Abstract
The mycobacterial heparin-binding hemagglutinin (HBHA) protein induces a potent gamma interferon (IFN-γ) response in latent tuberculosis (TB) infection and is a candidate vaccine and diagnostic antigen. We have assessed HBHA-specific intracellular IFN-γ, interleukin-2 (IL-2), and IL-17 production by CD4+ T cells in TB cases and household contacts (HHCs) as well as the level of secreted IFN-γ in whole-blood culture supernatant. HHCs were further classified as tuberculin skin test (TST) positive or negative, and the group was also divided as HIV positive or negative. Our study revealed that HBHA induces multifunctional IFN-γ-, IL-2-, and IL-17-coexpressing CD4+ T cells in HHCs but not in active TB cases; however, IFN-γ levels in culture supernatant did not differ between participant groups. Further studies are needed to completely understand how HBHA induces immune responses in different disease groups.
INTRODUCTION
With 9 million new tuberculosis (TB) infections per year (22), a vaccine is urgently needed. The Mycobacterium bovis BCG vaccine was developed more than 100 years ago and is currently the only vaccine against tuberculosis available. BCG has been shown to be protective against disseminated forms of TB in children, but vaccine efficacy decreases over time (10, 11, 22). Recombinant forms of BCG are currently being developed and tested in healthy controls in the developing world and Europe (13, 14, 37).
The heparin-binding hemagglutinin (HBHA) of Mycobacterium tuberculosis, a dissemination factor (19, 29) that has been identified as an immunodominant antigen able to induce gamma interferon (IFN-γ), is associated with protection against TB (15). This surface protein interacts with nonphagocytic cells, attaches to sulfated glycoconjugates, and has the ability to agglutinate red blood cells (19). The specific methylation of the antigen was shown to be important for generating an immune response to the antigen (5), and it has been suggested that HBHA can be used in gamma interferon release assays (IGRAs) to diagnose M. tuberculosis infection or to discriminate between the active and latent forms of the disease (15). Current preclinical strategies are to administer the HBHA vaccine as a booster following vaccination with BCG (35).
IGRAs that are based on the principle that the antigens ESAT-6, CFP10, and TB7.7 are specific to the M. tuberculosis complex offer an attractive alternative to the tuberculin skin test (TST) (26, 28, 38). IGRAs work on the principle that IFN-γ released upon T-cell stimulation is collected and measured by a standard enzyme-linked immunosorbent assay (ELISA). Although IGRAs are now commonly used in research studies to identify latent M. tuberculosis infection, there is no strong evidence to suggest that the in vitro IFN-γ response to mycobacterial antigens is associated with disease resistance, representing a delicate balance between mycobacterial burden and the immune response. IGRAs are not prognostic tests but rather imply previous exposure.
A number of recent studies have shed light on the complex immunological networks and cytokine exchanges that result from infection with M. tuberculosis (17, 30), including an increase in the number of circulating pro- and anti-inflammatory cytokines from the different T helper cells (34). Scriba et al. (33) have previously shown that CD4+ T cells that specifically secrete interleukin-17 (IL-17) and high levels of IL-22 in bronchoalveolar lavage (BAL) fluid contribute to the immune response against M. tuberculosis in healthy adults. Another recent South African study demonstrated that BCG vaccination in children induces cells with a central memory phenotype that express IFN-γ and IL-2 (34). A study conducted in Germany has shown that M. tuberculosis antigen restimulation of peripheral blood mononuclear cells (PBMCs) isolated from children with latent and active TB leads to the production of multifunctional memory CD4+ T cells that coexpress granulocyte-macrophage colony-stimulating factor (GM-CSF) (25).
Based on previous studies demonstrating the immunogenicity of HBHA in the context of IFN-γ secretion and the diagnostic potential of this antigen, we have evaluated the ability of this M. tuberculosis surface antigen to induce multiple cytokines in peripheral blood mononuclear cell and whole-blood assays. We have performed intracellular cytokine staining and flow cytometry to investigate the phenotype and pattern of cytokine production of HBHA-stimulated cells from TB index cases and household contacts (HHCs).
MATERIALS AND METHODS
Study participants and area of recruitment.
Data in the present study are based on individuals recruited as part of the Bill and Melinda Gates Foundation-supported initiative “Biomarkers of protective immunity against TB in the context of HIV/AIDS in Africa.” The local research and ethics committees approved the study (ethics reference no. N05/11/187), and study participants gave written informed consent for venipuncture and immunological assays.
