Abstract
Current oral cholera vaccines induce lower protective efficacy and shorter duration of protection against cholera than wild-type infection provides, and this difference is most pronounced in young children. Despite this, there are limited data comparing immune responses in children following wild-type disease versus vaccination, especially with regard to memory responses associated with long-term immunity. Here, we report a comparison of immune responses in young children (2 to 5 years of age; n = 20) and older children (6 to 17 years of age; n = 20) given two doses of an oral killed cholera vaccine containing recombinant cholera toxin B subunit (CtxB) 14 days apart and compare these responses to those induced in similarly aged children recovering from infection with Vibrio cholerae O1 Ogawa in Bangladesh. We found that the two vaccine groups had comparable vibriocidal and lipopolysaccharide (LPS)-specific plasma antibody responses. Vaccinees developed lower levels of IgG memory B cell (MBC) responses against CtxB but no significant MBC responses against LPS. In contrast, children recovering from natural cholera infection developed prominent LPS IgG and IgA MBC responses, as well as CtxB IgG MBC responses. Plasma LPS IgG, IgA, and IgM responses, as well as vibriocidal responses, were also significantly higher in children following disease than after vaccination. Our findings suggest that acute and memory immune responses following oral cholera vaccination in children are significantly lower than those observed following wild-type disease, especially responses targeting LPS. These findings may explain, in part, the lower efficacy of oral cholera vaccination in children.
INTRODUCTION
Cholera is a dehydrating illness caused by infection with the O1 or O139 serogroup of Vibrio cholerae. It is a disease of poverty, made worse in urban areas of endemicity by the overcrowding of informal housing settlements or slums in high-risk populations (27). Each year around the world, 2 to 3 million people are infected, and up to 130,000 die from cholera (41, 43). Although cholera is seen in all age groups, in areas of endemicity, children under 5 years of age have a large burden of disease (10).
The World Health Organization has recently updated its position statement on the use of cholera vaccines (42), and cholera vaccination is now recommended for inclusion in integrated cholera control programs. Two whole-cell oral killed cholera vaccines (OCVs) are currently internationally available: Dukoral (WC-rBS; Crucell, Sweden), a mono-serogroup killed V. cholerae O1 supplemented with 1 mg/dose of recombinant cholera toxin B subunit (CtxB), and Shanchol (bivWC; Sanofi Aventis/Shantha Biotechnics, India), a bivalent serogroup killed V. cholerae O1/O139 vaccine not supplemented with additional CtxB (35). WC-rBS is licensed in over 60 countries while bivWC is licensed in India and was recently prequalified by the WHO. A Cochrane review of killed whole-cell OCV studies estimates that vaccine efficacy in the second year after vaccination was 66% for all ages but only 38% for children of <5 years of age (36). A recent field study of bivWC in Kolkata, India, demonstrated 66% protective efficacy over 3 years of follow-up; however, the youngest children (<5 years of age) had only 43% protective efficacy, with no significant protection in the third year of follow-up (37). The mechanism behind these differences in vaccine efficacy between age groups remains to be elucidated and better understood.
Vibriocidal, lipopolysaccharide (LPS) IgA-specific, and CtxB IgA-specific responses have been correlated with protection from V. cholerae O1 infection in an observational study of household contacts of cholera patients in Bangladesh (14); however, such serological responses wane rapidly in the months after infection and vaccination (32) and are unlikely to be determinants of long-term protection. It is hypothesized that memory B cells (MBCs) are the mediators of a rapid anamnestic immune response on reexposure and that they are associated with duration of protection following infection and vaccination (17). We have previously demonstrated that in adults hospitalized with V. cholerae infection, MBC responses to V. cholerae antigens persist up to 1 year, the last period evaluated, longer than that of any other known markers of cholera immunity (13). In individuals with wild-type V. cholerae infection, we have recently shown that levels of V. cholerae antigen-specific memory B cells are comparable between younger children and older children and adults (22), suggesting that an optimal vaccination strategy could theoretically induce protective immunity in both younger and older children. Despite a number of studies evaluating serological parameters of OCV immunogenicity in children (5, 12, 39) and one of MBC responses to OCV in adults (2), there are currently no data on memory B cell responses in children receiving oral cholera vaccination. Therefore, the aims of this study were to evaluate acute-phase immune responses in pediatric recipients of an OCV, including characterizing memory B cell responses, and to compare these responses with those seen in children with cholera in Bangladesh.
MATERIALS AND METHODS
Study design and subject enrollment.
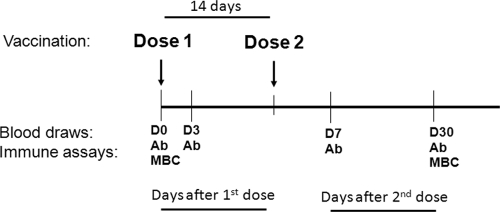
Following a process of informed consent of parents/guardians, we enrolled 20 healthy young children (aged 2 to 5 years) and 20 healthy older children (aged 6 to 17 years) from an urban informal settlement area of Dhaka, Bangladesh. Study participants were administered two doses of the licensed OCV Dukoral (WC-rBS; Crucell, Sweden) 14 days apart. Children were excluded if they had a Z score of less than −2 (for those of <5 years of age), a history of gastrointestinal disorder, any history of parasitic infection, suffered from any diarrheal disease in the past 2 weeks, or had any febrile illness or antibiotic usage in the past week. Prior to vaccination, subjects were screened for evidence of recent enteric infections. Stool specimens were collected and screened for the presence of V. cholerae O1, enterotoxigenic Escherichia coli (ETEC), Salmonella spp., and Shigella spp. using standard microbiological techniques (6). If stool and symptom screenings were negative, participants were administered the vaccine. We obtained blood samples before vaccination (day 0), at 3 days after the first dose of vaccine, and at 7 and 30 days after the second dose of vaccine (Fig. 1). At each time point, we assayed vibriocidal antibodies and responses against V. cholerae O1 antigens in plasma. We also assessed antigen-specific IgG and IgA memory B cells before vaccination and 30 days after the second dose of vaccination, as described below.
