Abstract
Toxoplasma gondii is distributed worldwide and infects most species of warm-blooded animals, including humans. The heavy incidence and severe or lethal damage caused by T. gondii infection clearly indicates the need for the development of a vaccine. To evaluate the protective efficacy of a multiantigenic DNA vaccine expressing GRA7 and ROP1 of T. gondii with or without a plasmid encoding murine interleukin-12 (pIL12), we constructed DNA vaccines using the eukaryotic plasmids pGRA7, pROP1, and pGRA7-ROP1. Mice immunized with pGRA7, pROP1, or pGRA7-ROP1 showed significantly increased serum IgG2a titers; production of gamma interferon (IFN-γ), IL-10, and tumor necrosis factor alpha (TNF-α); in vitro T cell proliferation; and survival, as well as decreased cyst burdens in the brain, compared to mice immunized with either the empty plasmid, pIL12, or vector with pIL12 (vector+pIL12). Moreover, mice immunized with the multiantigenic DNA vaccine pGRA7-ROP1 had higher IgG2a titers, production of IFN-γ and TNF-α, survival time, and cyst reduction rate compared to those of mice vaccinated with either pGRA7 or pROP1 alone. Furthermore, mice immunized with either a pGRA7-ROP1+pIL12 or a single-gene vaccine combined with pIL12 showed greater Th1 immune response and protective efficacy than the single-gene-vaccinated groups. Our data suggest that the multiantigenic DNA antigen pGRA7-ROP1 was more effective in stimulating host protective immune responses than separately injected single antigens, and that IL-12 serves as a good DNA adjuvant.
INTRODUCTION
Toxoplasma gondii is an obligate intracellular protozoan that infects one-third of the world's population. Although 80 to 90% of individuals with primary infection are asymptomatic, toxoplasmosis is a significant cause of morbidity and mortality in immunocompromised and congenitally infected individuals (6). In veterinary medicine, T. gondii infection has economic importance due to abortion and neonatal loss in livestock (mainly sheep and goats), and it is a source of transmission to humans (8). Thus, a vaccine against T. gondii would be valuable for reducing the high incidence of and preventing both fetal infection and reactivation in immunocompromised individuals. The development of a vaccine might also reduce economic losses in the livestock industry.
The live vaccine has a short shelf life, and there is a risk of the vaccine reversing to the pathogenic phenotype. DNA vaccines have become a major focus, because they promote the specific expression of an encoded vaccine antigen by host cells and have the ability to deliver multivalent vaccines to a host in a single dose. Additionally, DNA vaccines can also elicit potent, long-lasting humoral and cell-mediated immunity (1). The family of vaccine candidate antigens includes T. gondii membrane-associated surface antigens SAG1 (10, 18) and SAG2 (4); excreted-secreted dense-granule proteins GRA1 (8, 9), GRA2 (11), GRA4, GRA6 (8), and GRA7 (3, 8, 9, 16); rhoptry proteins ROP1 (3, 10) and ROP2 (9, 18); and micronemal proteins MIC1, MIC2, MIC3 (14), and MIC6 (13). GRA proteins are potent antigens that trigger strong T and B cell responses upon infection, and GRA7 is expressed by all infectious stages of T. gondii (6, 8). ROP1 is released at the time of invasion into the forming parasitophorous vacuole, and it is related to the T. gondii penetrating enhancing factor (2).
DNA cocktail vaccinations have been reported to enhance protection against toxoplasmosis in a mouse model compared to single-gene vaccines (4, 5, 12). In this study, we selected T. gondii GRA7 and ROP1 as targets for DNA antigenicity generation, because they are known to be important during invasion and penetration into host cells (2, 6, 8). In addition, they are potent stimulators of humoral and cellular immune responses (3, 8, 9, 10). However, no evaluation of the protective efficacy of multiantigenic DNA vaccine expressing GRA7 and ROP1 of T. gondii with or without a plasmid encoding murine interleukin-12 (pIL12) has been published. To evaluate protective immunity, we constructed DNA vaccines expressing the GRA7 and ROP1 antigens (pGRA7 and pROP1) and a fusion of both (pGRA7-ROP1). We then examined their expression in eukaryotic cells and investigated the immunogenicity and protective efficacy of these DNA vaccines with or without the coadministration of pIL12 as a genetic adjuvant to protect BALB/c mice against toxoplasmosis.
MATERIALS AND METHODS
Animals and Toxoplasma gondii strains.
Female BALB/c mice were purchased from DaeHan BioLink Co. (Chungcheongbuk-do, South Korea). All mice were maintained under specific-pathogen-free conditions and were 6 to 8 weeks of age when first immunized. Animal studies were carried out under the authority of the Chungnam National University Animal Ethnics Committee (application number 2010-2-33). Two T. gondii strains were used. The tachyzoites of the virulent RH strain were used for plasmid construction and the preparation of soluble tachyzoite antigen (STAg), and the cyst-forming Me49 strain was used to orally infect mice to evaluate the length of survival (in days) and brain cyst numbers.
Cell culture.
Human retinal pigment epithelial cells (ARPE-19; ATCC, Rockville, MD) were maintained in a 1:1 mixture of Dulbecco's modified Eagle medium (DMEM; Invitrogen Life Technologies, Carlsbad, CA) and nutrient mixture F12 (DMEM-F12) containing 10% heat-inactivated fetal bovine serum (FBS), 0.348% sodium bicarbonate, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cell cultures were maintained at 37°C and 5% CO2, and the medium was changed every 3 to 4 days.
Isolation of T. gondii tachyzoites and cysts.
Tachyzoites of T. gondii strain RH were infected into ARPE-19 cells (parasite/cell ratio, 5:1) and incubated at 37°C with 5% CO2 for 2 to 3 days. Following spontaneous host cell rupture, lysed tachyzoites and host cellular debris were centrifuged at 900 × g for 10 min using Percoll (Sigma Chemical Co., St. Louis, MO) to pellet parasites. The final pellet was suspended in cold phosphate-buffered saline (PBS), and the suspension was passed through a 5.0-μm-pore-size filter (Millipore, Bedford, MA). Purified tachyzoites were used in all experiments.
The cysts of T. gondii strain Me49 were obtained from the brains of infected mice. Each brain was suspended in PBS to a final volume of 5 ml and homogenized by serial passages through 18-gauge syringe needles. Mean cyst numbers in the brain were determined in triplicate by counting 10-μl samples of the homogenate under a microscope at ×200 magnification.