Individuals with active TB were recruited from community clinics in Ravensmead and Uitsig, Cape Town, South Africa. TB index cases were all newly diagnosed, untreated, and confirmed with sputum cultures. Household contacts (HHCs) with known recent exposure to smear-positive active TB were also recruited but had no symptoms, signs, positive sputum cultures, or chest X-ray findings indicative of active TB disease. BCG scar status suggested that all participants had received a BCG vaccination, and all were HIV negative, as confirmed by a rapid HIV test (Determine HIV-1/2; Abbott). Recruitment of HIV-positive participants was done through the infectious diseases clinic at Tygerberg Hospital, Cape Town, South Africa. The members of the HIV-positive group had no active TB disease (confirmed in the same manner as for HHCs) and were not on antiretroviral treatment at time of enrollment. For all participants, heparinized whole blood was collected and transported to the immunology laboratory at Stellenbosch University, Cape Town, South Africa, where immunological assays were performed within 2 h of blood collection. The HHC and HIV-positive participants also underwent a tuberculin skin test (TST) using 2 tuberculin units of M. tuberculosis purified protein derivative (PPD) (Statens Serum Institut [SSI], Denmark), which was administered on the volar aspect of the left forearm and read between 48 and 72 h later by the experienced study nurses. An induration of >10 mm was considered positive for HIV-uninfected HHCs, and an induration of >5 mm was considered positive for HIV-infected participants.
The communities of Ravensmead and Uitsig have an extremely high prevalence of TB infection (1,000/100,000) (6). The community consists mainly of mixed-race individuals with an HIV infection prevalence of approximately 2% (18). The demographic and laboratory information on the study participants is presented in Tables 1 and 2.
Table 1.
Characteristics of the study group for the PBMC stimulation assays
| Characteristic | Value for each study participant group |
|
|---|---|---|
| TB index casesa | HHCs | |
| Sample size | 11 | 11 |
| Mean age (yr) (range) | 32.5 (22–45) | 18 (10–37) |
| Sex (male:female) | 4:7 | 8:3 |
| BCG vaccinated | Yes | Yes |
| No. with each TST response | ||
| 0–10 mm | NA | 5 |
| >10 mm | NA | 6 |
TB index cases did not undergo TST tests. NA, not applicable.
Table 2.
Demographic and clinical/laboratory characteristics of the study group used for the whole-blood stimulation assays
| Characteristic | Value for each study participant group |
|||
|---|---|---|---|---|
| HHCa |
TB indexb | HIV+c | ||
| TST− | TST+ | |||
| Sample size | 4 | 12 | 5 | 5 |
| Mean age (yr) (range) | 34 (10–61) | 32.1 (12–56) | 31.2 (22–38) | 32.8 (27–44) |
| No. (%) of males | 2 (50%) | 3 (25%) | 3 (60%) | 3 (60%) |
| BCG vaccinated | Yes | Yes | Yes | Yes |
| No. with each TST response | ||||
| 0–10 mm | 4 | 0 | NA | 2 |
| 11–15 mm | 0 | 4 | NA | 0 |
| >15 mm | 0 | 8 | NA | 2 |
HHC, household contact; TST, tuberculin skin test; TST−, negative TST; TST+, positive TST.
TB index cases do not undergo TST tests. NA, not applicable.
One HIV-positive (HIV+) patient was not tested with the TST.
Whole-blood assay and IFN-γ ELISA.
The whole-blood assay was done as described by Black et al. (3). In brief, heparinized whole blood diluted (1:10) in RPMI 1640 (Sigma) was incubated with M. tuberculosis antigens at a final concentration of 10 μg/ml in a 96-well plate (Nunclon, Gibco) format and cultured for 7 days at 37°C and 5% CO2. The antigens included PPD and TB10.4 (SSI), TesatCFP10 (an ESAT6/CFP10 fusion protein), and TB10.3, Ag85A, and HspX (kindly provided by Tom Ottenhof and Michel Klein, Leiden University Medical Center, The Netherlands), all at a final concentration of 10 μg/ml. Staphylococcus enterotoxin B (SEB) (Sigma; final concentration, 0.1 μg/ml) was added as a positive control. RPMI 1640 was used as a negative control. Supernatants were harvested after 7 days and frozen at −80°C until analysis. The methylated native HBHA (nHBHA) antigen was kindly provided by Camille Locht (Institut Pasteur de Lille) as a methylated protein purified from BCG.
