Abstract
Melioidosis is a severe disease caused by the Gram-negative bacterium Burkholderia pseudomallei. Previously we showed that pretreatment of mice with CpG oligodeoxynucleotide (CpG ODN) 2 to 10 days prior to B. pseudomallei challenge conferred as high as 90% protection, but this window of protection was rather short. In the present study, we therefore aimed to prolong this protective window and to gain further insight into the mechanisms underlying the protection induced by CpG ODN against B. pseudomallei infection. It was found that the CpG ODN incorporated with cationic liposomes (DOTAP) but not zwitterionic liposomes (DOPC) provided complete protection against bacterial challenge. Although marked elevation of gamma interferon (IFN-γ) was found in the infected animals 2 days postinfection, it was significantly lowered by the DOTAP-plus-CpG ODN pretreatment. When appropriately activated, the phagocytic index and oxidative burst responses of neutrophils appeared not to be elevated. However, macrophages from stimulated mice showed higher levels of nitric oxide production and exhibited higher levels of antimicrobial activities, judging from lower numbers of viable intracellular bacteria. Taken together, our results demonstrate that DOTAP can enhance the protective window period of CpG ODN to at least 30 days and provide 100% protection against B. pseudomallei infection. The protective effect of DOTAP plus CpG ODN could provide an alternative approach to preventing this lethal infection, for which no vaccine is yet available.
INTRODUCTION
Melioidosis is an infectious disease caused by Burkholderia pseudomallei. It is a major public health problem in Southeast Asia and tropical Australia. Melioidosis patients show a broad spectrum of disease, ranging from asymptomatic latent infection to fatal septicemia to chronic abscess formation (8, 29). In northeast Thailand, melioidosis shows a 50% mortality rate. The average incidence in 2006 was estimated to be 8.63 per 100,000 people (5, 30), and 10% of the infected individuals showed relapse even after prolonged antibiotic treatment (44). Currently, there is no vaccine available for this disease.
Study of B. pseudomallei infection in a murine model demonstrated that C57BL/6 mice were more resistant to infection and showed lower bacteremia than BALB/c mice. The strain difference may be related to the high Th1 response in C57BL/6 mice (21). Th1 cytokines, i.e., gamma interferon (IFN-γ), interleukin 12 (IL-12), and tumor necrosis factor alpha (TNF-α), appeared to be important early mediators of protection, since the administration of neutralizing monoclonal antibodies against these cytokines increased susceptibility to infection (35). Neutrophils and macrophages have been reported to play major roles in host defense against this infection (3, 12). However, the relative importance of the cell-mediated and humoral arms of both the innate and the adaptive immune responses in B. pseudomallei infection remains unclear (45).
Several antigens have been used for immunization against experimental B. pseudomallei infection in mice, including lipopolysaccharide (LPS), capsule polysaccharide, flagella (6, 36, 37), and several other bacterial proteins, including the lipoprotein releasing system transmembrane protein (LolC), an ATP–binding cassette transporter protein (20), and the type III secretion system protein (11). More recently, two outer membrane proteins (OmpAs) of B. pseudomallei that reacted with melioidosis patients' convalescent-phase sera have been identified: BPSL2522 (Omp3) and BPSL2765 (Omp7) (19). Live attenuated B. pseudomallei has also been shown to confer some degree of protection (4, 18, 37). For passive protection, monoclonal antibodies against capsular polysaccharide and lipopolysaccharide did not give complete protection, since abscesses could be found in the spleen and liver (23). Because surface proteins on the outer membrane of bacteria may play a role in bacterial adhesion to and invasion of host cells, these components could also be potential vaccine candidates (9, 43).
The immunomodulator CpG oligodeoxynucleotide (CpG ODN) is a synthetic oligodeoxynucleotide that has been shown to promote a Th1 immune response by interacting with Toll-like receptor 9 (TLR9) via endosome maturation, resulting in improved antigen uptake and presentation by antigen-presenting cells (APCs) (25, 26). Moreover, several reports indicate that CpG ODN can protect mice against many bacterial, viral, and parasitic infections (10, 27, 32). Our group previously demonstrated that CpG ODN given 2 to 10 days before challenge could induce significant protection in mice against B. pseudomallei (47). However, the protection was not significant when the CpG ODN was given 30 days before challenge. Prolonging the bioavailability and duration of action of CpG ODN may improve its therapeutic efficacy. Liposome encapsulation has been successfully used to protect CpG ODNs from degradation while increasing their uptake by cells of the immune system (17). Toward this goal, we therefore compared various liposomes to extend the window period of CpG ODN delivery before challenge. These combinations were then evaluated for protective efficacy in BALB/c mice.
MATERIALS AND METHODS
Animals.
Male BALB/c mice (6 to 8 weeks old) were obtained from the National Animal Centre, Mahidol University (Bangkok, Thailand). All mice were maintained in the animal unit at the Faculty of Medicine, Khon Kaen University (Khon Kaen, Thailand), and fed with water and a standard laboratory diet ad libitum. These animals were used for B. pseudomallei infection under the approved protocol (no. AEKKU13/2550) from the animal ethics committee of the Faculty of Medicine, Khon Kaen University.
CpG ODN.
The CpG oligodeoxynucleotide (CpG ODN) used in this study was CpG ODN 1826 (5′ TCCATGACGTTCCTGACGTT 3′), which possesses a nuclease-resistant phosphorothioate backbone and contained only a trace of endotoxin (less than 0.001 EU/μg; Invivogen, California).
