus 4%). In contrast, a study of 69 U.S. children, when adjusted for underlying diseases and delays in appropriate antibiotic therapy, found MRSA not to be associated with increased mortality (280). The impact of treatment is similarly unclear, with no large comparative studies of children being reported. Although vancomycin treatment failure was documented in a small pediatric series (n = 22) of MRSA-B, the mortality rate seemed similar to those of other reported data, at13.6%. Treatment failure was associated with prematurity and pvl-positive infections but not a high vancomycin MIC (by either the Etest or broth microdilution) (315).
In summary, pediatric SAB mortality rates were low in recent series from developed countries and, similarly to adults, are declining with better management in children, from 20% (1990 to 1994) to 11% (1993 to 2007) and from 19.5% (1971 to 1976) to 2.5% (1996 to 2000), based on data from Argentina (215, 216) and Denmark (71), respectively. Although outcomes are influenced by the presence of comorbidities and infection manifestations, the impact of other variables remains unclear and warrants further study.
CLINICAL IMPLICATIONS, FUTURE RESEARCH, AND CONCLUSIONS
The most consistent predictor of mortality in patients with SAB is age. The impacts of other host factors, except for the presence of comorbidities, are unclear. Pathogen-host interactions, especially the presence of shock, and the source of SAB are strong predictors of outcome. Although antibiotic resistance may be associated with increased mortality, questions remain as to whether this reflects pathogen-specific factors or poorer responses to antibiotic therapy, namely, vancomycin. Optimal management relies on starting appropriate antibiotics in a timely fashion, with outcomes being improved in certain patient subgroups.
Of all these factors, the treating clinician can only influence patient management. These remain uncertain, however, with no real indication of superior outcomes with any alternative antibiotic, especially for the treatment of MRSA-B. With the advent of “personalized” care, hopefully, better treatment algorithms can be developed (e.g., the need for early surgery or a certain antibiotic for line-related SAB). For this to occur, a greater understanding of predictors of mortality is required, which possibly necessitates the inclusion of genomic approaches in future studies. Furthermore, more international collaborative studies should be encouraged, similar to the International Collaboration of Infective Endocarditis or HIV cohort studies. This would allow the standardization of data collection and adequate power to tease out some of the controversies in SAB management.
In conclusion, SAB remains a common infection with significant associated mortality. Its impact and significance remain underestimated in the community. A greater recognition of the impact of SAB will hopefully lead to more research on and a better understanding of predictors of mortality, which subsequently would assist SAB management and optimize patient outcomes.
ACKNOWLEDGMENT
We thank Ann Hofmeyr for her insightful and critical appraisal of the manuscript.
Biographies

Sebastian J. van Hal obtained his medical degree from The University of Cape Town, South Africa, in 1995. After working extensively in Africa and Europe, he moved to Australia and completed his training in Infectious Diseases and Microbiology. His research interests include the study of the consequences and mechanisms of antibiotic resistance, especially in Staphylococcus aureus. He is currently enrolled as a research scholar at The University of Queensland, UQ Centre for Clinical Research, in Brisbane, Australia, examining the consequences of reduced vancomycin susceptibility on patient outcomes.

Slade O. Jensen is a Senior Lecturer in the Department of Infectious Diseases and Microbiology at the School of Medicine, University of Western Sydney (UWS), Australia. He obtained his Ph.D. at the University of Sydney, where he worked as a research-only academic until he joined UWS in 2010. Dr. Jensen's research interests include understanding the molecular mechanisms that contribute to the capacity of staphylococcal plasmids to acquire and maintain antimicrobial resistance genes in the absence of selective pressure.

Vikram L. Vaska obtained his medical degree from The University of Adelaide, Australia, in 1997. He subsequently trained in Paediatrics, Paediatric Infectious Diseases, and Clinical Microbiology in Adelaide and Brisbane. Currently, he is a research scholar at The University of Queensland, UQ Centre for Clinical Research, in Brisbane, Australia, examining aspects of community-associated methicillin-resistant S. aureus infections.

Björn A. Espedido completed his Ph.D. in Medicine at The University of Sydney, Australia. His doctoral thesis examined the effect of outer membrane permeability defects and β-lactamase production on carbapenem resistance in the Enterobacteriaceae. He is currently a postdoctoral scientific officer in the Antibiotic Resistance and Mobile Elements Group (ARMEG) within the Microbiology and Infectious Diseases Unit of the School of Medicine, University of Western Sydney. Dr. Espedido's research interests are in the area of molecular bacteriology with a primary focus on using comparative whole-genome approaches to identify genetic determinants that confer not only antibiotic resistance but also increased virulence in Staphylococcus aureus and other clinically important bacteria. Additionally, he is interested in plasmid partitioning systems in S. aureus with a view to understanding the biology of these mobile elements that play an important role in the spread of antibiotic resistance.

David L. Paterson is a Professor of Medicine at The University of Queensland Centre for Clinical Research (UQCCR) as well as Consultant Infectious Diseases Physician, Consultant Microbiologist, and Medical Advisor for the Centre for Healthcare Related Infection Surveillance and Prevention (CHRISP). He received his medical degree and Ph.D. from The University of Queensland. In 2007, he returned to Brisbane after spending 10 years at The University of Pittsburgh School of Medicine. His research interests include the study of the molecular and clinical epidemiology of infections with antibiotic-resistant organisms. The focus of this work is the translation of knowledge into optimal prevention and treatment of these infections.

Iain B. Gosbell is the Foundation Professor of Infectious Diseases and Microbiology at the School of Medicine, University of Western Sydney, Australia. He described the emergence of community MRSA in southwestern Sydney and subsequently developed research interests in the epidemiology of MRSA in the community and also in hospitals. Since commencing at UWS, he has established the Antibiotic Resistance and Mobile Elements Group (ARMEG) at UWS, with Dr. Jensen, Dr. Espedido, and Associate Professor van Hal. The ARMEG examines the genetics responsible for antibiotic resistance, especially vancomycin and daptomycin resistance in MRSA, the plasmid biology of MRSA, and the contribution of bacterial biofilms to health care-associated infections.
REFERENCES
- 1. Aksoy O, et al. 2007. Early surgery in patients with infective endocarditis: a propensity score analysis. Clin. Infect. Dis. 44: 364–372 [DOI] [PubMed] [Google Scholar]
- 2. Albanese J, et al. 2000. Cerebrospinal fluid penetration and pharmacokinetics of vancomycin administered by continuous infusion to mechanically ventilated patients in an intensive care unit. Antimicrob. Agents Chemother. 44: 1356–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Albur MS, Bowker K, Weir I, Macgowan A. 14 June 2011, posting date Factors influencing the clinical outcome of methicillin-resistant Staphylococcus aureus bacteraemia. Eur. J. Clin. Microbiol. Infect. Dis. [Epub ahead of print.] doi:10.1007/s10096-011-1310-2 [DOI] [PubMed] [Google Scholar]
- 4. Aligholi M, et al. 2008. Emergence of high-level vancomycin-resistant Staphylococcus aureus in the Imam Khomeini Hospital in Tehran. Med. Princ. Pract. 17: 432–434 [DOI] [PubMed] [Google Scholar]
- 5. Allard C, et al. 2008. Secular changes in incidence and mortality associated with Staphylococcus aureus bacteraemia in Quebec, Canada, 1991–2005. Clin. Microbiol. Infect. 14: 421–428 [DOI] [PubMed] [Google Scholar]
- 6. Ammerlaan H, et al. 2009. Adequacy of antimicrobial treatment and outcome of Staphylococcus aureus bacteremia in 9 Western European countries. Clin. Infect. Dis. 49: 997–1005 [DOI] [PubMed] [Google Scholar]
- 7. Asgeirsson H, Kristjansson M, Kristinsson KG, Gudlaugsson O. 2011. Staphylococcus aureus bacteraemia—nationwide assessment of treatment adequacy and outcome. J. Infect. 62: 339–346 [DOI] [PubMed] [Google Scholar]
- 8. Bae IG, et al. 2009. Heterogeneous vancomycin-intermediate susceptibility phenotype in bloodstream methicillin-resistant Staphylococcus aureus isolates from an international cohort of patients with infective endocarditis: prevalence, genotype, and clinical significance. J. Infect. Dis. 200: 1355–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bae IG, et al. 2009. Presence of genes encoding the Panton-Valentine leukocidin exotoxin is not the primary determinant of outcome in patients with complicated skin and skin structure infections due to methicillin-resistant Staphylococcus aureus: results of a multinational trial. J. Clin. Microbiol. 47: 3952–3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bagger JP, Zindrou D, Taylor KM. 2004. Postoperative infection with meticillin-resistant Staphylococcus aureus and socioeconomic background. Lancet 363: 706–708 [DOI] [PubMed] [Google Scholar]