All frozen supernatants were analyzed simultaneously for IFN-γ by a sandwich ELISA using OPD (o-phenylenediamine dihydrochloride) Fast (Sigma) as the substrate. The optical densities (ODs) were read at 490 nm on a benchmark microplate reader (Bio-Rad), and a positive response was defined as a response above 32 pg/ml (the lower level of detection based on the IFN-γ standard curve) after subtraction of the assay blank.
PBMCs.
A total of 50 ml of blood from each participant was collected into 10-ml sodium heparin vacutainers (BD Biosciences). PBMCs were separated over Histopaque (Sigma-Aldrich) gradients by following standard procedures. Viability was assessed by trypan blue exclusion to ensure >90% viability in all samples used.
Intracellular cytokine staining and flow cytometric analysis.
A total of 106 PBMCs, resuspended in RPMI 1640 (Sigma) supplemented with 10% heat-inactivated human AB serum (Sigma), were aliquoted into 96-well U-bottom plates (Nunclon; Gibco). Stimulation was carried out in a final volume of 200 μl. M. bovis BCG (SSI) and nHBHA, at final concentrations of 1.9 × 105 CFU/ml and 10 μg/ml, respectively, were used as stimulants, RPMI 1640 was used as a negative control, and SEB, at a final concentration of 1 μg/ml (Sigma), was used as a positive control. Costimulatory antibodies (anti-CD28/49d at 1 μg/ml; BD Biosciences) were used in all culture conditions. Brefeldin A (10 μg/ml; Sigma-Aldrich) was added after 2 h, and the samples were incubated overnight for 16 h. Following the incubation, the cells were washed several times and then pelleted by centrifugation. For intracellular staining, the cells were permeabilized with Fix/Perm buffer (e-Bioscience) and then labeled with the monoclonal antibodies CD4-PerCP, IFN-γ-FITC, IL-2-APC (all BD Biosciences), and IL-17-PE (e-Bioscience) for 30 min. Isotype controls were included for each antibody. Cells were washed and fixed in 1% formaldehyde and analyzed within 1 h on a FACSCalibur flow cytometer (BD Biosciences). We acquired at least 100,000 cells in the lymphocyte gate using the CellQuest software (version 3.3; BD Biosciences). The analysis of the cytokine expression was done using the FlowJo software (version 7.2.5; Tree Star). Background values obtained for the unstimulated conditions were subtracted from the antigen-stimulated responses to yield antigen-specific cytokine production.
Statistical analysis.
All analyses were performed with the GraphPad Prism version 5 statistical package (GraphPad software, Inc., San Diego, CA). A P value of 0.05 was considered statistically significant for all comparisons. For the ELISA analysis, antigen response values were log transformed. Multiple comparisons of the antigen responses between participant groups were done using a two-way analysis of variance (ANOVA).
RESULTS
Characteristics of the study population.
For the PBMC stimulation (Table 1), followed by intracellular cytokine analysis, 22 HIV-uninfected participants, of whom 11 were TB index cases and 11 were HHCs, were included. The mean age of the TB index cases was higher than that of the HHCs (32.5 years versus 18 years). For the whole-blood assay (Table 2), followed by ELISA, 26 subjects were included and were stratified into four different groups based on TB disease, TST result, and HIV status. Two HIV-infected participants had TST readings of 0 mm, and the other two had readings above 20 mm. One HIV-positive participant was not tested for TST.
Cytokine production by antigen-stimulated cells.