Bacterial strains and culture conditions.
Virulent B. pseudomallei strain 1909a was isolated from the blood of a Thai patient from northeast Thailand with septicemic melioidosis. The 50% lethal dose (LD50) was 20 cells. Bacteria were streaked on Ashdown agar (1) and incubated at 37οC for single-colony isolation. To prepare mid-log-phase bacteria, one colony was inoculated into 3 ml tryptic soy broth (TSB) incubated at 37οC overnight, and then a 1% inoculum was added to 50 ml TSB and allowed to grow for 3 h with rotation at 250 rpm in a 37οC incubator. The bacterial cells were then diluted in phosphate-buffered saline (pH 7.2) to a desired concentration. The exact number of viable bacteria in the suspension was subsequently determined by plating the bacteria on nutrient agar and counting after 30 to 48 h of incubation at 37οC and is expressed in CFU/ml. All procedures described were carried out in a biosafety biohazard hood.
Preparation of liposomes containing CpG ODN.
Cationic liposomes were prepared from dioleoyl trimethylammonium propane (DOTAP) (Sigma, Missouri). The lipid was dissolved in chloroform, dried under a gentle stream of nitrogen, and hydrated with injectable-grade water. The suspension was then sonicated in a bath-type sonicator until translucent, creating a small unilamellar vesicle (SUV) suspension. To prepare dehydration-rehydration vesicles (DRV), the CpG ODN (100 μg CpG ODN/1,000 μg liposomes) was added to the SUV suspension and the mixture was frozen on dry ice and dried in a freeze-drier, after which 1 ml of phosphate-buffered saline (PBS) was added to rehydrate the lipid-antigen mixture (24).
Zwitterionic liposomes were prepared as DRV as described above using 1 mg phosphatidylcholine and 4 mg cholesterol (DOPC) (Sigma, Missouri) hydrated with injectable-grade water containing CpG ODN (100 μg/mice). The suspensions were freshly prepared before immunization.
When liposomes containing CpG were prepared, the unencapsulated CpG ODN was removed by centrifugation at 14,000 × g for 15 min at 4°C. The efficiency of incorporation (% entrapment) of CpG ODN in liposomes was determined using UV absorption at 260 nm. The analysis was performed on supernatants following centrifugation (22).
The percent entrapment was calculated as follows: % entrapment = [(OD260 of CpG before entrapment − OD260 of CpG after entrapment)/OD260 of CpG before entrapment] × 100, where OD260 is the optical density at 260 nm.
The percentages of entrapment of CpG ODN in DOTAP (cationic) liposomes and in DOPC (zwitterionic) liposomes were 96% and 25%, respectively. The concentration of the CpG ODN in DOTAP and DOPC liposomes was accordingly adjusted to 100 μg/μl.
Immunization and challenge.
Ten BALB/c mice (per group) were injected intramuscularly with either 100 μg CpG ODN per mouse on day −2 (as a positive control) or 100 μg CpG ODN plus 1,000 μg cationic (DOTAP) or 100 μg CpG ODN plus 1,000 μg zwitterionic (DOPC) liposomes in 100 μl of PBS per mouse on day −30 prior to bacterial challenge. After immunization, the animals were challenged with 5 LD50 of B. pseudomallei via the intraperitoneal (i.p.) route on day 0 and were monitored daily for mortality for the next 30 days. For i.p. and intramuscular (i.m.) administration, the injection volume was adjusted to 100 μl in PBS. PBS was used for injection in the negative-control group. Individual blood samples obtained by orbital puncture on days −23, −16, 2, and 7 were used for IFN-γ determination by enzyme-linked immunosorbent assay (ELISA).
Preparation of mouse neutrophils.
EDTA-treated blood was collected by orbital puncture from BALB/c mice, and neutrophils were isolated by a method modified from that described previously (2). The blood was laid on a three-layer Percoll density gradient (Amersham Biosciences) and centrifuged (1,500 × g, 30 min, room temperature). After centrifugation, neutrophils were harvested into RPMI-containing tubes. The remaining red cells were eliminated by hypotonic lysis. Neutrophil purity and viability were evaluated by differential counting of blood smears and by trypan blue staining. The purity and viability of the cells were 90% and 95%, respectively. The neutrophils were suspended in RPMI medium and used within 6 h.
Mouse macrophage isolation.
Mouse splenocytes from each experiment were obtained by passing spleen fragments through a steel mesh with a plunger. Mononuclear cells were isolated by Ficoll gradient (GE Health Sciences, New Jersey) and centrifuged at 800 × g for 20 min. The mononuclear cells were washed twice with RPMI containing 10% fetal bovine serum and allowed to adhere to a tissue culture plate at 37οC, 5% CO2, for 90 min. The adherent cells were counted with trypan blue and adjusted to 1 × 106 cells/ml in RPMI containing 10% fetal bovine serum (FBS). The macrophage nature of the adherent cells was ascertained by staining with CD14-fluorescein isothiocyanate (FITC) (Becton, Dickinson and Company, New Jersey), and only the specimens with >90% CD14+ were used in the experiments.
Determination of respiratory burst and bacterial counts.