Preparation of STAg.
Purified tachyzoites were centrifuged at 5,000 × g for 3 min and disrupted by three cycles of freezing at −20°C and thawing at 4°C. Finally, the lysate was sonicated on ice at 60 W/s and centrifuged for 40 min at 100,000 × g. The supernatants were pooled and sterile filtered (Gelman Sciences, Ann Arbor, MI), and the protein concentration was determined via the Bradford method using bovine serum albumin (BSA) as the standard. STAg was stored in aliquots at −70°C until use.
Construction of DNA vaccine plasmid.
The mammalian expression vector pCMV-Tag2B was used as a DNA vaccine vector. To construct the GRA7-ROP1 fusion expression plasmid, the GRA7 gene (786 bp, no stop codon; amino acid [aa] residues 17 to 225) and ROP1 gene (1,023 bp; aa residues 22 to 381) were amplified by PCR from cDNA of T. gondii (RH strain) using the following primers: GRA7 forward, 5′-CGCGGATCCGCGGCGGCTTTGCCCCAGTT-3′, and reverse, 5′-AATCTGCAGAGGCACCTCTTGCTCGAGTG-3′ (recognition sites for BamHI and PstI, respectively, are underlined); ROP1 forward, 5′-CCCAAGCTTGCCGCCCTTTCGAGCCACAA-3′, and reverse, 5′-CCGCTCGAG GCCCTCCTCGCCATTAGTTC-3′ (HindIII and XhoI recognition sites, respectively, are underlined). PCR products were cloned into the pGEM-T Easy vector (Promega Corporation, Madison, WI), digested with the appropriate restriction enzyme, and purified from agarose gels. GRA7 and ROP1 gene fragments were inserted into the mammalian expression vector pCMV-Tag2B, generating pCMV-Tag2B-GRA7 (pGRA7) and pCMV-Tag2B-ROP1 (pROP1). The BamHI/PstI fragment encoding GRA7 was excised and cloned into the BamHI/PstI sites of the pROP1 vector to produce pCMV-Tag2B-GRA7-ROP1 (designated pGRA7-ROP1). All recombinant plasmids were propagated in Escherichia coli DH5α and confirmed by restriction analysis and PCR sequencing (Solgent, Daejeon, South Korea). The murine IL-12 expression plasmid containing the p35 and p40 sequences, designated pUMVC3-mIL-12 (pIL12), was provided by Alexander Rakhmilevich (University of Wisconsin–Madison).
Plasmid extraction and purification.
Large-scale plasmid DNA was prepared using the Endotoxin-Free Mega kit according to the manufacturer's instructions (Qiagen, Hilden, Germany), and concentrations were determined by A260/A280 measurement. The ratios of the optical densities at 260 and 280 nm (OD260 and OD280, respectively) were 1.8 to 2.0, indicating no major protein contamination. Plasmid DNA was dissolved (1 mg/ml) in sterile endotoxin-free PBS and stored at −20°C until use.
Expression of the compound gene in vitro and in vivo.
ARPE-19 cells were transfected with pGRA7, pROP1, pGRA7-ROP1, or pCMV-Tag2B (control) using Lipofectamine LTX and Plus reagents (Invitrogen Life Technologies, Carlsbad, CA). Six-well culture plates were seeded with ARPE-19 cells (2 × 105 cells/well) and then cultured to 50 to 80% confluence. DNA (2.5 μg) in 500 μl DMEM and 2 μl Plus reagent was mixed gently and incubated at room temperature for 5 min. Lipofectamine LTX was mixed with diluted DNA and incubated at room temperature for 30 min. DNA-lipid complexes were added to cells that had been rinsed with serum-free medium. After incubation for 6 h at 37°C in a 5% CO2 incubator, medium was exchanged with medium containing 10% FBS. Following a further 48 h of incubation, cells were washed with PBS and harvested.
To examine the level of in vivo expression, total RNA was isolated from spleens of DNA-vaccinated mice 4 weeks postinoculation and were analyzed by multiplex reverse transcription-PCR (M-RT-PCR) with GRA7- and ROP1-specific primers. PCRs were carried out in a 50-μl total volume. Each PCR mixture contained 0.25 μl of TaKaRa Ex Taq (5 U/μl), 5 μl of 10× Ex Taq buffer, 4 μl of deoxynucleoside triphosphate (dNTP) mixture (2.5 mM each), 2 μl of each primer (10 pmol/μl), and 3 μl of cDNA. All PCRs were performed in a MyCycler (Bio-Rad Laboratories, CA). The denaturation of DNA (94°C for 30 s) was followed by 30 cycles of amplification (98°C for 10 s, 60°C for 30 s, and 72°C for 2 min) and ended by a 10-min extension at 72°C. Amplified products were electrophoresed in 1.2% agarose gel and visualized by ethidium bromide staining. These PCR products also were confirmed by sequencing.
SDS-PAGE and Western blot analysis.
After 48 h, pellets of transfected cells were suspended in 150 μl SDS-PAGE sample buffer and then sonicated and heated at 100°C for 5 min. Lysates were separated on a 12% polyacrylamide gel, transferred to polyvinylidene difluoride membranes, and then blocked with Tris-buffered saline (20 mM Tris, 137 mM NaCl, pH 7.6) containing 0.1% Tween 20 (TBST) and 5% skim milk. Membranes were washed three times with TBST and then incubated with diluted mouse anti-FLAG primary antibody (Sigma-Aldrich, St. Louis, MO) for 2 h at room temperature. Unreacted antibody was washed out with TBST, followed by incubation with goat anti-mouse IgG-peroxidase conjugate (Santa Cruz Biotechnology Inc., Santa Cruz, CA) diluted 1:10,000 in 5% skim milk–TBST. Peroxidase activity was detected using the enhanced chemiluminescence (ECL) Western blotting detection system (Amersham-Pharmacia, Freiburg, Germany).
Construction of bacterial expression plasmids and purification of recombinant protein.
