Abstract
Host cell invasion by Toxoplasma gondii is tightly related to microneme protein 6 (MIC6) and T. gondii perforin-like protein 1 (TgPLP1). In this study, we constructed a DNA vaccine expressing a TgPLP1/MIC6 fusion protein using the pIRESneo vector, and we evaluated the immune response induced by this vaccine in Kunming mice. Levels of IgG antibody, gamma interferon (IFN-γ), interleukin 2 (IL-2), IL-12, IL-4, and IL-10 were examined. Five mice were chosen randomly from every group (vaccinated groups or the nonvaccinated control group) and were challenged intragastrically with 80 cysts of T. gondii strain PRU (genotype II) in order to observe mortality daily. To analyze protection against a less-virulent challenge, eight mice of each group were orally infected with 20 cysts of strain PRU at the 14th day after the last immunization. The brain parasite load was evaluated 6 weeks after infection. The results demonstrated that immunization with pIRESneo/MIC6/PLP1 resulted in the lowest brain cyst count and prolonged the survival time of immunized mice. The levels of Toxoplasma-specific IgG, IFN-γ, IL-2, and IL-12 increased significantly, and the numbers of cysts in brains decreased more obviously, in the group immunized with plasmid pIRESneo/MIC6/PLP1 than in the other groups (P < 0.05). Compared with pIRESneo/MIC6/PLP1, coimmunization with pIRESneo/MIC6/PLP1 and adjuvant murine IL-18 promoted cellular and humoral immune responses but did not contribute significantly to cyst reduction (65.43% versus 61.60%) or the survival of immunized mice (45.0 ± 2.9 days versus 42.8 ± 2.9 days) (P > 0.05). Furthermore, the study also showed that the immune efficacy induced by pIRESneo/MIC6/PLP1 was better than that induced by pVAX/PLP1 or pVAX/MIC6 alone.
INTRODUCTION
Toxoplasma gondii is an obligately intracellular parasite that infects many species of warm-blooded animals, including humans, worldwide (11). Humans are infected either by eating infected meat or contaminated foods, by accidental ingestion of oocysts or sporozoites from cat feces, for instance, through contaminated drinking water (20), or by vertical transmission from acutely infected mothers. Primary infection during pregnancy can result in severe neonatal malformations and ocular complications in the fetus (16). Moreover, toxoplasmosis can cause considerable economic losses to the livestock industry (6, 27). Vaccination would be the ideal way to control human toxoplasmosis effectively.
Recent studies have focused on the identification, purification, and molecular cloning of T. gondii antigens that are potentially able to evoke host protective responses. So far, well-defined antigens studied include surface antigens (SAGs), dense-granule antigens (GRAs), and rhoptry antigens (ROPs). There has been increasing research on microneme proteins (MICs) as potential antigen targets for inducing an effective host immune response against T. gondii. The transmembrane microneme protein MIC6 and its partners MIC1 and MIC4 constitute an adhesive complex that plays important roles in host cell attachment by T. gondii (26). A recent study demonstrated that T. gondii MIC6 is a potential vaccine candidate, which elicits a broad range of immune responses and can prolong the survival time of immunized mice after challenge with T. gondii (18).
A previous study found that T. gondii perforin-like protein 1 (TgPLP1)-deficient parasites failed to exit normally after intracellular growth, resulting in entrapment within host cells (10). This defect was due to an inability to rapidly permeabilize the parasitophorous vacuole membrane (PVM) and host plasma membrane during exit. We have reported previously on the protective immunity triggered with a plasmid expressing TgPLP1 against a lethal challenge infection with the virulent T. gondii strain RH and have demonstrated that TgPLP1 is a potential vaccine candidate against toxoplasmosis. The addition of murine interleukin 18 (IL-18) enhanced the effect of the TgPLP1 plasmid vaccine, prolonging the survival time of immunized mice (22).
Because of the complicated life history and diverse morphology of T. gondii, as well as its wide range of hosts, the immunity induced by different T. gondii antigens was inconsistent. Vaccines based on a single antigen have few lymphocyte binding sites and are restricted largely by the major histocompatibility complex (MHC); therefore, it is difficult to mount an efficient immune response against T. gondii (5). Tests on animals and humans have shown that the immune efficacy of univalent vaccines is not ideal (1, 2). Therefore, bivalent DNA vaccines that target different T. gondii stages have been developed (5, 7).
In the present study, we constructed the eukaryotic plasmid pIRESneo/MIC6/TgPLP1, coexpressing T. gondii MIC6 and TgPLP1, and examined the immunogenicity and protective effect of this DNA vaccine in Kunming mice against infection with the PRU strain (genotype II) of T. gondii. The immune-enhancing effect of IL-18 codelivered with plasmid pIRESneo/MIC6/TgPLP1 was also evaluated.