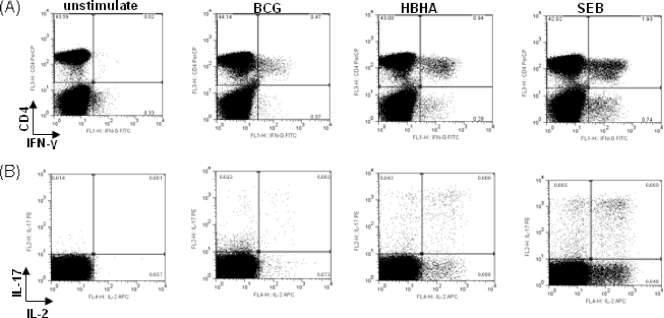
PBMCs were stimulated overnight with BCG, HBHA, and SEB and evaluated for the production of intracellular cytokines by flow cytometric analysis. In participants (11 HHCs versus 11 TB index cases) who responded to the antigen stimulation, we found CD4+ T cells positive for IL-2, IL-17, and IFN-γ (Fig. 1). Distinct populations of cytokine-positive cells were identified, and all the participants had positive cytokine responses to SEB. In the TB index cases, BCG stimulation induced IFN-γ responses (median response, percentage of CD4+ IFN-γ-positive [IFN-γ+] cells = 0.2113%, 95% confidence interval [CI] = 0.06462 to 0.9160) but not to the same level as observed in HHCs (median response, percentage of CD4+ IFN-γ+ cells = 0.2912%; maximum response, percentage of CD4+ IFN-γ+ cells = 5.95%), although this difference was not statistically significant (P = 0.3928; data not shown).
Fig 1.
A representative flow cytometric plot of the cytokine production of CD4 T cells from a healthy household contact stimulated with BCG, HBHA, or SEB. PBMCs from the HHC were stimulated for 16 h and then analyzed by flow cytometry for the production of Th1 and Th17 cytokines. The plot series in panel A are gated on the lymphocyte population in forward (FCS-A) and side (SSC-A) scatter plots of PBMCs, and the series in panel B are gated on CD4+ cells.
BCG- and HBHA-stimulated CD4+ IFN-γ+ cells produce IL-2 and IL-17.
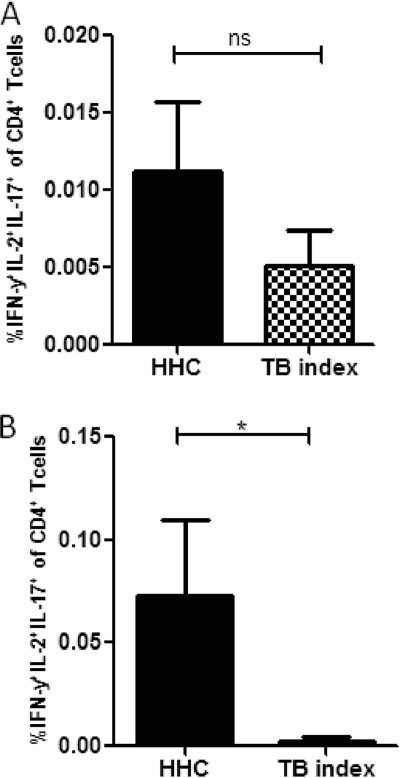
The cytokine response to recombinant HBHA was assessed in PBMCs after overnight stimulation of cells from TB index cases and HHCs. There were no differences observed in the background values for IFN-γ, IL-2, or IL-17 between HHCs and TB index cases (data not shown). Stimulation with BCG induced IFN-γ- and interleukin-2 and -17-positive (IFN-γ+ IL-2+ IL-17+) CD4 T cells in both the HHCs and TB index cases; the higher levels seen in HHCs were not statistically significant (Fig. 2A). In contrast, stimulation with HBHA induced a higher level of this cell phenotype in HHCs than in TB index cases (P = 0.05), with the multiple-cytokine response in TB index cases almost completely abrogated (Fig. 2B). The HBHA-specific CD4+ IL-2+ IL-17+ IFN-γ+ phenotype had a median value of 0.03% (range, 0.01 to 0.39%) in HHCs. Stimulation with SEB induced significantly more triple-positive cells than HBHA or BCG (highest observed frequencies were 0.04% for BCG, 0.39% for HBHA, and 1.11% for SEB) (data not shown).
Fig 2.
Frequency of IFN-γ+ CD4 T cells expressing IL-2 and IL-17. Lymphocytes were identified by light scatter properties (FCS-A versus SSC-A) followed by a gate on the CD4+ cells. IFN-γ+ cells were further gated to analyze IL-2+ and IL-17+ cells. Coexpression of cytokines after 16 h of BCG stimulation (A) or HBHA stimulation (B) is shown. Background cytokine values from the unstimulated PBMCs were subtracted from BCG- and HBHA-specific responses. The asterisk indicates a statistically significant difference between groups when compared using the Mann-Whitney U test. Bars show the mean and the standard error of the mean (SEM).
Distinct patterns of cytokine production in HHCs.