To construct pGEX-GRA7 and pGEX-ROP1 expression plasmids, the coding sequence of the T. gondii GRA7 and ROP1 genes (GenBank accession no. DQ459443.2 and M71274.1, respectively) were amplified by PCR (using Pfu-X DNA Polymerase; Solgent, Daejeon, South Korea) from cDNA of T. gondii strain RH with a pair of oligonucleotide primers (GRA7 forward primer, 5′-CGCGGATCCATGGCCCGACACGCAATTTT-3′, and reverse primer, 5′-TCCCCCGGGCTACTGGCGGGCATCCTCCC-3′; ROP1 forward primer, 5′-CGCGGATCCATGGAGCAAAGGCTGCCAA-3′; ROP1 reverse primer, 5′-GGCCTCGAGTTATTGCGATCCATCATCC-3′; recognition sites [GRA7, BamHI and SmaI; ROP1, BamHI and XhoI] are underlined). The PCR products were digested with the appropriate restriction enzyme and cloned into the BamHI/SmaI or BamHI/XhoI sites of the pGEX-4T-1 expression vector containing an N-terminal glutathione S-transferase (GST). The resulting plasmids were named pGEX-GRA7 and pGEX-ROP1.
Bacterially expressed recombinant T. gondii soluble proteins were purified with a Pierce GST spin purification kit (Pierce Biotechnology, Rockford, IL) according to the manufacturer's instructions. Purified GST fusion proteins were dialyzed against a dialysis buffer (PBS containing 2 mM EDTA and 1 mM dithiothreitol [DTT]), the protein concentration was determined by the Bradford assay method using BSA as the standard, and the solution was stored at −70°C until use.
DNA immunization and experimental design.
Nine groups (23 mice per group) were vaccinated twice at 2-week intervals with empty vector, pIL12, empty vector with pIL12 (vector+pIL12), pGRA7, pROP1, pGRA7-ROP1, pGRA7 with pIL12 (pGRA7+pIL12), pROP1 with pIL12 (pROP1+pIL12), or pGRA7-ROP1 with pIL12 (pGRA7-ROP1+pIL12). Mice were inoculated with an injection of 50 μg plasmid DNA (in 50 μl sterile endotoxin-free PBS) into the tibialis anterior muscles of both hind legs (100 μg/per mouse) using a 26-gauge needle. Blood and spleens were collected to assess serum IgG, in vitro T cell proliferation, and cytokine levels at 0, 2, 4, and 8 weeks after immunization. Four weeks after the final immunization, mice were challenged with Me49 strains to determine the number of days of survival of mice and parasite burdens in the brain.
Evaluation of survival length and parasite burdens in the brain.
Four weeks after the final immunization, six mice were challenged with a lethal dose (1,500 cysts per mouse) of T. gondii strain Me49 according to previous reports (10), and mortality was monitored daily for 4 weeks. In addition, five mice per group were orally challenged with a nonlethal dose of strain Me49 (20 cysts per mouse) 4 weeks after final immunization. Four weeks after the challenge, mice were euthanized and the brain cysts were counted as indicated above. All samples were counted in triplicate. Cyst reduction rate to vector control was calculated with the formula [1 − (brain cyst numbers of vaccinated mice/brain cyst number of vector control)] × 100.
Determination of T. gondii-specific IgG and IgG subclass titers.
T. gondii-specific serum IgG, IgG1, and IgG2a antibody levels were determined by enzyme-linked immunosorbent assay (ELISA). The 96-well plates were coated with 10 μg/ml of STAg, purified GST, and recombinant GRA7 and/or ROP1 (5 μg/ml) in 50 mM carbonate buffer (pH 9.6) (100 μl per well) at 4°C overnight. Plates were washed twice with PBS (pH 7.4) containing 0.05% Tween 20 (PBS-T20) and blocked with PBS containing 1% BSA (PBS–1% BSA) for 2 h at room temperature. Sera were diluted in PBS–0.1% BSA and incubated for 2 h. After being washed with PBS-T20, plates were incubated with horseradish peroxidase (HRP)-conjugated anti-mouse IgG (diluted 1:8,000 in PBS–1% BSA), IgG1, and IgG2a (1:2,000) for 2 h. After washing with PBS-T20, 200 μl of substrate solution (10 mg of O-phenylenediamine and 10 μl of 30% H2O2 in 25 ml of 0.1 M citrate-phosphate buffer, pH 5.0) was added. Plates then were incubated in the dark for 30 min, and the reaction was stopped by the addition of 3N HCl (50 μl). The OD then was measured by an ELISA reader at 490 nm.
We also determined the IgG2a titers against recombinant T. gondii GRA7 and ROP1 by endpoint dilution. Each serum sample was subjected to conventional 2-fold serial dilutions starting at 1:100, and we determined the OD levels using the same ELISA methods as those described above. The mean OD plus 5 standard deviations (SD) from 10 uninfected healthy mouse sera was used as the cutoff between anti-T. gondii IgG2a antibody-positive and -negative results, which made significant differences of IgG2a titers between uninfected mouse sera and T. gondii-infected mouse sera. The endpoint titer was the highest serum dilution that yielded an OD greater than the value that defined the cutoff. For convenience, titers are expressed as reciprocal values.
In vitro T cell proliferation assay.
In vitro T cell proliferation was measured using a chemiluminescent bromodeoxyuridine (BrdU) ELISA kit (Roche Molecular Biochemicals, Indianapolis, IN). Spleens from immunized mice were collected under aseptic conditions in RPMI 1640 medium (Invitrogen Life Technologies, Carlsbad, CA). Erythrocytes were removed by lysis, and the remaining cells were washed and suspended in RPMI 1640 medium supplemented with 10% FBS, HEPES (10 mM), l-glutamine (2 mM), sodium pyruvate (1 mM), β-mercaptoethanol (50 mM), gentamicin (50 mg/ml), penicillin (100 U/ml), and streptomycin (100 mg/ml). Cells then were seeded in triplicate in flat-bottomed 96-well microtiter plates (Corning Inc., Corning, NY) at 5 × 106 cell/ml in 100 μl complete medium; thereafter, 10 μg/ml of STAg was added. The plates were incubated at 37°C in 5% CO2. After 72 h, BrdU labeling solution (10 μl) was added to each well and incubated for 2 h. The plates were centrifuged, and the labeling medium was removed. The cells were dried, FixDenat (200 μl) was added, and the plates were incubated for 30 min at room temperature. After removing the FixDenat solution, 100 μl of anti-BrdU-POD (peroxidase conjugate) working solution was added, followed by incubation for 90 min. After removing antibody conjugate, the wells were rinsed three times with PBS (pH 7.4). After 30 min of incubation with 100 μl substrate solution, the absorbance of the samples was evaluated by an ELISA reader at 370 nm with a 492-nm reference.
