Abstract
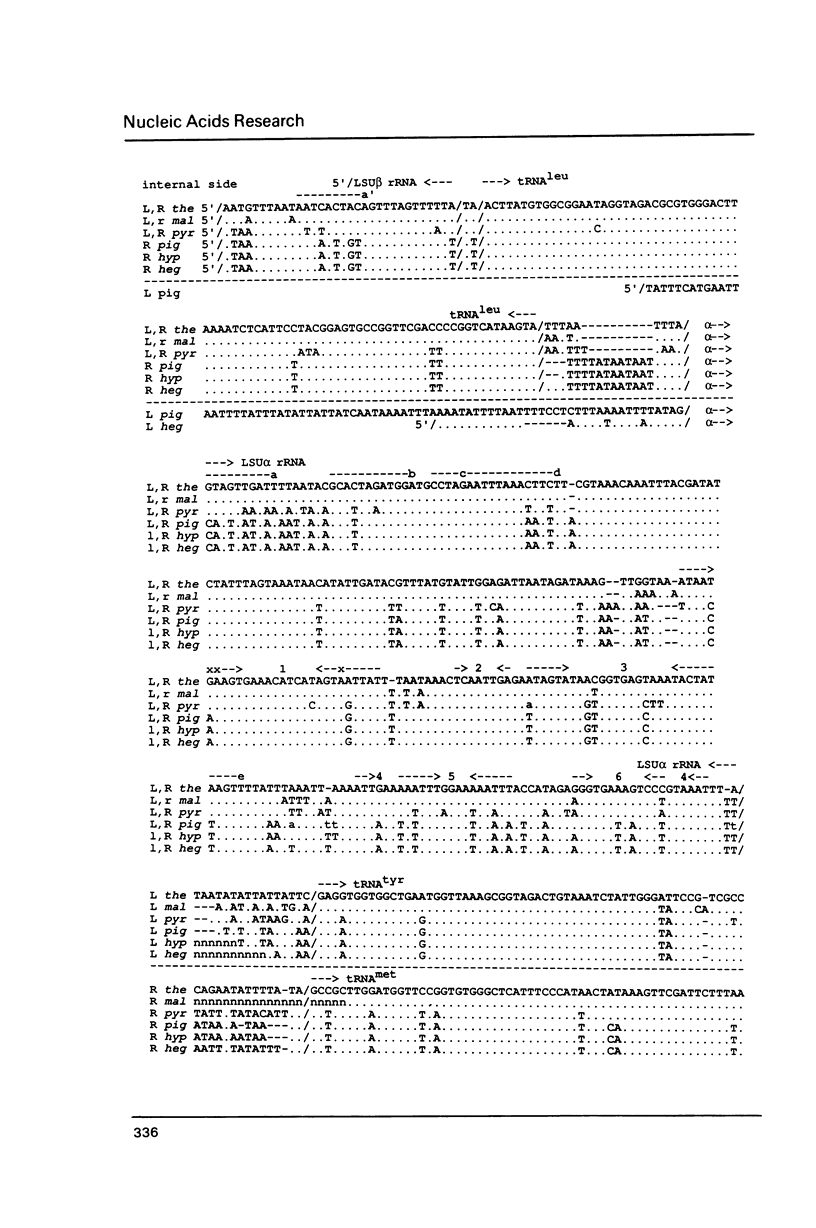
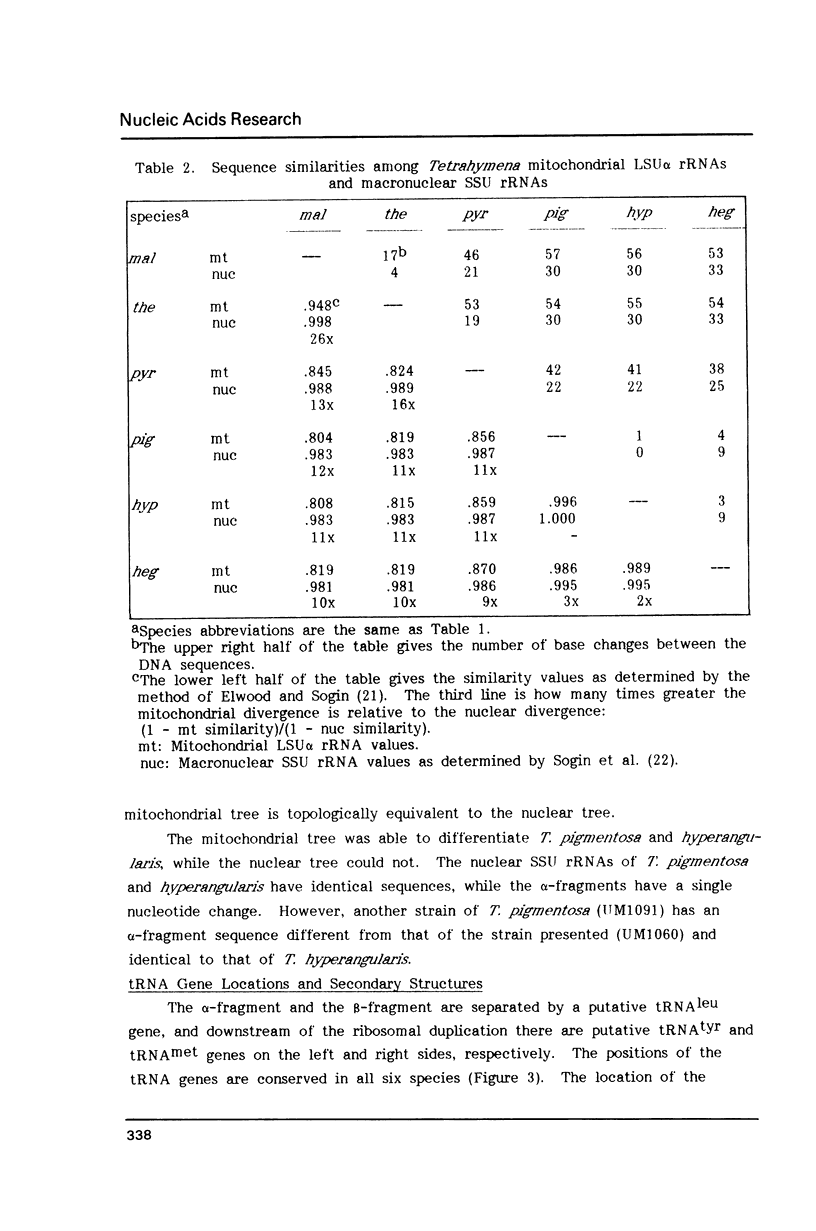
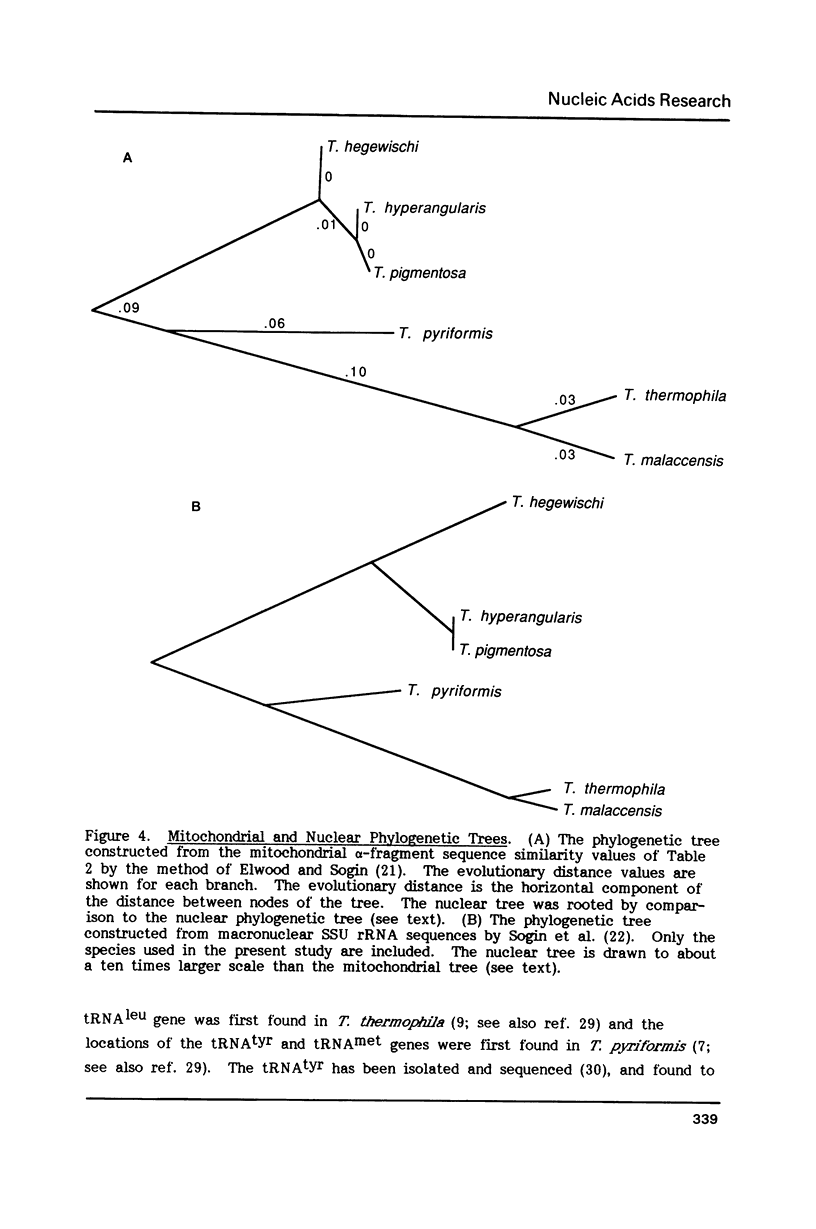
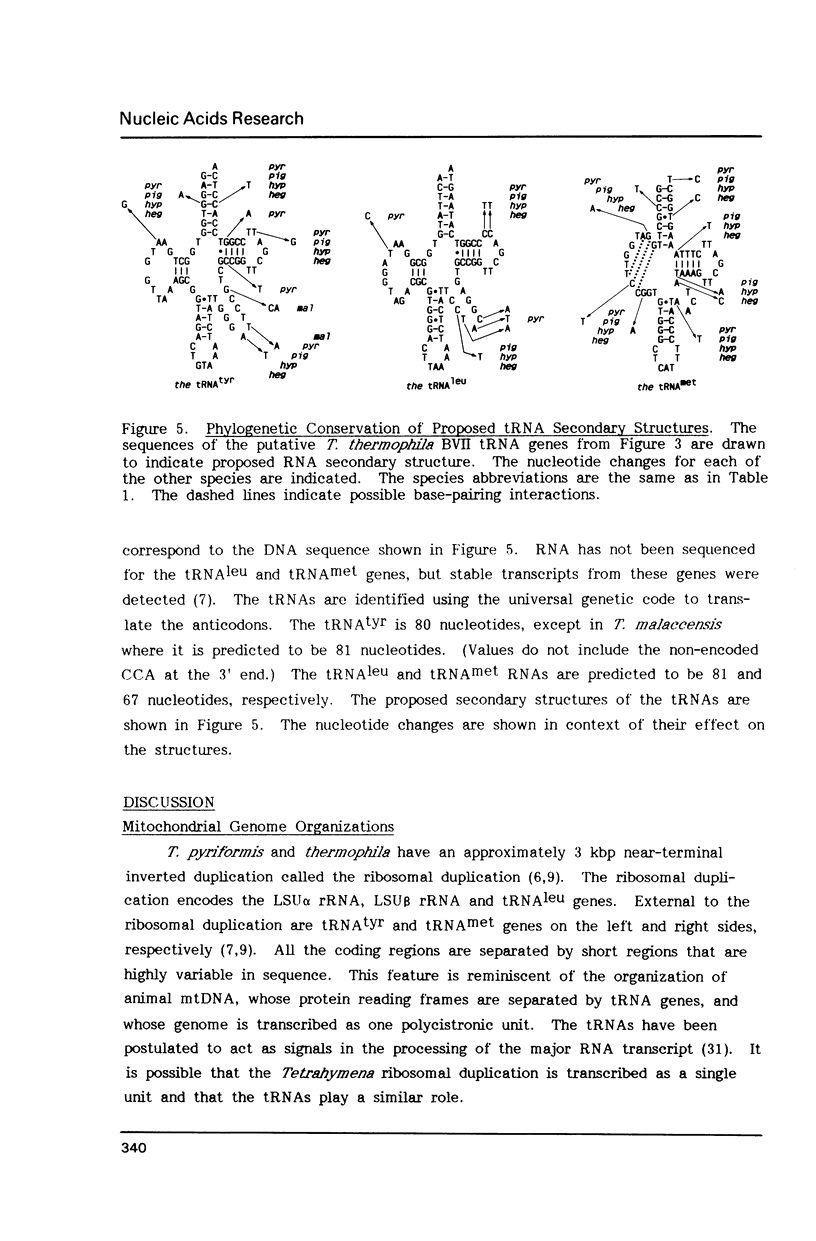
Tetrahymena thermophila mitochondrial DNA is a linear molecule with two tRNAs, large subunit beta (LSU beta) rRNA (21S rRNA) and LSU alpha rRNA (5.8S-like RNA) encoded near each terminus. The DNA sequence of approximately 550 bp of this region was determined in six species of Tetrahymena. In three species the LSU beta rRNA and tRNA(leu) genes were not present on one end of the DNA, demonstrating a mitochondrial genome organization different from that of T. thermophila. The DNA sequence of the LSU alpha rRNA was used to construct a mitochondrial phylogenetic tree, which was found to be topologically equivalent to a phylogenetic tree based on nuclear small subunit rRNA sequences (Sogin et al. (1986) EMBO J. 5, 3625-3630). The mitochondrial rRNA gene was found to accumulate base-pair substitutions considerably faster than the nuclear rRNA gene, the rate difference being similar to that observed for mammals.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcari P., Brownlee G. G. The nucleotide sequence of a small (3S) seryl-tRNA (anticodon GCU) from beef heart mitochondria. Nucleic Acids Res. 1980 Nov 25;8(22):5207–5212. doi: 10.1093/nar/8.22.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnberg A. C., Goldbach R. W., van Bruggen E. F., Borst P. The structure of Tetrahymena pyriformis mitochondrial DNA. II. The complex structure of strain GL mitochondrial DNA. Biochim Biophys Acta. 1977 Jul 5;477(1):51–69. doi: 10.1016/0005-2787(77)90160-5. [DOI] [PubMed] [Google Scholar]