- 11. Bahl D, et al. 1997. In vitro activities of ciprofloxacin and rifampin alone and in combination against growing and nongrowing strains of methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 41: 1293–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beibei L, et al. 2010. Linezolid versus vancomycin for the treatment of gram-positive bacterial infections: meta-analysis of randomised controlled trials. Int. J. Antimicrob. Agents 35: 3–12 [DOI] [PubMed] [Google Scholar]
- 13. Bendig EA, Singh J, Butler TJ, Arrieta AC. 2008. The impact of the central venous catheter on the diagnosis of infectious endocarditis using Duke criteria in children with Staphylococcus aureus bacteremia. Pediatr. Infect. Dis. J. 27: 636–639 [DOI] [PubMed] [Google Scholar]
- 14. Benfield T, et al. 2007. Increasing incidence but decreasing in-hospital mortality of adult Staphylococcus aureus bacteraemia between 1981 and 2000. Clin. Microbiol. Infect. 13: 257–263 [DOI] [PubMed] [Google Scholar]
- 15. Big C, Malani PN. 2010. Staphylococcus aureus bloodstream infections in older adults: clinical outcomes and risk factors for in-hospital mortality. J. Am. Geriatr. Soc. 58: 300–305 [DOI] [PubMed] [Google Scholar]
- 16. Blaine KP, et al. 2010. Progression to bacteremia in critical care patients colonized with methicillin-resistant Staphylococcus aureus expressing Panton-Valentine leukocidin. Diagn. Microbiol. Infect. Dis. 68: 28–33 [DOI] [PubMed] [Google Scholar]
- 17. Blomberg B, et al. 2007. Antimicrobial resistance predicts death in Tanzanian children with bloodstream infections: a prospective cohort study. BMC Infect. Dis. 7: 43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blot SI, Vandewoude KH, Hoste EA, Colardyn FA. 2002. Outcome and attributable mortality in critically ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Arch. Intern. Med. 162: 2229–2235 [DOI] [PubMed] [Google Scholar]
- 19. Blyth MJ, Kincaid R, Craigen MA, Bennet GC. 2001. The changing epidemiology of acute and subacute haematogenous osteomyelitis in children. J. Bone Joint Surg. Br. 83: 99–102 [DOI] [PubMed] [Google Scholar]
- 20. Boakes E, et al. 2011. Distinct bacteriophages encoding Panton-Valentine leukocidin (PVL) among international methicillin-resistant Staphylococcus aureus clones harboring PVL. J. Clin. Microbiol. 49: 684–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boucher H, Miller LG, Razonable RR. 2010. Serious infections caused by methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 51 (Suppl 2): S183–S197 [DOI] [PubMed] [Google Scholar]
- 22. Boyle-Vavra S, Daum RS. 2007. Community-acquired methicillin-resistant Staphylococcus aureus: the role of Panton-Valentine leukocidin. Lab. Invest. 87: 3–9 [DOI] [PubMed] [Google Scholar]
- 23. Bryant RE, Alford RH. 1977. Unsuccessful treatment of staphylococcal endocarditis with cefazolin. JAMA 237: 569–570 [PubMed] [Google Scholar]
- 24. Burke RE, Halpern MS, Baron EJ, Gutierrez K. 2009. Pediatric and neonatal Staphylococcus aureus bacteremia: epidemiology, risk factors, and outcome. Infect. Control Hosp. Epidemiol. 30: 636–644 [DOI] [PubMed] [Google Scholar]
- 25. Burkey MD, et al. 2008. The incidence of and risk factors for MRSA bacteraemia in an HIV-infected cohort in the HAART era. HIV Med. 9: 858–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cantoni L, Glauser MP, Bille J. 1990. Comparative efficacy of daptomycin, vancomycin, and cloxacillin for the treatment of Staphylococcus aureus endocarditis in rats and role of test conditions in this determination. Antimicrob. Agents Chemother. 34: 2348–2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Castaldo ET, Yang EY. 2007. Severe sepsis attributable to community-associated methicillin-resistant Staphylococcus aureus: an emerging fatal problem. Am. Surg. 73: 684–687; discussion, 687–688 [PubMed] [Google Scholar]
- 28. Chamis AL, et al. 2001. Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter-defibrillators. Circulation 104: 1029–1033 [DOI] [PubMed] [Google Scholar]
- 29. Chang FY, et al. 2003. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore) 82: 322–332 [DOI] [PubMed] [Google Scholar]
- 30. Charles PG, Ward PB, Johnson PD, Howden BP, Grayson ML. 2004. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin. Infect. Dis. 38: 448–451 [DOI] [PubMed] [Google Scholar]
- 31. Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40: 373–383 [DOI] [PubMed] [Google Scholar]
- 32. Chen SY, et al. 2010. Differences between methicillin-resistant Staphylococcus aureus bacteremic isolates harboring type IV and type V staphylococcal cassette chromosome mec genes based on prior patient healthcare exposure. Eur. J. Clin. Microbiol. Infect. Dis. 29: 1539–1546 [DOI] [PubMed] [Google Scholar]
- 33. Chen SY, et al. 2010. Impact of traditional hospital strain of methicillin-resistant Staphylococcus aureus (MRSA) and community strain of MRSA on mortality in patients with community-onset S aureus bacteremia. Medicine (Baltimore) 89: 285–294 [DOI] [PubMed] [Google Scholar]
- 34. Cheung AL, Bayer AS, Zhang G, Gresham H, Xiong Y-Q. 2004. Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 40: 1–9 [DOI] [PubMed] [Google Scholar]
- 35. Chia JW, Hsu LY, Chai LY, Tambyah PA. 2008. Epidemiology and outcomes of community-onset methicillin-susceptible Staphylococcus aureus bacteraemia in a university hospital in Singapore. BMC Infect. Dis. 8: 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chihara S, Popovich KJ, Weinstein RA, Hota B. 2010. Staphylococcus aureus bacteriuria as a prognosticator for outcome of Staphylococcus aureus bacteremia: a case-control study. BMC Infect. Dis. 10: 225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cohen-Wolkowiez M, et al. 2007. Mortality and neurodevelopmental outcome after Staphylococcus aureus bacteremia in infants. Pediatr. Infect. Dis. J. 26: 1159–1161 [DOI] [PubMed] [Google Scholar]
- 38. Collignon P, Nimmo GR, Gottlieb T, Gosbell IB. 2005. Staphylococcus aureus bacteremia, Australia. Emerg. Infect. Dis. 11: 554–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Conterno LO, Wey SB, Castelo A. 1998. Risk factors for mortality in Staphylococcus aureus bacteremia. Infect. Control Hosp. Epidemiol. 19: 32–37 [DOI] [PubMed] [Google Scholar]
- 40. Cosgrove SE, Fowler VG., Jr 2008. Management of methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 46 (Suppl 5): S386–S393 [DOI] [PubMed] [Google Scholar]
- 41. Cosgrove SE, et al. 2005. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect. Control Hosp. Epidemiol. 26: 166–174 [DOI] [PubMed] [Google Scholar]
- 42. Cosgrove SE, et al. 2003. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin. Infect. Dis. 36: 53–59 [DOI] [PubMed] [Google Scholar]
- 43. Cosgrove SE, et al. 2009. Initial low-dose gentamicin for Staphylococcus aureus bacteremia and endocarditis is nephrotoxic. Clin. Infect. Dis. 48: 713–721 [DOI] [PubMed] [Google Scholar]
- 44. Dajcs JJ, Thibodeaux BA, Girgis DO, O'Callaghan RJ. 2002. Corneal virulence of Staphylococcus aureus in an experimental model of keratitis. DNA Cell Biol. 21: 375–382 [DOI] [PubMed] [Google Scholar]
- 45. Darouiche RO, Hamill RJ. 1994. Antibiotic penetration of and bactericidal activity within endothelial cells. Antimicrob. Agents Chemother. 38: 1059–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Denniston S, Riordan FA., I 2006. Staphylococcus aureus bacteraemia in children and neonates: a 10 year retrospective review. J. Infect. 53: 387–393 [DOI] [PubMed] [Google Scholar]
- 47. Deresinski S. 2007. Counterpoint: vancomycin and Staphylococcus aureus—an antibiotic enters obsolescence. Clin. Infect. Dis. 44: 1543–1548 [DOI] [PubMed] [Google Scholar]
- 48. Desachy A, et al. 2007. Role of superantigenic strains in the prognosis of community-acquired methicillin-susceptible Staphylococcus aureus bacteraemia. Clin. Microbiol. Infect. 13: 1131–1133 [DOI] [PubMed] [Google Scholar]
- 49. de Sanctis JT, et al. 2011. Is there a clinical association of vancomycin MIC creep, agr group II locus, and treatment failure in MRSA bacteremia? Diagn. Mol. Pathol. 20: 184–188 [DOI] [PubMed] [Google Scholar]
- 50. Diep BA, Otto M. 2008. The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol. 16: 361–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Diep BA, et al. 2008. Contribution of Panton-Valentine leukocidin in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. PLoS One 3: e3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dinges MM, Orwin PM, Schlievert PM. 2000. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 13: 16–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dugdale DC, Ramsey PG. 1990. Staphylococcus aureus bacteremia in patients with Hickman catheters. Am. J. Med. 89: 137–141 [DOI] [PubMed] [Google Scholar]
- 54. Ehni WF, Reller B. 1989. Short-course therapy for catheter-associated Staphylococcus aureus bacteraemia. Arch. Intern. Med. 149: 533–536 [PubMed] [Google Scholar]
- 55. Ejlertsen T, Jensen A, Lester A, Rosdahl VT. 1994. Epidemiology of toxic shock syndrome toxin-1 production in Staphylococcus aureus strains isolated in Denmark between 1959 and 1990. Scand. J. Infect. Dis. 26: 599–604 [DOI] [PubMed] [Google Scholar]
- 56. El Atrouni WI, et al. 2009. Temporal trends in the incidence of Staphylococcus aureus bacteremia in Olmsted County, Minnesota, 1998 to 2005: a population-based study. Clin. Infect. Dis. 49: e130–e138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ellington MJ, et al. 2007. Is Panton-Valentine leucocidin associated with the pathogenesis of Staphylococcus aureus bacteraemia in the UK? J. Antimicrob. Chemother. 60: 402–405 [DOI] [PubMed] [Google Scholar]