Spleen cell cultures and cytokine quantification.
Four weeks after the last vaccination, 3 mice per group were euthanized, and their spleens were isolated. Single-cell suspensions of splenocytes were stimulated by incubation with purified GST antigen alone or in combination with recombinant GRA7 and ROP1 antigens (5 μg/ml). Cell-free supernatants were harvested and assayed for IL-4 activity at 24 h, for IL-10 activity at 72 h, for gamma interferon (IFN-γ) activity at 96 h, and for tumor necrosis factor alpha (TNF-α) activity at 48 h. The IL-4, IL-10, IFN-γ, and TNF-α concentrations were evaluated using a commercial ELISA kit according to the manufacturer's instructions (R&D Systems, Minneapolis, MN). Cytokine concentrations were determined by reference to standard curves constructed with known amounts of mouse recombinant IL-4, IL-10, IFN-γ, or TNF-α. The sensitivity limits for the assays were 2 pg/ml for IL-4, 4.0 pg/ml for IL-10, 2 pg/ml for IFN-γ, and 1.88 pg/ml for TNF-α.
Statistical analysis.
Results are expressed as the means ± SD for each group. The statistical evaluation of the differences in survival rates was checked by Kaplan-Meier test, and then a log-rank test was done. Statistical differences in parasite burdens, antibody titers, in vitro T cell proliferation, and cytokine levels were determined using the Kruskal-Wallis test. Differences among the various groups were considered significant when P < 0.05.
RESULTS
Expression of recombinant T. gondii ROP1 and GRA7 in ARPE-19 cells in vitro and in BALB/c mice in vivo.
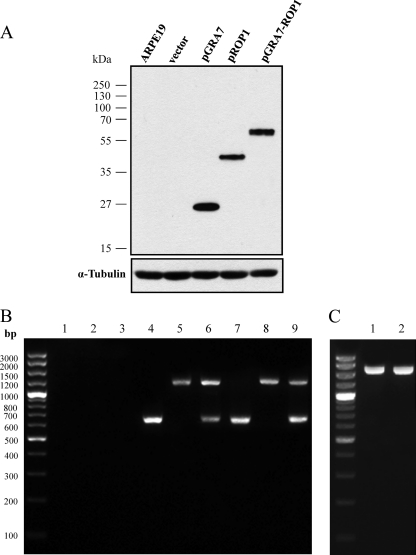
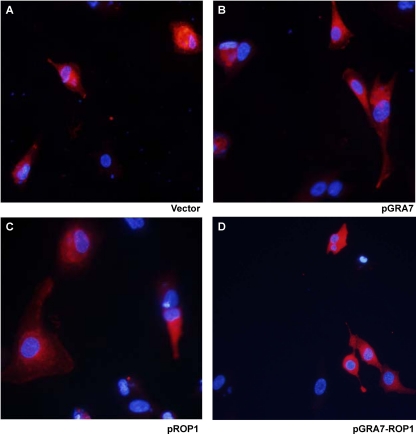
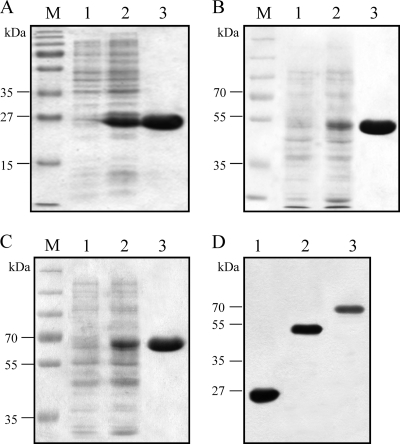
We constructed the recombinant plasmids pGRA7, pROP1, and pGRA7-ROP1 and confirmed that they expressed the T. gondii GRA7 and/or ROP1 proteins in vitro by Western blotting (Fig. 1A). The lysate of ARPE-19 cells transfected with pGRA7, pROP1, and pGRA7-ROP1 showed the specific expression of GRA7 and ROP1 proteins recognized by anti-Flag antibody (pGRA7, 27 kDa; pROP1, 43 kDa; and pGRA7-ROP1, 66 kDa) as expected based on their secretory protein property. In addition, we also confirmed that pGRA7, pROP1, and pGRA7-ROP1 were localized to the cytoplasm of transfected cells (Fig. 2). To identify the expression of recombinant plasmid in vivo, the presence of GRA7 and/or ROP1 from spleen of vaccinated mice was analyzed using the M-RT-PCR method. Figure 1B shows that expressions of GRA7 and ROP1 were not detected in the vaccinated mice with only vector, only pIL12, or vector+pIL12 DNA, whereas expressions of GRA7 (645 bp) and/or ROP1 (1,098 bp) were detected in the pGRA7- and/or pROP1-injected mice with M-RT-PCR. In the case of pGRA7-ROP1, the sequence containing the fused region (1,749 bp) was amplified by PCR using GRA7 forward and ROP1 reverse primers (Fig. 1C). These RT-PCR products were further sequenced to ensure their identity. Thus, it was proven that pGRA7, pROP1, and pGRA7-ROP1 were transfected and expressed their own products in the immunized mice.
Fig 1.
Detection of plasmid expression in vitro and in vivo. (A) Western blot analysis of ARPE-19 cells transfected with recombinant plasmid. Lysates of ARPE-19 cells transfected with empty vector, pGRA7, pROP1, or pGRA7-ROP1 recombinant plasmid DNA were electrophoresed in a 12% SDS-polyacrylamide gel, transferred to polyvinylidene difluoride membranes, and reacted with an anti-flag primary antibody. (B) In vivo expression of pGRA7, pROP1, and pGRA7-ROP1. Total RNA was isolated from spleens of DNA-vaccinated mice (lane 1, vector; lane 2, pIL12; lane 3, vector+pIL12; lane 4, pGRA7; lane 5, pROP1; lane 6, pGRA7-ROP1; lane 7, pGRA7+pIL12; lane 8, pROP1+pIL12; lane 9, pGRA7-ROP1+pIL12) 4 weeks postinoculation and were analyzed by multiplex reverse transcription-PCR (M-RT-PCR) using both GRA7 and ROP1 primers. (C) In vivo expression of pGRA7-ROP1 in pGRA7-ROP1 (lane 1)- and pGRA7-ROP1+pIL12 (lane 2)-immunized mice by RT-PCR using GRA7 forward and ROP1 reverse primer. The positions of molecular size standards are indicated on the left.