MATERIALS AND METHODS
Mice, cells, and parasites.
Specific-pathogen-free (SPF)-grade female inbred Kunming mice, 6 to 8 weeks old, were purchased from the Sun Yat-Sen University Laboratory Animal Center. All mice were handled in strict accordance with good animal practice according to the Animal Ethics Procedures and Guidelines of the People's Republic of China, and the study was approved by the Animal Ethics Committee of South China Agricultural University (permit SCAUAEM-2010-03). All mice were maintained under standard conventional conditions, with food and water ad libitum.
Marc-145 cells (a monkey kidney cell line) purchased from the Chinese Institute of Veterinary Drug Control, Beijing, China, were grown and maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with 10% (vol/vol) heat-inactivated fetal calf serum (FCS), 100 mg/ml streptomycin, and 100 IU/ml penicillin at 37°C under 5% CO2.
Two T. gondii strains (RH and PRU) were used. Strain RH (type I) is highly virulent for mice; it was used to produce the TgPLP1 and MIC6 clones. Strain PRU was used to challenge mice. This strain was selected because it produces many tissue cysts in the brain and is mildly virulent for mice. Cysts of T. gondii strain PRU were kindly provided by Xiao-Guang Chen (Department of Parasitology, School of Public Health and Tropical Medicine, Southern Medical University, Guangzhou, China) and were preserved in our laboratory (Laboratory of Parasitology, College of Veterinary Medicine, South China Agricultural University). Brain tissue cysts of strain PRU were obtained from the brains of Kunming mice 1 month after intraperitoneal infection with 20 cysts. Cysts were counted under an optical microscope. Strain RH tachyzoites were collected from the peritoneal fluids, washed by centrifugation, suspended in sterile phosphate-buffered saline (PBS), and sonicated. The supernatant containing soluble tachyzoite antigens (STAg) was kept at −80°C until further use.
Construction of the eukaryotic expression plasmid.
To construct the pIRESneo/MIC6/TgPLP1 expression plasmid, the coding sequence of the TgPLP1 gene (3,453 bp; sequence positions 1 to 3453) (GenBank accession no. EF102772) was amplified by PCR from T. gondii strain RH cDNA with a pair of oligonucleotide primers (forward primer PLP1F, 5′-ATCGATATGAGGTCACTCACACATGG-3′; reverse primer PLP1R, 5′-GGATCCTTACAGGTCTAACAGCTTGACG-3′) into which ClaI and BamHI recognition sites (underlined) were introduced. The MIC6 gene (1,050 bp; sequence positions 142 to 1191) (GenBank accession no. AF110270) was amplified by PCR from T. gondii strain RH genomic DNA with a pair of oligonucleotide primers (forward primer MIC6F, 5′-AAACCCGGGATGAGGCTCTTCCGGTGCT-3′; reverse primer MIC6R, 5′-CGTCTAGATTAATCCCATGTTTTGCTATCC-3′) into which SmaI and XbaI recognition sites (underlined) were introduced. The PCR product of MIC6 was cloned into the pGEM-T Easy vector (Promega) and was sequenced in both directions to ensure fidelity, generating pGEM-MIC6. The MIC6 fragment was cleaved by SmaI/XbaI from pGEM-MIC6, purified from an agarose gel (Sangon Biotech, China), and then cloned into the SmaI/XbaI sites of pIRESneo (Clontech), generating recombinant plasmid pIRESneo/MIC6, which was sequenced. The TgPLP1 fragment was digested by ClaI and NotI, purified from an agarose gel (Sangon Biotech, China), cloned into the corresponding sites of pIRESneo/MIC6 in frame, and sequenced. The resulting plasmid was named pIRESneo/MIC6/TgPLP1.
Plasmid pVAX/IL-18 (21) was kindly provided by Quan Liu (Veterinary Institute, Academy of Military Medical Sciences, Jilin Province, China). Plasmids pVAX/PLP1 (22) and pVAX/MIC6 (18) were preserved in our laboratory.
Expression of MIC6/TgPLP1 in vitro.
Marc-145 cells were transfected with pIRESneo/MIC6/TgPLP1, and with an empty vector (pIRESneo) as a negative control, by using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. Forty-eight hours after transfection, the cells were fixed with cool acetone for 15 min, and expression of the MIC6/TgPLP1 fusion protein was detected by the indirect immunofluorescence assay (IFA), with a goat anti-T. gondii polyclonal antiserum and fluorescein isothiocyanate (FITC)-labeled donkey anti-goat IgG (Proteintech Group Inc., Chicago, IL). Evans blue (Fisher) was included in the secondary antibody solution as a counterstain. Coverslips were rinsed three times with PBS. The monolayers binding the marker were covered with glycerin and were examined for specific fluorescence under a Zeiss Axioplan fluorescence microscope (Carl Zeiss, Germany).