Mouse neutrophils or macrophages (106 cells) and bacteria were diluted with PBS and mixed to give a multiplicity of infection (MOI) of 10:1. Bacteria were opsonized by incubation with 10% autologous mouse sera (without heat inactivation) for 30 min at 37οC in 5% CO2 incubator. Opsonized zymosan was used as a positive control. The neutrophils or macrophages were mixed with opsonized zymosan or B. pseudomallei in the presence of 200 μM luminol. Chemiluminescence was immediately measured using a luminometer (Turner BioSystem) every 1 min for a total time of 1 h. To compare the killing of B. pseudomallei by neutrophils, the bacterial colonies were counted before (0 min) and after (1, 2, 3, 4, and 6 h) adding to the suspension. The reaction mixtures were then diluted in distilled water and vigorously vortexed to disrupt cells. Viable colony counts were determined following an overnight culture on nutrient agar at 37οC (14). Statistical analysis was performed at the time points of each experiment giving the highest signals (15 min).
Phagocytic index assay.
Neutrophils were obtained from the day 2 blood of mice pretreated with CpG ODNs or PBS. The bacteria were opsonized by incubation with 10% autologous mouse sera (without heat inactivation) for 30 min at 37οC in a 5% CO2 incubator. After the neutrophil concentration was adjusted to 106 cells with PBS, they were incubated with opsonized B. pseudomallei at an MOI of 10:1 at 37οC for 1 h. After incubation, the suspension was centrifuged and resuspended in 10 μl of PBS and applied to glass slides for Giemsa staining. The neutrophils and bacteria were examined under bright microscopy, counting at least 200 cells in low-power fields. The phagocytic index was calculated and expressed as the ratio of phagocytosed bacteria to total bacterium-laden neutrophils.
Intracellular survival of B. pseudomallei in macrophages.
Ten BALB/c mice (per group) were injected intramuscularly with a total volume of DOTAP (100 μl/mouse) plus CpG ODN (100 μg/mouse) or DOTAP alone on day −30 or with CpG ODN alone or PBS control on day −2. On day 0, splenic macrophages were removed and cultured with RPMI medium containing 10% FBS. Aliquots of B. pseudomallei were coincubated with mouse macrophages at an MOI of 10:1 for 1 h at 37οC in 5% CO2 (41). After 1 h of incubation (total time [T] = 1), excess bacteria were removed by washing with PBS, and cells were incubated at 37οC in prewarmed medium containing kanamycin (250 μg/ml) for another 2 h to kill extracellular B. pseudomallei (T = 3). The medium was then replaced with medium containing kanamycin at a lower concentration (20 μg/ml) to suppress residual extracellular bacteria. Cells were further incubated for 5 more hours (T = 8) before the total number of intracellular bacteria expressed as CFU/ml was determined (41).
Nitric oxide assay.
The production of NO in macrophage supernatants was determined by nitrite quantification using the Griess reagent (Sigma, Missouri).
Measurement of mouse IFN-γ.
A mouse IFN-γ ELISA was carried out using commercial mouse IFN-γ MAX Deluxe ELISA kits (Biolegend, California) according to the manufacturer's instructions.
Statistical analysis.
For parametric statistics, the comparisons between two groups were analyzed by independent t test, and the comparisons of more than two groups were analyzed by one-way analysis of variance (ANOVA), followed by post hoc intergroup comparison. For nonparametric statistics, differences between two groups were analyzed by Mann-Whitney U test, and the comparison of more than two groups was analyzed by Kruskal-Wallis analysis. Survival times were compared using Kaplan-Meier curves and the log-rank test from the software program GraphPad Prism version 4.0 (GraphPad Software, San Diego, CA). Data were considered statistically significant if P values were <0.05.
RESULTS
Protective effect of CpG ODN-DOTAP cationic liposomes against B. pseudomallei.
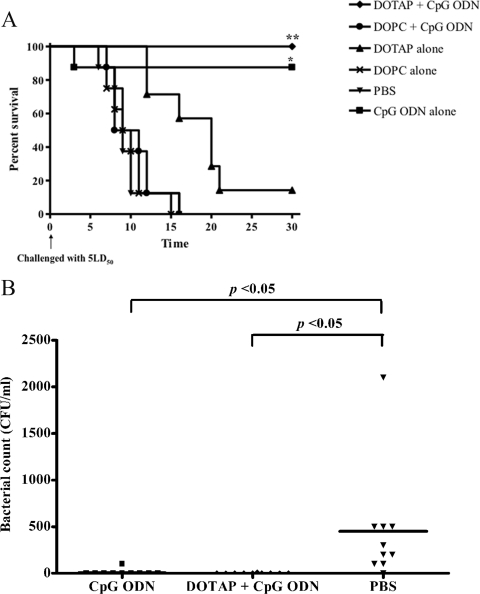
In an attempt to extend the window period for protection (from 2 to 10 days before challenge), mice were injected with CpG ODN incorporated in either cationic or zwitterionic liposomes on day −30 prior to challenge with 5 LD50 of B. pseudomallei on day 0. Mouse survival profiles in different groups are shown in Fig. 1A. The mean and standard error (SE) of survival times of CpG ODN alone (on day −2 as a positive control), CpG ODN incorporated in cationic (DOTAP) and zwitterionic (DOPC) liposomes, DOTAP alone, DOPC alone, and PBS were 26.65 ± 3.38, 30 ± 0, 10.25 ± 1.08, 18.71 ± 2.36, 9.75 ± 0.94, and 9.62 ± 1.01 days, respectively. These results demonstrated that mice administered CpG CpG ODN or CpG ODN with cationic (DOTAP) liposomes gave the highest protection against B. pseudomallei infection (100%). On the contrary, no enhancing effect could be observed with zwitterionic liposomes (DOPC) with CpG ODN (Fig. 1A). The results presented in Fig. 1B clearly demonstrate that the bacteremia on day 2 was significantly lower in both the CpG ODN and DOTAP-plus-CpG groups than for the PBS control. At the end of the experiment, all animals administered DOTAP plus CpG ODN appeared healthy and active.
