Abstract
Summary: Human papillomavirus (HPV) infection of the genital tract is common in young sexually active individuals, the majority of whom clear the infection without overt clinical disease. Most of those who do develop benign lesions eventually mount an effective cell-mediated immune (CMI) response, and the lesions regress. Regression of anogenital warts is accompanied histologically by a CD4+ T cell-dominated Th1 response; animal models support this and provide evidence that the response is modulated by antigen-specific CD4+ T cell-dependent mechanisms. Failure to develop an effective CMI response to clear or control infection results in persistent infection and, in the case of the oncogenic HPVs, an increased probability of progression to high-grade intraepithelial neoplasia and invasive carcinoma. Effective evasion of innate immune recognition seems to be the hallmark of HPV infections. The viral infectious cycle is exclusively intraepithelial: there is no viremia and no virus-induced cytolysis or cell death, and viral replication and release are not associated with inflammation. HPV globally downregulates the innate immune signaling pathways in the infected keratinocyte. Proinflammatory cytokines, particularly the type I interferons, are not released, and the signals for Langerhans cell (LC) activation and migration, together with recruitment of stromal dendritic cells and macrophages, are either not present or inadequate. This immune ignorance results in chronic infections that persist over weeks and months. Progression to high-grade intraepithelial neoplasia with concomitant upregulation of the E6 and E7 oncoproteins is associated with further deregulation of immunologically relevant molecules, particularly chemotactic chemokines and their receptors, on keratinocytes and endothelial cells of the underlying microvasculature, limiting or preventing the ingress of cytotoxic effectors into the lesions. Recent evidence suggests that HPV infection of basal keratinocytes requires epithelial wounding followed by the reepithelization of wound healing. The wound exudate that results provides a mechanistic explanation for the protection offered by serum neutralizing antibody generated by HPV L1 virus-like particle (VLP) vaccines.
INTRODUCTION
Human papillomaviruses (HPVs) are a large family of small, nonenveloped, double-stranded DNA viruses that are the cause of benign epithelial proliferations or warts. Until the early 1970s, it was assumed that there was only one HPV and that it was the cause of the various warty lesions that decorated a range of tissue sites; HPV was seen, except in rare circumstances (34), as causing unsightly but essentially trivial excrescences that, given time, would regress spontaneously. The advent of recombinant DNA technology and molecular cloning reversed this view, and within a decade, it became clear that there were multiple HPV types and that the warts on different tissue locations were caused by different HPV types with tropisms for mucosal or cutaneous squamous surfaces (56). It also became evident that HPV did not cause trivial disease only but that some members of the HPV family, particularly a subset infecting the anogenital tract, were true human carcinogens and were the cause of carcinoma of the cervix, the second most common cancer in women worldwide (33, 84).
At present, there are at least 180 HPV genotypes, numbered sequentially, that have been cloned from clinical lesions (6). HPVs are not classified into serotypes but into genotypes on the basis of DNA sequence. In vitro growth of HPV is problematic, and HPV infection is determined by the detection of HPV DNA in biopsy specimens, swabs, or scrapes from mucosal or cutaneous surfaces, using sensitive molecular hybridization methods. HPVs have a predilection for either cutaneous or mucosal epithelial surfaces and fall into two groups: low-risk types that predominantly cause benign warts and high-risk types that may result in malignant disease as an uncommon consequence of infection. This risk profile is shown clearly in the genital tract, where 30 to 40 HPVs regularly or sporadically infect the mucosal epithelium in men and women. The two most common low-risk mucosal HPVs are HPV6 and -11, which together cause about 90% of genital warts and almost all recurrent respiratory papillomas (RRP), as well as a proportion of low-grade cervical intraepithelial neoplasms (CIN1), vulval and vaginal intraepithelial neoplasms of grade 1 (VIN1 and VAIN1, respectively), and anal intraepithelial neoplasms of grade 1 (AIN1) (42).
High-risk HPVs are strongly associated with anogenital cancers (particularly carcinoma of the cervix), with a subset of head and neck cancers (59), and with the high-grade intraepithelial precursor lesions of anogenital cancers, such as CIN2/3, VIN2/3, and AIN2/3. Overall, it is estimated that 5.2% of all cancers are attributable to HPV. There are 15 recognized high-risk or oncogenic genital HPVs; HPV16 is the most prevalent type detected in HPV-associated cancers, followed by HPV18. Together, HPV16 and -18 are the cause of 70% of cervical cancers worldwide (8).
HPV, A SUCCESSFUL PATHOGEN
HPVs are very successful infectious agents. They induce chronic infections that have virtually no systemic sequelae, rarely kill the host, and periodically shed large amounts of infectious virus, over weeks and months, for transmission to naive individuals. To achieve this evolutionarily successful lifestyle, HPVs must avoid host defense systems, and the key to understanding how this is achieved is the virus replication cycle, which is itself an immune evasion mechanism that inhibits and delays the host immune response to HPV infection.
HPV Infectious Cycle
HPVs are exclusively intraepithelial pathogens, and infection and vegetative virus growth are absolutely dependent upon the expression of the complete program of keratinocyte differentiation (23). In simple terms, it is thought that virus infects primitive basal keratinocytes, probably wound keratinocytes, which probably assume the stem cell phenotype during the wounding process. However, high-level viral gene expression with viral protein production and virus assembly occurs only in the upper differentiated layers of the stratum spinosum and granulosum of squamous epithelia (12). Viral gene expression is confined to the keratinocyte; there is no evidence that viral genes are expressed in any cell other than keratinocytes, and importantly, there is a differential spatial and temporal pattern of HPV gene expression in the infected epithelium (Fig. 1 and 2) (72). After infection of wound basal cells, it is thought that there is a round of viral DNA replication that appears to be independent of the cell cycle and amplifies the viral copy number to around 50 to 100 copies per cell. The infected cell is thought to then leave this primitive compartment and enter the transit amplifying proliferative compartment of the epithelium, where there is a phase of plasmid or episomal maintenance when the viral copy number remains constant and viral gene expression is minimal. In this phase of the viral life cycle of the high-risk HPVs, the expression of the potent oncogenes E6 and E7 is under very tight control, and E6 and E7 transcripts of high-risk HPV types are barely detectable in the proliferating compartment of the epithelium. When the infected keratinocyte enters the differentiating compartment, in the stratum spinosum exiting the cell cycle, then there is a massive upregulation of viral gene expression and viral DNA replication, with amplification of the viral copy number to many thousands of copies per cell, abundant expression of the E6 and E7 early genes, and expression of late genes from the late promoter (12, 24).