- Brown W. M., George M., Jr, Wilson A. C. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. M., Prager E. M., Wang A., Wilson A. C. Mitochondrial DNA sequences of primates: tempo and mode of evolution. J Mol Evol. 1982;18(4):225–239. doi: 10.1007/BF01734101. [DOI] [PubMed] [Google Scholar]
- Cann R. L., Brown W. M., Wilson A. C. Polymorphic sites and the mechanism of evolution in human mitochondrial DNA. Genetics. 1984 Mar;106(3):479–499. doi: 10.1093/genetics/106.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann R. L., Stoneking M., Wilson A. C. Mitochondrial DNA and human evolution. Nature. 1987 Jan 1;325(6099):31–36. doi: 10.1038/325031a0. [DOI] [PubMed] [Google Scholar]
- Cummings D. J. Evolutionary divergence of mitochondrial DNA from Paramecium aurelia. Mol Gen Genet. 1980;180(1):77–84. doi: 10.1007/BF00267354. [DOI] [PubMed] [Google Scholar]
- DeSalle R., Giddings L. V. Discordance of nuclear and mitochondrial DNA phylogenies in Hawaiian Drosophila. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6902–6906. doi: 10.1073/pnas.83.18.6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris S. D., Wilson A. C., Brown W. M. Evolutionary tree for apes and humans based on cleavage maps of mitochondrial DNA. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2432–2436. doi: 10.1073/pnas.78.4.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J. M., Cummings D. J. Structure and replication of mitochondrial DNA from Paramecium aurelia. J Mol Biol. 1975 Oct 5;97(4):593–609. doi: 10.1016/s0022-2836(75)80061-1. [DOI] [PubMed] [Google Scholar]