Fig 2.
Vector, pGRA7, pROP1, and pGRA7-ROP1 were localized to the cytoplasm of transfected cells. ARPE-19 cells were transfected with vector (A), pGRA7 (B), pROP1 (C), or pGRA7-ROP1 (D). Cells were fixed 48 h after transfection, anti-flag antibody (red) was used to evaluate the expression of protein levels, and nuclear DNA was stained with DAPI (shown in blue).
T. gondii-specific IgG2a titers were higher in pGRA7-ROP1-immunized mice than in pGRA7- or pROP1-immunized mice.
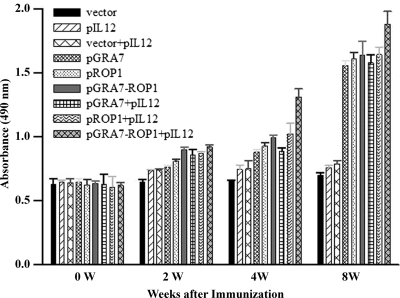
As shown in Fig. 3, T. gondii IgG titers increased with time in mice immunized with pGRA7 and/or pROP1, with or without pIL12. In particular, high IgG titers were detected in mice immunized with pGRA7-ROP1 combined with pIL12 at 4 weeks after the first immunization (P < 0.01 versus the other groups). However, there were no significant differences in the IgG titers of mice immunized with the single-gene vaccines (pGRA7 and pROP1) with or without pIL12 and pGRA7-ROP1, nor were there any significant differences between mice immunized with pGRA7 and/or pROP1 throughout the study period. Mice immunized with empty vector, pIL12, or vector+pIL12 did not produce IgG against STAg.
Fig 3.
Detection of total IgG level in the vaccinated mouse sera by ELISA. Anti-T. gondii IgG titers in sera (diluted 1:100) of mice immunized with empty vector, pIL12, pGRA7, and/or pROP1. Immune sera were collected at each time point, and IgG titers were determined by ELISA. Results were expressed as means ± SD (3 mice per group). Three independent experiments were performed, and data from one representative experiment are shown.
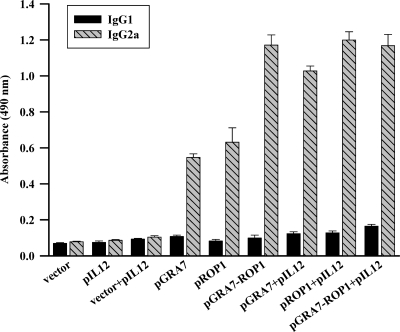
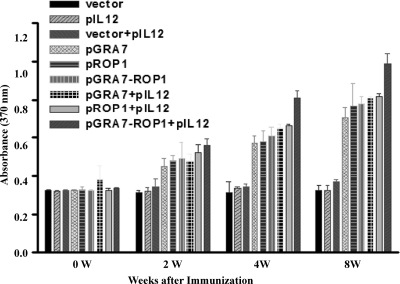
To determine whether a Th1 or Th2 response was elicited in immunized mice, T. gondii-specific IgG1 and IgG2a titers of immunized mice sera (8 weeks after the first immunization) were analyzed using STAg (Fig. 4). IgG2a titers were significantly higher than IgG1 titers in mice immunized with pGRA7, pROP1, and pGRA7-ROP1 (IgG2a OD, >0.5; IgG1 OD, <0.2); however, there were no significant differences in mice immunized with vector, pIL12, and vector+pIL12. Furthermore, the preponderance of IgG2a over IgG1 was markedly higher in mice immunized with either a single-gene vaccine combined with pIL12 or the multiple-gene vaccine compared to mice immunized with the single-gene vaccine (P < 0.05).
Fig 4.
Detection of IgGl and IgG2a levels in the vaccinated mouse sera by ELISA. Anti-T. gondii IgG1 and IgG2a titers in the sera (diluted 1:100) of mice immunized with empty vector, pIL12, pGRA7, and/or pROP1. Immune sera were collected at 8 weeks after the first immunization and determined by ELISA. Results were expressed as means ± SD (3 mice per group). Three independent experiments were performed, and data from one representative experiment are shown.
To measure the IgG2a titer of T. gondii antigen-specific antibody in immunized mice, we purified bacterially expressed recombinant T. gondii GRA7 and ROP1 protein (Fig. 5), and the presence of specific antibodies against each recombinant protein in the sera were examined by the endpoint dilution method. There was a good correlation (r2 = 0.76) between the OD (antibody titers) and the serum dilution factor of T. gondii-infected mouse sera, and calculated endpoint titers were determined by using the straight line through the OD-dilution points to find the serum dilution at which the OD would equal the cutoff value. In the endpoint titration of specific IgG2a antibody against T. gondii GRA7 and ROP1, mice immunized with pGRA7 and/or pROP1 produced high levels of specific IgG2a antibodies against rGRA7 and rROP1 (Table 1). Moreover, mice immunized with a single gene plus pIL12 showed higher endpoint titration than the mice immunized with the single-gene vaccine. These results indicated that pGRA7- and/or pROP1-immunized mice induced specific antibodies against T. gondii GRA7 and/or ROP1 and induced a predominant Th1-type immune response.
Fig 5.
Expression and purification of recombinant proteins. Proteins were submitted to SDS-PAGE and revealed by Coomassie blue staining. (A) Lane 1, pGEX-4T-1-transfomed E. coli; lane 2, IPTG-induced, pGEX-4T-1-transfomed E. coli soluble fraction; lane 3, purifed GST protein (26 kDa). (B) Lane 1, pGEX-GRA7 transfomed E. coli; lane 2, IPTG-induced, pGEX-GRA7-transfomed E. coli soluble fraction; lane 3, purifed GRA7 protein (53 kDa). (C) Lane 1, pGEX-ROP1-transfomed E. coli; lane 2, IPTG-induced, pGEX-ROP1-transfomed E. coli soluble fraction; lane 3, purifed ROP1 protein (69 kDa). (D) Western blot analysis of purified recombinant proteins with anti-GST primary Ab. Lane 1, GST; lane 2, GRA7; lane 3, ROP1. M, protein molecular size marker.