DNA immunization and challenge.
Seven groups of mice (25 mice per group) were injected intramuscularly with 100 μg of plasmid DNA suspended in 100 μl sterile PBS (100 μl in one thigh skeletal muscle of each mouse) (Table 1). Group I mice were injected with PBS as a blank control; group II to group VI mice were injected with the empty vector pVAX I or pIRESneo, pVAX/IL-18, pVAX/TgPLP1, or pVAX/MIC6, respectively, also as controls; and groups VII and VIII were injected with pIRESneo/MIC6/TgPLP1 and pIRESneo/MIC6/TgPLP1+pVAX/IL-18 (100 μg each), respectively. Two booster injections were administered, one at the 2nd week and one at the 4th week after the first immunization. Five mice were randomly chosen from every group (vaccinated and nonvaccinated groups), challenged intragastrically with 80 cysts of strain PRU, and observed daily for mortality. In the present study, death was used as an endpoint for evaluating the protective efficacy induced by the DNA vaccines, because the ultimate goal of any vaccination study is to develop a vaccine that can protect the hosts (humans and/or animals) against lethal infection with T. gondii. It has been a general practice to use death as an endpoint for Toxoplasma vaccination studies (7, 13, 14, 25). To analyze protection against nonlethal challenge, eight mice of each group were orally infected with 20 cysts of strain PRU at the 14th day after the last immunization. Brain parasite loads were evaluated 6 weeks after infection. After removal, the whole brain from each mouse was individually homogenized in 3 ml PBS, and 1 μl of the homogenized brain was examined in order to calculate the number of T. gondii tissue cysts under an optical microscope. This procedure was carried out in triplicate, and the mean of three counts was obtained and was then used to calculate the total number of T. gondii tissue cysts in each brain sample.
Table 1.
Summary of treatments performed on micea
| Group | Treatment |
|---|---|
| I | PBS |
| II | pVAX I |
| III | pIRESneo |
| IV | pVAX/IL-18 |
| V | pVAX/TgPLP1 |
| VI | pVAX/MIC6 |
| VII | pIRESneo/MIC6/TgPLP1 |
| VIII | pIRESneo/MIC6/TgPLP1+pVAX/IL-18 |
Each group consisted of 25 mice. All treatments were injected into the thigh muscle and were given in 100 μl of PBS. For groups II through VIII, 100 μg of plasmid DNA was given. Two weeks after the final inoculation, five mice in each group were challenged intragastrically with 80 cysts of T. gondii strain PRU, and eight mice in each group were orally infected with 20 cysts of strain PRU.
Blood was collected from the mouse tail vein prior to each immunization, four times in total. Sera were separated and stored at −20°C until use.
IgG determination.
An anti-T. gondii IgG enzyme-linked immunosorbent assay (ELISA) kit was used for the measurement of antigen-specific antibodies according to the manufacturer's instructions (Combined Biotech Co., Ltd., Shenzhen, China). Briefly, plates were coated overnight at 4°C with 1 μg of STAg in 100 μl of carbonate buffer, pH 9.2. Mouse sera diluted 1:100 in PBS were applied to the wells. The bound antibodies were detected by horseradish peroxidase-conjugated anti-mouse IgG, diluted 1:2,000. Immune complexes were revealed by incubation with ortho-phenylenediamine and 0.15% H2O2 for 30 min. The reaction was stopped by the addition of 1 M H2SO4, and the absorbance at 450 nm was measured using an ELISA reader (EL×800; BioTek). All samples were run in triplicate.
Lymphoproliferation assay.
The lymphoproliferation assay was carried out according to a method described previously (18). Briefly, splenocyte suspensions from three mice of each group were prepared by pushing the spleens through a wire mesh. After the red blood cells (RBC) were removed using RBC lysis solution (Sigma), splenocytes were resuspended in DMEM supplemented with 10% FCS. Cells were then plated in 96-well Costar plates at a density of 5 × 105 per well and were cultured with either STAg (10 μg/ml), concanavalin A (ConA) (5 μg/ml; Sigma), or medium alone (negative control) in the incubator for 60 h at 37°C under 5% CO2. Proliferative activity was measured using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (5 mg/ml; Sigma) dye assay (17). The stimulation index (SI) was calculated as the ratio of the average optical density at 570 nm (OD570) of wells containing antigen-stimulated cells to the average OD570 of wells containing only cells with medium. All assays were performed in triplicate.
Cytokine assays.