- Goldbach R. W., Arnberg A. C., van Bruggen E. F., Defize J., Borst P. The structure of Tetrahymena pyriformis mitochondrial DNA. I. Strain differences and occurrence of inverted repetitions. Biochim Biophys Acta. 1977 Jul 5;477(1):37–50. doi: 10.1016/0005-2787(77)90159-9. [DOI] [PubMed] [Google Scholar]
- Goldbach R. W., Borst P., Bollen-de Boer J. E., van Bruggen E. F. The organization of ribosomal RNA genes in the mitochondrial DNA of Tetrahymena pyriformis strain ST. Biochim Biophys Acta. 1978 Nov 21;521(1):169–186. doi: 10.1016/0005-2787(78)90260-5. [DOI] [PubMed] [Google Scholar]
- Gray M. W. Mitochondrial genome diversity and the evolution of mitochondrial DNA. Can J Biochem. 1982 Mar;60(3):157–171. doi: 10.1139/o82-022. [DOI] [PubMed] [Google Scholar]
- Gray M. W., Sankoff D., Cedergren R. J. On the evolutionary descent of organisms and organelles: a global phylogeny based on a highly conserved structural core in small subunit ribosomal RNA. Nucleic Acids Res. 1984 Jul 25;12(14):5837–5852. doi: 10.1093/nar/12.14.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen T. Y., Schnare M. N., Young P. G., Gray M. W. Rearranged coding segments, separated by a transfer RNA gene, specify the two parts of a discontinuous large subunit ribosomal RNA in Tetrahymena pyriformis mitochondria. J Biol Chem. 1987 Feb 25;262(6):2879–2887. [PubMed] [Google Scholar]
- Johnson M. J., Wallace D. C., Ferris S. D., Rattazzi M. C., Cavalli-Sforza L. L. Radiation of human mitochondria DNA types analyzed by restriction endonuclease cleavage patterns. J Mol Evol. 1983;19(3-4):255–271. doi: 10.1007/BF02099973. [DOI] [PubMed] [Google Scholar]