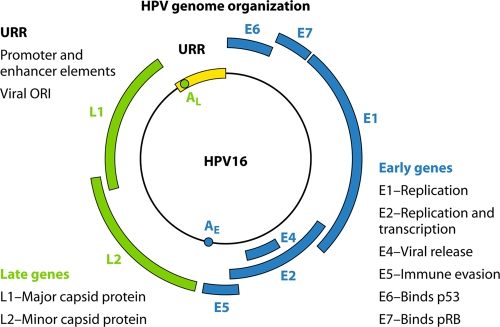
Fig 1.
Cartoon illustrating the genomic organization of a typical mucosal high-risk HPV. The genome contains early and late regions, which relate to the positions of the genes within the genome and their timing of expression during the viral life cycle. The early region carries a number of genes which function at the level of viral replication and transcription, i.e., E2, E1, E6, and E7. E2 encodes a protein which has an auxiliary role in viral replication and also functions at the level of transcriptional regulation of the viral early genes. Many studies have shown that it is a negative regulator of viral gene expression. The E6 and E7 genes encode the major transforming proteins of the oncogenic HPVs, but it should be noted that although they are transforming proteins, they also have important roles in the normal viral life cycle. The late region encodes viral structural proteins, with L1 being the major capsid protein and L2 being the minor capsid protein. Control of early gene transcription and replication is conferred by the upstream regulatory region (URR), which contains promoter and enhancer elements as well as the viral origin of replication. Here the transcriptional apparatus assembles and generates polycistronic transcripts which utilize the early polyadenylation signal.
Fig 2.
Papillomaviruses are absolutely species specific and tissue specific. HPV will infect and replicate in a fully differentiating squamous epithelium only. The virus infectious cycle is rather complex and can explain the duration of an HPV infection. It involves both temporal and spatial separation of viral protein expression. The virus first infects a keratinocyte in the basal layer of the epithelium as a consequence of microtrauma, i.e., an abrasion of the epithelium that exposes the basement membrane and basal cells. In the proliferative compartments of the epithelium, there is a phase of plasmid maintenance, the virus and cell replicate together, and the viral copy number is maintained at around 50 to 100 copies in the daughter cells. For the oncogenic viruses, in particular, viral gene expression is very tightly controlled during this phase. As long as the cell is dividing, the high-risk HPVs control the expression of their viral proteins very tightly. The E6 and E7 oncogenes are thus expressed at very low levels. When the host cell stops dividing and begins to differentiate into a mature keratinocyte, this provides a signal to the virus to activate all of its genes to increase the viral genome copy number to the thousands. In the case of incipient malignancy, control of E6 and E7 expression is lost, and gene expression in the cell becomes deregulated. In the top layers of the epithelium, all of the viral genes, including those encoding the L1 and L2 proteins, are expressed, and many thousands of viral genomes are encapsidated; these exit the cell as infectious virus particles. The time taken from infection to the generation of infectious virus is at least 3 weeks. HPV thus has a very long infectious cycle, has no blood-borne phase, and does not cause cell death.
HPV gene expression.
It is important to recognize that these events occur in cells that are differentiating and have, to all intents and purposes, exited the cell cycle. The papillomavirus genome is small, and the viruses encode only one DNA replication enzyme, E1; apart from this and the viral E2 protein, replication is totally dependent upon the cellular DNA synthetic machinery (Fig. 1 and 2). The challenge for the virus is that the cellular DNA polymerases and other replication factors are produced only in mitotically active cells. To solve this problem, HPVs encode proteins that, in the context of the viral life cycle, initiate cellular DNA synthesis in noncycling cells, inhibit the apoptosis that would otherwise ensue, and delay the differentiation program of the infected keratinocyte, creating an environment permissive for viral DNA replication (Fig. 1). Central to these functions are the E6 and E7 genes (49). The E7 gene of the high-risk viruses binds the unphosphorylated form of the retinoblastoma protein, overriding the G1/S checkpoint of the cell cycle. The E6 gene of the high-risk HPVs binds p53 and targets p53 for ubiquitination. The combination of these events overrides cell cycle checkpoints and allows for viral DNA replication in noncycling cells. An unfortunate but rare by-product of this is the deregulation of growth control in the infected cell and the development of cancer.
HPV IMMUNE EVASION STRATEGIES
The infectious cycle of HPVs is tailored to the differentiation program of the target cell, the keratinocyte, which raises several important issues with respect to immune recognition. First, infection and vegetative growth are completely dependent upon the program of keratinocyte differentiation, from basal cell to terminally differentiated superficial squames. The full infectious cycle takes a long time, and even in the optimal scenario the time from infection to virus release takes about 3 weeks, since this is the time taken for the basal keratinocyte to move up through the epithelium, undergo complete differentiation, and desquamate. In reality, the period between infection and the appearance of lesions is highly variable and can range from weeks to months, suggesting that the virus does effectively evade host defenses. In addition, there is no cytolysis or cytopathic death as a consequence of virus replication and assembly. These key events for the virus occur in the fully differentiating keratinocyte, a cell destined for death and desquamation far from the sites of immune activity. Thus, there is no virus-induced cell death and therefore no inflammation, and for most of the duration of the HPV infectious cycle, there appears to be little or no release of proinflammatory cytokines, important for activation and migration of antigen-presenting cells (APCs), into the local milieu (69). HPV is an exclusively intraepithelial pathogen, there is no blood-borne or viremic phase of the life cycle, and only minimal amounts of virus are exposed to immune defenses (Fig. 3). In effect, the virus is practically invisible to the host defenses, which remain ignorant of the presence of the pathogen for long periods.
Fig 3.
HPV is efficient at evading recognition. The virus can globally downregulate keratinocyte innate immune sensors and suppress the type I interferon response, which is critical for the control of viral infection. There is no viremia and no virus-induced cell death; hence, there is no inflammation or danger signal to the immune system.