For the detection of cytokines (18), splenocytes from nine immunized mice were cultured with different stimuli as described for the lymphoproliferation assay. Cell supernatants were harvested and assayed for interleukin-2 (IL-2) and IL-4 activities at 24 h, for IL-10 and IL-12 activities at 72 h, and for gamma interferon (IFN-γ) activity at 96 h. The IL-2, IL-4, IL-10, IL-12, and IFN-γ concentrations were evaluated by using a commercial ELISA kit according to the manufacturer's instructions (R&D Systems). Cytokine concentrations were determined by reference to standard curves constructed with known amounts of mouse recombinant IFN-γ, IL-2, IL-4, IL-10, or IL-12. The sensitivity limits for the assays were 20 pg/ml for IFN-γ, 50 pg/ml for IL-2 and IL-12, and 10 pg/ml for IL-4 and IL-10.
Statistical analysis.
All data were processed and analyzed by the SPSS data editor (version 13.0; SPSS Inc., Chicago, IL). In comparisons between groups, the results were considered different if P was <0.05.
RESULTS
Identification of the expressed product by IFA.
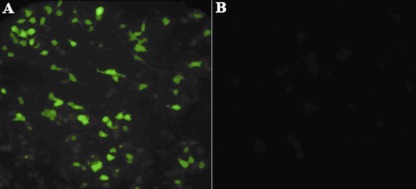
In vitro expression of pIRESneo/MIC6/TgPLP1 was evaluated by IFA at 48 h posttransfection. As shown in Fig. 1, green fluorescence was observed in Marc-145 cells transfected with pIRESneo/MIC6/TgPLP1, whereas no fluorescence was observed in pIRESneo-transfected cells, by use of an anti-T. gondii polyclonal antiserum.
Fig 1.
Indirect immunofluorescence detection of the MIC6/TgPLP1 fusion protein in Marc-145 cells transfected with pIRESneo/MIC6/TgPLP1 (A) or an empty vector (pIRESneo) (B).
Humoral response.
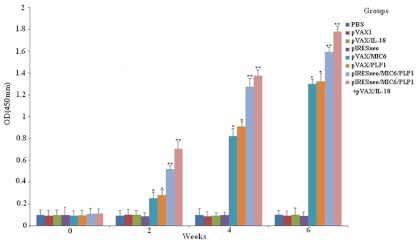
To determine the levels of anti-T. gondii antibodies, all sera were tested by ELISA. As shown in Fig. 2, significantly higher levels of T. gondii-specific IgG antibodies were detected in the sera of mice immunized with pIRESneo/MIC6/TgPLP1 or pIRESneo/MIC6/TgPLP1+pVAX/IL-18, especially after the third immunization. In contrast, mice injected with pVAX/PLP1 or pVAX/MIC6 alone also generated anti-TgPLP1/MIC6 antibodies, but at levels significantly lower than those of mice immunized with pIRESneo/MIC6/TgPLP1 or pIRESneo/MIC6/TgPLP1+pVAX/IL-18 (P < 0.05). Mice injected with PBS, pVAX I, pIRESneo, or pVAX/IL-18 alone did not generate anti-TgPLP1 antibodies, and there were significant differences between these four groups and the groups mentioned above (P < 0.05).
Fig 2.
Determination of levels of specific anti-T. gondii antibodies in the sera of Kunming mice immunized with 100 μg of PBS, pVAX I, pIRESneo, pVAX I/IL-18, pVAX/MIC6, pVAX I/TgPLP1, or pIRESneo/MIC6/TgPLP1 alone, or with a combination of pVAX I/IL-18 and pVAX I/TgPLP1 (100 μg each) on weeks 0, 2, and 4. Sera were collected 1 day prior to each immunization and were tested by ELISA using STAg. Results are means ± standard errors for three independent experiments. Extremely statistically significant differences (**, P < 0.01) and significant differences (*, P < 0.05) from the control are indicated.
Cellular proliferative responses induced by DNA vaccination.
Splenocytes from 8 groups of mice were prepared 2 weeks after the third immunization to assess the proliferative immune responses to the MIC6/TgPLP1 fusion protein. As shown in Table 2, the group of mice immunized with pIRESneo/MIC6/TgPLP1 had higher lymphocyte responses than the controls (P < 0.05). When the mice were coinjected with pIRESneo/MIC6/TgPLP1 and pVAX/IL-18, the level of splenocyte proliferation was further increased over those of the other groups (P < 0.05). Meanwhile, the groups of mice immunized with pVAX/TgPLP1, pVAX/MIC6, or pVAX/IL-18 alone had significantly higher lymphocyte responses than the rest of the controls (P < 0.05). In addition, splenocytes from all experimental and control groups proliferated to comparable levels in response to the mitogen ConA (data not shown).