Fig 1.
Effect of CpG ODN with cationic or zwitterionic liposomes against B. pseudomallei infection. BALB/c mice (10 mice/group) were injected intramuscularly with either CpG ODN with cationic (DOTAP) or with zwitterionic (DOPC) liposomes, liposomes alone, or PBS (negative control) on day −30 or CpG ODN alone on day −2 (positive control) and then challenged intraperitoneally with 5 LD50 B. pseudomallei (100 CFU) on day 0. Mice were observed daily for 30 days, and percent survival was plotted against time (A). The bacteremia in mice treated with CpG ODN, DOTAP plus CpG ODN, and PBS control was determined on day 2 after infection (B). Survival times were compared using Kaplan-Meier curves and the log-rank test from GraphPad Prism version 4.0 (GraphPad Software, San Diego, CA). The asterisks (** or *) indicate significant differences between the DOTAP-plus-CpG ODN or CpG ODN group with the DOTAP, DOPC, or PBS group, respectively (P < 0.01).
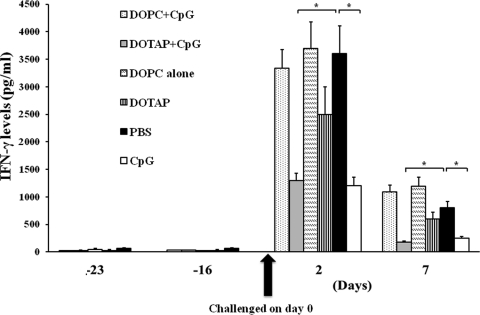
Attenuation of robust IFN-γ levels by CpG ODN-DOTAP liposomes.
Since IFN-γ is a critical factor in controlling B. pseudomallei infection, we measured IFN-γ levels on days −23 and −16 (before challenge) and 2 and 7 (after challenge). Results shown in Fig. 2 demonstrated that before challenge all groups had low IFN-γ levels. Similar to our previously reported findings (47), mice infected with B. pseudomallei showed a marked increase in IFN-γ levels on day 2 (Fig. 2). In contrast, mice administered DOTAP plus CpG or CpG ODN, which conferred 90 to 100% protection (Fig. 1), showed significantly lower IFN-γ levels after infection than PBS control mice. These 2 groups showed no significant difference. Moreover, animals dosed with DOPC, DOTAP alone, and DOPC plus CpG ODN, which provided lower protection, showed high levels of IFN-γ, similar to those observed in the PBS controls (Fig. 2). Although DOTAP alone gave some protection, the level of IFN-γ for mice treated with DOTAP alone was not significantly different from that for mice treated with DOPC alone or PBS control.
Fig 2.
Effects of CpG ODN administration with cationic or zwitterionic liposomes on IFN-γ serum profiles of B. pseudomallei-infected animals. BALB/c mice (10 mice/group) were treated as described in the legend to Fig. 1. The animals in each group were bled serially as shown (the numbers of specimens at each time point were not equal, since some animals in the control group died before day 7). Differences between groups at each time point were calculated by the Mann-Whitney U test, and those indicated by “*” were statistically significant (P < 0.01) from results for the PBS control. Error bars represent SEM.
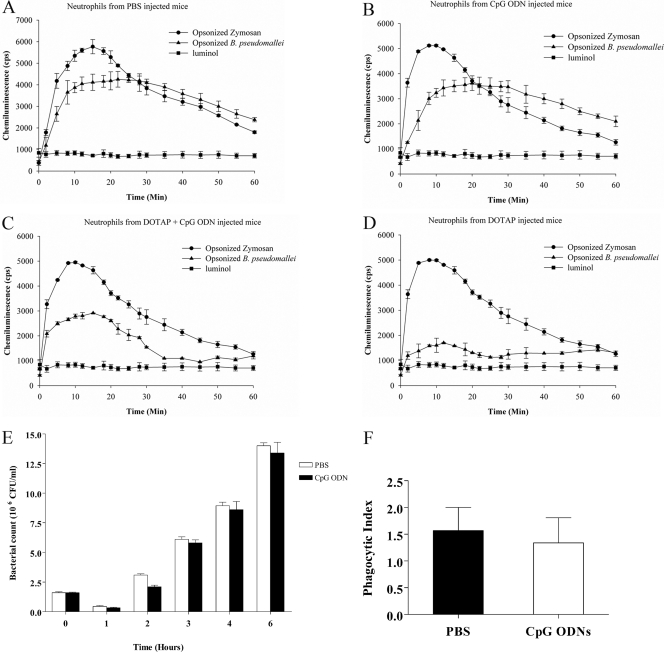
Effect of CpG ODN and CpG ODN liposomes on macrophages and neutrophils.