HPV Compromises Innate Defenses in Keratinocytes
Central to this achievement is the ability of HPVs, particularly the high-risk HPVs, to compromise the role of keratinocytes as innate immune sentinels. Keratinocytes can respond to cell injury and cell stress and can sense pathogens, thus mediating immune responses (53). Eukaryotic cells express germ line-encoded receptors of the innate immune system, pathogen recognition receptors (PRRs), that recognize invariant molecular motifs known as pathogen-associated molecular patterns (PAMPs) (46). Toll-like receptors (TLRs) are perhaps the best studied of PRRs, and ligation of TLRs results in the activation of host signaling pathways mediated via Mal/Myd88 or TRIM/TRIF adaptor molecules and in the initiation of both innate and adaptive immune responses (1). Genital tract keratinocytes express several TLRs, located either on the cell surface (TLR1, TLR2, TLR4, TLR5, and TLR6) or in the endosome (TLR3 and TLR9) (52). TLR7 expression is induced on keratinocytes by triggering TLR3 with double-stranded RNA (a feature of viral infections), thus activating interferon (IFN) response genes (36). Crucially, activation of TLRs on keratinocytes leads to the production of type I interferons and to predominantly Th1-type cytotoxic responses (48).
Downregulation of interferon responses.
The type I interferons, principally IFN-α and IFN-β, have antiviral, antiproliferative, antiangiogenic, and immunostimulatory properties and act as a bridge between innate and adaptive immunity (43). Most DNA viruses have mechanisms for inhibiting interferon synthesis and signaling, and the papillomaviruses are no exception. High-risk HPV infection downregulates IFN-α-inducible gene expression, and the HPV16 E6 and E7 oncoproteins interact directly with components of the interferon signaling pathways. Thus, E7 inhibits IFN-α-mediated signal transduction by binding to P48/IRF-9, preventing translocation to the nucleus and thereby inhibiting the formation of the ISGF-3 transcription complex that binds the interferon-specific response element (ISRE) in the nucleus (3). E7 also interferes with intermediate IFN-mediated signals by physically associating with IRF-1, inhibiting IRF-1-mediated activation of the IFN-β promoter for recruitment of histone deacetylase to the promoter, thereby preventing transcription (58). In vivo expression of HPV18 E7 results in reduced expression of IRF-1 target genes, such as the TAP1, IFN-β, and MCP-1 genes, by inhibition of the transactivating function of IRF-1 (75). The E6 protein of HPV also targets the interferon pathway. E6 binds to IRF-3 and inhibits its transcriptional activation function, thereby preventing transcription of IFN-α mRNA (65). E6 binds to TYK2, preventing binding to the cytoplasmic portion of the IFN receptor and inhibiting phosphorylation of TYK2, STAT1, and STAT2, impairing JAK-STAT activation and therefore specifically inhibiting IFN-α-mediated signaling (37).
Interferon response and progression in cervical neoplasia.
In productive viral infection, HPV exists as a nuclear episome, but the integration of high-risk HPV DNA into the host genome is an important step in neoplastic progression in the cervix (60, 76, 78). Integration usually causes deletion or disruption of the viral regulatory E2 gene while retaining a variable segment of the HPV genome but always including the E6 and E7 oncogenes and the upstream regulatory region (2, 25). In the HPV16-containing W12 cervical keratinocyte cell line, which mimics the CIN-invasive carcinoma spectrum in vitro, HPV16 integration with consequent disruption/deletion of E2 leads to increased expression of the viral oncogenes (62). Cells containing integrated high-risk HPV acquire a strong growth advantage over cells harboring episomal high-risk HPV, and they undergo clonal expansion (30, 32). These cells also show increased genomic instability and therefore have a greater probability of acquiring the secondary genomic abnormalities that may drive malignant progression (61). Episome loss in these cells is closely associated with endogenous activation of antiviral response genes that are also inducible by the type I IFN pathway. Exogenous IFN-β can dramatically hasten the transition from episomal to integrated HPV16 in W12 keratinocytes, with clearance of episomes through noncytolytic mechanisms permitting the emergence of clones with integrated HPV16; these cells are resistant to IFN-β-mediated growth inhibition (32).
HPV globally downregulates keratinocyte cytokine responses in episome-containing keratinocytes.
A second group of PRRs, encoded by the nucleotide binding domain-, leucine rich repeat-containing (NLR) gene family, recognize PAMPs and endogenous signals or damage-associated molecular patterns (DAMPs), elicited during cell injury and stress. Activation of the NLRs results in the activation of proinflammatory signaling pathways and procaspase 1 (44). The assembly of the inflammasome leads to the activation of caspase 1, which then cleaves pro-interleukin 1β (pro-IL-1β) and pro-IL-18. Keratinocytes constitutively secrete or can be induced to secrete several cytokines, including interleukin 1, interleukin 6, interleukin 10, interleukin 18, and tumor necrosis factor (4, 53). Interleukin 1 is a key keratinocyte cytokine with a broad range of pleiotropic effects, including activation of T helper cells and dendritic cells (DCs) and the promotion of B cell maturation and clonal expansion. Under normal conditions, keratinocytes synthesize both pro-IL-1-β and pro-IL-1α but cannot process and secrete them in their activated form. After inflammasome activation, processing and secretion of IL-1β (as the activated cytokine) occur. The situation with respect to IL-1α is less clear, with evidence that secretion of the mature cytokine does not require inflammasome activation (4). The secretion of proinflammatory cytokines by keratinocytes is central to the activation of tissue resident immune cells, such as Langerhans cells (LCs) and macrophages, and the recruitment of effector T cells (7), all of which kick start adaptive immune responses to the local injury or infection.