- Miyata T., Hayashida H., Kikuno R., Hasegawa M., Kobayashi M., Koike K. Molecular clock of silent substitution: at least six-fold preponderance of silent changes in mitochondrial genes over those in nuclear genes. J Mol Evol. 1982;19(1):28–35. doi: 10.1007/BF02100221. [DOI] [PubMed] [Google Scholar]
- Morin G. B., Cech T. R. The telomeres of the linear mitochondrial DNA of Tetrahymena thermophila consist of 53 bp tandem repeats. Cell. 1986 Sep 12;46(6):873–883. doi: 10.1016/0092-8674(86)90069-3. [DOI] [PubMed] [Google Scholar]
- Nanney D. L., McCoy J. W. Characterization of the species of the Tetrahymena pyriformis complex. Trans Am Microsc Soc. 1976 Oct;95(4):664–682. [PubMed] [Google Scholar]
- Noller H. F. Structure of ribosomal RNA. Annu Rev Biochem. 1984;53:119–162. doi: 10.1146/annurev.bi.53.070184.001003. [DOI] [PubMed] [Google Scholar]
- Ojala D., Montoya J., Attardi G. tRNA punctuation model of RNA processing in human mitochondria. Nature. 1981 Apr 9;290(5806):470–474. doi: 10.1038/290470a0. [DOI] [PubMed] [Google Scholar]
- Pace N. R., Olsen G. J., Woese C. R. Ribosomal RNA phylogeny and the primary lines of evolutionary descent. Cell. 1986 May 9;45(3):325–326. doi: 10.1016/0092-8674(86)90315-6. [DOI] [PubMed] [Google Scholar]