Fig 1.
Vaccination and blood draw time line. Ab, plasma for vibriocidal and antigen-specific antibody assays; MBC, PBMC isolation for memory B cell assay; D, day.
We compared immune responses of the vaccinees to those of children, matched by age group, hospitalized with severe acute watery diarrhea and stool culture positive for V. cholerae O1 Ogawa (22). All study participants were of the same socio-economic status. We assessed immune responses in patients on days 2, 7, and 30 following presentation.
These studies were approved by the Research Review Committee and Ethical Review Committee of the International Centre for Diarrheal Disease Research, Bangladesh (ICDDR,B), Dhaka, Bangladesh, and the Institutional Review Board of the Massachusetts General Hospital, Boston, MA.
Isolation of PBMCs.
From diluted heparinized blood, we separated peripheral blood mononuclear cells (PBMCs) and plasma by density gradient centrifugation on Ficoll-Isopaque (Pharmacia, Piscataway, NJ). We stored plasma at −70°C for immunological analyses. We washed and suspended the isolated PBMCs at a concentration of 107 cells/ml in RPMI complete medium (Gibco, Carlsbad, CA) containing 10% heat-inactivated fetal bovine serum (FBS; HyClone, Logan, UT) and assessed memory B cell responses in a culture-based assay as described below.
Vibriocidal antibody assay.
For both vaccinees and patients, we measured vibriocidal antibody titers in plasma using guinea pig complement and V. cholerae O1 Ogawa (X-25049) as the target organism, as previously described (31). We defined the vibriocidal titer as the reciprocal of the highest dilution resulting in >50% reduction of the optical density compared to that of control wells without plasma. We considered individuals showing a ≥4-fold increase in vibriocidal titer compared to their baseline to be responders.
Detection of cholera antigen-specific antibody levels in plasma.
We measured plasma CtxB- and LPS-specific IgA, IgG, and IgM antibody responses using a standardized enzyme-linked immunosorbent assay (ELISA) technique as previously described (31). In brief, we coated 96-well polystyrene plates (Nunc F) with either V. cholerae O1 Ogawa LPS (2.5 μg/ml) or 0.3 nM ganglioside GM1 overnight, followed by recombinant CtxB (rCtxB) subunit (0.5 μg/ml; gifts from A. M. Svennerholm, University of Gothenburg, Sweden). We added 100 μl of plasma (diluted 1:25 for LPS and 1:100 for CtxB in 0.1% bovine serum albumin in phosphate-buffered saline–Tween) per well and used horseradish peroxidase-conjugated secondary antibodies to human IgA, IgG, and IgM (Jackson ImmunoResearch, West Grove, PA), followed by ortho-phenylenediamine (Sigma, St. Louis, MO) in 0.1 M sodium citrate buffer (pH 4.5) and 0.1% hydrogen peroxide for development. We read plates kinetically at 450 nm for 5 min and normalized the maximal rate of change in optical density in milliabsorbance units per minute across plates by calculating the ratio of the test sample to a standard of pooled convalescent-phase sera from previously infected cholera patients included on each plate (15). We considered individuals with a ≥2-fold increase in anti-CtxB and LPS responses compared to their baseline to be responders.
Detection of memory B cell (MBC) responses by culture-based assay using ELISPOT.
We assessed MBC responses to V. cholerae O1 antigens as previously described (13). In brief, we plated freshly harvested PBMCs in 24-well cell culture plates (BD Biosciences, San Jose, CA) at a concentration of 5 × 105 PBMC/well. To each well, we added culture medium consisting of RPMI 1640 medium, 10% FBS, 200 U of penicillin/ml, 200 μg of streptomycin/ml, 2 mM l-glutamine, and 50 μM 2-mercaptoethanol, with or without a stimulatory cocktail optimized to stimulate antigen-independent proliferation and differentiation of memory B cells into antibody-secreting cells (ASCs) (9). The mitogens used for stimulation were 6 μg of CpG oligonucleotide/ml (Operon, Huntsville, AL), a 1/100,000 dilution of crude pokeweed mitogen extract, and a 1/10,000 dilution of fixed Staphylococcus aureus Cowan (Sigma, St. Louis, MO). We incubated the plates at 37°C in a 5% CO2 incubator for 6 days, after which the cells were harvested and washed. For the enzyme-linked immunosorbent spot (ELISPOT) assay, we coated nitrocellulose-bottom plates (Mahan-4550; Millipore, Bedford, MA) with GM1 ganglioside (3 nM) followed by recombinant CtxB (2.5 μg/ml) or with V. cholerae O1 Ogawa LPS (25 μg/ml). As a negative control, we coated plates with keyhole limpet hemocyanin ([KLH] 2.5 μg/ml; Pierce Biotechnology, Rockford, IL). We also coated plates with affinity-purified goat anti-human immunoglobulin (5 μg/ml; Jackson Immunology Research West Grove, PA) to detect total IgG and IgA MBC responses. After the plates were blocked with RPMI 1640 medium containing 10% FBS, we used 20% of the cells from each well to assess total IgG and IgA ASC and 80% to detect antigen-specific IgG and IgA ASC. We detected IgG and IgA ASC using horseradish peroxidase-conjugated mouse anti-human IgG and IgA (Hybridoma Reagent Laboratory, Baltimore, MD), respectively, followed by 3-amino-9-ethyl carbazole (AEC) for developing the plates. We quantified the number of ASC per well by using a stereomicroscope. Data were collected independently by two individuals and then averaged. For inclusion in analyses, we required sufficient stimulation of total antibody isotype-specific memory B cells (defined as a ≥3-fold increase comparing stimulated to nonstimulated wells), fewer than four antigen-specific cells in the corresponding sample's unstimulated wells, and fewer than four antigen-specific cells in the corresponding sample's KLH well, as previously described (2, 22). Application of these criteria resulted in the inclusion of approximately 90% of all data. We expressed ELISPOT counts as the percentage of antigen-specific memory B cells out of the total IgG or IgA memory B cell population.