Recent evidence shows that HPV infection dampens these crucial responses almost from the start of the infectious cycle and that interleukin 1β and interleukin 6 are central to this. Using genomewide expression profiling of foreskin and cervical keratinocytes in which HPV16 or -18 episomes were maintained, Karim and colleagues (38) provided evidence of downregulation of an array of proinflammatory and chemotactic cytokines as well as antigen-processing and -presenting molecules. Interleukin 1β and interleukin 6 were central to the HPV-associated gene networks. Importantly, HPV episomal maintenance in this system did not abolish these key responses but significantly reduced them. Paradoxically, several antiviral response genes were upregulated in the HPV episome-maintaining cells, and these phenomena emphasize the importance of recognizing the stage of the life cycle that the various experimental systems reflect. The in vitro HPV-infected cells used in these studies represent the phase in the infectious cycle in which HPV episomes are maintained at a constant copy number in actively dividing basal and parabasal cells, with minimal early gene expression and rigorous control of E6/E7 expression. An important question to be addressed is what consequences the upregulation of early gene expression, particularly that of E6 and E7, has on innate immune responses in the upper and intermediate layers of the stratum spinosum. It may be that in these cells the downregulation of interferon expression becomes extremely important in determining recognition of HPV infection.
HPV Interactions with DCs (Professional APCs)
Since HPV infections are exclusively intraepithelial, HPV antigens theoretically should be processed and presented by the professional APCs of squamous epithelia, the LCs, which reside in the parabasal and lower suprabasal layers of squamous epithelia. Viral capsid entry is usually an activating signal for dendritic cells, but there is evidence that LCs are not activated by the uptake of HPV capsids. LCs incubated with HPV16 L1 virus-like particles (VLPs) do not initiate epitope-specific immune responses against L1-derived antigens and, in effect, are tolerized by VLP uptake (26). In contrast, stromal DCs are activated by VLPs and stimulate HPV-specific T cells (16). As far as it is known, HPV gene expression is confined to keratinocytes, and therefore cross presentation of HPV antigens by LCs or other dendritic cells is critical for induction of effector T cell responses to nonstructural HPV proteins. Human LCs have been shown to prime and cross-prime naive CD8+ cells (47), but recent data from the mouse suggest that in the skin the important cross-presenting APCs are the langerin+ CD103+ DCs (5), a subset most likely of dermal origin. Dermal DCs and macrophages recruited to HPV-infected epithelium may be key players in the recognition of HPV antigens and the induction of effector responses. However, the suboptimal codon usage by HPV (83) that results in very low protein levels in infected cells could provide a further constraint on the effectiveness of cross presentation by intraepithelial DCs.
IMMUNE RESPONSE TO HPV IN NATURAL INFECTIONS
Despite the best efforts of the virus to evade host defenses, at least 80 to 90% of genital HPV infections will resolve with time (50). Anogenital warts and CIN1 lesions regress as a result of a successful cell-mediated immune response directed against early viral proteins, specifically the E2 and E6 proteins (21, 77, 80). Immunohistochemical studies clearly show that regression of oral warts in natural papillomavirus infections in dogs (35, 55) and of anogenital warts in humans (13) is accompanied by a massive infiltration into the lesion of mononuclear cells (CD4+ CD8+ CD56+ macrophages) and expression of Th1 cytokines (70). However, despite this intense local response, systemic antigen-specific T cell responses are weak and often transient (35).
The cellular effectors in these responses are still not identified unequivocally. In a 12-month prospective study of histologically confirmed CIN1 lesions, regression during the study period was strongly correlated with the presence at study entry of intraepithelial granzyme B+ CD8+ and granzyme B+ CD56+ cells (79). Immunohistochemical studies showed CD8+ T cells expressing the α4/β7 integrin to be present in CIN1/koilocytic cervical lesions but absent or present in reduced numbers in CIN3 lesions (45). In a recent, very elegant study directly analyzing cervical lymphocytes in lesions by both fluorescence-activated cell sorting (FACS) and immunohistochemistry, it was shown that virtually all intraepithelial cervical lymphocytes express α4/β7, the mucosal homing receptor for lymphocytes. Lesion regression assessed retrospectively in this study correlated with the presence at study entry of intraepithelial CD8+ T cells (74). Importantly, lesion regression could be predicted retrospectively by the expression of the ligand for α4/β7, mucosal addressin cell adhesion molecule 1 (MadCAM1), on the vascular endothelium in dysplastic lesions.
Immune Responses Are Deregulated during HPV-Associated Neoplastic Progression
A more complete picture of events leading to progression of HPV-infected lesions in the cervix is emerging from these data. About 10 to 20% of individuals develop persistent cervical HPV infection and remain HPV DNA positive; it is this group that are at high risk for progression to CIN2/3 (50). In these persistent HPV infections, the absence of cell death means that the inflammatory signals that would activate intraepithelial APCs such as LCs and recruit stromal DCs and macrophages to the epithelium and plasmacytoid DCs to the infected focus are absent. Furthermore, HPVs downregulate innate sensing signaling pathways in the infected keratinocyte (38); proinflammatory cytokines, particularly the type I interferons, are not released; and again, the signals for Langerhans cell activation and migration and the recruitment of stromal dendritic cells and macrophages are either not present or inadequate. In this scenario, there are long periods of uninterrupted virus replication in the epithelium, during which the host is ignorant of virus. This is a high-risk strategy for the host when the infection is with an oncogenic genital HPV, as it increases the risk of “accidents” in virus replication that result in the deregulated expression of the viral E6 and E7 oncoproteins, the bypassing of cell cycle checkpoints, and neoplastic transformation. With neoplastic transformation and genomic instability, the expression of key cytokines, adhesion molecules, chemokines, and chemokine receptors on the infected epithelium and on the underlying microvascular endothelium of the stroma is deregulated (14, 15), resulting in the downregulation of key receptors essential for the ingress of antigen-specific T cells and other cytotoxic effectors into the epithelium (74). Thus, even if HPV antigen-specific cytotoxic cells have been generated, their ingress into the epithelium is poor and regulatory T cells increasingly dominate the lesions and abrogate the killer effector response (40, 41, 79).
HUMORAL RESPONSE TO HPV INFECTION
HPV-induced lesion regression is due to a cell-mediated immune response to early proteins. In animal infections, this cell-mediated immune response is closely followed by seroconversion and production of antibodies to the major coat protein, L1 (54), and this is probably also true in humans (11). Antibody concentrations achieved in animals and humans are low, and many women do not seroconvert, although this observation should be tempered by the recognition that the current methods of measuring HPV antibody concentration are not standardized and are relatively insensitive, with low signal-to-noise ratios. There is no viremia in natural infections, and free virus particles are shed from the surfaces of squamous epithelia, with poor access to vascular and lymphatic channels and thus to lymph nodes, where immune responses would be initiated. This is reflected in the time taken for seroconversion, which for an HPV16 infection is 9 months, on average, after the first detection of HPV DNA in the cervical scrape (10).