- Powell J. R., Caccone A., Amato G. D., Yoon C. Rates of nucleotide substitution in Drosophila mitochondrial DNA and nuclear DNA are similar. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9090–9093. doi: 10.1073/pnas.83.23.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnare M. N., Heinonen T. Y., Young P. G., Gray M. W. Phenylalanine and tyrosine transfer RNAs encoded by Tetrahymena pyriformis mitochondrial DNA: primary sequence, post-transcriptional modifications, and gene localization. Curr Genet. 1985;9(5):389–393. doi: 10.1007/BF00421610. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Elwood H. J. Primary structure of the Paramecium tetraurelia small-subunit rRNA coding region: phylogenetic relationships within the Ciliophora. J Mol Evol. 1986;23(1):53–60. doi: 10.1007/BF02100998. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Ingold A., Karlok M., Nielsen H., Engberg J. Phylogenetic evidence for the acquisition of ribosomal RNA introns subsequent to the divergence of some of the major Tetrahymena groups. EMBO J. 1986 Dec 20;5(13):3625–3630. doi: 10.1002/j.1460-2075.1986.tb04691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solignac M., Monnerot M., Mounolou J. C. Mitochondrial DNA evolution in the melanogaster species subgroup of Drosophila. J Mol Evol. 1986;23(1):31–40. doi: 10.1007/BF02100996. [DOI] [PubMed] [Google Scholar]
- Suyama Y., Jenney F., Okawa N. Two transfer RNA sequences abut the large ribosomal RNA gene in Tetrahymena mitochondrial DNA: tRNA(leu) (anticodon UAA) and tRNA(met) (anticodon CAU). Curr Genet. 1987;11(4):327–330. doi: 10.1007/BF00355408. [DOI] [PubMed] [Google Scholar]