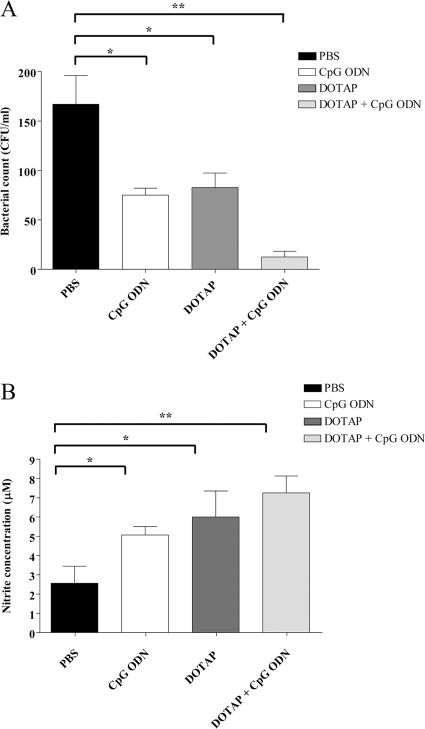
Our present data and previously reported study (47) clearly demonstrated that the elevated IFN-γ levels detected in the day 2 blood of infected mice were not be able to protect them from lethal melioidosis. The elevated IFN-γ levels on day 2 after B. pseudomallei infection are the result of bacterial infection and may occur too late to stimulate the immune response against the bacteria. In contrast, the elevated IFN-γ levels before bacterial infection are immunostimulatory, leading to control of infection. Since an important protective mechanism against bacterial infection is the ability of innate immune cells to produce reactive oxygen species or NO, we investigated these responses in neutrophils and macrophages. Oxidative burst responses to B. pseudomallei in murine neutrophils isolated from CpG ODN-treated, DOTAP-plus-CpG ODN-treated, or control (PBS-treated) mice were investigated. The results shown in Fig. 3A to E demonstrated that there were no differences in reactive oxygen intermediates (ROI) in all groups or in the numbers of intracellular bacteria between neutrophils from CpG ODN- or PBS-treated mice. Furthermore, the phagocytic indices from these two groups were similar (Fig. 3F). Macrophages have been found to play an important role in killing of B. pseudomallei (40). The killing mechanism is mediated through NO production. In this experiment, we therefore investigated whether ROI or NO production in mice treated with CpG ODN or DOTAP plus CpG ODN was increased, leading to reduced levels of intracellular bacteria. The ROI response to B. pseudomallei in the macrophages isolated from DOTAP-plus-CpG ODN-treated mice was increased within 15 min, whereas that of the CpG-ODN group was maximal at 30 min (Fig. 4). However, the ROI levels were not significantly different from those for the DOTAP-alone and PBS control groups. The number of intracellular bacteria (mean ± standard deviation [SD]) at 8 h after infection in mice treated with CpG ODN alone was 75 ± 7.07 CFU/ml, which was significantly lower than that of the PBS control group (167 ± 28.86 CFU/ml) but similar to that of the DOTAP-alone group. Moreover, since DOTAP plus CpG ODN given on day −30 before challenge conferred 100% protection (Fig. 1), the number of intracellular bacteria in macrophages from this group was also analyzed and was found to be significantly less (12.5 ± 5.77 CFU/ml) than that of the control group (Fig. 5A). In addition, NO production (as nitrite) in the supernatants from macrophages from mice treated with DOTAP alone (6 ± 1.35 μM) was higher than that for mice treated with CpG ODN alone (5 ± 0.5 μM). Macrophages from mice given DOTAP plus CpG ODN showed higher NO production. (7.25 ± 0.88 μM). The NO production from all those groups was significantly higher than that for the PBS-treated control group (2.7 μM) (Fig. 5B).
Fig 3.
Profiles of generation of ROI by neutrophils. Luminol-augmented ROI generation in neutrophils was measured using neutrophils isolated from BALB/c mice treated with PBS (A), CpG ODN for 2 days (B), or DOTAP plus CpG ODN (C) or DOTAP alone (D) for 30 days before exposure to opsonized B. pseudomallei at an MOI of 10:1 or zymosan (positive control) in the presence of 200 μM luminol. The chemiluminescence signal was measured at various times after incubation of neutrophils with bacteria or zymosan. To compare the killing of bacteria by neutrophils, bacterial counts were performed in lysed cells at 0 and 1, 2, 3, 4, and 6 h after mixing (E). For the phagocytic index, the cells were infected with B. pseudomallei at an MOI of 10:1 at 37°C for 1 h. After incubation, the suspension was centrifuged and resuspended for Giemsa staining. The neutrophils and bacteria were examined under bright microscopy, counting at least 200 cells in different fields. The phagocytic index was calculated and expressed as a ratio of the number of phagocytosed bacteria to the number of total B. pseudomallei-containing neutrophils (F). The results represent means ± SD from 3 independent experiments, and each test was performed in duplicate.
Fig 4.
Profiles of generation of ROI by macrophages. Luminol-augmented ROI generation in macrophages was measured using macrophages isolated from BALB/c mice injected with PBS (A), with CpG ODN (B) for 2 days, or with DOTAP plus CpG ODN (C) or DOTAP alone (D) for 30 days before exposure to opsonized B. pseudomallei at an MOI of 10:1 or zymosan (positive control) in the presence of 200 μM luminol. The chemiluminescence signal was measured at various times after incubation of macrophages with bacteria or zymosan. The results represent means ± SD from 3 independent experiments, and each test was performed in duplicate.
Fig 5.
Intracellular bacterial survival and nitric oxide production of macrophages. Numbers of intracellular bacteria (A) or nitrite concentration (B) of splenic macrophages from mice treated with PBS or CpG ODN on day −2 or with DOTAP alone or ODN plus DOTAP on day −30 is shown. Macrophages (1 × 106 cells/ml) were infected with B. pseudomallei at an MOI of 10:1 for 1 h. Extracellular bacteria were removed by incubation with medium containing 250 μg/ml kanamycin for 2 h. The medium was then replaced with new medium containing 20 μg/ml kanamycin and further incubated for 5 h. At 8 h, the supernatants were assayed for nitrite, and macrophages were lysed with 0.1%Triton X-100 and cultured on nutrient agar to determine numbers of intracellular bacteria. The levels of nitrite were determined using the Griess reagent. Each bar represents means ± SD. Results from 3 independent experiments are shown. The asterisks (* and **) indicated significant differences from results for the PBS control (P < 0.05 and P < 0.01, respectively).