Table 1.
IgG2a endpoint titers determined by ELISA against Toxoplasma gondii recombinant GRA7 and/or ROP1 protein
| Group | Endpoint titer against recombinant T. gondii antigensa |
||
|---|---|---|---|
| rGRA7 | rROP1 | rGRA7 and rROP1 | |
| pGRA7 | 8,533 | 0 | 10,666 |
| pROP1 | 0 | 10,666 | 11,733 |
| pGRA7-ROP1 | 14,933 | 17,066 | 21,333 |
| pGRA7+pIL12 | 17,066 | 0 | 14,933 |
| pROP1+pIL12 | 0 | 19,200 | 17,066 |
| pGRA7-ROP1+pIL12 | 17,066 | 19,200 | 21,333 |
Data represent the average endpoint titers from 3 mice per group. Three independent experiments were performed, and data from one representative are shown. All mice seroconverted to the antigens with which they were vaccinated. Mice vaccinated with the empty vector, pIL12, and vector+pIL12 did not develop antibodies to the recombinant antigens.
pGRA7-ROP1-immunized mice had higher IFN-γ and TNF-α production than pGRA7- or pROP1-immunized mice.
To determine whether single- or multiple-gene immunization augments the Th1 or Th2 cytokine response, purified GRA7- and ROP1-treated culture supernatants of splenocytes were obtained from immunized mice 4 weeks following the final immunization. As shown in Table 2, IL-10, IFN-γ, and TNF-α, but not IL-4, levels in mice immunized with single- or multiple-gene vaccines were significantly higher than those of mice immunized with empty vector, pIL12, or vector+pIL12. Moreover, IFN-γ and TNF-α levels of mice immunized with pROP1 or pGRA7-ROP1 were further increased by pIL12 coadministration. However, IL-4 levels in all immunized mice failed to show significant differences.
Table 2.
Cytokine concentrations of culture supernatants of the splenocytes from mice immunized with empty vector, pIL12, pGRA7, and/or pROP1 by ELISA
| Group | Cytokine concna (pg/ml) |
|||||||
|---|---|---|---|---|---|---|---|---|
| GST antigen stimulated |
GRA7 and ROP1 antigen stimulated |
|||||||
| IL-4 | IL-10 | IFN-γ | TNF-α | IL-4 | IL-10 | IFN-γ | TNF-α | |
| Vector | 34 ± 4 | 36 ± 14 | 39 ± 5 | 20 ± 8 | 39 ± 3 | 37 ± 22 | 42 ± 4 | 36 ± 2 |
| pIL12 | 35 ± 7 | 36 ± 8 | 41 ± 4 | 27 ± 4 | 46 ± 8 | 41 ± 10 | 43 ± 6 | 35 ± 4 |
| Vector+pIL12 | 33 ± 15 | 34 ± 13 | 40 ± 6 | 26 ± 5 | 50 ± 5 | 42 ± 7 | 44 ± 5 | 38 ± 6 |
| pGRA7 | 38 ± 12 | 68 ± 16 | 89 ± 14* | 54 ± 11* | 42 ± 7 | 191 ± 29* | 317 ± 51* | 323 ± 59* |
| pROP1 | 35 ± 2 | 77 ± 13 | 83 ± 8* | 65 ± 7* | 36 ± 2 | 184 ± 33* | 327 ± 37* | 286 ± 31* |
| pGRA7-ROP1 | 36 ± 3 | 94 ± 11* | 124 ± 26* | 88 ± 12* | 36 ± 3 | 250 ± 70* | 420 ± 34* | 471 ± 73* |
| pGRA7+pIL12 | 34 ± 7 | 86 ± 11* | 107 ± 16* | 65 ± 10* | 34 ± 5 | 214 ± 25* | 345 ± 29* | 343 ± 68* |
| pROP1+pIL12 | 36 ± 7 | 77 ± 23 | 118 ± 13* | 71 ± 12* | 38 ± 3 | 249 ± 33* | 440 ± 20* | 451 ± 47* |
| pGRA7-ROP1 + pIL12 | 35 ± 3 | 101 ± 21* | 140 ± 17* | 109 ± 18* | 36 ± 4 | 410 ± 42* | 494 ± 42* | 641 ± 52* |
Values for IL-4, IL-10, IFN-γ, and TNF-α are for 24, 72, 96, and 48 h, respectively. Data represent the means ± SD from 3 mice per group. Splenocytes from mice were harvested 4 weeks after the last immunization. Single-cell suspensions of splenocytes were stimulated by incubation with purified GST antigen alone or in combination with recombinant GRA7 and ROP1 antigens (5 μg/ml). Three independent experiments were performed, and data from one representative experiment are shown. *,P < 0.05 compared to mice immunized with empty vector.
In vitro splenocyte proliferation was significantly higher in pGRA7- and/or pROP1-immunized mice.
As shown in Fig. 6, in vitro splenocyte proliferation was significantly higher in mice immunized with pGRA7 and/or pROP1 with or without pIL12 from 2 weeks after immunization compared to mice immunized with empty vector, pIL12, or vector+pIL12 (P < 0.05). However, there were no significant differences between single-gene immunizations with or without pIL12 codelivery and immunization with multiple genes. The coadministration of pGRA7-ROP1 and pIL12 at 4 and 8 weeks after immunization augmented spleen cell proliferation compared to that of spleen cells from mice immunized with pGRA7-ROP1 alone or the single gene with pIL12 (P < 0.05).
Fig 6.
In vitro proliferation of splenocytes from mice after stimulation with the soluble tachyzoite antigen of T. gondii (STAg). Splenocytes were cultured with 10 μg/ml STAg for 72 h, and proliferation was measured using a chemiluminescent BrdU ELISA kit. Absorbance was evaluated by an ELISA reader at 370 nm with a 492-nm reference. Results are expressed as means ± SD (3 mice per group). Three independent experiments were performed, and data from one representative experiment are shown.