HPV VACCINES
One might well ask why, if natural antibody responses are so poor, should vaccines that generate serum neutralizing antibodies be protective? However, Shope showed more than 60 years ago that neutralizing antibodies protected rabbits against high-dose viral challenge with cottontail rabbit papillomavirus (CRPV) (68). In Shope's experiments, if rabbits were infected systemically with CRPV by direct injection of virus into the muscle or into the bloodstream, papillomas did not arise on the skin but neutralizing antibodies were generated in these animals. If the immunized animals were then challenged with a high viral dose by abrasion of the epithelium, the animals were completely resistant and no papillomas arose. This and other data suggested very strongly that generating serum neutralizing antibody to the virus capsid protein would be an effective prophylactic vaccine strategy, and this has proved to be so. The currently available HPV vaccines are subunit vaccines consisting of VLPs assembled from the major coat proteins (L1) of HPV16 and HPV18 only or of HPV16/18/6 and -11. These prophylactic HPV vaccines have been shown to be highly efficacious in randomized controlled trials (27, 57). HPV VLPs induce very high concentrations of neutralizing antibodies to L1 (at least 2 to 4 log higher than those in natural infections) (31). The vaccines are delivered intramuscularly, resulting in rapid access to the local lymph nodes and thus circumventing the immune avoidance strategies of the viral intraepithelial infectious cycle. As a result of the repeat structure of capsomers across the particle surface, VLPs are highly immunogenic, inducing potent antibody responses in the absence of adjuvant due to their ability to activate both innate and adaptive immune responses (81, 82).
Mechanism of Protection
The mechanism of protection afforded by these vaccines is assumed to be via antibody. However, at present, there is no immune correlate of protection, though virtually all vaccinated individuals have seroconverted and there have been no obvious vaccine breakthroughs. The most unequivocal evidence that the mechanism of protection elicited by VLPs is by serum antibody comes from experiments with rabbits and dogs. In these experiments, it was shown that naive animals passively immunized with purified serum IgG from either VLP-immunized (9, 73) or naturally infected (28) animals were completely protected against high-dose viral challenge. The mechanism by which VLP-induced serum antibodies can effect protection against an exclusively intraepithelial infection is not immediately apparent. HPVs infect cells in the basal layer of squamous epithelium at multiple sites in the anogenital tract, including the cervical squamo-columnar junction, the portio surface of the cervix, the upper and lower epithelia of the vagina, multiple sites on the vulva, the perianal and intra-anal mucosa, the penile shaft, and scrotal skin. The squamo-columnar junction is bathed in cervical mucous secretions which contain antibody, and it could be argued that surface neutralization by antibody in these secretions would be the mechanism of protection. This mechanism must certainly contribute to protection against HPV infection in the cervix and vagina but cannot explain vaccine-mediated protection of the well-keratinized and comparatively dry surfaces of the vulva, penis, and perianal skin (22, 29).
Virus entry into basal keratinocytes.
Virus neutralizing antibody prevents virus entry into cells. Thus, the questions to be addressed are as follows: how does HPV access and infect the basal cell of stratified squamous epithelium, and how do neutralizing antibodies to L1 prevent this? These questions have been addressed in a series of elegant experiments with a cervicovaginal model of infection using a surrogate virus or pseudovirion (64). Pseudovirions are VLPs comprising both coat proteins (L1 and L2) that have packaged a DNA plasmid encoding a reporter molecule, such as an enzyme or a fluorescent protein, whose expression allows the pseudovirion to be tracked in cells or tissues. In these experiments, female mice (and macaque monkeys) were treated with progesterone for 4 days. The squamo-columnar junction was abraded with a cytobrush, HPV pseudovirions were applied to the junction, and the fate of these was tracked by confocal microscopy. These studies showed that only microabrasion that resulted in the removal of the full thickness of the epithelium but retention of the epithelial basement membrane (BM) permitted infection of basal cells, since pseudovirions attached first to the BM before entering the basal cells. This sequence of events provides a mechanistic explanation for antibody protection and potentially for a recall response in natural infection. The microwound from epithelial denudation would almost immediately induce serous exudation into the wound bed (63). This would be rich in large serum proteins, including IgGs, together with phagocytes and immunocytes, including B memory cells, of which there is a small circulating population allowing for both virus neutralization and memory recall.
Both capsid proteins L1 and L2 mediate virus entry into keratinocytes.
The virus capsid consists of two proteins, L1 and L2; the L1 protein is assembled into 72 pentamers that stud the surface of the particle. Protective serum neutralizing antibody responses in natural infections in animals and humans are specific to L1 and are type specific. The L2 protein is deep within the pentamer, and antibody responses to L2 have not been reported for natural infections. However, it turns out that both L1 and L2 are necessary for virus entry, and details on this mechanism have come from experiments with L1 and L2 HPV16 pseudovirions in vitro and with the in vivo cervicovaginal challenge model (17, 19, 20, 39). The sequence of events and virus entry appears to be as follows. Virus binds to heparin sulfate proteoglycans in the epithelial basement membrane via L1. The virus capsid then undergoes a conformational change allowing the exposure of L2, which is then cleaved at a specific furin site at the N terminus. This newly exposed site on L2 binds to surface molecules on the wound keratinocyte, and it is speculated that there the capsid undergoes a further conformational change and that the cellular receptor binding site on L1 is exposed or made more accessible. The virus binds via L1 to the cellular receptor, and cell entry is achieved. In vivo experiments indicate that viral entry is a very protracted process requiring 24 to 48 h before entry into the wound keratinocyte, and this is supported by in vitro data (67). Certainly, if the in vivo challenge model is an accurate reflection of HPV entry, and if wounding followed by reepithelization is a prerequisite, then several hours will elapse between viral binding to the BM and entry into the wound epithelial cell.
How and when do neutralizing antibodies prevent viral entry?