Table 2.
Splenocyte proliferation responses of mice immunized with various plasmidsa
| Group | Proliferation SIb |
|---|---|
| pIRESneo/MIC6/PLP1+pVAX/IL-18 | 8.63 ± 0.15 A |
| pIRESneo/MIC6/PLP1 | 6.74 ± 0.14 B |
| pVAX/PLP1 | 4.20 ± 0.27 C |
| pVAX/MIC6 | 4.29 ± 0.18 C |
| pVAX/IL-18 | 4.18 ± 0.20 C |
| pIRESneo | 0.86 ± 0.03 D |
| pVAX I | 0.90 ± 0.07 D |
| PBS | 0.95 ± 0.11 D |
Splenocytes from three mice were harvested 2 weeks after the last immunization.
SI, stimulation index. Data followed by different capital letters are significantly different from each other (P < 0.05).
Cytokine production.
The supernatants of splenocytes cultured from mice from 8 groups were harvested at different times after stimulation with STAg and were assessed for the production of IFN-γ, IL-2, IL-12, IL-4, and IL-10 (Table 3). Very large amounts of specific IFN-γ, IL-2, and IL-12 were produced in the supernatants of restimulated splenocyte cultures from mice immunized with pIRESneo/MIC6/TgPLP1+pVAX/IL-18, pIRESneo/MIC6/TgPLP1, pVAX/IL-18, pVAX/PLP1, or pVAX/MIC6, but no specific production of IFN-γ was observed in the other groups (the PBS, pIRESneo, and pVAX I groups). Specific amounts of IL-4 and IL-10 were also synthesized by restimulated splenocytes from mice immunized with pIRESneo/MIC6/TgPLP1 or pIRESneo/MIC6/TgPLP1+pVAX/IL-18. There were no significant differences in the production of IL-4 and IL-10 between the pVAX/IL-18 group and the groups immunized with pIRESneo, pVAX I, or PBS.
Table 3.
Cytokine production by splenocytes of immunized Kunming mice after stimulation by STAga
| Group | Cytokine production (pg/ml)b |
||||
|---|---|---|---|---|---|
| IFN-γ | IL-2 | IL-12 | IL-4 | IL-10 | |
| pIRESneo/MIC6/PLP1+pVAX/IL-18 | 886.5 ± 9.5 A | 413.5 ± 9.5 A | 329.8 ± 18.8 A | 150.8 ± 9.5 A | 103.5 ± 4.0 B |
| pIRESneo/MIC6/PLP1 | 763.5 ± 12.3 B | 304.0 ± 9.4 B | 239.3 ± 16.7 B | 164.0 ± 15.1 A | 151.8 ± 11.4 A |
| pVAX/PLP1 | 471.5 ± 28.9 D | 206.3 ± 28.2 D | 130.3 ± 17.7 D | 118.5 ± 6.4 B | 67.3 ± 2.9 C |
| pVAX/MIC6 | 475.8 ± 21.2 D | 208.0 ± 7.2 D | 130.5 ± 7.51 D | 115.5 ± 7.9 B | 68.3 ± 1.9 C |
| pVAX/IL-18 | 616.8 ± 50.7 C | 259.8 ± 6.0 C | 190.0 ± 6.8 C | 57.5 ± 3.1 C | 33.8 ± 2.3 D |
| pIRESneo | 57.0 ± 2.4 E | 59.0 ± 0.9 E | 57.5 ± 2.3 E | 50.5 ± 1.2 C | 40.5 ± 1.2 D |
| pVAX I | 56.5 ± 4.2 E | 56.3 ± 3.9 E | 54.5 ± 3.4 E | 49.0 ± 1.6 C | 40.8 ± 1.0 D |
| PBS | 55.8 ± 1.8 E | 55.5 ± 12.9 E | 55.5 ± 3.6 E | 50.0 ± 2.7 C | 39.0 ± 0.8 D |
Splenocytes from nine mice were harvested 2 weeks after the last immunization.
Values for IFN-γ are for 96 h poststimulation; values for IL-2 and IL-4 are for 24 h; values for IL-10 and IL-12 are for 72 h. Data followed by different capital letters in the same column are significantly different from each other (P < 0.05).
Protection of mice against challenge with T. gondii following DNA vaccination.