- 58. Espersen F, Frimodt-Moller N, Thamdrup Rosdahl V, Jessen O. 1989. Staphylococcus aureus bacteraemia in children below the age of one year. A review of 407 cases. Acta Paediatr. Scand. 78: 56–61 [DOI] [PubMed] [Google Scholar]
- 59. Fang CT, et al. 2006. Early empirical glycopeptide therapy for patients with methicillin-resistant Staphylococcus aureus bacteraemia: impact on the outcome. J. Antimicrob. Chemother. 57: 511–519 [DOI] [PubMed] [Google Scholar]
- 60. Ferreras M, et al. 1998. The interaction of Staphylococcus aureus bi-component γ-hemolysins and leucocidins with cells and lipid membranes. Biochim. Biophys. Acta 1414: 108–126 [DOI] [PubMed] [Google Scholar]
- 61. Filice GA, Van Etta LL, Darby CP, Fraser DW. 1986. Bacteremia in Charleston County, South Carolina. Am. J. Epidemiol. 123: 128–136 [DOI] [PubMed] [Google Scholar]
- 62. Finks J, et al. 2009. Vancomycin-resistant Staphylococcus aureus, Michigan, USA, 2007. Emerg. Infect. Dis. 15: 943–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fong RK, Low J, Koh TH, Kurup A. 2009. Clinical features and treatment outcomes of vancomycin-intermediate Staphylococcus aureus (VISA) and heteroresistant vancomycin-intermediate Staphylococcus aureus (hVISA) in a tertiary care institution in Singapore. Eur. J. Clin. Microbiol. Infect. Dis. 28: 983–987 [DOI] [PubMed] [Google Scholar]
- 64. Fortunov RM, Hulten KG, Hammerman WA, Mason EO, Jr, Kaplan SL. 2006. Community-acquired Staphylococcus aureus infections in term and near-term previously healthy neonates. Pediatrics 118: 874–881 [DOI] [PubMed] [Google Scholar]
- 65. Fowler VG, Jr, et al. 2006. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 355: 653–665 [DOI] [PubMed] [Google Scholar]
- 66. Fowler VG, Jr, et al. 2005. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 293: 3012–3021 [DOI] [PubMed] [Google Scholar]
- 67. Fowler VG, Jr, et al. 2003. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch. Intern. Med. 163: 2066–2072 [DOI] [PubMed] [Google Scholar]
- 68. Fowler VG, Jr, et al. 1999. Infective endocarditis due to Staphylococcus aureus: 59 prospectively identified cases with follow-up. Clin. Infect. Dis. 28: 106–114 [DOI] [PubMed] [Google Scholar]
- 69. Fowler VG, Jr, et al. 1998. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin. Infect. Dis. 27: 478–486 [DOI] [PubMed] [Google Scholar]
- 70. Fowler VG, et al. 2004. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J. Infect. Dis. 190: 1140–1149 [DOI] [PubMed] [Google Scholar]
- 71. Frederiksen MS, et al. 2007. Changing epidemiology of pediatric Staphylococcus aureus bacteremia in Denmark from 1971 through 2000. Pediatr. Infect. Dis. J. 26: 398–405 [DOI] [PubMed] [Google Scholar]
- 72. Fridkin SK. 2001. Vancomycin-intermediate and -resistant Staphylococcus aureus: what the infectious disease specialist needs to know. Clin. Infect. Dis. 32: 108–115 [DOI] [PubMed] [Google Scholar]
- 73. Fridkin SK, et al. 2003. Epidemiological and microbiological characterization of infections caused by Staphylococcus aureus with reduced susceptibility to vancomycin, United States, 1997–2001. Clin. Infect. Dis. 36: 429–439 [DOI] [PubMed] [Google Scholar]
- 74. Fridkin SK, et al. 2005. Methicillin-resistant Staphylococcus aureus disease in three communities. N. Engl. J. Med. 352: 1436–1444 [DOI] [PubMed] [Google Scholar]
- 75. Friedland IR, du Plessis J, Cilliers A. 1995. Cardiac complications in children with Staphylococcus aureus bacteremia. J. Pediatr. 127: 746–748 [DOI] [PubMed] [Google Scholar]
- 76. Gafur OA, et al. 2008. The impact of the current epidemiology of pediatric musculoskeletal infection on evaluation and treatment guidelines. J. Pediatr. Orthop. 28: 777–785 [DOI] [PubMed] [Google Scholar]
- 77. Ganga R, et al. 2009. Role of SCCmec type in outcome of Staphylococcus aureus bacteremia in a single medical center. J. Clin. Microbiol. 47: 590–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gillet Y, et al. 2002. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359: 753–759 [DOI] [PubMed] [Google Scholar]
- 79. Gillet Y, et al. 2007. Factors predicting mortality in necrotizing community-acquired pneumonia caused by Staphylococcus aureus containing Panton-Valentine leukocidin. Clin. Infect. Dis. 45: 315–321 [DOI] [PubMed] [Google Scholar]
- 80. Goergens ED, McEvoy A, Watson M, Barrett IR. 2005. Acute osteomyelitis and septic arthritis in children. J. Paediatr. Child Health 41: 59–62 [DOI] [PubMed] [Google Scholar]
- 81. Goldberg E, et al. 2010. Co-trimoxazole versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus bacteraemia: a retrospective cohort study. J. Antimicrob. Chemother. 65: 1779–1783 [DOI] [PubMed] [Google Scholar]
- 82. Gomez J, et al. 2007. Predictors of mortality in patients with methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia: the role of empiric antibiotic therapy. Eur. J. Clin. Microbiol. Infect. Dis. 26: 239–245 [DOI] [PubMed] [Google Scholar]
- 83. Gonzalez BE, et al. 2005. Pulmonary manifestations in children with invasive community-acquired Staphylococcus aureus infection. Clin. Infect. Dis. 41: 583–590 [DOI] [PubMed] [Google Scholar]
- 84. Gonzalez BE, et al. 2005. Severe staphylococcal sepsis in adolescents in the era of community-acquired methicillin-resistant Staphylococcus aureus. Pediatrics 115: 642–648 [DOI] [PubMed] [Google Scholar]
- 85. Gonzalez C, Rubio M, Romero-Vivas J, Gonzalez M, Picazo JJ. 1999. Bacteremic pneumonia due to Staphylococcus aureus: a comparison of disease caused by methicillin-resistant and methicillin-susceptible organisms. Clin. Infect. Dis. 29: 1171–1177 [DOI] [PubMed] [Google Scholar]
- 86. Gould IM. 2007. MRSA bacteraemia. Int. J. Antimicrob. Agents 30 (Suppl 1): S66–S70 [DOI] [PubMed] [Google Scholar]
- 87. Grayson ML. 2006. The treatment triangle for staphylococcal infections. N. Engl. J. Med. 355: 724–727 [DOI] [PubMed] [Google Scholar]
- 88. Graziani AL, Lawson LA, Gibson GA, Steinberg MA, MacGregor RR. 1988. Vancomycin concentrations in infected and noninfected human bone. Antimicrob. Agents Chemother. 32: 1320–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Guilarde AO, Turchi MD, Martelli CM, Primo MG. 2006. Staphylococcus aureus bacteraemia: incidence, risk factors and predictors for death in a Brazilian teaching hospital. J. Hosp. Infect. 63: 330–336 [DOI] [PubMed] [Google Scholar]
- 90. Hakim H, Mylotte JM, Faden H. 2007. Morbidity and mortality of staphylococcal bacteremia in children. Am. J. Infect. Control 35: 102–105 [DOI] [PubMed] [Google Scholar]
- 91. Harbarth S, Rutschmann O, Sudre P, Pittet D. 1998. Impact of methicillin resistance on the outcome of patients with bacteremia caused by Staphylococcus aureus. Arch. Intern. Med. 158: 182–189 [DOI] [PubMed] [Google Scholar]
- 92. Hartman BJ, Tomasz A. 1984. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J. Bacteriol. 158: 513–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hawkins C, et al. 2007. Persistent Staphylococcus aureus bacteremia: an analysis of risk factors and outcomes. Arch. Intern. Med. 167: 1861–1867 [DOI] [PubMed] [Google Scholar]
- 94. Healy CM, Hulten KG, Palazzi DL, Campbell JR, Baker CJ. 2004. Emergence of new strains of methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit. Clin. Infect. Dis. 39: 1460–1466 [DOI] [PubMed] [Google Scholar]
- 95. Herman RA, Kee VR, Moores KG, Ross MB. 2008. Etiology and treatment of community-associated methicillin-resistant Staphylococcus aureus. Am. J. Health Syst. Pharm. 65: 219–225 [DOI] [PubMed] [Google Scholar]
- 96. Hewagama S, Einsiedel L, Spelman T. 10 February 2011, posting date. Staphylococcus aureus bacteraemia at Alice Springs Hospital, Central Australia, 2003–2006. Intern. Med. J. [Epub ahead of print.] doi:10.1111/j.1445-5994.2011.02449.x [DOI] [PubMed] [Google Scholar]
- 97. Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. 2006. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch. Intern. Med. 166: 2138–2144 [DOI] [PubMed] [Google Scholar]
- 98. Hill PC, et al. 2001. Prospective study of 424 cases of Staphylococcus aureus bacteraemia: determination of factors affecting incidence and mortality. Intern. Med. J. 31: 97–103 [PubMed] [Google Scholar]
- 99. Hiramatsu K, et al. 1997. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 350: 1670–1673 [DOI] [PubMed] [Google Scholar]
- 100. Holland TL, Fowler VG., Jr 2011. Vancomycin minimum inhibitory concentration and outcome in patients with Staphylococcus aureus bacteremia: pearl or pellet? J. Infect. Dis. 204: 329–331 [DOI] [PubMed] [Google Scholar]
- 101. Holmes NE, et al. 2011. Antibiotic choice may not explain poorer outcomes in patients with Staphylococcus aureus bacteremia and high vancomycin minimum inhibitory concentrations. J. Infect. Dis. 204: 340–347 [DOI] [PubMed] [Google Scholar]
- 102. Horan TC, Gaynes RP. 2004. Surveillance of nosocomial infections, p 1659–1702 In Mayhall CG. (ed), Hospital epidemiology and infection control, 3rd ed. Lippincott Williams & Wilkins, Philadelphia, PA: [Google Scholar]
- 103. Horne KC, et al. 2009. Prospective comparison of the clinical impacts of heterogeneous vancomycin-intermediate methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-susceptible MRSA. Antimicrob. Agents Chemother. 53: 3447–3452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Howden BP, Davies JK, Johnson PD, Stinear TP, Grayson ML. 2010. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin. Microbiol. Rev. 23: 99–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Howden BP, et al. 2004. Treatment outcomes for serious infections caused by methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility. Clin. Infect. Dis. 38: 521–528 [DOI] [PubMed] [Google Scholar]