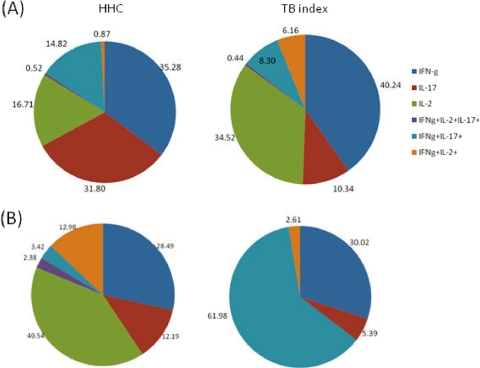
Stimulation with BCG induced similar cytokine patterns in HHCs and TB index cases (Fig. 3A). The median response to BCG was higher in the CD4+ IFN-γ+ subset of HHCs than in TB index cases (0.97% versus 0.4%, respectively). The proportion of cells producing both IFN-γ and IL-2 was markedly increased in the TB index cases (6% of CD4 T cells) when stimulated with BCG compared to that for the HHCs. In both study groups, BCG stimulation induced 6 distinct cytokine-producing cell subsets. HBHA stimulation induced a completely different cytokine pattern in HHCs than in TB index cases (Fig. 3B). The cytokine profile of the HHCs was much broader and resembled the 6 BCG-induced cell subsets. The majority of responses to HBHA seen in HHCs were absent in TB index cases. The few cytokine responses present in TB index cases after HBHA stimulation were comprised mainly of IFN-γ+ IL-17+ (61%) and IFN-γ+ cells (30%). Interestingly, no CD4+ IL-2+ T cells were induced by HBHA in the TB index cases.
Fig 3.
Proportions of IL-2 and IL-17 cytokine-producing IFN-γ+ cells after stimulation with mycobacterial antigens. The pie charts illustrate the median frequencies of BCG-specific (A) and HBHA-specific (B) cytokine responses in healthy household contacts (n = 11) and TB index cases (n = 11). Responses are divided into 6 cytokine-positive cell subsets: cells producing (i) IL-2, IL-17 and IFN-γ, (ii) IL-17 and IFN-γ, (iii) IL-2 and IFN-γ, (iv) IFN-γ only, (v) IL-17 only, or (vi) IL-2 only.
Classical TB antigens induce an IFN-γ response in a whole-blood assay.
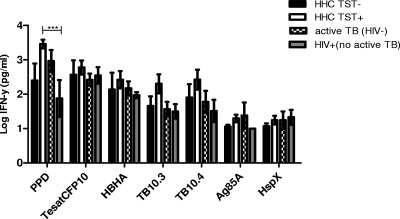
We used a seven-day whole-blood assay to assess the IFN-γ responses to a panel of 6 classical TB antigens in the 4 participant groups: TST-negative (0 mm) HHCs (control group), TST-positive HHCs (latent TB), active TB (HIV uninfected), and HIV positive (no active TB). In Fig. 4, the log-transformed responses to all of the tested antigens are shown for the different groups. The mean IFN-γ responses to each of the tested antigens were compared between the study groups. When the TST-negative group (n = 4) was compared to the other patient groups, none of the antigen responses were found to be significantly different. The IFN-γ response to PPD in the latent TB group (mean, 4,175 pg/ml) was significantly higher than that in the HIV-positive (no active TB) group (mean, 631 pg/ml; P < 0.001). Within the latent TB group, the mean IFN-γ response to PPD was also significantly higher than that to TB10.3 (1,045 pg/ml; P < 0.05). Within the active TB group (n = 5), the IFN-γ response to HspX was much lower than the response to PPD (44 pg/ml versus 2,245 pg/ml, respectively; P < 0.05). None of the classical antigens induced noteworthy responses in the HIV-positive patients (n = 4).
Fig 4.
Intergroup comparison of IFN-γ levels after whole-blood stimulation with mycobacterial antigens. After a 7-day incubation, supernatant levels of IFN-γ were measured by an ELISA of whole-blood culture supernatants from TST-negative HHCs (n = 4), TST-positive HHCs (n = 12), HIV-uninfected active TB cases (n = 5), and HIV-positive participants without active TB (n = 4). IFN-γ levels were log10 transformed. Graphs show the mean and the standard error of the mean (SEM). ***, P < 0.001.
HBHA-induced IFN-γ does not differentiate between latent infection and active TB disease.