DISCUSSION
Synthetic oligodeoxynucleotides containing unmethylated CpG dinucleotide motifs (CpG ODN) are capable of mimicking the immunostimulatory effects of bacterial DNA on B cells, monocytes/macrophages, NK cells, and dendritic cells. After activation, these cells secrete cytokines such as TNF-α, IL-12, and type-1 IFNs, which promote a Th1 immune response (7, 26, 48). The effects of CpG ODN are therefore useful in clearing certain pathogens that are susceptible to a strong Th1 response. For example, there are numerous reports showing that CpG ODN administration can protect mice from Listeria monocytogenes (27), Klebsiella pneumoniae (10), Francisella tularensis (15), and Burkholderia pseudomallei (47). For melioidosis, CpG ODN has been successfully used for prophylaxis in both BALB/c and C57BL/6 mice (34). In addition, combining preexposure vaccination with CpG treatment at the time of infection provided significantly greater protection than CpG treatment alone (13). As shown in our previous study, the administration of CpG ODN to mice 2 to 10 days before B. pseudomallei challenge conferred better than 90% protection (47). To increase the duration of protection conferred by CpG ODN, we utilized liposomes which are known to enhance different biological and immunological activities of various active molecules and compounds (39). Several studies have demonstrated that liposomes are an effective delivery vehicle which can protect their internal contents from nuclease activity, thus enhancing their half-life and distribution within the animal host. Liposome-encapsulated CpG ODN can also increase the CpG ODN half-life, making it suitable as an adjuvant for Th1 immune responses (38). Using two types of liposomes, we found interesting differences in innate immune responses elicited by CpG ODN. For example, cationic liposomes (DOTAP) were found to be more effective than zwitterionic liposomes in enhancing CpG ODN-mediated protection in mice that were subsequently challenged with B. pseudomallei (Fig. 1). The superiority of cationic liposomes in this regard is likely attributable to electrostatic interactions between the negatively charged CpG ODN and positively charged liposomes (38). The percent entrapment of CpG ODN in DOTAP or DOPC liposomes was 96% or 25%, respectively. The difference of entrapment might have some effect on immune activation and mouse survival in our study. Compared to CpG ODN used in our previous study, DOTAP plus CpG ODN could extend the window period from 2 to 10 days to −30 days before infection. It should be noted, however, that a different bacterial strain and dose (5 LD50 instead of 10 to 50 LD50) were used in the present study.
It has been reported that high levels of IFN-γ and other proinflammatory cytokines have been correlated with severity of melioidosis (28). On the other hand, IFN-γ has also been found to be an early mediator of protection in mice experimentally infected with B. pseudomallei, since neutralizing monoclonal antibody against this cytokine increased susceptibility to infection (35). Nevertheless, high and uncontrolled production of proinflammatory cytokines may lead to host illness, septic shock, and death. The DOTAP-plus-CpG ODN intervention in the present study was found to not only extend the protective effect of CpG ODN (Fig. 1) but to also attenuate the levels of IFN-γ after infection (Fig. 2), similar to those of CpG ODN in our previous report (47). These low IFN-γ levels might be due to the low number of bacteria in DOTAP-plus-CpG-ODN-treated animals. This hypothesis correlated with the low bacteremia found on day 2 in the CpG ODN and DOTAP-plus-CpG ODN groups (Fig. 1B). Although the percentages of survival of the DOTAP-alone and PBS control groups were different (Fig. 1), the IFN-γ levels were similar (Fig. 2). We hypothesize that DOTAP preactivated mouse immunity, giving some protection, leading to reduced numbers of bacteria and IFN-γ levels on days 2 and 7. The numbers of surviving animals on day 7 in the two groups was 7 and 10 in the PBS and DOTAP groups, respectively. However, this protection was not significantly different from that for the control group. It is possible that other types (e.g., type I) of IFNs may play a role in the protection observed in our study. However, it has been reported that B. pseudomallei fails to stimulate beta IFN (IFN-β) production in mouse macrophages (40).