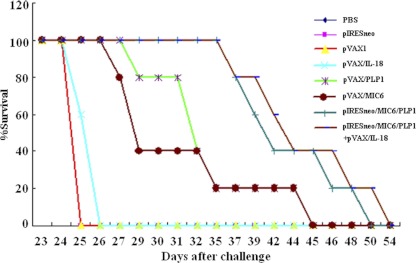
To examine protective immunity, 5 mice of each group were challenged with 80 cysts of strain PRU at 2 weeks after the final immunization. Mortality was checked daily. The percentages of survival in the different groups of mice are shown in Fig. 3. Interestingly, all mice immunized with PBS, pIRESneo, or pVAX I died at day 25, giving a value of 0% for the survival of these three control groups at day 25 (Fig. 3). Immunization of Kunming mice with pIRESneo/MIC6/TgPLP1 dramatically increased the survival time (42.8 ± 2.9 days) (P, <0.05 versus controls) of these mice following infection with T. gondii. Coimmunization with pIRESneo/MIC6/TgPLP1 and pVAX/IL-18 enhanced the survival time of immunized mice even further (45.0 ± 2.9 days), although the difference was not statistically significant (P, >0.05).
Fig 3.
Survival curves of immunized Kunming mice after lethal challenge with 80 cysts of T. gondii strain PRU 2 weeks after the last immunization. Death was used as an endpoint for these data. The three control groups (PBS, pVAXI, and pIRESneo) had 0% survival at day 25; thus, the lines representing these control groups overlap on the graph.
Immunoprotective value was also determined in a chronic model by using 20 cysts of strain PRU. The rest of the mice were immunized, and 2 weeks after the last booster, they were orally infected with 20 cysts of PRU. Cysts in the brain were counted 6 weeks after challenge. The lowest brain cyst burdens were observed in mice immunized with pIRESneo/MIC6/PLP1+pVAX/IL-18 (1,085.67 ± 49.32 cysts per brain) or pIRESneo/MIC6/PLP1 (1,206.00 ± 10.02 cysts per brain) and were significantly lower than those in the PBS group (3,140.33 ± 96.72 cysts per brain) and the other five groups (P < 0.05). Brain cyst burdens were significantly lower (P < 0.05) in mice immunized with pVAX/PLP1 (1,759.00 ± 60.47) or pVAX/MIC6 (1,890.00 ± 46.36) than in the control animals (Table 4).
Table 4.
Mean cyst burden per mouse brain 6 weeks after challenge with 20 cysts of T. gondii strain PRU per mouse
| Group | No. of brain cysts (mean ± SE)a | % reductionb |
|---|---|---|
| PBS | 3,140.33 ± 96.72 A | |
| pVAX I | 3,130.67 ± 42.98 A | 0.31 |
| pIRESneo | 3,101.67 ± 121.48 A | 1.23 |
| pVAX/IL-18 | 2,976.67 ± 68.49 A | 5.21 |
| pVAX/MIC6 | 1,890.00 ± 46.36 B | 39.81 |
| pVAX/PLP1 | 1,759.00 ± 60.47 B | 43.99 |
| pIRESneo/MIC6/PLP1 | 1,206.00 ± 10.02 C | 61.6 |
| pIRESneo/MIC6/PLP1+pVAX/IL-18 | 1,085.67 ± 49.32 C | 65.43 |
Data followed by different capital letters are significantly different from each other (P < 0.05).
From the values for the control group (PBS).
DISCUSSION
The present study shows that the fusion of TgPLP1 with MIC6 elicited a strong humoral and cellular immune response against T. gondii, with significant levels of specific IgG antibodies, IFN-γ, IL-2, and IL-12 production, and lymphoproliferation (P < 0.05) relative to those of the control groups. Moreover, this study demonstrated that the vaccination was capable of decreasing the mortality of animals infected with T. gondii strain PRU and of reducing the levels of tissue cysts in the brains of infected animals (P < 0.05). Murine IL-18 markedly enhanced the humoral and cellular response in that it played a functional role in the immune reaction against T. gondii. These results are consistent with those of previous studies (8, 9, 12, 13, 22). In previous studies, we demonstrated that DNA vaccines individually encoding the target antigens MIC4, ROP16, and ROP18 can elicit a broad range of immune responses that are capable of extending the survival time of animals acutely infected with T. gondii (15, 23, 24).
PLPs are expressed by many bacterial and protozoan pathogens and are important virulence factors of many pathogenic bacteria and parasites. A previous study (22) evaluated the protective effect of the novel target antigen TgPLP1 in DNA vaccination against T. gondii infection in mice and showed that TgPLP1 induces a strong protective humoral and cellular response against T. gondii, indicating that it is a potential vaccine candidate. MIC6 has also been shown to be effective at increasing the survival time of immunized mice after lethal challenge with T. gondii strain RH (18). However, a single-gene DNA vaccine can induce only partial immunity. Therefore, in this study, we constructed double-gene recombinant plasmid pIRESneo/MIC6/PLP using TgPLP1 and MIC6 from different life stages, and we detected high levels of specific IgG antibodies after immunization (Fig. 2). This result demonstrated that the immune efficacy of pIRESneo/MIC6/PLP was dramatically better than that of the one-gene plasmid pVAX/MIC6 or pVAX/TgPLP1 (P < 0.05).