The ability of recombinant methylated HBHA to discriminate between TST-negative HHCs, TST-positive HHCs, and active TB cases (Fig. 4) was investigated. Log10-transformed IFN-γ responses to the HBHA antigen were compared for the different participant groups after 7-day whole-blood assays. All of the participants in the active TB and HIV-positive groups responded to HBHA, and only 2 TST-positive HHCs and 1 TST-negative HHC did not respond to the antigen. The mean responses to HBHA were not different between any of the groups. The magnitude of the response to HBHA was higher in the TST-positive HHCs (mean, 992 pg/ml) than in the active TB group (mean, 218 pg/ml); however, the difference was not significant. There was no difference in the responses to TesatCFP10.
DISCUSSION
This pilot study evaluates the cytokine response to HBHA in a high-prevalence TB setting. We have assessed the profile of HBHA-stimulated lymphocytes in TB index cases and household contact controls. Our study shows that the methylated HBHA induces multifunctional CD4 T cells that produced IFN-γ, IL-2, and IL-17 at significantly higher frequencies in HHCs than in TB index cases. The patterns of cytokine production are also clearly different between HHCs and TB index cases when stimulated with HBHA. Cytokine responses to HBHA consisted mainly of IFN-γ+- and IL-17+-secreting CD4 T cells for the TB index group. Data from several studies in countries with low incidence of TB have shown that the IFN-γ response to HBHA is much lower in patients with active TB disease than in latently infected people (15, 19). Masungi and colleagues (20) have shown that the IFN-γ that is produced after stimulation with HBHA is the consequence of T-cell stimulation rather than nonspecific stimulation of monocytes. We also found low levels of background cytokine production (data not shown) in the PBMC stimulation assays. The fact that HHCs produced significantly more multifunctional CD4 T cells in our study implicates this cell phenotype in a protective response against TB even though longitudinal studies will have to be performed to confirm this result. Similar findings have been reported for nondisease progression in HIV (2) and Leishmania (4). Recent studies on patients with active TB found that specific IL-17- and IL-22-producing CD4 T cells are present in the anti-TB response (33). Our study underscores the importance of IL-17 in TB disease but did not find that IL-17-producing cells are distinct from IFN-γ-producing CD4 T cells. We have shown that the cytokine response to HBHA in the active TB participant group consists mainly of IFN-γ+ IL-17+ CD4 T cells and that the multifunctional cytokine response is almost completely abrogated. Our data are similar to those of Annunziato et al. (1), who found that in patients with Crohn's disease, the CD4+ T cells coexpressed IFN-γ and IL-17.
In contrast to our results that show that the broad and multifunctional cytokine pattern induced by HBHA is indicative of protection/nondisease progression, others have shown increased cytokine production by multiplex cytokine arrays in active TB disease (36). However, the techniques employed in this study and those in the Sutherland paper measure different parameters and are not directly comparable. In the intracellular cytokine whole-blood staining assays reported by Scriba et al. (33), increased levels of IL-17 were found in HHCs, a result which is similar to our findings in PBMC stimulation assays.
Many studies have now focused on other cytokines, in addition to IFN-γ, in the search for protective host factors (21, 23, 24). Although numerous studies define protection against active TB as dependent upon an upregulation of IFN-γ levels (31), others have questioned its aptness (9, 12). Qiu et al. (30) showed that during severe TB disease, there is an uneven upregulation of gene networks and the overexpression of several cytokines and chemokines. For example, in patients with active TB, anti-TB treatment results in the differential expression of IL-4 and the IL-4 splice variant IL-4Δ2 (8, 31). In addition, experiments in BCG-vaccinated rhesus macaques revealed a 600-fold increase in the expression of 78 genes among the 138 assessed (16), highlighting the importance of other genes in the discrimination between active TB disease and latent infection.
The diagnosis of latent TB infection currently relies on the IGRAs and/or TST. The IGRAs are based on the principle that IFN-γ is released upon in vitro stimulation of whole blood with TB-specific antigens (27). The current IGRAs do not distinguish between latent and active TB. Several scientists have evaluated HBHA as a potential diagnostic antigen (15, 19, 32). In Finland, a country with a low incidence of TB (6.1/100,000) (5) in which routine BCG vaccination is performed, the antigen could not discriminate between people with TB infection and nondiseased people who were previously vaccinated with BCG. Our data from a high-TB-incidence area concur with this and other findings from South Africa (7).