- 106. Hsu RB. 2005. Risk factors for nosocomial infective endocarditis in patients with methicillin-resistant Staphylococcus aureus bacteremia. Infect. Control Hosp. Epidemiol. 26: 654–657 [DOI] [PubMed] [Google Scholar]
- 107. Huang JH, Hsu RB. 2008. Treatment of infective endocarditis caused by methicillin-resistant Staphylococcus aureus: teicoplanin versus vancomycin in a retrospective study. Scand. J. Infect. Dis. 40: 462–467 [DOI] [PubMed] [Google Scholar]
- 108. Huggan PJ, Murdoch DR, Gallagher K, Chambers ST. 2008. Concomitant Staphylococcus aureus bacteriuria is associated with poor clinical outcome in adults with S. aureus bacteraemia. J. Hosp. Infect. 69: 345–349 [DOI] [PubMed] [Google Scholar]
- 109. Huggan PJ, et al. 2010. Population-based epidemiology of Staphylococcus aureus bloodstream infection in Canterbury, New Zealand. Intern. Med. J. 40: 117–125 [DOI] [PubMed] [Google Scholar]
- 110. Hurley JC. 2003. Comparison of mortality associated with methicillin-susceptible and methicillin-resistant Staphylococcus aureus bacteremia: an ecological analysis. Clin. Infect. Dis. 37: 866–868; author reply, 868–869 [DOI] [PubMed] [Google Scholar]
- 111. Hurley JC. 2002. Risk of death from methicillin-resistant Staphylococcus aureus bacteraemia: a meta-analysis. Med. J. Aust. 176: 188; author reply, 189 [DOI] [PubMed] [Google Scholar]
- 112. Hyams PJ, et al. 1975. Staphylococcal bacteremia and hexachlorophene bathing. Epidemic in a newborn nursery. Am. J. Dis. Child. 129: 595–599 [DOI] [PubMed] [Google Scholar]
- 113. Iannini PB, Crossley K. 1976. Therapy of Staphylococcus aureus bacteraemia associated with a removable focus of infection. Ann. Intern. Med. 84: 558–560 [DOI] [PubMed] [Google Scholar]
- 114. Ippolito G, Leone S, Lauria FN, Nicastri E, Wenzel RP. 2010. Methicillin-resistant Staphylococcus aureus: the superbug. Int. J. Infect. Dis. 14 (Suppl 4): S7–S11 [DOI] [PubMed] [Google Scholar]
- 115. Isaacs D, Fraser S, Hogg G, Li HY. 2004. Staphylococcus aureus infections in Australasian neonatal nurseries. Arch. Dis. Child. Fetal Neonatal Ed. 89: F331–F335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ito T, Katayama Y, Hiramatsu K. 1999. Cloning and nucleotide sequence determination of the entire mec DNA of pre-methicillin-resistant Staphylococcus aureus N315. Antimicrob. Agents Chemother. 43: 1449–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Iwatsuki K, Yamasaki O, Morizane S, Oono T. 2006. Staphylococcal cutaneous infections: invasion, evasion and aggression. J. Dermatol. Sci. 42: 203–214 [DOI] [PubMed] [Google Scholar]
- 118. Jacobsson G, Colque-Navarro P, Gustafsson E, Andersson R, Mollby R. 2010. Antibody responses in patients with invasive Staphylococcus aureus infections. Eur. J. Clin. Microbiol. Infect. Dis. 29: 715–725 [DOI] [PubMed] [Google Scholar]
- 119. Jacobsson G, Dashti S, Wahlberg T, Andersson R. 2007. The epidemiology of and risk factors for invasive Staphylococcus aureus infections in western Sweden. Scand. J. Infect. Dis. 39: 6–13 [DOI] [PubMed] [Google Scholar]
- 120. Jacobsson G, Gustafsson E, Andersson R. 2008. Outcome for invasive Staphylococcus aureus infections. Eur. J. Clin. Microbiol. Infect. Dis. 27: 839–848 [DOI] [PubMed] [Google Scholar]
- 121. Jahamy H, et al. 2008. Staphylococcus aureus skin/soft-tissue infections: the impact of SCCmec type and Panton-Valentine leukocidin. Scand. J. Infect. Dis. 40: 601–606 [DOI] [PubMed] [Google Scholar]
- 122. Jang HC, et al. 2009. Salvage treatment for persistent methicillin-resistant Staphylococcus aureus bacteremia: efficacy of linezolid with or without carbapenem. Clin. Infect. Dis. 49: 395–401 [DOI] [PubMed] [Google Scholar]
- 123. Jarraud S, et al. 2001. egc, a highly prevalent operon of enterotoxin gene, forms a putative nursery of superantigens in Staphylococcus aureus. J. Immunol. 166: 669–677 [DOI] [PubMed] [Google Scholar]
- 124. Jenkins TC, et al. 2009. Epidemiology of healthcare-associated bloodstream infection caused by USA300 strains of methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Infect. Control Hosp. Epidemiol. 30: 233–241 [DOI] [PubMed] [Google Scholar]
- 125. Jenkins TC, Price CS, Sabel AL, Mehler PS, Burman WJ. 2008. Impact of routine infectious diseases service consultation on the evaluation, management, and outcomes of Staphylococcus aureus bacteremia. Clin. Infect. Dis. 46: 1000–1008 [DOI] [PubMed] [Google Scholar]
- 126. Jensen AG, Espersen F, Skinhoj P, Rosdahl VT, Frimodt-Moller N. 1993. Staphylococcus aureus meningitis. A review of 104 nationwide, consecutive cases. Arch. Intern. Med. 153: 1902–1908 [DOI] [PubMed] [Google Scholar]
- 127. Jensen AG, et al. 2002. Treatment and outcome of Staphylococcus aureus bacteremia: a prospective study of 278 cases. Arch. Intern. Med. 162: 25–32 [DOI] [PubMed] [Google Scholar]
- 128. Jensen VF, et al. 2009. DANMAP 2009—use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, foods and humans in Denmark. http://www.danmap.org/Downloads/Rep.aspx
- 129. Jin T. 2003. Fatal outcome of bacteraemic patients caused by infection with staphylokinase-deficient Staphylococcus aureus strains. J. Med. Microbiol. 52: 919–923 [DOI] [PubMed] [Google Scholar]
- 130. Jones LB, et al. 2008. Chronic granulomatous disease in the United Kingdom and Ireland: a comprehensive national patient-based registry. Clin. Exp. Immunol. 152: 211–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kaech C, et al. 2006. Course and outcome of Staphylococcus aureus bacteraemia: a retrospective analysis of 308 episodes in a Swiss tertiary-care centre. Clin. Microbiol. Infect. 12: 345–352 [DOI] [PubMed] [Google Scholar]
- 132. Kain KC, Schulzer M, Chow AW. 1993. Clinical spectrum of nonmenstrual toxic shock syndrome (TSS): comparison with menstrual TSS by multivariate discriminant analyses. Clin. Infect. Dis. 16: 100–106 [DOI] [PubMed] [Google Scholar]
- 133. Kang C-I, et al. 2010. Clinical impact of methicillin resistance on outcome of patients with Staphylococcus aureus infection: a stratified analysis according to underlying diseases and sites of infection in a large prospective cohort. J. Infect. 61: 299–306 [DOI] [PubMed] [Google Scholar]
- 134. Kang CI, Song JH, Ko KS, Chung DR, Peck KR. 2011. Clinical features and outcome of Staphylococcus aureus infection in elderly versus younger adult patients. Int. J. Infect. Dis. 15: e58–e62 [DOI] [PubMed] [Google Scholar]
- 135. Kaplan SL, et al. 2003. Linezolid versus vancomycin for treatment of resistant Gram-positive infections in children. Pediatr. Infect. Dis. J. 22: 677–686 [DOI] [PubMed] [Google Scholar]
- 136. Katayama Y, Ito T, Hiramatsu K. 2000. A new class of genetic element, Staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 44: 1549–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Kechrid A, et al. 2011. Molecular analysis of community-acquired methicillin-susceptible and resistant Staphylococcus aureus isolates recovered from bacteraemic and osteomyelitis infections in children from Tunisia. Clin. Microbiol. Infect. 17: 1020–1026 [DOI] [PubMed] [Google Scholar]
- 138. Kempker RR, Farley MM, Ladson JL, Satola S, Ray SM. 2010. Association of methicillin-resistant Staphylococcus aureus (MRSA) USA300 genotype with mortality in MRSA bacteremia. J. Infect. 61: 372–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Khatib R, et al. 2006. Persistence in Staphylococcus aureus bacteremia: incidence, characteristics of patients and outcome. Scand. J. Infect. Dis. 38: 7–14 [DOI] [PubMed] [Google Scholar]
- 140. Kim SH, et al. 2008. Outcome of vancomycin treatment in patients with methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 52: 192–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Kim SH, et al. 2006. Outcome of inappropriate empirical antibiotic therapy in patients with Staphylococcus aureus bacteraemia: analytical strategy using propensity scores. Clin. Microbiol. Infect. 12: 13–21 [DOI] [PubMed] [Google Scholar]
- 142. Kim SH, et al. 2003. Outcome of Staphylococcus aureus bacteremia in patients with eradicable foci versus noneradicable foci. Clin. Infect. Dis. 37: 794–799 [DOI] [PubMed] [Google Scholar]
- 143. Klevens RM, et al. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298: 1763–1771 [DOI] [PubMed] [Google Scholar]
- 144. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. 1985. APACHE II: a severity of disease classification system. Crit. Care Med. 13: 818–829 [PubMed] [Google Scholar]
- 145. Kochanek KD, Xu J, Murphy SL, Minino AM, Kung H-C. 2012. Deaths: final data for 2009. National Vital Statistics Report, vol 60, no 3. National Center for Health Statistics, Hyattsville, MD: http://www.cdc.gov/nchs/data_access/vitalstatsonline.htm [PubMed] [Google Scholar]
- 146. Kolata J, et al. 2011. Distinctive patterns in the human antibody response to Staphylococcus aureus bacteremia in carriers and non-carriers. Proteomics 11: 3914–3927 [DOI] [PubMed] [Google Scholar]
- 147. Krakauer T. 1999. Immune response to staphylococcal superantigens. Immunol. Res. 20: 163–173 [DOI] [PubMed] [Google Scholar]
- 148. Kuikka A, Valtonen VV. 1994. Improved outcome of Staphylococcus aureus bacteremia. Infect. Dis. Clin. Pract. 3: 282–287 [Google Scholar]
- 149. Kuint J, et al. 2007. Comparison of community-acquired methicillin-resistant Staphylococcus aureus bacteremia to other staphylococcal species in a neonatal intensive care unit. Eur. J. Pediatr. 166: 319–325 [DOI] [PubMed] [Google Scholar]
- 150. Kumar A, et al. 2006. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 34: 1589–1596 [DOI] [PubMed] [Google Scholar]