Statistical analyses.
We assessed normality of the data using a Shapiro-Wilk test. We compared baseline characteristics with a Fisher exact test for categorical variables and a Mann-Whitney U test for continuous variables. We assessed differences in the magnitude of immune responses between two groups with a Mann-Whitney U test. All reported P values are two-tailed, with a cutoff of a P value of <0.05 considered a threshold for statistical significance. We performed analyses using GraphPad Prism, version 5.0 (GraphPad Software, Inc., La Jolla, CA) and SPSS, version 17.0 (SPSS Inc., Chicago, IL).
RESULTS
Study population.
We enrolled 20 young children (aged 2 to 5 years; mean, 56 months old; median, 60 months old) and 20 older children (aged 6 to 17 years; mean, 10.5 years old; median, 10 years old) for administration of two doses of the WC-rBS oral cholera vaccine 14 days apart. Ninety percent of the younger children and 100% of the older children completed 30 days of follow-up. We compared the immune responses of these vaccinees to those of 38 patients matched for age group presenting with acute dehydrating diarrhea and stool culture positive only for V. cholerae O1 Ogawa. All patients were treated per clinical protocol with intravenous and oral fluid replacement therapy and azithromycin. Other than age, there were no significant differences between older and younger vaccinee age groups in gender or blood group, and there were no differences between vaccinees and the age group-matched patients by gender or blood group (Table 1). Infected children had a mean weight-to-height Z score of −1.4.
Table 1.
Demographic and clinical characteristics of study subjects
| Subject characteristic | Value for subjects 2 to 5 yr old |
Value for subjects 6 to 17 yr old |
||||
|---|---|---|---|---|---|---|
| WC-rBS vaccineesa (n = 20) | Cholera patients (n = 20) | P | WC-rBS vaccineesa (n = 20) | Cholera patients (n = 18) | P | |
| No. that completed 30-day follow-up | 18 | 18 | 20 | 17 | ||
| Mean age (yr [range]) | 4.7 (3–5) | 4.2 (2–5) | NSb | 10.5 (7–14) | 9.9 (6–16) | NS |
| Female (no. [%]) | 9 (45) | 10 (50) | NS | 10 (50) | 7 (39) | NS |
| ABO blood group (no. [%]) | ||||||
| A | 5 (25) | 3 (15) | NS | 4 (20) | 5 (28) | NS |
| B | 4 (20) | 4 (20) | NS | 6 (30) | 7 (39) | NS |
| AB | 2 (10) | 1 (5) | NS | 3 (15) | 1 (6) | NS |
| O | 9 (45) | 12 (60) | NS | 7 (35) | 5 (28) | NS |
WC-rBS, whole-cell recombinant B subunit oral cholera vaccine (Dukoral, Crucell, Sweden); n, number of participants.
NS, nonsignificant.
Vibriocidal response in plasma.
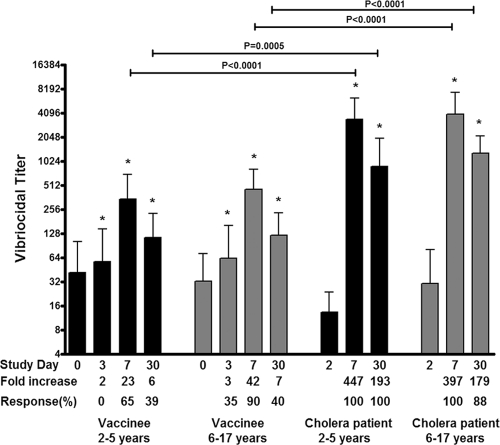
We measured vibriocidal responses to V. cholerae O1 Ogawa prior to and after vaccination. The baseline geometric mean titer (GMT) in the younger and older child vaccinee cohorts were 37 and 32, respectively. These were not significantly different from baseline titers of age-matched patients (15 and 34, respectively). Vibriocidal responses were significantly higher by day 3 after the first dose of vaccine in both younger (P = 0.007) and older (P = 0.008) children and reached a peak at day 7 after the second dose (Fig. 2). Sixty-five percent of the younger vaccinees and 90% of older pediatric vaccinees achieved a ≥4-fold increase by day 7 after the second dose of vaccine compared to baseline (P = 0.13 for the comparison between age groups). Vibriocidal titers were comparable between vaccinee age groups at all days examined although a subanalysis disclosed that vibriocidal responses in vaccinees of ≤4 years of age (n = 5) were significantly lower on day 7 after the second dose of vaccine than responses in older vaccinees (n = 35, P = 0.01). In comparison, age-matched patients had significantly higher vibriocidal titers at both day 7 and day 30 after infection than vaccinees (P < 0.001 for both age groups). A subanalysis showed that in both vaccinees and patients, the baseline vibriocidal titer negatively correlated with fold change in titer at day 7 (P < 0.001).