Our study, even though it reports on only a small number of participants, sheds light on the ability of the methylated HBHA antigen to induce different multicytokine profiles in active TB patients and their HHCs. This profile and pattern of cytokines may be useful in future discriminatory evaluations of latent and active TB and reinforce the need for a biosignature other than just IFN-γ. At the same time, it also raises the question as to whether the multifunctional CD4 T cells that we detect are the cause or effect of the HBHA antigenic response. In our setting, HBHA holds no discriminatory potential as a stimulant for an assay that measures only IFN-γ levels in active TB and latent TB infection. The biological contextualization of the HBHA-induced differences needs further clarification in future studies.
ACKNOWLEDGMENTS
We thank the field staff and all the study participants for taking part in the project. We are grateful to Henrik Mueller and Teri Roberts for critical reading of the manuscript and to Hawa Golakai and Nelita du Plessis for isolating PBMCs.
A.G.L. is supported by a DST/NRF Innovation Postdoctoral Fellowship.
The authors declare no conflict of interest.
Footnotes
Published ahead of print 29 March 2012
REFERENCES
- 1. Annunziato F, et al. 2007. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 204:1849–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Betts MR, et al. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Black GF, et al. 2001. Patterns and implications of naturally acquired immune responses to environmental and tuberculous mycobacterial antigens in northern Malawi. J. Infect. Dis. 184:322–329 [DOI] [PubMed] [Google Scholar]
- 4. Darrah PA, et al. 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13:843–850 [DOI] [PubMed] [Google Scholar]
- 5. Delogu G, et al. 2004. Expression and purification of recombinant methylated HBHA in Mycobacterium smegmatis. FEMS Microbiol. Lett. 239:33–39 [DOI] [PubMed] [Google Scholar]
- 6. den Boon S, et al. 2007. High prevalence of tuberculosis in previously treated patients, Cape Town, South Africa. Emerg. Infect. Dis. 13:1189–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dheda K, et al. 2009. Utility of quantitative T cell responses versus unstimulated IFN-γ for the diagnosis of pleural tuberculosis. Eur. Respir. J. 34:1118–1126 [DOI] [PubMed] [Google Scholar]
- 8. Djoba Siawaya JF, et al. 2008. Differential expression of interleukin-4 (IL-4) and IL-4Δ2 mRNA, but not transforming growth factor beta (TGF-β), TGF-βRII, Foxp3, gamma interferon, T-bet, or GATA-3 mRNA, in patients with fast and slow responses to antituberculosis treatment. Clin. Vaccine Immunol. 15:1165–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dockrell H. 2007. Gamma interferon—key, but not sufficient for protection against TB? Microbiol. Today November 2007:172–175 [Google Scholar]
- 10. Fine PE. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339–1345 [DOI] [PubMed] [Google Scholar]
- 11. Fine PE, Vynnycky E. 1998. The effect of heterologous immunity upon the apparent efficacy of (e.g., BCG) vaccines. Vaccine 16:1923–1928 [DOI] [PubMed] [Google Scholar]
- 12. Flynn JL, Chan J. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93–129 [DOI] [PubMed] [Google Scholar]
- 13. Hoft DF, et al. 2008. A new recombinant bacille Calmette-Guerin vaccine safely induces significantly enhanced tuberculosis-specific immunity in human volunteers. J. Infect. Dis. 198:1491–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horwitz MA, Harth G, Dillon BJ, Maslesa-Galic S. 2000. Recombinant bacillus Calmette-Guerin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc. Natl. Acad. Sci. U. S. A. 97:13853–13858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hougardy JM, et al. 2007. Heparin-binding-hemagglutinin-induced IFN-gamma release as a diagnostic tool for latent tuberculosis. PLoS One 2:e926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang D, et al. 2007. Immune gene networks of mycobacterial vaccine-elicited cellular responses and immunity. J. Infect. Dis. 195:55–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jacobsen M, et al. 2007. Candidate biomarkers for discrimination between infection and disease caused by Mycobacterium tuberculosis. J. Mol. Med. 85:613–621 [DOI] [PubMed] [Google Scholar]