- 151. Ladhani S, Konana OS, Mwarumba S, English MC. 2004. Bacteraemia due to Staphylococcus aureus. Arch. Dis. Child. 89: 568–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lalani T, et al. 2010. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation 121: 1005–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Lalani T, et al. 2008. Associations between the genotypes of Staphylococcus aureus bloodstream isolates and clinical characteristics and outcomes of bacteremic patients. J. Clin. Microbiol. 46: 2890–2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Lalueza A, et al. 2010. Is high vancomycin minimum inhibitory concentration a good marker to predict the outcome of methicillin-resistant Staphylococcus aureus bacteremia? J. Infect. Dis. 201: 311–312; author reply, 312-313 [DOI] [PubMed] [Google Scholar]
- 155. Lamagni TL, et al. 2011. Mortality in patients with meticillin-resistant Staphylococcus aureus bacteraemia, England 2004-2005. J. Hosp. Infect. 77: 16–20 [DOI] [PubMed] [Google Scholar]
- 156. Lambert ML, et al. 2011. Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: a cohort study. Lancet Infect. Dis. 11: 30–38 [DOI] [PubMed] [Google Scholar]
- 157. Lamer C, et al. 1993. Analysis of vancomycin entry into pulmonary lining fluid by bronchoalveolar lavage in critically ill patients. Antimicrob. Agents Chemother. 37: 281–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. LaPlante KL, Rybak MJ. 2004. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 48: 4665–4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Larsen M, et al. 2012. Major but differential decline in the incidence of Staphylococcus aureus bacteraemia in HIV-infected individuals from 1995 to 2007: a nationwide cohort study. HIV Med. 13: 45–53 [DOI] [PubMed] [Google Scholar]
- 160. Laupland KB, Ross T, Gregson DB. 2008. Staphylococcus aureus bloodstream infections: risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000-2006. J. Infect. Dis. 198: 336–343 [DOI] [PubMed] [Google Scholar]
- 161. Lautenschlager S, Herzog C, Zimmerli W. 1993. Course and outcome of bacteremia due to Staphylococcus aureus: evaluation of different clinical case definitions. Clin. Infect. Dis. 16: 567–573 [DOI] [PubMed] [Google Scholar]
- 162. Lee S, et al. 2011. Is cefazolin inferior to nafcillin for treatment of methicillin-susceptible Staphylococcus aureus bacteremia? Antimicrob. Agents Chemother. 55: 5122–5126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Leibovici L, et al. 1998. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J. Intern. Med. 244: 379–386 [DOI] [PubMed] [Google Scholar]
- 164. Lesens O, et al. 2003. Role of comorbidity in mortality related to Staphylococcus aureus bacteremia: a prospective study using the Charlson weighted index of comorbidity. Infect. Control Hosp. Epidemiol. 24: 890–896 [DOI] [PubMed] [Google Scholar]
- 165. Levine DP, Fromm BS, Reddy BR. 1991. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann. Intern. Med. 115: 674–680 [DOI] [PubMed] [Google Scholar]
- 166. Li M, et al. 2009. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 106: 5883–5888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Liao C-H, Chen S-Y, Huang Y-T, Hsueh P-R. 2008. Outcome of patients with meticillin-resistant Staphylococcus aureus bacteraemia at an emergency department of a medical centre in Taiwan. Int. J. Antimicrob. Agents 32: 326–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Libert M, et al. 2008. Risk factors for meticillin resistance and outcome of Staphylococcus aureus bloodstream infection in a Belgian university hospital. J. Hosp. Infect. 68: 17–24 [DOI] [PubMed] [Google Scholar]
- 169. Libman H, Arbeit RD. 1984. Complications associated with Staphylococcus aureus bacteraemia. Arch. Intern. Med. 144: 541–545 [PubMed] [Google Scholar]
- 170. Lin SH, Lai CC, Tan CK, Liao WH, Hsueh PR. 2011. Comparative efficacy of vancomycin and teicoplanin in the treatment of hospitalised elderly patients with persistent meticillin-resistant Staphylococcus aureus (MRSA) bacteraemia. Int. J. Antimicrob. Agents 37: 179–181 [DOI] [PubMed] [Google Scholar]
- 171. Lin SH, et al. 2010. Risk factors for mortality in patients with persistent methicillin-resistant Staphylococcus aureus bacteraemia in a tertiary care hospital in Taiwan. J. Antimicrob. Chemother. 65: 1792–1798 [DOI] [PubMed] [Google Scholar]
- 172. Liu C, et al. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52: e18–e55 [DOI] [PubMed] [Google Scholar]
- 173. Lodise TP, et al. 2008. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob. Agents Chemother. 52: 3315–3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Lodise TP, Jr, McKinnon PS, Levine DP, Rybak MJ. 2007. Impact of empirical-therapy selection on outcomes of intravenous drug users with infective endocarditis caused by methicillin-susceptible Staphylococcus aureus. Antimicrob. Agents Chemother. 51: 3731–3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Lodise TP, McKinnon PS. 2005. Clinical and economic impact of methicillin resistance in patients with Staphylococcus aureus bacteremia. Diagn. Microbiol. Infect. Dis. 52: 113–122 15964499 [Google Scholar]
- 176. Lodise TP, McKinnon PS, Swiderski L, Rybak MJ. 2003. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin. Infect. Dis. 36: 1418–1423 [DOI] [PubMed] [Google Scholar]
- 177. Lustberg ME. 2008. Shock as a covariate in a study of treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 46: 1483; author reply, 1484-1485 [DOI] [PubMed] [Google Scholar]
- 178. Lyon GJ, Mayville P, Muir TW, Novick RP. 2000. Rational design of a global inhibitor of the virulence response in Staphylococcus aureus, based in part on localization of the site of inhibition to the receptor-histidine kinase, AgrC. Proc. Natl. Acad. Sci. U. S. A. 97: 13330–13335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Lyon GJ, Wright JS, Christopoulos A, Novick RP, Muir TW. 2002. Reversible and specific extracellular antagonism of receptor-histidine kinase signaling. J. Biol. Chem. 277: 6247–6253 [DOI] [PubMed] [Google Scholar]
- 180. Lyytikainen O, Ruotsalainen E, Jarvinen A, Valtonen V, Ruutu P. 2005. Trends and outcome of nosocomial and community-acquired bloodstream infections due to Staphylococcus aureus in Finland, 1995-2001. Eur. J. Clin. Microbiol. Infect. Dis. 24: 399–404 [DOI] [PubMed] [Google Scholar]
- 181. Maclayton DO, Suda KJ, Coval KA, York CB, Garey KW. 2006. Case-control study of the relationship between MRSA bacteremia with a vancomycin MIC of 2 microg/mL and risk factors, costs, and outcomes in inpatients undergoing hemodialysis. Clin. Ther. 28: 1208–1216 [DOI] [PubMed] [Google Scholar]
- 182. MacNeal WJ, Frisbee FC. 1936. One hundred patients with Staphyloccocus septicemia receiving bacteriophage service. Am. J. Med. Sci. 191: 179–189 [Google Scholar]
- 183. Malachowa N, et al. 2011. Global changes in Staphylococcus aureus gene expression in human blood. PLoS One 6: e18617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Malani PN, Rana MM, Banerjee M, Bradley SF. 2008. Staphylococcus aureus bloodstream infections: the association between age and mortality and functional status. J. Am. Geriatr. Soc. 56: 1485–1489 [DOI] [PubMed] [Google Scholar]
- 185. Maor Y, et al. 2009. Clinical features of heteroresistant vancomycin-intermediate Staphylococcus aureus bacteremia versus those of methicillin-resistant S. aureus bacteremia. J. Infect. Dis. 199: 619–624 [DOI] [PubMed] [Google Scholar]
- 186. Maor Y, et al. 2007. Prevalence and characteristics of heteroresistant vancomycin-intermediate Staphylococcus aureus bacteremia in a tertiary care center. J. Clin. Microbiol. 45: 1511–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Marchaim D, et al. 2010. Case-control study to identify factors associated with mortality among patients with methicillin-resistant Staphylococcus aureus bacteraemia. Clin. Microbiol. Infect. 16: 747–752 [DOI] [PubMed] [Google Scholar]
- 188. Markowitz N, Quinn EL, Saravolatz LD. 1992. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Ann. Intern. Med. 117: 390–398 [DOI] [PubMed] [Google Scholar]
- 189. Marrack P, Kappler J. 1990. The staphylococcal enterotoxins and their relatives. Science 248: 705–711 [DOI] [PubMed] [Google Scholar]
- 190. Matsuhashi M, et al. 1986. Molecular cloning of the gene of a penicillin-binding protein supposed to cause high resistance to beta-lactam antibiotics in Staphylococcus aureus. J. Bacteriol. 167: 975–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190a. McCabe WR, Jackson GG. 1967. Gram-negative bacteremia. II. Clinical laboratory, and therapeutic observations. Arch. Intern. Med. 110: 856–864 [Google Scholar]
- 191. McCallum N, et al. 2006. In vivo survival of teicoplanin-resistant Staphylococcus aureus and fitness cost of teicoplanin resistance. Antimicrob. Agents Chemother. 50: 2352–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. McClelland RS, et al. 1999. Staphylococcus aureus bacteremia among elderly vs younger adult patients: comparison of clinical features and mortality. Arch. Intern. Med. 159: 1244–1247 [DOI] [PubMed] [Google Scholar]
- 193. McCormick JK, Yarwood JM, Schlievert PM. 2001. Toxic shock sydnrome and bacterial superantigens: an update. Annu. Rev. Microbiol. 55: 77–104 [DOI] [PubMed] [Google Scholar]
- 194. McDonald E, Bailie R, Brewster D, Morris P. 2008. Are hygiene and public health interventions likely to improve outcomes for Australian Aboriginal children living in remote communities? A systematic review of the literature. BMC Public Health 8: 153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Melzer M, Eykyn SJ, Gransden WR, Chinn S. 2003. Is methicillin-resistant Staphylococcus aureus more virulent than methicillin-susceptible S. aureus? A comparative cohort study of British patients with nosocomial infection and bacteremia. Clin. Infect. Dis. 37: 1453–1460 [DOI] [PubMed] [Google Scholar]