Although cellular immunity could not be studied, the cytokines can be classified into the Th1 type and the Th2 type. The former produces mainly IFN-γ, IL-2, and IL-12, but the latter produces IL-4, IL-5, IL-6, and IL-10. IFN-γ and IL-4 play a guiding role in the dynamic balance between Th1 and Th2. Immunization with pIRESneo/MIC6/PLP enhanced Th1 cell-mediated immunity over that for control groups, with high levels of IFN-γ, IL-2, and IL-12 but low levels of IL-4 and IL-10 (Table 3). These findings demonstrate that intramuscular immunization with pIRESneo/MIC6/PLP potentiates Th1-type cellular immune responses in Kunming mice. Several factors have been proposed to determine whether an immune response is Th1 or Th2 biased, including the features of antigens (expressed by eukaryotic/procaryotic systems), doses of antigen, sites of immunization, and different delivery routes in the host (3, 19). All these will be considered in further studies.
The challenge dose of T. gondii may be an important impact factor in analyzing the immune protective effect (14). A higher dosage of T. gondii was used to challenge mice in an early DNA vaccine study. Zhang et al. (25) used 1 × 104 tachyzoites for evaluation of the immune response of mice to a DNA vaccine and assessed that the dosage was too high to allow observation of a longer survival time. Even though the vaccine induced some immune response, the mice were not capable of fighting too high a dosage of the lethal T. gondii parasite strain RH. However, it is easier to evaluate the survival rate by using less virulent strains to challenge mice. Dautu et al. (4) observed the survival rate induced by a DNA vaccine in C57BL/6 and BALB/c mice challenged with 20 cysts of strain Beverley. Therefore, this study used the low-virulence strain PRU administered orally at 20 cysts per mouse, and the immune effects of the recombinant plasmid vaccine were observed. At the same time, 80 cysts of strain PRU per mouse were administered intragastrically in order to study survival after challenge.
In conclusion, immunization with the recombinant plasmid DNA encoding T. gondii TgPLP1 and MIC6 offers protective efficacy, and this is a promising vaccine candidate against chronic toxoplasmosis. The application of targeted stage-specific immunization strategies and/or combination with other effective antigens should improve the protective effect of TgPLP1 or MIC6 and potentially eliminate or significantly mitigate the risks of brain cyst reactivation during chronic infection by T. gondii.
ACKNOWLEDGMENTS
This study was supported, in part, by grants from the National Natural Science Foundation of China (grants 30901067, 31172316, and 31101812), the National Program for High Technology Research (2011AA10A215), the Open Funds of the State Key Laboratory of Veterinary Etiological Biology, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences (SKLVEB2009KFKT014, SKLVEB2010KFKT010, SKLVEB2011KF KT010, and SKLVEB2011KFKT004), the Scientific and Technological Planning Project of Guangdong Province (grant 2010B020307006), the Specialized Research Fund for the Doctoral Program of Higher Education (grant 20094404120016), and the Program for Outstanding Scientists in Agricultural Research.
Footnotes
Published ahead of print 29 February 2012
REFERENCES
- 1. Alexander J, Jebbari H, Bluethmann H, Satoskar A, Roberts CW. 1996. Immunological control of Toxoplasma gondii and appropriate vaccine design. Curr. Top. Microbiol. Immunol. 219:183–195 [DOI] [PubMed] [Google Scholar]
- 2. Araujo FG. 1994. Immunization against Toxoplasma gondii. Parasitol. Today 10:358–360 [DOI] [PubMed] [Google Scholar]
- 3. Baca-Estrada ME, Foldvari M, Snider M, van Drunen Littel-van den Hurk S, Babiuk LA. 1997. Effect of IL-4 and IL-12 liposomal formulations on the induction of immune response to bovine herpesvirus type-1 glycoprotein D. Vaccine 15:1753–1760 [DOI] [PubMed] [Google Scholar]
- 4. Dautu G, et al. 2007. Toxoplasma gondii: DNA vaccination with genes encoding antigens MIC2, M2AP, AMA1 and BAG1 and evaluation of their immunogenic potential. Exp. Parasitol. 116:273–282 [DOI] [PubMed] [Google Scholar]