Fig 2.
Geometric mean reciprocal vibriocidal antibody titers by age group and vaccination status, with error bars representing 95% confidence intervals. An asterisk denotes a significant difference (P < 0.05) from the baseline (day 0 or day 2) titer.
V. cholerae-specific antibody responses in plasma.
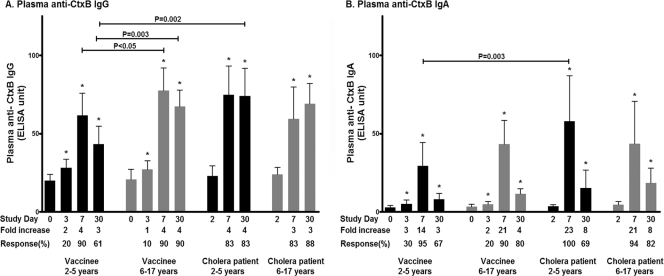
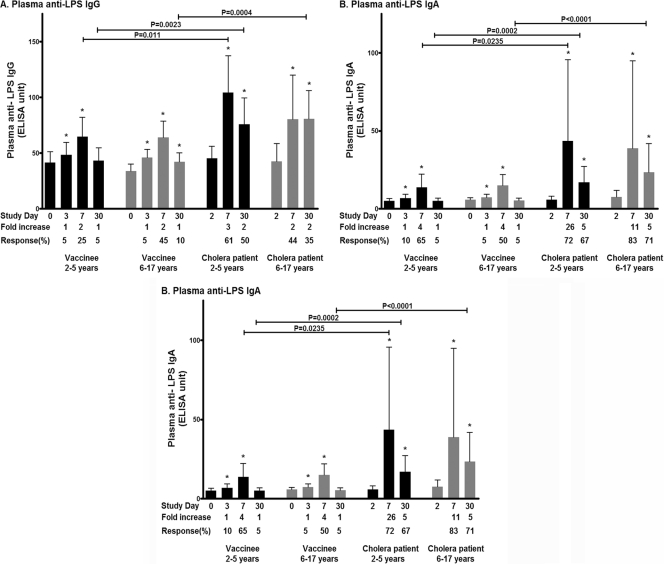
We assessed antibody responses to the V. cholerae antigens LPS and CtxB in plasma. Both vaccinee age groups had IgA and IgG plasma responses to CtxB and IgA, IgG, and IgM responses to LPS that were significantly higher than baseline values at day 7 after the second dose of vaccine (Fig. 3 and 4). No differences were detected in magnitude or responder frequency in a comparison of anti-LPS responses in older and younger vaccine recipients, but older children had higher day 7 and day 30 IgG responses against CtxB than younger children (magnitude of response, P < 0.05 at day 7 and P = 0.003 at day 30; responder frequency at day 30, 90% of older children and 61% younger children, P = 0.06). In general, levels of CtxB antibodies did not significantly differ between vaccinees and matched patients, except in younger children, where patients had higher day 30 CtxB-specific IgG and day 7 CtxB-specific IgA than vaccinees (Fig. 3). Younger patients also had higher peak levels of IgG and IgA against LPS than their age-matched vaccine cohort on day 7, and older patients had higher IgG, IgA, and IgM responses against LPS than their age-matched vaccine cohort on day 30 (Fig. 4). There was no significant difference in the magnitude of anti-LPS responses between younger and older children recovering from wild-type disease at any time point.
Fig 3.
Mean plasma antibody IgG (A) and IgA (B) responses to CtxB by age group as measured by ELISA, with error bars representing standard errors of the means. An asterisk denotes a significant difference (P ≤ 0.05) from the baseline (day 2).
Fig 4.
Mean plasma antibody IgG (A), IgA (B), and IgM (C) responses to LPS by age group as measured by ELISA, with error bars representing standard errors of the means. An asterisk denotes a significant difference (P ≤ 0.05) from the baseline (day 2).
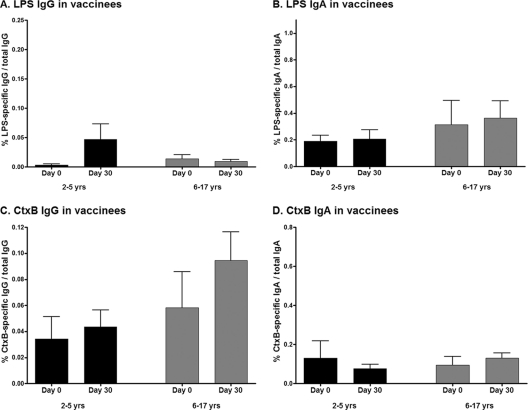
Antigen-specific memory B cell responses.