The question of how and when neutralizing antibodies prevent viral entry has been addressed using the cervicovaginal infection model, and in essence, the data suggest that after HPV16 L1 VLP immunization, antibodies that prevent both the initial binding to the BM and binding to the keratinocyte cell surface are generated (18). Passive immunization experiments with this model suggest that these neutralizing antibodies to L1 are effective at very low concentrations, consistent with data from animal papillomavirus models (71) and from natural infections in humans (66).
The protracted presence of extracellular virus during infection raises the question of why seroconversion occurs so late in natural infections when there apparently is adequate opportunity for priming via capsid recognition and transport, if not by LCs, then by stromal DCs and macrophages entering the microwound bed. The retention of the basement membrane, a characteristic of blister wounds, is probably important in this scenario. Wounds retaining the BM are characterized by a complex cytokine milieu in which transforming growth factor beta (TGF-β), an immunosuppressive cytokine, is expressed by keratinocytes in the first 12 to 24 h postwounding (51). This cytokine milieu may contribute to the poor priming in HPV infections.
Biography

Margaret A. Stanley is Professor of Epithelial Biology, Department of Pathology, University of Cambridge. She attended the Universities of London, Bristol, and Adelaide. She was made a Fellow of the Academy of Medical Sciences in 2005 and an Honorary Fellow of the Royal College of Obstetricians and Gynaecologists in 2008. She has served on several research council committees and was a member of the Biology and Biotechnology Science Research Council from 2000 to 2003. She was a member of the Spongiform Encephalopathies Advisory Committee (SEAC) that advised the United Kingdom government on prion diseases and in 2004 was awarded the Order of the British Empire (OBE) for services to virology. Her research interests concern the biology of cervical epithelium and how and why cancer of the cervix develops. Her current research focuses on mechanisms of host defense and the development of vaccines and immunotherapies against human papillomaviruses, the cause of cervical cancer. She has published extensively in peer-reviewed journals (162 papers) and in the past 5 years has given more than 90 keynote or plenary presentations at national and international meetings on these topics. At present, she is on the editorial boards of Sexually Transmitted Infections, Journal of Clinical Virology, and Reviews in Medical Virology and is a member of the Council of the International Papillomavirus Society.
REFERENCES
- 1.Akira S, Hemmi H. 2003. Recognition of pathogen-associated molecular patterns by TLR family. Immunol. Lett. 85:85–95 [DOI] [PubMed] [Google Scholar]
- 2.Alazawi W, et al. 2002. Changes in cervical keratinocyte gene expression associated with integration of human papillomavirus 16. Cancer Res. 62:6959–6965 [PubMed] [Google Scholar]
- 3.Arany I, Goel A, Tyring SK. 1995. Interferon response depends on viral transcription in human papillomavirus-containing lesions. Anticancer Res. 15:2865–2869 [PubMed] [Google Scholar]
- 4.Arend WP, Palmer G, Gabay C. 2008. IL-1, IL-18, and IL-33 families of cytokines. Immunol. Rev. 223:20–38 [DOI] [PubMed] [Google Scholar]
- 5.Bedoui S, et al. 2009. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 10:488–495 [DOI] [PubMed] [Google Scholar]
- 6.Bernard HU, et al. 2010. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 401:70–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black AP, et al. 2007. Human keratinocyte induction of rapid effector function in antigen-specific memory CD4+ and CD8+ T cells. Eur. J. Immunol. 37:1485–1493 [DOI] [PubMed] [Google Scholar]
- 8.Bosch FX, et al. 2008. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine 26(Suppl 10):K1–K16 [DOI] [PubMed] [Google Scholar]
- 9.Breitburd F, et al. 1995. Immunization with virus-like particles from cotton tail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J. Virol. 69:3959–3963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter JJ, et al. 2000. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J. Infect. Dis. 181:1911–1919 [DOI] [PubMed] [Google Scholar]
- 11.Carter JJ, et al. 1996. The natural history of human papillomavirus type 16 capsid antibodies among a cohort of university women. J. Infect. Dis. 174:927–936 [DOI] [PubMed] [Google Scholar]
- 12.Chow LT, Broker TR, Steinberg BM. 2010. The natural history of human papillomavirus infections of the mucosal epithelia. APMIS 118:422–449 [DOI] [PubMed] [Google Scholar]
- 13.Coleman N, et al. 1994. Immunological events in regressing genital warts. Am. J. Clin. Pathol. 102:768–774 [DOI] [PubMed] [Google Scholar]
- 14.Coleman N, et al. 1993. Characterization and functional analysis of the expression of intercellular adhesion molecule-1 in human papillomavirus-related disease of cervical keratinocytes. Am. J. Pathol. 143:355–367 [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman N, Stanley MA. 1994. Characterization and functional analysis of the expression of vascular adhesion molecules in human papillomavirus related disease of the cervix. Cancer 74:884–892 [DOI] [PubMed] [Google Scholar]
- 16.Da Silva DM, Fausch SC, Verbeek JS, Kast WM. 2007. Uptake of human papillomavirus virus-like particles by dendritic cells is mediated by Fcgamma receptors and contributes to acquisition of T cell immunity. J. Immunol. 178:7587–7597 [DOI] [PubMed] [Google Scholar]
- 17.Day PM, Gambhira R, Roden RB, Lowy DR, Schiller JT. 2008. Mechanisms of human papillomavirus type 16 neutralization by l2 cross-neutralizing and l1 type-specific antibodies. J. Virol. 82:4638–4646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Day PM, et al. 2010. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe 8:260–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Day PM, Schiller JT. 2009. The role of furin in papillomavirus infection. Future Microbiol. 4:1255–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day PM, et al. 2007. Neutralization of human papillomavirus with monoclonal antibodies reveals different mechanisms of inhibition. J. Virol. 81:8784–8792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Jong A, et al. 2002. Frequent detection of human papillomavirus 16 E2-specific T-helper immunity in healthy subjects. Cancer Res. 62:472–479 [PubMed] [Google Scholar]