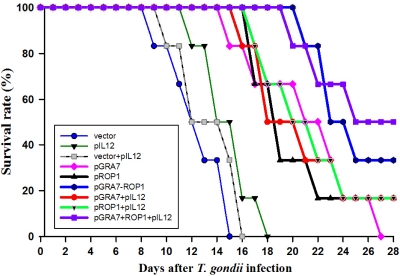
pGRA7-ROP1-immunized mice showed enhanced protection against T. gondii challenge compared to pGRA7- or pROP1-immunized mice.
The number of days of survival after challenge with a lethal dose of T. gondii cysts are shown in Fig. 7. Mice immunized with empty vector, pIL12, or vector+pIL12 died beginning 8 days after T. gondii infection, and all mice were dead by day 18 postinfection. Significantly longer survival times were obtained for single-gene- or multiple-gene-immunized mice compared to mice immunized with empty vector, pIL12, or vector+pIL12 (P < 0.01). pGRA7+ROP1- and pGRA7-ROP1+pIL12-immunized mice survived significantly longer than single-gene-immunized mice, and the survival rates of them were 33.3 and 50.0%, respectively, at 4 weeks after infection.
Fig 7.
Survival curves of mice immunized with empty vector, pIL12, pGRA7, and/or pROP1 after oral challenge with 1,500 cysts of T. gondii Me49 strain 4 weeks after the final immunization (6 mice per group). Two independent experiments were performed; data from one representative experiment were shown.
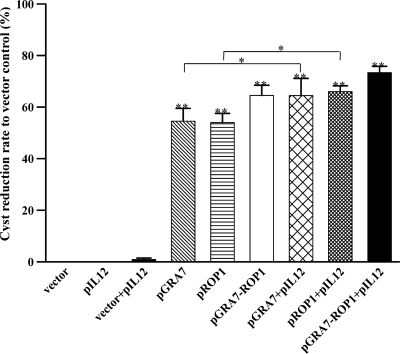
Four weeks after oral challenge with T. gondii cysts, the number of cysts in the brain was determined microscopically, and the cyst reduction rates to vector control were higher in the multiple-gene-immunized mice than in single-gene-immunized mice (Fig. 8). The reduction rates of single- or multiple-gene-immunized mice were further increased with pIL12 codelivery, and the most reduced rates were observed in pGRA7-ROP1+IL-12-immunized mice.
Fig 8.
Reduction rate to vector control of T. gondii cyst numbers in the brain of mice immunized with empty vector, pIL12, pGRA7, and/or pROP1. Four weeks after the last immunization, mice were orally challenged with a nonlethal dose of the T. gondii Me49 strain (20 cysts per mouse) and evaluated 4 weeks after infection. Cyst reduction rate to vector control was calculated with the formula [1 − (brain cyst numbers of vaccinated mice/brain cyst number of vector control)] × 100. Data are represented as means ± SD. Three independent experiments were performed, and data from one representative experiment are shown. **, Significance compared to mice immunized with empty vector (P < 0.01). *, Signficiance compared to mice immunized with single-gene vaccines (pGRA7 or pROP1) only (P < 0.05).
DISCUSSION
DNA vaccination can protect animals and human beings against pathogenic microorganisms, particularly intracellular parasites (1). In the present study, BALB/c mice immunized intramuscularly with pGRA7, pROP1, or pGRA7-ROP1 produced specific antibodies against T. gondii GRA7 and/or ROP1, and protective immunity was induced. Moreover, immunization with the multiantigenic gene plasmid pGRA7-ROP1 induced a greater tendency toward Th1-type immune responses than did immunization with the single-antigen gene plasmid pGRA7 or pROP1. This type of immune response was further enhanced by the codelivery of pIL12 as a genetic adjuvant.
It has been demonstrated that a Th1-biased response is required for effective protection in naturally occurring T. gondii infection (6). To determine the T-helper response of immunized mice, we evaluated the IgG subclass induced by DNA immunization. The ratio of IgG2a to IgG1 titers against STAg, which was characteristic of the Th1-type response, was markedly higher in pGRA7-ROP1 immunization than in single-gene vaccination, which was also confirmed by IgG2a endpoint titration against T. gondii recombinant GRA7 and/or ROP1. Furthermore, immunization with a single-gene vaccine with pIL12 augmented the predominance of IgG2a over IgG1 induced by single-gene vaccine alone. Our data confirmed previous reports that seroconversion can readily be obtained by DNA vaccination (16), and the preponderance of anti-Toxoplasma IgG2a over IgG1 was greater in multiple-gene than in single-gene vaccination (18). It is well known that IFN-γ is the central cytokine that is responsible for resistance against T. gondii during both the early and late stages of infection (6). We also detected both Th1 (IFN-γ and TNF-α) and Th2 (IL-4 and IL-10) cytokines in splenocytes from pGRA7-, pROP1-, and pGRA7-ROP1-immunized mice. In this study, IFN-γ production was increased by about 8- to 12-fold in single- and multiple-gene-immunized mice; however, IL-4 production was not changed significantly after GRA7 and/or ROP1 immunization. Our results show that immunization with pGRA7, pROP1, or pGRA7-ROP1 elicits a Th1 immune response, and the multiple-gene vaccine induced greater Th1 immune responses than the single-gene vaccine. This phenomenon of a single-gene vaccine is further enhanced by the addition of pIL12. These results were similar to previous data that found that immunization with a plasmid encoding the ROP1 genes enhanced the production of IFN-γ in vitro and ROP1-specific IgG2a antibody in sheep (10).
Generally, cell-mediated immunity, and especially the T cell immune response, plays a key role in protection against T. gondii infection. It was reported that multiantigenic gene plasmids are more protective against T. gondii challenge than single-antigen gene plasmids (4, 5, 12). In contrast, a significant suppression of immune responses was shown when the plasmids were pooled in a cocktail and injected into a single site (15). In this study, specific T cell responses, GRA7- or ROP1-specific lymphoproliferation based on BrdU assay, were observed in spleen cell cultures from mice immunized with either pGRA7 or pROP1 with or without pIL12 but not in mice immunized with empty vector, pIL12, or vector+pIL12. Also, pGRA7- and/or pROP1-vaccinated mice produced significantly higher levels of IFN-γ, and these mice also had significantly reduced rates of parasite burden in the brain and longer survival times compared to those of the empty vector-, pIL12-, or vector+pIL12-vaccinated mice. These data indicate that the pGRA7, pROP1, and pGRA7-ROP1 DNA vaccines, either alone or in combination with pIL12, provide an appropriate epitope for presentation on major histocompatibility complex class I (MHC-I) (17) and evoke a cellular immune response to control the multiplication and spreading of a T. gondii infection (7, 11). These results were similar to the previous study (9) showing that the combination of GRA1 and GRA7 reduced brain cyst burden up to 89% in C3H/HeN mice after challenge with 76K. However, Vercammen et al. (16) reported that BALB/c mice vaccinated with genes encoding T. gondii ROP2, GRA1, and GRA7 led to mortality rates of at least 80% after challenge with strain IPB-G, so no protection was observed in BALB/c-vaccinated mice. These results were somewhat similar to and somewhat different from ours. The mortality rate of single-gene-vaccinated mice (80 to 100%) was similar to ours (survival rate, 0 to 16%); however, they did not evaluate the protective effects of multiantigenic vaccine and adjuvant, and they also used a different parasite strain.