- 196. Mendell TH. 1939. Staphylococcic septicemia—a review of thirty-five cases, with six recoveries, twenty-nine deaths and sixteen autopsies. Arch. Intern. Med. 63: 1068–1083 [Google Scholar]
- 197. Miro JM, et al. 2005. Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis Merged Database. Clin. Infect. Dis. 41: 507–514 [DOI] [PubMed] [Google Scholar]
- 198. Mohr JF, Murray BE. 2007. Vancomycin is not obsolete for the treatment of infection caused by methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 44: 1536–1542 [DOI] [PubMed] [Google Scholar]
- 199. Moise-Broder PA, et al. 2004. Accessory gene regulator group II polymorphism in methicillin-resistant Staphylococcus aureus is predictive of failure of vancomycin therapy. Clin. Infect. Dis. 38: 1700–1705 [DOI] [PubMed] [Google Scholar]
- 200. Morgan MS. 2007. Diagnosis and treatment of Panton-Valentine leukocidin (PVL)-associated staphylococcal pneumonia. Int. J. Antimicrob. Agents 30: 289–296 [DOI] [PubMed] [Google Scholar]
- 201. Morin CA, Hadler JL. 2001. Population-based incidence and characteristics of community-onset Staphylococcus aureus infections with bacteremia in 4 metropolitan Connecticut areas, 1998. J. Infect. Dis. 184: 1029–1034 [DOI] [PubMed] [Google Scholar]
- 202. Musta AC, et al. 2009. Vancomycin MIC plus heteroresistance and outcome of methicillin-resistant Staphylococcus aureus bacteremia: trends over 11 years. J. Clin. Microbiol. 47: 1640–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Muttaiyah S, et al. 2010. Incidence, risk factors, and outcomes of Panton-Valentine leukocidin-positive methicillin-susceptible Staphylococcus aureus infections in Auckland, New Zealand. J. Clin. Microbiol. 48: 3470–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Mylotte JM, Aeschlimann JR, Rotella DL. 1996. Staphylococcus aureus bacteremia: factors predicting hospital mortality. Infect. Control Hosp. Epidemiol. 17: 165–168 [DOI] [PubMed] [Google Scholar]
- 205. Mylotte JM, McDermott C. 1987. Staphylococcus aureus bacteraemia caused by infected intravenous catheters. Am. J. Infect. Control 15: 1–6 [DOI] [PubMed] [Google Scholar]
- 206. Mylotte JM, Tayara A. 2000. Staphylococcus aureus bacteremia: predictors of 30-day mortality in a large cohort. Clin. Infect. Dis. 31: 1170–1174 [DOI] [PubMed] [Google Scholar]
- 207. Naber CK, et al. 2009. Clinical consensus conference: survey on Gram-positive bloodstream infections with a focus on Staphylococcus aureus. Clin. Infect. Dis. 48 (Suppl 4): S260–S270 [DOI] [PubMed] [Google Scholar]
- 208. Nannini EC, et al. 2009. Inoculum effect with cefazolin among clinical isolates of methicillin-susceptible Staphylococcus aureus: frequency and possible cause of cefazolin treatment failure. Antimicrob. Agents Chemother. 53: 3437–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Neoh HM, et al. 2007. Impact of reduced vancomycin susceptibility on the therapeutic outcome of MRSA bloodstream infections. Ann. Clin. Microbiol. Antimicrob. 6: 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Neuner EA, Casabar E, Reichley R, McKinnon PS. 2010. Clinical, microbiologic, and genetic determinants of persistent methicillin-resistant Staphylococcus aureus bacteremia. Diagn. Microbiol. Infect. Dis. 67: 228–233 [DOI] [PubMed] [Google Scholar]
- 211. Nguyen HM, Graber CJ. 2010. Limitations of antibiotic options for invasive infections caused by methicillin-resistant Staphylococcus aureus: is combination therapy the answer? J. Antimicrob. Chemother. 65: 24–36 [DOI] [PubMed] [Google Scholar]
- 212. Nickerson EK, et al. 2009. Factors predicting and reducing mortality in patients with invasive Staphylococcus aureus disease in a developing country. PLoS One 4: e6512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Nilsson IM, Hartford O, Foster T, Tarkowski A. 1999. Alpha-toxin and gamma-toxin jointly promote Staphylococcus aureus virulence in murine septic arthritis. Infect. Immun. 67: 1045–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Osrin D, Vergnano S, Costello A. 2004. Serious bacterial infections in newborn infants in developing countries. Curr. Opin. Infect. Dis. 17: 217–224 [DOI] [PubMed] [Google Scholar]
- 215. Paganini HR, et al. 2010. Community-acquired Staphylococcus aureus bacteremia: 17 years of experience in Argentine children. Arch. Argent. Pediatr. 108: 311–317 [DOI] [PubMed] [Google Scholar]
- 216. Paganini HR, Gonzalez F, Casimir L, Rosanova MT. 1997. Community-acquired Staphylococcus aureus bacteremia in children: analysis of mortality risk factors. Medicina (B. Aires) 57: 281–286 (In Spanish.) [PubMed] [Google Scholar]
- 217. Panton PN, Valentine FCO. 1932. Staphylococcal toxin. Lancet 219: 506–508 [Google Scholar]
- 218. Park SY, Son JS, Oh IH, Choi JM, Lee MS. 2011. Clinical impact of methicillin-resistant Staphylococcus aureus bacteremia based on propensity scores. Infection 39: 141–147 [DOI] [PubMed] [Google Scholar]
- 219. Patel N, et al. 2011. Vancomycin: we can't get there from here. Clin. Infect. Dis. 52: 969–974 [DOI] [PubMed] [Google Scholar]
- 220. Paul M, et al. 2010. Importance of appropriate empirical antibiotic therapy for methicillin-resistant Staphylococcus aureus bacteraemia. J. Antimicrob. Chemother. 65: 2658–2665 [DOI] [PubMed] [Google Scholar]
- 221. Paul M, et al. 2010. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob. Agents Chemother. 54: 4851–4863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Paul M, et al. 2011. Are all beta-lactams similarly effective in the treatment of methicillin-sensitive Staphylococcus aureus bacteraemia? Clin. Microbiol. Infect. 17: 1581–1586 [DOI] [PubMed] [Google Scholar]
- 223. Pedersen M, Benfield TL, Skinhoej P, Jensen AG. 2006. Haematogenous Staphylococcus aureus meningitis. A 10-year nationwide study of 96 consecutive cases. BMC Infect. Dis. 6: 49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Perencevich EN. 2000. Excess shock and mortality in Staphylococcus aureus related to methicillin resistance. Clin. Infect. Dis. 31: 1311–1313 [DOI] [PubMed] [Google Scholar]
- 225. Perez-Jorge EV, Burdette SD, Markert RJ, Beam WB. 2010. Staphylococcus aureus bacteremia (SAB) with associated S. aureus bacteriuria (SABU) as a predictor of complications and mortality. J. Hosp. Med. 5: 208–211 [DOI] [PubMed] [Google Scholar]
- 226. Perlroth J, Kuo M, Tan J, Bayer AS, Miller LG. 2008. Adjunctive use of rifampin for the treatment of Staphylococcus aureus infections: a systematic review of the literature. Arch. Intern. Med. 168: 805–819 [DOI] [PubMed] [Google Scholar]
- 227. Perovic O, et al. 2006. Staphylococcus aureus bacteraemia at two academic hospitals in Johannesburg. S. Afr. Med. J. 96: 714–717 [PubMed] [Google Scholar]
- 228. Pintado V, et al. 2002. Clinical study of 44 cases of Staphylococcus aureus meningitis. Eur. J. Clin. Microbiol. Infect. Dis. 21: 864–868 [DOI] [PubMed] [Google Scholar]
- 229. Popovich KJ, Weinstein RA, Hota B. 2008. Are community-associated methicillin-resistant Staphylococcus aureus (MRSA) strains replacing traditional nosocomial MRSA strains? Clin. Infect. Dis. 46: 787–794 [DOI] [PubMed] [Google Scholar]
- 230. Porath AD, Brooks GD. 2008. Vancomycin minimum inhibitory concentration as a predictor of mortality in methicillin-resistant Staphylococcus aureus bacteremia: a second look. Clin. Infect. Dis. 46: 1483–1484; author reply, 1484-1485 [DOI] [PubMed] [Google Scholar]
- 231. Prevost G, et al. 1995. Panton-Valentine leucocidin and gamma-hemolysin from Staphylococcus aureus ATCC 49775 are encoded by distinct genetic loci and have different biological activities. Infect. Immun. 63: 4121–4129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Price J, Atkinson S, Llewelyn M, Paul J. 2009. Paradoxical relationship between the clinical outcome of Staphylococcus aureus bacteremia and the minimum inhibitory concentration of vancomycin. Clin. Infect. Dis. 48: 997–998 [DOI] [PubMed] [Google Scholar]
- 233. Public Health Laboratory Service Communicable Diseases Surveillance Centre 2003. Staphylococcus aureus bacteraemia: England, Wales, and Northern Ireland, January to December 2002. CDR Wkly. 13 (12): 1203 http://www.hpa.org.uk/cdr/archives/2003/cdr1203.pdf [Google Scholar]
- 234. Quinn EL, et al. 1973. Clinical experiences with cefazolin and other cephalosporins in bacterial endocarditis. J. Infect. Dis. 128 (Suppl): S386–S389 [DOI] [PubMed] [Google Scholar]
- 235. Raad, Sabbagh MF. 1992. Optimal duration of therapy for catheter-related Staphylococcus aureus bacteremia: a study of 55 cases and review. Clin. Infect. Dis. 14: 75–82 [DOI] [PubMed] [Google Scholar]
- 236. Riedel DJ, Weekes E, Forrest GN. 2008. Addition of rifampin to standard therapy for treatment of native valve infective endocarditis caused by Staphylococcus aureus. Antimicrob. Agents Chemother. 52: 2463–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Rieg S, et al. 2009. Mortality of S. aureus bacteremia and infectious diseases specialist consultation—a study of 521 patients in Germany. J. Infect. 59: 232–239 [DOI] [PubMed] [Google Scholar]
- 238. Ringberg H, Thoren A, Lilja B. 2000. Metastatic complications of Staphylococcus aureus septicemia. To seek is to find. Infection 28: 132–136 [DOI] [PubMed] [Google Scholar]
- 239. Robinson JL, Rennie RP. 2002. Outcome of bacteremia and fungemia in paediatric oncology patients. Can. J. Infect. Dis. 13: 375–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Robinson JO, Pearson JC, Christiansen KJ, Coombs GW, Murray RJ. 2009. Community-associated versus healthcare-associated methicillin-resistant Staphylococcus aureus bacteraemia: a 10-year retrospective review. Eur. J. Clin. Microbiol. Infect. Dis. 28: 353–361 [DOI] [PubMed] [Google Scholar]