- 22.Dillner J, et al. 2010. Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: randomised controlled trial. BMJ 341:c3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doorbar J. 2005. The papillomavirus life cycle. J. Clin. Virol. 32:S7–S15 [DOI] [PubMed] [Google Scholar]
- 24.Doorbar J. 2007. Papillomavirus life cycle organization and biomarker selection. Dis. Markers 23:297–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dowen SE, et al. 2003. Amplification of chromosome 5p correlates with increased expression of Skp2 in HPV-immortalized keratinocytes. Oncogene 22:2531–2540 [DOI] [PubMed] [Google Scholar]
- 26.Fausch SC, Da Silva DM, Rudolf MP, Kast WM. 2002. Human papillomavirus virus-like particles do not activate Langerhans cells: a possible immune escape mechanism used by human papillomaviruses. J. Immunol. 169:3242–3249 [DOI] [PubMed] [Google Scholar]
- 27.Garland SM, et al. 2007. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N. Engl. J. Med. 356:1928–1943 [DOI] [PubMed] [Google Scholar]
- 28.Ghim S, et al. 2000. Spontaneously regressing oral papillomas induce systemic antibodies that neutralize canine oral papillomavirus. Exp. Mol. Pathol. 68:147–151 [DOI] [PubMed] [Google Scholar]
- 29.Giuliano AR, et al. 2011. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N. Engl. J. Med. 364:401–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gray E, et al. 2010. In vitro progression of human papillomavirus 16 episome-associated cervical neoplasia displays fundamental similarities to integrant-associated carcinogenesis. Cancer Res. 70:4081–4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harro CD, et al. 2001. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J. Natl. Cancer Inst. 93:284–292 [DOI] [PubMed] [Google Scholar]
- 32.Herdman MT, et al. 2006. Interferon-beta treatment of cervical keratinocytes naturally infected with human papillomavirus 16 episomes promotes rapid reduction in episome numbers and emergence of latent integrants. Carcinogenesis 27:2341–2353 [DOI] [PubMed] [Google Scholar]
- 33.International Agency for Research on Cancer 1996. Human papillomaviruses, vol 64. Meeting of IARC Working Group on 6 to 13 June 1995. International Agency for Research on Cancer, Lyon, France. [Google Scholar]
- 34.Jablonska S, Dabrowski J, Jakubowicz K. 1972. Epidermodysplasia verruciformis as a model in studies on the role of papovaviruses in oncogenesis. Cancer Res. 32:583–589 [PubMed] [Google Scholar]
- 35.Jain S, Moore RA, Anderson DM, Gough GW, Stanley MA. 2006. Cell-mediated immune responses to COPV early proteins. Virology 356:23–34 [DOI] [PubMed] [Google Scholar]
- 36.Kalali BN, et al. 2008. Double-stranded RNA induces an antiviral defense status in epidermal keratinocytes through TLR3-, PKR-, and MDA5/RIG-I-mediated differential signaling. J. Immunol. 181:2694–2704 [DOI] [PubMed] [Google Scholar]
- 37.Kanodia S, Fahey LM, Kast WM. 2007. Mechanisms used by human papillomaviruses to escape the host immune response. Curr. Cancer Drug Targets 7:79–89 [DOI] [PubMed] [Google Scholar]
- 38.Karim R, et al. 2011. Human papillomavirus deregulates the response of a cellular network comprising of chemotactic and proinflammatory genes. PLoS One 6:e17848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. 2009. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc. Natl. Acad. Sci. U. S. A. 106:20458–20463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobayashi A, et al. 2004. Functional attributes of mucosal immunity in cervical intraepithelial neoplasia and effects of HIV infection. Cancer Res. 64:6766–6774 [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi A, Weinberg V, Darragh T, Smith-McCune K. 2008. Evolving immunosuppressive microenvironment during human cervical carcinogenesis. Mucosal Immunol. 1:412–420 [DOI] [PubMed] [Google Scholar]
- 42.Lacey CJ, Lowndes CM, Shah KV. 2006. Chapter 4: burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine 24(Suppl 3):S35–S41 [DOI] [PubMed] [Google Scholar]
- 43.Le Bon A, Tough DF. 2002. Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 14:432–436 [DOI] [PubMed] [Google Scholar]
- 44.Martinon F, Mayor A, Tschopp J. 2009. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27:229–265 [DOI] [PubMed] [Google Scholar]
- 45.McKenzie J, et al. 1991. Immunocytochemical characterization of large granular lymphocytes in normal cervix and HPV associated disease. J. Pathol. 165:75–80 [DOI] [PubMed] [Google Scholar]
- 46.Medzhitov R, Janeway CAJ. 1997. Innate immunity: the virtues of a non-clonal system of recognition. Cell 91:295–298 [DOI] [PubMed] [Google Scholar]
- 47.Merad M, Ginhoux F, Collin M. 2008. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat. Rev. Immunol. 8:935–947 [DOI] [PubMed] [Google Scholar]
- 48.Miller LS, Modlin RL. 2007. Human keratinocyte Toll-like receptors promote distinct immune responses. J. Invest. Dermatol. 127:262–263 [DOI] [PubMed] [Google Scholar]
- 49.Moody CA, Laimins LA. 2010. Human papillomavirus oncoproteins: pathways to transformation. Nat. Rev. Cancer 10:550–560 [DOI] [PubMed] [Google Scholar]
- 50.Moscicki AB, Schiffman M, Kjaer S, Villa LL. 2006. Chapter 5: updating the natural history of HPV and anogenital cancer. Vaccine 24(Suppl 3):S42–S51 [DOI] [PubMed] [Google Scholar]
- 51.Myers SR, Leigh IM, Navsaria H. 2007. Epidermal repair results from activation of follicular and epidermal progenitor keratinocytes mediated by a growth factor cascade. Wound Repair Regen. 15:693–701 [DOI] [PubMed] [Google Scholar]