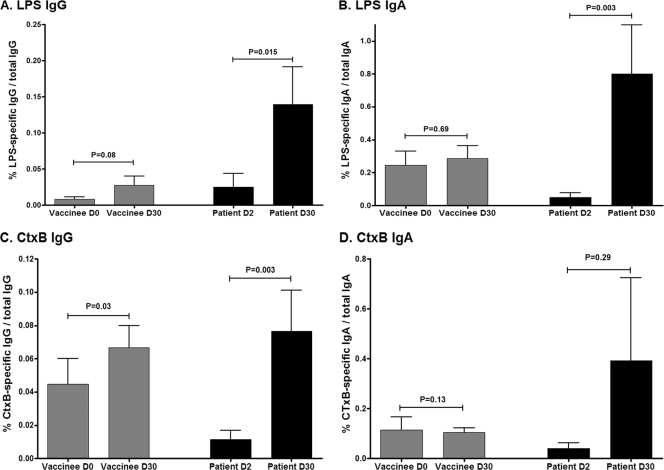
We assessed memory B cell (MBC) responses to LPS and CtxB on day 0 (prevaccination) and day 30 after the second dose of vaccine compared to day 2 (acute phase) and day 30 (convalescence), respectively, following wild-type disease. On each day examined, younger and older child vaccinees had comparable levels of detectable memory B cells, and when results were analyzed by age cohort, no significant MBC responses to CtxB or LPS were detected following vaccination with OCV (Fig. 5). When the data were analyzed combining all pediatric vaccinees into one cohort, we were able to detect a small but significant increase in CtxB-specific IgG memory B cell responses in vaccinees on day 30 following the second immunization (P = 0.03) (Fig. 6C), with a trend toward an LPS-specific IgG memory B cell response (P = 0.08) (Fig. 6A). In comparison, we were able to detect significant increases in CtxB-specific IgG memory B cell responses 30 days following infection in cholera patients when we analyzed data both in aggregate (P = 0.003) (Fig. 6C) and by age cohort (young children day 30 CtxB-specific IgG MBC, P = 0.02 compared to day 2) (data not shown). Similarly, we detected significant LPS-specific memory B cell responses in children recovering from cholera when we analyzed data both in aggregate (LPS-specific day 30 IgG MBC, P = 0.015; LPS-specific day 30 IgA MBC, P = 0.003) (Fig. 6A and B) and by age cohort (LPS-specific day 30 IgA MBC compared to day 2, P = 0.03 for young children and P = 0.06 for older children) (data not shown) but not in vaccinees, irrespective of the analysis approach.
Fig 5.
Mean antigen-specific IgG (A and C) and IgA (B and D) memory B cell responses, as percentages of total memory B cells, with error bars representing standard errors of the means, by vaccinee age group.
Fig 6.
Mean antigen-specific IgG (A and C) and IgA (B and D) memory B cell responses, as percentages of total memory B cells, with error bars representing standard errors of the means. Data represent combined age group analysis.
DISCUSSION
Cholera affects all age groups. In zones of endemicity, young children are vulnerable (10), and by adolescence most have serological evidence of previous exposure to V. cholerae O1 (25). During outbreaks of cholera in zones where cholera is not endemic, children and adults are equally affected by cholera (21, 24). As such, children bear a very large burden of cholera globally (10). Despite this, current oral cholera vaccines are associated with lower protective efficacy and a shorter duration of protection in younger children than observed following vaccination of adults (7, 38, 40). In addition, vaccination against cholera is also associated with lower levels of protection and a shorter duration of protection than wild-type infection with V. cholerae O1 provides (19, 36), despite the fact that protection by wild-type infection against subsequent cholera appears to be similar in younger children and older persons (4). The immunologic correlates of these differences are poorly understood, especially in children. Here, we show that immune responses to an oral killed cholera vaccine (WC-rBS) are generally comparable in younger and older children in an area where cholera is endemic; however, these responses are much lower than those detected following wild-type cholera infection, most notably in anti-LPS responses in plasma, the vibriocidal response, and memory B cell responses to LPS.
The vibriocidal antibody is the most-studied immunologic marker of cholera infection and is an indirect/surrogate marker of protection. While vibriocidal titers correlate with protection against cholera, there is no threshold at which protection is complete (34). The vibriocidal response can be largely removed by adsorption of plasma to LPS, strongly suggesting that antibodies reacting with LPS are a critical component of the vibriocidal response (26). Few direct comparisons have been made of the differences in vibriocidal responses to OCV between younger and older children. In immunogenicity studies of WC-rBS in Peruvian children, younger and older children had comparable seroconversion rates 14 days after the second dose of vaccine (39). In our study, we similarly found no differences in vibriocidal responses between younger and older vaccinated children although a subanalysis disclosed that the youngest children (defined as ≤4 years of age) were less able to mount a vibriocidal response than older children. However, we did find that age-matched patients with V. cholerae O1 Ogawa infection had significantly higher responses (both in magnitude and responder frequency) than vaccinees at all days evaluated.
We have previously shown that LPS and CtxB plasma IgA antibody responses correlate with protection against cholera in household contacts of cholera patients (14). In our current study, we now show that two doses of WC-rBS vaccination induce prominent IgA and IgG anti-CtxB responses in younger and older pediatric vaccine recipients and that these responses are generally comparable to those induced in children with wild-type cholera infection, differing only in day 30 IgG and day 7 IgA responses in the younger children. These similarities may in part reflect that each dose of WC-rBS is supplemented with 1 mg of recombinant CtxB. It should be noted that an alternative OCV, bivWC (Shanchol), does not contain supplemental CtxB, and the anti-CtxB responses induced by WC-rBS (Dukoral) and bivWC (Shanchol) may well be different. Despite our ability to detect prominent anti-CtxB responses following vaccination of children with WC-rBS and after wild-type disease, we found a marked difference between anti-LPS plasma responses in vaccinees versus children recovering from wild-type cholera disease in both age groups. Specifically, although vaccination induced low-level plasma IgG, IgA, and IgM responses against LPS that were similar in younger and older children, these responses were much lower than those observed following wild-type disease in age-matched controls.