- 241. Røder BL, et al. 1995. No difference in enterotoxin production among Staphylococcus aureus strains isolated from blood compared with strains isolated from healthy carriers. J. Med. Microbiol. 42: 43–47 [DOI] [PubMed] [Google Scholar]
- 242. Rogers BA, Drake AK, Spelman D. 2009. Methicillin resistant Staphylococcus aureus endocarditis in an Australian tertiary hospital: 1991-2006. Heart Lung Circ. 18: 208–213 [DOI] [PubMed] [Google Scholar]
- 243. Roghmann MC. 2000. Predicting methicillin resistance and the effect of inadequate empiric therapy on survival in patients with Staphylococcus aureus bacteremia. Arch. Intern. Med. 160: 1001–1004 [DOI] [PubMed] [Google Scholar]
- 244. Romero-Vivas J, Rubio M, Fernandez C, Picazo JJ. 1995. Mortality associated with nosocomial bacteremia due to methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 21: 1417–1423 [DOI] [PubMed] [Google Scholar]
- 245. Rose WE, Rybak MJ, Tsuji BT, Kaatz GW, Sakoulas G. 2007. Correlation of vancomycin and daptomycin susceptibility in Staphylococcus aureus in reference to accessory gene regulator (agr) polymorphism and function. J. Antimicrob. Chemother. 59: 1190–1193 [DOI] [PubMed] [Google Scholar]
- 246. Ruokonen EM, Takala JMP, Kari AMP, Alhava E. 1991. Septic shock and multiple organ failure. Crit. Care Med. 19: 1146–1151 [DOI] [PubMed] [Google Scholar]
- 247. Ruotsalainen E, et al. 2006. Levofloxacin does not decrease mortality in Staphylococcus aureus bacteraemia when added to the standard treatment: a prospective and randomized clinical trial of 381 patients. J. Intern. Med. 259: 179–190 [DOI] [PubMed] [Google Scholar]
- 248. Sabath LD, Garner C, Wilcox C, Finland M. 1975. Effect of inoculum and of beta-lactamase on the anti-staphylococcal activity of thirteen penicillins and cephalosporins. Antimicrob. Agents Chemother. 8: 344–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Saha B, Singh AK, Ghosh A, Bal M. 2008. Identification and characterization of a vancomycin-resistant Staphylococcus aureus isolated from Kolkata (South Asia). J. Med. Microbiol. 57: 72–79 [DOI] [PubMed] [Google Scholar]
- 250. Sakoulas G, et al. 2005. Reduced susceptibility of Staphylococcus aureus to vancomycin and platelet microbicidal protein correlates with defective autolysis and loss of accessory gene regulator (agr) function. Antimicrob. Agents Chemother. 49: 2687–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Sakoulas G, et al. 2003. Staphylococcus aureus accessory gene regulator (agr) group II: is there a relationship to the development of intermediate-level glycopeptide resistance? J. Infect. Dis. 187: 929–938 [DOI] [PubMed] [Google Scholar]
- 252. Sakoulas G, et al. 2004. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J. Clin. Microbiol. 42: 2398–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Salgado CD, Farr BM, Calfee DP. 2003. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin. Infect. Dis. 36: 131–139 [DOI] [PubMed] [Google Scholar]
- 254. Schaffer AC, Lee JC. 2009. Staphylococcal vaccines and immunotherapies. Infect. Dis. Clin. North Am. 23: 153–171 [DOI] [PubMed] [Google Scholar]
- 255. Schlievert PM, McCormick JK, Bohach GA, Ohlendorf DH. 2009. Exotoxins, p 125–146 In Crossley KB, Jefferson KK, Archer GL, Fowler J, Vance G. (ed), Staphylococci in human disease, 2nd ed. Wiley-Blackwell, West Sussex, United Kingdom: [Google Scholar]
- 256. Schramm GE, Johnson JA, Doherty JA, Micek ST, Kollef MH. 2006. Methicillin-resistant Staphylococcus aureus sterile-site infection: the importance of appropriate initial antimicrobial treatment. Crit. Care Med. 34: 2069–2074 [DOI] [PubMed] [Google Scholar]
- 257. Schweizer ML, et al. 2010. Empiric antibiotic therapy for Staphylococcus aureus bacteremia may not reduce in-hospital mortality: a retrospective cohort study. PLoS One 5: e11432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Schweizer ML, et al. 2011. Increased mortality with accessory gene regulator (agr) dysfunction in Staphylococcus aureus among bacteremic patients. Antimicrob. Agents Chemother. 55: 1082–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Seidl K, et al. 2011. Combinatorial phenotypic signatures distinguish persistent from resolving methicillin-resistant Staphylococcus aureus bacteremia isolates. Antimicrob. Agents Chemother. 55: 575–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Senthilkumar A, Kumar S, Sheagren JN. 2001. Increased incidence of Staphylococcus aureus bacteremia in hospitalized patients with acquired immunodeficiency syndrome. Clin. Infect. Dis. 33: 1412–1416 [DOI] [PubMed] [Google Scholar]
- 261. Seybold U, et al. 2006. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin. Infect. Dis. 42: 647–656 [DOI] [PubMed] [Google Scholar]
- 262. Shime N, Kosaka T, Fujita N. 2010. The importance of a judicious and early empiric choice of antimicrobial for methicillin-resistant Staphylococcus aureus bacteraemia. Eur. J. Clin. Microbiol. Infect. Dis. 29: 1475–1479 [DOI] [PubMed] [Google Scholar]
- 263. Shinefield H, et al. 2002. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N. Engl. J. Med. 346: 491–496 [DOI] [PubMed] [Google Scholar]
- 264. Shopsin B, et al. 2003. Prevalence of agr specificity groups among Staphylococcus aureus strains colonizing children and their guardians. J. Clin. Microbiol. 41: 456–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Shorr AF, et al. 2006. Healthcare-associated bloodstream infection: a distinct entity? Insights from a large U.S. database. Crit. Care Med. 34: 2588–2595 [DOI] [PubMed] [Google Scholar]
- 266. Shurland S, Zhan M, Bradham DD, Roghmann M-C. 2007. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect. Control Hosp. Epidemiol. 28: 273–279 [DOI] [PubMed] [Google Scholar]
- 267. Siegman-Igra Y, Reich P, Orni-Wasserlauf R, Schwartz D, Giladi M. 2005. The role of vancomycin in the persistence or recurrence of Staphylococcus aureus bacteraemia. Scand. J. Infect. Dis. 37: 572–578 [DOI] [PubMed] [Google Scholar]
- 268. Sievert DM, et al. 2008. Vancomycin-resistant Staphylococcus aureus in the United States, 2002-2006. Clin. Infect. Dis. 46: 668–674 [DOI] [PubMed] [Google Scholar]
- 269. Sigauque B, et al. 2009. Community-acquired bacteremia among children admitted to a rural hospital in Mozambique. Pediatr. Infect. Dis. J. 28: 108–113 [DOI] [PubMed] [Google Scholar]
- 270. Skov R, et al. 1995. Staphylococcus aureus bacteremia: a 14-year nationwide study in hematological patients with malignant disease or agranulocytosis. Scand. J. Infect. Dis. 27: 563–568 [DOI] [PubMed] [Google Scholar]
- 271. Small PM, Chambers HF. 1990. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob. Agents Chemother. 34: 1227–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Song MD, Wachi M, Doi M, Ishino F, Matsuhashi M. 1987. Evolution of an inducible penicillin-target protein in methicillin-resistant Staphylococcus aureus by gene fusion. FEBS Lett. 221: 167–171 [DOI] [PubMed] [Google Scholar]
- 273. Soriano A, et al. 2008. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 46: 193–200 [DOI] [PubMed] [Google Scholar]
- 274. Soriano A, Martinez JA, Mensa J. 2008. Reply to Lustberg and Porath and Brooks. Clin. Infect. Dis. 46: 1484–1485 [Google Scholar]
- 275. Soriano A, et al. 2000. Pathogenic significance of methicillin resistance for patients with Staphylococcus aureus bacteremia. Clin. Infect. Dis. 30: 368–373 [DOI] [PubMed] [Google Scholar]
- 276. Soriano A, et al. 2000. Reply. Clin. Infect. Dis. 31: 1311–1313 [DOI] [PubMed] [Google Scholar]
- 277. Srinivasan A, et al. 2010. Staphylococcus aureus bacteremia in pediatric patients with cancer. Pediatr. Infect. Dis. J. 29: 172–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Stevens DL, et al. 2002. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin. Infect. Dis. 34: 1481–1490 [DOI] [PubMed] [Google Scholar]
- 279. Stoll BJ, et al. 2002. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 110: 285–291 [DOI] [PubMed] [Google Scholar]
- 280. Storch GA, Rajagopalan L. 1986. Methicillin-resistant Staphylococcus aureus bacteremia in children. Pediatr. Infect. Dis. 5: 59–67 [DOI] [PubMed] [Google Scholar]
- 281. Stryjewski ME, et al. 2007. Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clin. Infect. Dis. 44: 190–196 [DOI] [PubMed] [Google Scholar]
- 282. Suryati BA, Watson M. 2002. Staphylococcus aureus bacteraemia in children: a 5-year retrospective review. J. Paediatr. Child Health 38: 290–294 [DOI] [PubMed] [Google Scholar]
- 283. Svetitsky S, Leibovici L, Paul M. 2009. Comparative efficacy and safety of vancomycin versus teicoplanin: systematic review and meta-analysis. Antimicrob. Agents Chemother. 53: 4069–4079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Tacconelli E, Pop-Vicas AE, D'Agata EM. 2006. Increased mortality among elderly patients with meticillin-resistant Staphylococcus aureus bacteraemia. J. Hosp. Infect. 64: 251–256 [DOI] [PubMed] [Google Scholar]
- 285. Takesue Y, et al. 2011. Clinical characteristics of vancomycin minimum inhibitory concentration of 2 mug/ml methicillin-resistant Staphylococcus aureus strains isolated from patients with bacteremia. J. Infect. Chemother. 17: 52–57 [DOI] [PubMed] [Google Scholar]
- 286. Talon D, et al. 2002. The impact of resistance to methicillin in Staphylococcus aureus bacteremia on mortality. Eur. J. Intern. Med. 13: 31–36 [DOI] [PubMed] [Google Scholar]
- 287. Tao M, Yamashita H, Watanabe K, Nagatake T. 1999. Possible virulence factors of Staphylococcus aureus in a mouse septic model. FEMS Immunol. Med. Microbiol. 23: 135–146 [DOI] [PubMed] [Google Scholar]
- 288. Tenover FC, Moellering RC., Jr 2007. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin. Infect. Dis. 44: 1208–1215 [DOI] [PubMed] [Google Scholar]