- Suyama Y., Miura K. Size and structural variations of mitochondrial DNA. Proc Natl Acad Sci U S A. 1968 May;60(1):235–242. doi: 10.1073/pnas.60.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bell C. T. 5S and 5.8S ribosomal RNA evolution in the suborder Tetrahymenina (Ciliophora: Hymenostomatida). J Mol Evol. 1985;22(3):231–236. doi: 10.1007/BF02099752. [DOI] [PubMed] [Google Scholar]
- Vawter L., Brown W. M. Nuclear and mitochondrial DNA comparisons reveal extreme rate variation in the molecular clock. Science. 1986 Oct 10;234(4773):194–196. doi: 10.1126/science.3018931. [DOI] [PubMed] [Google Scholar]
- Walker T. A., Pace N. R. 5.8S ribosomal RNA. Cell. 1983 Jun;33(2):320–322. doi: 10.1016/0092-8674(83)90413-0. [DOI] [PubMed] [Google Scholar]
- Wolstenholme D. R., Clary D. O. Sequence evolution of Drosophila mitochondrial DNA. Genetics. 1985 Apr;109(4):725–744. doi: 10.1093/genetics/109.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Macfarlane J. L., Okimoto R., Clary D. O., Wahleithner J. A. Bizarre tRNAs inferred from DNA sequences of mitochondrial genomes of nematode worms. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1324–1328. doi: 10.1073/pnas.84.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaug A. J., Cech T. R. In vitro splicing of the ribosomal RNA precursor in nuclei of Tetrahymena. Cell. 1980 Feb;19(2):331–338. doi: 10.1016/0092-8674(80)90507-3. [DOI] [PubMed] [Google Scholar]
- de Bruijn M. H., Schreier P. H., Eperon I. C., Barrell B. G., Chen E. Y., Armstrong P. W., Wong J. F., Roe B. A. A mammalian mitochondrial serine transfer RNA lacking the "dihydrouridine" loop and stem. Nucleic Acids Res. 1980 Nov 25;8(22):5213–5222. doi: 10.1093/nar/8.22.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]