Immunity against cholera following wild-type disease is believed to last at least 3 to 10 years (4, 19, 23). Since acute-phase immune responses including vibriocidal and plasma antibody responses fall to baseline within 6 to 12 months of infection (13), longer-term protection against cholera is probably afforded by the ability to mount anamnestic responses facilitated by long-term memory responses (17). We have previously found that anti-LPS and anti-CtxB memory B cell responses are induced following wild-type cholera infection and that these responses persist even after the vibriocidal and plasma antibody responses have returned to baseline (13). In adults, we have also demonstrated that oral cholera vaccination with WC-rBS induces anti-CtxB memory B cell responses but not anti-LPS memory responses (2).
In our current analyses, we have now extended this work. When results were analyzed by age cohort, we were unable to detect anti-LPS or anti-CtxB memory B cell responses in both younger and older vaccine recipients although when we analyzed in aggregate, we did detect IgG memory B cell responses against CtxB in children receiving WC-rBS. In comparison, we were able to detect significant MBC responses against CtxB and LPS in children recovering from cholera when responses were analyzed both by age cohort and in aggregate. The ability of both naturally infected and vaccinated children to generate anti-CtxB memory responses may in part reflect that the cholera vaccine used in this study contains 1 mg of recombinant CtxB per dose, as well as the potential boosting of preexisting immune responses to the immunologically cross-reactive heat labile toxin (LT) of enterotoxigenic E. coli (ETEC) (11), an infection that is endemic in resource-limited areas of the world including Bangladesh (30). However, the significance of these CtxB responses in protecting against cholera is uncertain since there is no heterologous protection against cholera between V. cholerae O1 and O139, despite the fact that that these serogroups express identical enterotoxins (29), and since protective efficacy of oral cholera vaccines containing and not containing supplemental CtxB are comparable (7).
No difference has been noted in the degree of protection against subsequent disease afforded by previous cholera infection in younger and older children in areas of endemicity (4); however, following vaccination, young children have a significantly shorter duration and lower level of protection than older persons (7, 38, 40). In our current study, we found that the plasma antibody against LPS, the memory B cell response against LPS, and vibriocidal responses (largely targeting LPS) are significantly lower in child vaccinees than in children recovering from wild-type cholera infection. This is particularly significant when we consider that responses against LPS may be a prime mediator of protection against cholera (11, 14, 29).
LPS is a T cell-independent antigen (16), and young children are less able to mount T cell-independent responses than older children and adults (18). Our inability to detect differences in acute and MBC responses against LPS in comparing cohorts of younger and older children following vaccination in this analysis may in part reflect the relatively small size of our vaccine cohorts and the fact that the mean age of our cohort of ≤5 years of age was 4.7 years. As such, many of these young children in this area of high endemicity for cholera may already have been exposed to V. cholerae (33), mitigating our ability to detect differences in younger versus older vaccine recipients. This possibility is further supported by the fact that we were able to detect a significant increase in both plasma IgG and IgA to both CtxB and LPS within 3 days of administration of oral cholera vaccine even in the cohort of children ≤5 years old in this study, suggesting prior exposure and a primed response. Similarly, the observation in our subanalysis that the youngest vaccinees in this study were less able to mount a vibriocidal response than older vaccinees supports this hypothesis. Whether we would have found similar results if we had conducted the study in an area where cholera is not endemic or if we had focused our enrollment and analysis on children less than 3 years of age is uncertain. We also cannot exclude the possibility that vaccination induces low-level memory responses against LPS in children but that these responses are below the level of detection of our assay. Finally, our inability to specifically detect differences in anti-LPS memory B cell responses between older and younger vaccine recipients may also be due to the fact that oral cholera vaccines are particularly poor inducers of LPS-specific memory B cell responses, even in immunologically primed adults (2), and this reality complicates our ability to detect differences between younger and older children for this immunologic marker.
A potential shortcoming of our study is that our group and others have demonstrated the effect of zinc supplementation (1, 3, 28), small bowel bacterial overgrowth (20), and antiparasitic drug treatment (8) on immune responses to OCV in young children. We did not assess differences between age groups of such modifiers of immune responses in this study, and these factors deserve further investigation. Furthermore, difficulties in recruiting child participants, especially those less than 4 years of age, limited our sample size.
In conclusion, in this study we demonstrate that both younger and older children vaccinated with a WC-rBS oral killed cholera vaccine formulation mount comparable immune responses in this area of high endemicity for cholera where prior exposure may have occurred but that these responses, especially vibriocidal and LPS responses, including memory B cell responses, are significantly lower than the responses detected in age-matched children recovering from natural cholera infection requiring hospitalization. These observations are significant and may in part explain differences in protective efficacy and duration of protection observed between wild-type infection and vaccination with currently available oral cholera vaccines.