- 5. Donnelly JJ, Wahren B, Liu MA. 2005. DNA vaccines: progress and challenges. J. Immunol. 175:633–639 [DOI] [PubMed] [Google Scholar]
- 6. Dubey JP, et al. 2005. Prevalence of viable Toxoplasma gondii in beef, chicken, and pork from retail meat stores in the United States: risk assessment to consumers. J. Parasitol. 91:1082–1093 [DOI] [PubMed] [Google Scholar]
- 7. Fachado A, et al. 2003. Protective effect of a naked DNA vaccine cocktail against lethal toxoplasmosis in mice. Vaccine 21:1327–1335 [DOI] [PubMed] [Google Scholar]
- 8. Fehniger TA, et al. 1999. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J. Immunol. 162:4511–4520 [PubMed] [Google Scholar]
- 9. Gu YE, Tsutsui H, Ku G, Hsiao K, Fleming MA. 1997. Activation of interferon-gamma inducing factor mediated by interleukin-1β converting enzyme. Science 275:206–209 [DOI] [PubMed] [Google Scholar]
- 10. Kafsack BF, et al. 2009. Rapid membrane disruption by a perforin-like protein facilitates parasite exit from host cells. Science 323:530–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim K, Weiss LM. 2004. Toxoplasma gondii: the model apicomplexan. Int. J. Parasitol. 34:423–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Letscher-Bru V, et al. 1998. Protective effect of vaccination with a combination of recombinant surface antigen 1 and interleukin-12 against toxoplasmosis in mice. Infect. Immun. 66:4503–4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu Q, et al. 2010. The protective effect of a Toxoplasma gondii SAG1 plasmid DNA vaccine in mice is enhanced with IL-18. Res. Vet. Sci. 89:93–97 [DOI] [PubMed] [Google Scholar]
- 14. Liu S, et al. 2009. Evaluation of protective effect of multi-epitope DNA vaccine encoding six antigen segments of Toxoplasma gondii in mice. Parasitol. Res. 105:267–274 [DOI] [PubMed] [Google Scholar]
- 15. Liu MM, et al. 2010. Toxoplasma gondii microneme protein 8 (MIC8) is a potential vaccine candidate against toxoplasmosis. Parasitol. Res. 106:1079–1084 20177910 [DOI] [PubMed] [Google Scholar]
- 16. Montoya JG, Liesenfeld O. 2004. Toxoplasmosis. Lancet 363:1965–1976 [DOI] [PubMed] [Google Scholar]
- 17. Okamura H, et al. 1995. A novel costimulatory factor for gamma interferon induction found in the livers of mice causes endotoxic shock. Infect. Immun. 63:3966–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peng GH, et al. 2009. Toxoplasma gondii microneme protein 6 (MIC6) is a potential vaccine candidate against toxoplasmosis in mice. Vaccine 27:6570–6574 [DOI] [PubMed] [Google Scholar]
- 19. Romagnani S. 1992. Induction of TH1 and TH2 responses: a key role for the ‘natural’ immune response? Immunol. Today 13:379–381 [DOI] [PubMed] [Google Scholar]
- 20. Tenter AM, Heckeroth AR, Weiss LM. 2000. Toxoplasma gondii from animals to humans. Int. J. Parasitol. 30:1217–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wei F, et al. 2008. Enhancement by IL-18 of the protective effect of a Schistosoma japonicum 26kDa GST plasmid DNA vaccine in mice. Vaccine 26:4145–4149 [DOI] [PubMed] [Google Scholar]
- 22. Yan HK, et al. 2011. Toxoplasma gondii: protective immunity against experimental toxoplasmosis induced by a DNA vaccine encoding the perforin-like protein 1. Exp. Parasitol. 128:38–43 [DOI] [PubMed] [Google Scholar]
- 23. Yuan ZG, et al. 2011. Protective effect against toxoplasmosis in mice induced by DNA immunization with gene encoding Toxoplasma gondii ROP18. Vaccine 29:6614–6619 [DOI] [PubMed] [Google Scholar]
- 24. Yuan ZG, et al. 2011. Protective immunity induced by Toxoplasma gondii rhoptry protein 16 against toxoplasmosis in mice. Clin. Vaccine Immunol. 18:119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang J, et al. 2007. Evaluation of the immune response induced by multiantigenic DNA vaccine encoding SAG1 and ROP2 of Toxoplasma gondii and the adjuvant properties of murine interleukin-12 plasmid in BALB/c mice. Parasitol. Res. 101:331–338 [DOI] [PubMed] [Google Scholar]
- 26. Zheng B, et al. 2009. MIC6 associates with aldolase in host cell invasion by Toxoplasma gondii. Parasitol. Res. 105:441–445 [DOI] [PubMed] [Google Scholar]
- 27. Zou FC, et al. 2009. Seroprevalence of Toxoplasma gondii in pigs in Southwestern China. Parasitol. Int. 58:306–307 [DOI] [PubMed] [Google Scholar]