The mechanism of action of CpG ODN in protection against B. pseudomallei has been further elucidated in this study, in particular with regard to the role of neutrophils and macrophages. Following activation, macrophages produce nitric oxide (NO), which is generated by inducible nitric oxide synthases (iNOS) (31). Our study demonstrated that macrophages from mice stimulated with CpG ODN for 2 days showed lower levels of surviving intracellular B. pseudomallei (Fig. 5A). Activated macrophages are known to produce various factors, such as NO and superoxide, that can contribute to the elimination of certain pathogens (16, 41). Our results further demonstrate the protective activity of CpG ODN or CpG ODN plus DOTAP on macrophages against B. pseudomallei via NO production. B. pseudomallei has been previously reported to be susceptible to NO (33). Bacteria can evade immune mechanisms by downregulating iNOS expression (41), and interference with iNOS production can facilitate survival of intracellular bacteria (41). It is therefore likely that CpG ODN- or CpG ODN-plus-DOTAP-stimulated mice in our study undergo macrophage activation, leading to increased killing of intracellular bacteria and increased mouse survival. In contrast, neutrophils from CpG ODN- or CpG ODN-plus-DOTAP-stimulated mice showed similar ROI and phagocytic indices compared to results for PBS controls (Fig. 3 to 4). Although CpG ODN has been shown to be capable of stimulating mouse neutrophils (42), which are important in pulmonary bacterial clearance, neutrophil stimulation was not evident in our study with CpG ODN-treated mice. However, neutrophils may contribute to the protection through early production of cytokines or chemokines (12). The MyD88 signaling pathway (which could be stimulated by CpG ODN via TLR9) was found to provide significant protection against B. pseudomallei (46). However, MyD88 knockout mice showed no difference from wild-type mice in B. pseudomallei phagocytosis and killing (46). These data indicate that neutrophil-mediated killing capacity and phagocytosis in CpG ODN-stimulated mice may not play a major role in the clearance of B. pseudomallei. Further investigation is required to elucidate the precise role of neutrophils and macrophages in bacterial clearance. Although the purity of neutrophils and macrophages used in our study was around 90%, the contributions of other cells, such as other granulocytes and dendritic cells, in the experiment cannot be excluded.
In conclusion, our study suggests a role for macrophages but not neutrophils in protection of CpG ODN- and CpG ODN-plus-DOTAP-treated mice against B. pseudomallei infection and further that CpG ODN incorporated in cationic liposomes can extend the protective window from 2 to 10 days to 30 days before B. pseudomallei challenge. Whether the protective window might possibly be extended beyond 30 days is not known, although it seems unlikely due to the generally short-lived nature of the underlying innate immune processes. Nevertheless, since a vaccine is not available, the protective effect of DOTAP plus CpG ODN may serve as an alternative approach to preventing this potentially lethal infection.
ACKNOWLEDGMENTS
This work was supported by the Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (to A.P. and S.W. [grant no. PHD/0007/2548]), the Commission on Higher Education (CHE) Thailand, Melioidosis Research Center, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand, and Office of the Higher Education Commission, through the Health Cluster (SheP-GMS), Khon Kaen University.
Footnotes
Published ahead of print 21 March 2012
REFERENCES
- 1. Ashdown LR. 1979. An improved screening technique for isolation of Pseudomonas pseudomallei from clinical specimens. Pathology 11:293–297 [DOI] [PubMed] [Google Scholar]
- 2. Boxio R, Bossenmeyer-Pourie C, Steinckwich N, Dournon C, Nusse O. 2004. Mouse bone marrow contains large numbers of functionally competent neutrophils. J. Leukoc. Biol. 75:604–611 [DOI] [PubMed] [Google Scholar]
- 3. Breitbach K, et al. 2006. Role of inducible nitric oxide synthase and NADPH oxidase in early control of Burkholderia pseudomallei infection in mice. Infect. Immun. 74:6300–6309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Breitbach K, Kohler J, Steinmetz I. 2008. Induction of protective immunity against Burkholderia pseudomallei using attenuated mutants with defects in the intracellular life cycle. Trans. R. Soc. Trop. Med. Hyg. 102(Suppl. 1):S89–S94 [DOI] [PubMed] [Google Scholar]
- 5. Cheng AC, Currie BJ. 2005. Melioidosis: epidemiology, pathophysiology, and management. Clin. Microbiol. Rev. 18:383–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chua KL, Chan YY, Gan YH. 2003. Flagella are virulence determinants of Burkholderia pseudomallei. Infect. Immun. 71:1622–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cowdery JS, Chace JH, Yi AK, Krieg AM. 1996. Bacterial DNA induces NK cells to produce IFN-gamma in vivo and increases the toxicity of lipopolysaccharides. J. Immunol. 156:4570–4575 [PubMed] [Google Scholar]
- 8. Currie BJ, Fisher DA, Anstey NM, Jacups SP. 2000. Melioidosis: acute and chronic disease, relapse and re-activation. Trans. R. Soc. Trop. Med. Hyg. 94:301–304 [DOI] [PubMed] [Google Scholar]
- 9. Das M, Chopra AK, Cantu JM, Peterson JW. 1998. Antisera to selected outer membrane proteins of Vibrio cholerae protect against challenge with homologous and heterologous strains of V. cholerae. FEMS Immunol. Med. Microbiol. 22:303–308 [DOI] [PubMed] [Google Scholar]
- 10. Deng JC, et al. 2004. CpG oligodeoxynucleotides stimulate protective innate immunity against pulmonary Klebsiella infection. J. Immunol. 173:5148–5155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Druar C, et al. 2008. Evaluating Burkholderia pseudomallei Bip proteins as vaccines and Bip antibodies as detection agents. FEMS Immunol. Med. Microbiol. 52:78–87 [DOI] [PubMed] [Google Scholar]