ACKNOWLEDGMENTS
This work was supported by the ICDDR,B, grants from the National Institutes of Health, including the National Institute of Allergy and Infectious Diseases (U01 AI058935 to S.B.C. and E.T.R., R03 AI063079 to F.Q., U01 AI077883 to E.T.R.), Training Grant in Vaccine Development and Public Health (TW005572 to M.M.A. and F.Q.), an American Recovery and Reinvestment Act Postdoctoral Fellowship in Global Infectious Diseases (TW05572 to D.T.L.), a Career Development Award (K01 TW07409 to J.B.H., TW07144 to R.C.L., and K08 AI089721 to R.C.C.), and a Clinical Research Scholars award (R24 TW007988 to S.M.P. and F.K.) from the Fogarty International Center, the Swedish Agency for International Development and Cooperation (to F.Q. and A.S.), a Physician Scientist Early Career Award from the Howard Hughes Medical Institute (to R.C.L.), a Postdoctoral Fellowship in Tropical Infectious Diseases from the American Society for Tropical Medicine and Hygiene—Burroughs Wellcome Fund (to D.T.L.), and the Harvard Initiative for Global Health Postdoctoral Fellowship in Global Infectious Diseases (to D.T.L.).
Footnotes
Published ahead of print 21 March 2012
REFERENCES
- 1. Ahmed T, Svennerholm AM, Al Tarique A, Sultana GN, Qadri F. 2009. Enhanced immunogenicity of an oral inactivated cholera vaccine in infants in Bangladesh obtained by zinc supplementation and by temporary withholding breast-feeding. Vaccine 27:1433–1439 [DOI] [PubMed] [Google Scholar]
- 2. Alam MM, et al. 2011. Antigen-specific memory B-cell responses in Bangladeshi adults after one- or two-dose oral killed cholera vaccination and comparison with responses in patients with naturally acquired cholera. Clin. Vaccine Immunol. 18:844–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Albert MJ, et al. 2003. Supplementation with zinc, but not vitamin A, improves seroconversion to vibriocidal antibody in children given an oral cholera vaccine. J. Infect. Dis. 187:909–913 [DOI] [PubMed] [Google Scholar]
- 4. Ali M, Emch M, Park JK, Yunus M, Clemens J. 2011. Natural cholera infection-derived immunity in an endemic setting. J. Infect. Dis. 204:912–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chowdhury F, et al. 2008. A comparison of clinical and immunologic features in children and older patients hospitalized with severe cholera in Bangladesh. Pediatr. Infect. Dis. J. 27:986–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chowdhury F, et al. 2011. Impact of rapid urbanization on the rates of infection by Vibrio cholerae O1 and enterotoxigenic Escherichia coli in Dhaka, Bangladesh. PLoS Negl. Trop. Dis. 5:e999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clemens JD, et al. 1990. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet 335:270–273 [DOI] [PubMed] [Google Scholar]
- 8. Cooper PJ, et al. 2000. Albendazole treatment of children with ascariasis enhances the vibriocidal antibody response to the live attenuated oral cholera vaccine CVD 103-HgR. J. Infect. Dis. 182:1199–1206 [DOI] [PubMed] [Google Scholar]
- 9. Crotty S, Aubert RD, Glidewell J, Ahmed R. 2004. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J. Immunol. Methods 286:111–122 [DOI] [PubMed] [Google Scholar]
- 10. Deen JL, et al. 2008. The high burden of cholera in children: comparison of incidence from endemic areas in Asia and Africa. PLoS Negl Trop. Dis. 2:e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Glass RI, et al. 1985. Seroepidemiological studies of El Tor cholera in Bangladesh: association of serum antibody levels with protection. J. Infect. Dis. 151:236–242 [DOI] [PubMed] [Google Scholar]
- 12. Hallander HO, et al. 2002. Calibrated serological techniques demonstrate significant different serum response rates to an oral killed cholera vaccine between Swedish and Nicaraguan children. Vaccine 21:138–145 [DOI] [PubMed] [Google Scholar]
- 13. Harris AM, et al. 2009. Antigen-specific memory B-cell responses to Vibrio cholerae O1 infection in Bangladesh. Infect. Immun. 77:3850–3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harris JB, et al. 2008. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl. Trop. Dis. 2:e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. John M, Bridges EA, Miller AO, Calderwood SB, Ryan ET. 2002. Comparison of mucosal and systemic humoral immune responses after transcutaneous and oral immunization strategies. Vaccine 20:2720–2726 [DOI] [PubMed] [Google Scholar]
- 16. Kately JR, Patel CB, Friedman H. 1975. Involvement of T- and B-lymphocytes in the immune response to the protein exotoxin and the lipopolysaccharide antigens of Vibrio cholerae. Ann. N. Y. Acad. Sci. 249:404–412 [DOI] [PubMed] [Google Scholar]
- 17. Kelly DF, Pollard AJ, Moxon ER. 2005. Immunological memory: the role of B cells in long-term protection against invasive bacterial pathogens. JAMA 294:3019–3023 [DOI] [PubMed] [Google Scholar]
- 18. Klein Klouwenberg P, Bont L. 2008. Neonatal and infantile immune responses to encapsulated bacteria and conjugate vaccines. Clin. Dev. Immunol. 2008:628963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koelle K, Rodo X, Pascual M, Yunus M, Mostafa G. 2005. Refractory periods and climate forcing in cholera dynamics. Nature 436:696–700 [DOI] [PubMed] [Google Scholar]