- 18. Kritzinger FE, et al. 2009. No decrease in annual risk of tuberculosis infection in endemic area in Cape Town, South Africa. Trop. Med. Int. Health 14:136–142 [DOI] [PubMed] [Google Scholar]
- 19. Locht C, Hougardy JM, Rouanet C, Place S, Mascart F. 2006. Heparin-binding hemagglutinin, from an extrapulmonary dissemination factor to a powerful diagnostic and protective antigen against tuberculosis. Tuberculosis (Edinb.) 86:303–309 [DOI] [PubMed] [Google Scholar]
- 20. Masungi C, et al. 2002. Differential T and B cell responses against Mycobacterium tuberculosis heparin-binding hemagglutinin adhesin in infected healthy individuals and patients with tuberculosis. J. Infect. Dis. 185:513–520 [DOI] [PubMed] [Google Scholar]
- 21. Mistry R, et al. 2007. Gene-expression patterns in whole blood identify subjects at risk for recurrent tuberculosis. J. Infect. Dis. 195:357–365 [DOI] [PubMed] [Google Scholar]
- 22. Mittrucker HW, et al. 2007. Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 104:12434–12439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moller M, et al. 2007. Host susceptibility to tuberculosis: CARD15 polymorphisms in a South African population. Mol. Cell Probes. 21:148–151 [DOI] [PubMed] [Google Scholar]
- 24. Moller M, et al. 2009. Investigation of chromosome 17 candidate genes in susceptibility to TB in a South African population. Tuberculosis (Edinb.) 89:189–194 [DOI] [PubMed] [Google Scholar]
- 25. Mueller H, et al. 2008. Mycobacterium tuberculosis-specific CD4+, IFNγ+, and TNFα+ multifunctional memory T cells coexpress GM-CSF. Cytokine 43:143–148 [DOI] [PubMed] [Google Scholar]
- 26. Pai M, Dheda K, Cunningham J, Scano F, O'Brien R. 2007. T-cell assays for the diagnosis of latent tuberculosis infection: moving the research agenda forward. Lancet Infect. Dis. 7:428–438 [DOI] [PubMed] [Google Scholar]
- 27. Pai M, O'Brien R. 2008. New diagnostics for latent and active tuberculosis: state of the art and future prospects. Semin. Respir. Crit. Care Med. 29:560–568 [DOI] [PubMed] [Google Scholar]
- 28. Pai M, Zwerling A, Menzies D. 2008. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann. Intern. Med. 149:177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pethe K, et al. 2001. The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature 412:190–194 [DOI] [PubMed] [Google Scholar]
- 30. Qiu L, et al. 2008. Severe tuberculosis induces unbalanced up-regulation of gene networks and overexpression of IL-22, MIP-1α, CCL27, IP-10, CCR4, CCR5, CXCR3, PD1, PDL2, IL-3, IFN-β, TIM1, and TLR2 but low antigen-specific cellular responses. J. Infect. Dis. 198:1514–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roberts T, Beyers N, Aguirre A, Walzl G. 2007. Immunosuppression during active tuberculosis is characterized by decreased interferon-gamma production and CD25 expression with elevated forkhead box P3, transforming growth factor-beta, and interleukin-4 mRNA levels. J. Infect. Dis. 195:870–878 [DOI] [PubMed] [Google Scholar]
- 32. Savolainen L, et al. 2008. Pilot study of diagnostic potential of the Mycobacterium tuberculosis recombinant HBHA protein in a vaccinated population in Finland. PLoS One 3:e3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scriba TJ, et al. 2008. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J. Immunol. 180:1962–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Soares AP, et al. 2008. Bacillus Calmette-Guerin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J. Immunol. 180:3569–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stop TB Partnership 2008. TB vaccines pipeline. http://www.stoptb.org/assets/documents/global/retooling/StopTB%202008%20Vaccines%20Pipeline%20March%2008.pdf
- 36. Sutherland JS, Adetifa IM, Hill PC, Adegbola RA, Ota MO. 2009. Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. Eur. J. Immunol. 39:723–729 [DOI] [PubMed] [Google Scholar]
- 37. Tchilian EZ, et al. 2009. Immunogenicity and protective efficacy of prime-boost regimens with recombinant ΔureC hly+ Mycobacterium bovis BCG and modified vaccinia virus Ankara expressing M. tuberculosis antigen 85A against murine tuberculosis. Infect. Immun. 77:622–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Veerapathran A, et al. 2008. T-cell assays for tuberculosis infection: deriving cut-offs for conversions using reproducibility data. PLoS One 3:e1850. [DOI] [PMC free article] [PubMed] [Google Scholar]