In this study, we show that a DNA vaccine expressing the T. gondii GRA7 or ROP1 antigen (pGRA7 or pROP1) and a fusion of both (pGRA7-ROP1) induced specific humoral and cellular immune responses in BALB/c mice, and immunization with either a multiantigenic DNA vaccine (pGRA7-ROP1) or a single-gene vaccine combined with pIL12 induces greater Th1-type immune responses and protective efficacy against T. gondii infection than individual antigen. Nevertheless, further studies are needed to develop more effective multiantigenic DNA vaccines by varying the components thereof.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (no. 2011-0006228) and the Basic Science Research Program through the National Research Foundation of Korea (no. 2011-0023501), which is funded by the Ministry of Education, Science, and Technology.
We have no conflicts of interest.
Footnotes
Published ahead of print 14 March 2012
REFERENCES
- 1. Beláková J, Horynová M, Krupka M, Weigl E, Raska M. 2007. DNA vaccines are they still just a powerful tool for the future. Arch. Immunol. Ther. Exp. 55:387–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boothroyd JC, Dubremetz JF. 2008. Kiss and spit: the dual roles of Toxoplasma rhoptries. Nat. Rev. Microbiol. 6:79–88 [DOI] [PubMed] [Google Scholar]
- 3. Chen H, Chen G, Zheng H, Guo H. 2003. Induction of immune responses in mice by vaccination with Liposome-entrapped DNA complexes encoding Toxoplasma gondii SAG1 and ROP1 genes. Chin. Med. J. 16:1561–1566 [PubMed] [Google Scholar]
- 4. Cui YL, et al. 2008. Protective effect of a multiantigenic DNA vaccine against Toxoplasma gondii with co-delivery of IL-12 in mice. Parasite Immunol. 30:309–313 [DOI] [PubMed] [Google Scholar]
- 5. Fachado A, et al. 2003. Protective effect of a naked DNA vaccine cocktail against lethal toxoplasmosis in mice. Vaccine 21:1327–1335 [DOI] [PubMed] [Google Scholar]
- 6. Filisetti D, Candolfi E. 2004. Immune response to Toxoplasma gondii. Ann. Ist. Super. Sanita. 40:71–80 [PubMed] [Google Scholar]
- 7. Gazzinelli RT, Hakim FT, Hieny S, Shearer GM, Sher A. 1991. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-γ production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J. Immunol. 146:286–292 [PubMed] [Google Scholar]
- 8. Hiszczyńska-Sawicka E, et al. 2011. Evaluation of immune responses in sheep induced by DNA immunization with genes encoding GRA1, GRA4, GRA6 and GRA7 antigens of Toxoplasma gondii. Vet. Parasitol. 177:281–289 [DOI] [PubMed] [Google Scholar]
- 9. Jongert E, de Craeye S, Dewit J, Huygen K. 2007. GRA7 provides protective immunity in cocktail DNA vaccines against Toxoplasma gondii. Parasite Immunol. 29:445–453 [DOI] [PubMed] [Google Scholar]
- 10. Li B, et al. 2010. Immunological response of sheep to injections of plasmids encoding Toxoplasma gondii SAG1 and ROP1 genes. Parasite Immunol. 32:671–683 [DOI] [PubMed] [Google Scholar]
- 11. Liu S, et al. 2009. Evaluation of protective effect of multi-epitope DNA vaccine encoding six antigen segments of Toxoplasma gondii in mice. Parasitol. Res. 105:267–274 [DOI] [PubMed] [Google Scholar]
- 12. Mévélec MN, et al. 2005. Evaluation of protective effect of DNA vaccination with genes encoding antigens GRA4 and SAG1 associated with GM-CSF plasmid, against acute, chronical and congenital toxoplasmosis in mice. Vaccine 23:4489–4499 [DOI] [PubMed] [Google Scholar]
- 13. Peng GH, et al. 2009. Toxoplasma gondii microneme protein 6 (MIC6) is a potential vaccine candidate against toxoplasmosis in mice. Vaccine 27:6570–6574 [DOI] [PubMed] [Google Scholar]
- 14. Rosenberg C, et al. 2009. Induction of partial protection against infection with Toxoplasma gondii genotype II by DNA vaccination with recombinant chimeric tachyzoite antigens. Vaccine 27:2489–2498 [DOI] [PubMed] [Google Scholar]
- 15. Sedegah M, et al. 2004. Reduced immunogenicity of DNA vaccine plasmids in mixtures. Gene Ther. 11:448–456 [DOI] [PubMed] [Google Scholar]
- 16. Vercammen M, et al. 2000. DNA vaccination with genes encoding Toxoplasma gondii antigens GRA1, GRA7, and ROP2 induces partially protective immunity against lethal challenge in mice. Infect. Immun. 68:38–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang X, Claflin J, Kang H, Suzuki Y. 2005. Importance of CD8(+)Vβ8(+) T cells in IFN γ-mediated prevention of toxoplasmic encephalitis in genetically resistant BALB/c mice. J. Interferon Cytokine Res. 25:338–344 [DOI] [PubMed] [Google Scholar]
- 18. Zhang J, et al. 2007. Evaluation of the immune response induced by multiantigenic DNA vaccine encoding SAG1 and ROP2 of Toxoplasma gondii and the adjuvant properties of murine interleukin-12 plasmid in BALB/c mice. Parasitol. Res. 101:331–338 [DOI] [PubMed] [Google Scholar]