- 20. Lagos R, et al. 1999. Effect of small bowel bacterial overgrowth on the immunogenicity of single-dose live oral cholera vaccine CVD 103-HgR. J. Infect. Dis. 180:1709–1712 [DOI] [PubMed] [Google Scholar]
- 21. Legros D, et al. 2000. Epidemiology of cholera outbreak in Kampala, Uganda. East Afr. Med. J. 77:347–349 [DOI] [PubMed] [Google Scholar]
- 22. Leung DT, et al. 2011. Comparison of memory B cell, antibody-secreting cell, and plasma antibody responses in young children, older children, and adults with infection caused by Vibrio cholerae O1 El Tor Ogawa in Bangladesh. Clin. Vaccine Immunol. 18:1317–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Levine MM, et al. 1981. Duration of infection-derived immunity to cholera. J. Infect. Dis. 143:818–820 [DOI] [PubMed] [Google Scholar]
- 24. Luque Fernandez MA, et al. 2011. Descriptive spatial analysis of the cholera epidemic 2008–2009 in Harare, Zimbabwe: a secondary data analysis. Trans. R. Soc. Trop. Med. Hyg. 105:38–45 [DOI] [PubMed] [Google Scholar]
- 25. Mosley WH, Benenson AS, Barui R. 1968. A serological survey for cholear antibodies in rural east Pakistan. 1. The distribution of antibody in the control population of a cholera-vaccine field-trial area and the relation of antibody titre to the pattern of endemic cholera. Bull. World Health Organ. 38:327–334 [PMC free article] [PubMed] [Google Scholar]
- 26. Neoh SH, Rowley D. 1970. The antigens of Vibrio cholerae involved in the vibriocidal action of antibody and complement. J. Infect. Dis. 121:505–513 [DOI] [PubMed] [Google Scholar]
- 27. Penrose K, de Castro MC, Werema J, Ryan ET. 2010. Informal urban settlements and cholera risk in Dar es Salaam, Tanzania. PLoS Negl. Trop. Dis. 4:e631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qadri F, et al. 2004. Suppressive effect of zinc on antibody response to cholera toxin in children given the killed, B subunit-whole cell, oral cholera vaccine. Vaccine 22:416–421 [DOI] [PubMed] [Google Scholar]
- 29. Qadri F, et al. 1995. Comparison of the vibriocidal antibody response in cholera due to Vibrio cholerae O139 Bengal with the response in cholera due to Vibrio cholerae O1. Clin. Diagn. Lab. Immunol. 2:685–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Qadri F, Svennerholm AM, Faruque AS, Sack RB. 2005. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin. Microbiol. Rev. 18:465–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qadri F, et al. 1997. Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect. Immun. 65:3571–3576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sack DA, et al. 1991. Antibody responses after immunization with killed oral cholera vaccines during the 1985 vaccine field trial in Bangladesh. J. Infect. Dis. 164:407–411 [DOI] [PubMed] [Google Scholar]
- 33. Sack RB, et al. 2003. A 4-year study of the epidemiology of Vibrio cholerae in four rural areas of Bangladesh. J. Infect. Dis. 187:96–101 [DOI] [PubMed] [Google Scholar]
- 34. Saha D, et al. 2004. Incomplete correlation of serum vibriocidal antibody titer with protection from Vibrio cholerae infection in urban Bangladesh. J. Infect. Dis. 189:2318–2322 [DOI] [PubMed] [Google Scholar]
- 35. Shin S, Desai SN, Sah BK, Clemens JD. 2011. Oral vaccines against cholera. Clin. Infect. Dis. 52:1343–1349 [DOI] [PubMed] [Google Scholar]
- 36. Sinclair D, Abba K, Zaman K, Qadri F, Graves PM. 2011. Oral vaccines for preventing cholera. Cochrane Database Syst. Rev. 3:CD008603. doi:10.1002/14651858.CD008603.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sur D, et al. 2011. Efficacy of a low-cost, inactivated whole-cell oral cholera vaccine: results from 3 years of follow-up of a randomized, controlled trial. PLoS Negl. Trop. Dis. 5:e1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sur D, et al. 2009. Efficacy and safety of a modified killed-whole-cell oral cholera vaccine in India: an interim analysis of a cluster-randomised, double-blind, placebo-controlled trial. Lancet 374:1694–1702 [DOI] [PubMed] [Google Scholar]
- 39. Taylor DN, Cardenas V, Perez J, Puga R, Svennerholm AM. 1999. Safety, immunogenicity, and lot stability of the whole cell/recombinant B subunit (WC/rCTB) cholera vaccine in Peruvian adults and children. Am. J. Trop. Med. Hyg. 61:869–873 [DOI] [PubMed] [Google Scholar]
- 40. Taylor DN, et al. 2000. Two-year study of the protective efficacy of the oral whole cell plus recombinant B subunit cholera vaccine in Peru. J. Infect. Dis. 181:1667–1673 [DOI] [PubMed] [Google Scholar]
- 41. World Health Organization 2009. Cholera: global surveillance summary, 2008. Wkly. Epidemiol. Rec. 84:309–324 [PubMed] [Google Scholar]
- 42. World Health Organization 2010. Cholera vaccines: WHO position paper. Wkly. Epidemiol. Rec. 85:117–128 [PubMed] [Google Scholar]
- 43. Zuckerman JN, Rombo L, Fisch A. 2007. The true burden and risk of cholera: implications for prevention and control. Lancet Infect. Dis. 7:521–530 [DOI] [PubMed] [Google Scholar]