- 52.Nasu K, Narahara H. 2010. Pattern recognition via the Toll-like receptor system in the human female genital tract. Mediators Inflamm. 2010:976024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. 2009. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 9:679–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicholls PK, et al. 1999. Naturally occurring, nonregressing canine oral papillomavirus infection: host immunity, virus characterization, and experimental infection. Virology 265:365–374 [DOI] [PubMed] [Google Scholar]
- 55.Nicholls PK, et al. 2001. Regression of canine oral papillomas is associated with infiltration of CD4+ and CD8+ lymphocytes. Virology 283:31–39 [DOI] [PubMed] [Google Scholar]
- 56.Orth G, Jablonska S, Breitburd F, Favre M, Croissant O. 1978. The human papillomaviruses. Bull. Cancer 65:151–164 [PubMed] [Google Scholar]
- 57.Paavonen J, et al. 2009. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 374:301–314 [DOI] [PubMed] [Google Scholar]
- 58.Park JS, et al. 2000. Inactivation of interferon regulatory factor-1 tumor suppressor protein by HPV E7 oncoprotein. Implication for the E7-mediated immune evasion mechanism in cervical carcinogenesis. J. Biol. Chem. 275:6764–6769 [DOI] [PubMed] [Google Scholar]
- 59.Parkin DM, Bray F. 2006. Chapter 2: the burden of HPV-related cancers. Vaccine 24(Suppl 3):S11–S25 [DOI] [PubMed] [Google Scholar]
- 60.Pett M, Coleman N. 2007. Integration of high-risk human papillomavirus: a key event in cervical carcinogenesis? J. Pathol. 212:356–367 [DOI] [PubMed] [Google Scholar]
- 61.Pett MR, et al. 2004. Acquisition of high-level chromosomal instability is associated with integration of human papillomavirus type 16 in cervical keratinocytes. Cancer Res. 64:1359–1368 [DOI] [PubMed] [Google Scholar]
- 62.Pett MR, et al. 2006. Selection of cervical keratinocytes containing integrated HPV16 associates with episome loss and an endogenous antiviral response. Proc. Natl. Acad. Sci. U. S. A. 103:3822–3827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reusch MK, Mansbridge JN, Nickoloff BJ, Morhenn VB. 1991. Immunophenotyping of skin cells during healing of suction blister injury. Dermatologica 183:179–183 [DOI] [PubMed] [Google Scholar]
- 64.Roberts JN, et al. 2007. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat. Med. 13:857–861 [DOI] [PubMed] [Google Scholar]
- 65.Ronco LV, Karpova AY, Vidal M, Howley PM. 1998. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes Dev. 12:2061–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Safaeian M, et al. 2010. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. J. Natl. Cancer Inst. 102:1653–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sapp M, Bienkowska-Haba M. 2009. Viral entry mechanisms: human papillomavirus and a long journey from extracellular matrix to the nucleus. FEBS J. 276:7206–7216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shope RE. 1937. Immunization of rabbits to infectious papillomatosis. J. Exp. Med. 65:607–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stanley M. 2006. Immune responses to human papillomavirus. Vaccine 24(Suppl 1):S16–S22 [DOI] [PubMed] [Google Scholar]
- 70.Stanley MA. 2002. Imiquimod and the imidazoquinolones: mechanism of action and therapeutic potential. Clin. Exp. Dermatol. 27:571–577 [DOI] [PubMed] [Google Scholar]
- 71.Stanley MA, et al. 2001. Intra-epithelial vaccination with COPV L1 DNA by particle-mediated DNA delivery protects against mucosal challenge with infectious COPV in beagle dogs. Vaccine 19:2783–2792 [DOI] [PubMed] [Google Scholar]
- 72.Stanley MA, Pett M. 2007. Papillomaviruses, p 448–472. In Mahy BWJ, ter Meulen V. (ed), Topley & Wilson's microbiology & microbial infections. Virology, vol 2, 10th ed. Wiley-Blackwell, Hoboken, NJ [Google Scholar]
- 73.Suzich JA, et al. 1995. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc. Natl. Acad. Sci. U. S. A. 92:11553–11557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trimble CL, et al. 2010. Human papillomavirus 16-associated cervical intraepithelial neoplasia in humans excludes CD8 T cells from dysplastic epithelium. J. Immunol. 185:7107–7114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Um SJ, et al. 2002. Abrogation of IRF-1 response by high-risk HPV E7 protein in vivo. Cancer Lett. 179:205–212 [DOI] [PubMed] [Google Scholar]
- 76.Vinokurova S, et al. 2008. Type-dependent integration frequency of human papillomavirus genomes in cervical lesions. Cancer Res. 68:307–313 [DOI] [PubMed] [Google Scholar]
- 77.Welters MJ, et al. 2003. Frequent display of human papillomavirus type 16 E6-specific memory T-helper cells in the healthy population as witness of previous viral encounter. Cancer Res. 63:636–641 [PubMed] [Google Scholar]
- 78.Wentzensen N, Vinokurova S, von Knebel Doeberitz M. 2004. Systematic review of genomic integration sites of human papillomavirus genomes in epithelial dysplasia and invasive cancer of the female lower genital tract. Cancer Res. 64:3878–3884 [DOI] [PubMed] [Google Scholar]
- 79.Woo Y, et al. 2008. Characterising the local immune responses in cervical intraepithelial neoplasia: a cross-sectional and longitudinal analysis. BJOG 115:1616–1622 [DOI] [PubMed] [Google Scholar]
- 80.Woo YL, et al. 2010. A prospective study on the natural course of low-grade squamous intraepithelial lesions and the presence of HPV16 E2-, E6- and E7-specific T-cell responses. Int. J. Cancer 126:133–141 [DOI] [PubMed] [Google Scholar]
- 81.Yan M, et al. 2005. Activation of dendritic cells by human papillomavirus-like particles through TLR4 and NF-kappaB-mediated signalling, moderated by TGF-beta. Immunol. Cell Biol. 83:83–91 [DOI] [PubMed] [Google Scholar]
- 82.Yang R, et al. 2004. Human papillomavirus type-16 virus-like particles activate complementary defense responses in key dendritic cell subpopulations. J. Immunol. 173:2624–2631 [DOI] [PubMed] [Google Scholar]
- 83.Zhao KN, Gu W, Fang NX, Saunders NA, Frazer IH. 2005. Gene codon composition determines differentiation-dependent expression of a viral capsid gene in keratinocytes in vitro and in vivo. Mol. Cell. Biol. 25:8643–8655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.zur Hausen H, de Villiers EM, Gissmann L. 1981. Papillomavirus infections and human genital cancer. Gynecol. Oncol. 12:S124–S128 [DOI] [PubMed] [Google Scholar]