- 289. Tong SY, et al. 2009. Community-associated strains of methicillin-resistant Staphylococcus aureus and methicillin-susceptible S. aureus in indigenous Northern Australia: epidemiology and outcomes. J. Infect. Dis. 199: 1461–1470 [DOI] [PubMed] [Google Scholar]
- 290. Tong SY, et al. 2010. Clinical correlates of Panton-Valentine leukocidin (PVL), PVL isoforms, and clonal complex in the Staphylococcus aureus population of Northern Australia. J. Infect. Dis. 202: 760–769 [DOI] [PubMed] [Google Scholar]
- 291. Topeli A, Unal S, Akalin HE. 2000. Risk factors influencing clinical outcome in Staphylococcus aureus bacteraemia in a Turkish University Hospital. Int. J. Antimicrob. Agents 14: 57–63 [DOI] [PubMed] [Google Scholar]
- 292. Traber K, Novick R. 2006. A slipped-mispairing mutation in AgrA of laboratory strains and clinical isolates results in delayed activation of agr and failure to translate delta- and alpha-haemolysins. Mol. Microbiol. 59: 1519–1530 [DOI] [PubMed] [Google Scholar]
- 293. Tsuji BT, Harigaya Y, Lesse AJ, Sakoulas G, Mylotte JM. 2009. Loss of vancomycin bactericidal activity against accessory gene regulator (agr) dysfunctional Staphylococcus aureus under conditions of high bacterial density. Diagn. Microbiol. Infect. Dis. 64: 220–224 [DOI] [PubMed] [Google Scholar]
- 294. Tsuji BT, MacLean RD, Dresser LD, McGavin MJ, Simor AE. 2011. Impact of accessory gene regulator (agr) dysfunction on vancomycin pharmacodynamics among Canadian community and health-care associated methicillin-resistant Staphylococcus aureus. Ann. Clin. Microbiol. Antimicrob. 10: 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Tumbarello M, et al. 2002. Risk factors and predictors of mortality of methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia in HIV-infected patients. J. Antimicrob. Chemother. 50: 375–382 [DOI] [PubMed] [Google Scholar]
- 296. Turnidge JD, et al. 2009. Staphylococcus aureus bacteraemia: a major cause of mortality in Australia and New Zealand. Med. J. Aust. 191: 368–373 [DOI] [PubMed] [Google Scholar]
- 297. Valente AM, et al. 2005. Frequency of infective endocarditis among infants and children with Staphylococcus aureus bacteremia. Pediatrics 115: e15–e19 [DOI] [PubMed] [Google Scholar]
- 298. Van der Auwera P, Klastersky J, Thys JP, Meunier-Carpentier F, Legrand JC. 1985. Double-blind, placebo-controlled study of oxacillin combined with rifampin in the treatment of staphylococcal infections. Antimicrob. Agents Chemother. 28: 467–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Van der Auwera P, Meunier-Carpentier F, Klastersky J. 1983. Clinical study of combination therapy with oxacillin and rifampin for staphylococcal infections. Rev. Infect. Dis. 5 (Suppl 3): S515–S522 [DOI] [PubMed] [Google Scholar]
- 300. van Hal SJ, et al. 2011. Methicillin-resistant Staphylococcus aureus vancomycin susceptibility testing: methodology correlations, temporal trends and clonal patterns. J. Antimicrob. Chemother. 66: 2284–2287 [DOI] [PubMed] [Google Scholar]
- 301. van Hal SJ, Jones M, Gosbell IB, Paterson DL. 2011. Vancomycin heteroresistance is associated with reduced mortality in ST239 methicillin-resistant Staphylococcus aureus blood stream infections. PLoS One 6: e21217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. van Hal SJ, Paterson DL. 2011. Systematic review and meta-analysis of the significance of heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 55: 405–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. van Hal SJ, et al. 2011. Performance of various testing methodologies for detection of heteroresistant vancomycin-intermediate Staphylococcus aureus in bloodstream isolates. J. Clin. Microbiol. 49: 1489–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. van Leeuwen W, van Nieuwenhuizen W, Gijzen C, Verbrugh H, van Belkum A. 2000. Population studies of methicillin-resistant and -sensitive Staphylococcus aureus strains reveal a lack of variability in the agrD gene, encoding a staphylococcal autoinducer peptide. J. Bacteriol. 182: 5721–5729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Venditti M, et al. 2003. Staphylococcus aureus bacteremia in patients with hematologic malignancies: a retrospective case-control study. Haematologicaae 88: 923–930 [PubMed] [Google Scholar]
- 306. Verkaik NJ, et al. 2010. Heterogeneity of the humoral immune response following Staphylococcus aureus bacteremia. Eur. J. Clin. Microbiol. Infect. Dis. 29: 509–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Vikram HR, Buenconsejo J, Hasbun R, Quagliarello VJ. 2003. Impact of valve surgery on 6-month mortality in adults with complicated, left-sided native valve endocarditis: a propensity analysis. JAMA 290: 3207–3214 [DOI] [PubMed] [Google Scholar]
- 308. Wang F-D, Chen Y-Y, Chen T-L, Liu C-Y. 2008. Risk factors and mortality in patients with nosocomial Staphylococcus aureus bacteremia. Am. J. Infect. Control 36: 118–122 [DOI] [PubMed] [Google Scholar]
- 309. Wang J-T, et al. 2010. Risk factors for mortality of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) bloodstream infection: with investigation of the potential role of community-associated MRSA strains. J. Infect. 61: 449–457 [DOI] [PubMed] [Google Scholar]
- 310. Wang JL, et al. 2008. Comparison of both clinical features and mortality risk associated with bacteremia due to community-acquired methicillin-resistant Staphylococcus aureus and methicillin-susceptible S. aureus. Clin. Infect. Dis. 46: 799–806 [DOI] [PubMed] [Google Scholar]
- 311. Wang JL, et al. 2007. Adult methicillin-resistant Staphylococcus aureus bacteremia in Taiwan: clinical significance of non-multi-resistant antibiogram and Panton-Valentine leukocidin gene. Diagn. Microbiol. Infect. Dis. 59: 365–371 [DOI] [PubMed] [Google Scholar]
- 312. Wang JL, Wang JT, Sheng WH, Chen YC, Chang SC. 2010. Nosocomial methicillin-resistant Staphylococcus aureus (MRSA) bacteremia in Taiwan: mortality analyses and the impact of vancomycin, MIC = 2 mg/L, by the broth microdilution method. BMC Infect. Dis. 10: 159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Watanakunakorn C, Baird IM. 1977. Staphylococcus aureus bacteraemia and endocarditis associated with a removable infected intravenous device. Am. J. Med. 63: 253–256 [DOI] [PubMed] [Google Scholar]
- 314. Wehrhahn MC, et al. 2010. Clinical and laboratory features of invasive community-onset methicillin-resistant Staphylococcus aureus infection: a prospective case-control study. Eur. J. Clin. Microbiol. Infect. Dis. 29: 1025–1033 [DOI] [PubMed] [Google Scholar]
- 315. Welsh KJ, et al. 2010. Clinical characteristics, outcomes, and microbiologic features associated with methicillin-resistant Staphylococcus aureus bacteremia in pediatric patients treated with vancomycin. J. Clin. Microbiol. 48: 894–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Wertheim HF, et al. 2004. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 364: 703–705 [DOI] [PubMed] [Google Scholar]
- 317. Whitby M, McLaws ML, Berry G. 2001. Risk of death from methicillin-resistant Staphylococcus aureus bacteraemia: a meta-analysis. Med. J. Aust. 175: 264–267 [DOI] [PubMed] [Google Scholar]
- 318. Wilcox MH, et al. 2009. Complicated skin and skin-structure infections and catheter-related bloodstream infections: noninferiority of linezolid in a phase 3 study. Clin. Infect. Dis. 48: 203–212 [DOI] [PubMed] [Google Scholar]
- 319. Wilson AP, Gruneberg RN, Neu H. 1994. A critical review of the dosage of teicoplanin in Europe and the USA. Int. J. Antimicrob. Agents 4 (Suppl 1): 1–30 [DOI] [PubMed] [Google Scholar]
- 320. Wilson R, Hamburger M. 1957. Fifteen years' experience with Staphylococcus septicemia in a large city hospital; analysis of fifty-five cases in the Cincinnati General Hospital 1940 to 1954. Am. J. Med. 22: 437–457 [DOI] [PubMed] [Google Scholar]
- 321. Wolkewitz M, Frank U, Philips G, Schumacher M, Davey P. 2011. Mortality associated with in-hospital bacteraemia caused by Staphylococcus aureus: a multistate analysis with follow-up beyond hospital discharge. J. Antimicrob. Chemother. 66: 381–386 [DOI] [PubMed] [Google Scholar]
- 322. Wyllie DH, Crook DW, Peto TEA. 2006. Mortality after Staphylococcus aureus bacteraemia in two hospitals in Oxfordshire, 1997-2003: cohort study. BMJ 333: 281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Yoon YK, Kim JY, Park DW, Sohn JW, Kim MJ. 2010. Predictors of persistent methicillin-resistant Staphylococcus aureus bacteraemia in patients treated with vancomycin. J. Antimicrob. Chemother. 65: 1015–1018 [DOI] [PubMed] [Google Scholar]
- 324. Yzerman EPF, et al. 1996. ΔAPACHE II for predicting course and outcome of nosocomial Staphylococcus aureus bacteremia and its relation to host defense. J. Infect. Dis. 173: 914–919 [DOI] [PubMed] [Google Scholar]
- 325. Zaidi AK, et al. 2005. Hospital-acquired neonatal infections in developing countries. Lancet 365: 1175–1188 [DOI] [PubMed] [Google Scholar]
- 326. Zaidi AKM, Thaver D, Ali SA, Khan TA. 2009. Pathogens associated with sepsis in newborns and young infants in developing countries. Pediatr. Infect. Dis. J. 28: S10–S18 [DOI] [PubMed] [Google Scholar]
- 327. Zimmerli W, Frei R, Widmer AF, Rajacic Z. 1994. Microbiological tests to predict treatment outcome in experimental device-related infections due to Staphylococcus aureus. J. Antimicrob. Chemother. 33: 959–967 [DOI] [PubMed] [Google Scholar]
- 328. Zimmerli W, Trampuz A, Ochsner PE. 2004. Prosthetic-joint infections. N. Engl. J. Med. 351: 1645–1654 [DOI] [PubMed] [Google Scholar]
- 329. Zumla A. 1992. Superantigens, T cells, and microbes. Clin. Infect. Dis. 15: 313–320 [DOI] [PubMed] [Google Scholar]