- 12. Easton A, Haque A, Chu K, Lukaszewski R, Bancroft GJ. 2007. A critical role for neutrophils in resistance to experimental infection with Burkholderia pseudomallei. J. Infect. Dis. 195:99–107 [DOI] [PubMed] [Google Scholar]
- 13. Easton A, et al. 2011. Combining vaccination and post exposure CpG therapy provides optimal protection against lethal sepsis in a biodefense model of human melioidosis. J. Infect. Dis. 204:636–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Egan AM, Gordon DL. 1996. Burkholderia pseudomallei activates complement and is ingested but not killed by polymorphonuclear leukocytes. Infect. Immun. 64:4952–4959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elkins KL, Rhinehart-Jones TR, Stibitz S, Conover JS, Klinman DM. 1999. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J. Immunol. 162:2291–2298 [PubMed] [Google Scholar]
- 16. Gao JJ, et al. 1999. Cutting edge: bacterial DNA and LPS act in synergy in inducing nitric oxide production in RAW 264.7 macrophages. J. Immunol. 163:4095–4099 [PubMed] [Google Scholar]
- 17. Gursel M, Tunca S, Ozkan M, Ozcengiz G, Alaeddinoglu G. 1999. Immunoadjuvant action of plasmid DNA in liposomes. Vaccine 17:1376–1383 [DOI] [PubMed] [Google Scholar]
- 18. Haque A, et al. 2006. A live experimental vaccine against Burkholderia pseudomallei elicits CD4+ T cell-mediated immunity, priming T cells specific for 2 type III secretion system proteins. J. Infect. Dis. 194:1241–1248 [DOI] [PubMed] [Google Scholar]
- 19. Hara Y, Mohamed R, Nathan S. 2009. Immunogenic Burkholderia pseudomallei outer membrane proteins as potential candidate vaccine targets. PLoS One 4:e6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harland DN, et al. 2007. Identification of a LolC homologue in Burkholderia pseudomallei, a novel protective antigen for melioidosis. Infect. Immun. 75:4173–4180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoppe I, et al. 1999. Characterization of a murine model of melioidosis: comparison of different strains of mice. Infect. Immun. 67:2891–2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jaafari MR, et al. 2006. Immune response and protection assay of recombinant major surface glycoprotein of Leishmania (rgp63) reconstituted with liposomes in BALB/c mice. Vaccine 24:5708–5717 [DOI] [PubMed] [Google Scholar]
- 23. Jones SM, Ellis JF, Russell P, Griffin KF, Oyston PC. 2002. Passive protection against Burkholderia pseudomallei infection in mice by monoclonal antibodies against capsular polysaccharide, lipopolysaccharide or proteins. J. Med. Microbiol. 51:1055–1062 [DOI] [PubMed] [Google Scholar]
- 24. Kirby C, Gregoriadis G. 1984. Dehydration-rehydration vesicles: a simple method for high yield drug entrapment in liposomes. Biotechnology 2:979–984 [Google Scholar]
- 25. Klinman DM, Currie D, Gursel I, Verthelyi D. 2004. Use of CpG oligodeoxynucleotides as immune adjuvants. Immunol. Rev. 199:201–216 [DOI] [PubMed] [Google Scholar]
- 26. Krieg AM. 2002. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20:709–760 [DOI] [PubMed] [Google Scholar]
- 27. Krieg AM, Love-Homan L, Yi AK, Harty JT. 1998. CpG DNA induces sustained IL-12 expression in vivo and resistance to Listeria monocytogenes challenge. J. Immunol. 161:2428–2434 [PubMed] [Google Scholar]
- 28. Lauw FN, et al. 1999. Elevated plasma concentrations of interferon (IFN)-gamma and the IFN-gamma-inducing cytokines interleukin (IL)-18, IL-12, and IL-15 in severe melioidosis. J. Infect. Dis. 180:1878–1885 [DOI] [PubMed] [Google Scholar]
- 29. Leelarasamee A, Bovornkitti S. 1989. Melioidosis: review and update. Rev. Infect. Dis. 11:413–425 [DOI] [PubMed] [Google Scholar]
- 30. Limmathurotsakul D, et al. 2010. Increasing incidence of human melioidosis in Northeast Thailand. Am. J. Trop. Med. Hyg. 82:1113–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. MacMicking J, Xie QW, Nathan C. 1997. Nitric oxide and macrophage function. Annu. Rev. Immunol. 15:323–350 [DOI] [PubMed] [Google Scholar]
- 32. McCluskie MJ, Davis HL. 2000. Oral, intrarectal and intranasal immunizations using CpG and non-CpG oligodeoxynucleotides as adjuvants. Vaccine 19:413–422 [DOI] [PubMed] [Google Scholar]
- 33. Miyagi K, Kawakami K, Saito A. 1997. Role of reactive nitrogen and oxygen intermediates in gamma interferon-stimulated murine macrophage bactericidal activity against Burkholderia pseudomallei. Infect. Immun. 65:4108–4113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rozak DA, et al. 2010. CpG oligodeoxyribonucleotides protect mice from Burkholderia pseudomallei but not Francisella tularensis Schu S4 aerosols. J. Immune Based Ther. Vaccines 8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Santanirand P, Harley VS, Dance DA, Drasar BS, Bancroft GJ. 1999. Obligatory role of gamma interferon for host survival in a murine model of infection with Burkholderia pseudomallei. Infect. Immun. 67:3593–3600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sarkar-Tyson M, et al. 2007. Polysaccharides and virulence of Burkholderia pseudomallei. J. Med. Microbiol. 56:1005–1010 [DOI] [PubMed] [Google Scholar]
- 37. Stevens MP, et al. 2004. Attenuated virulence and protective efficacy of a Burkholderia pseudomallei bsa type III secretion mutant in murine models of melioidosis. Microbiology 150:2669–2676 [DOI] [PubMed] [Google Scholar]
- 38. Suzuki Y, et al. 2004. Liposome-encapsulated CpG oligodeoxynucleotides as a potent adjuvant for inducing type 1 innate immunity. Cancer Res. 64:8754–8760 [DOI] [PubMed] [Google Scholar]
- 39. Torchilin VP. 2005. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 4:145–160 [DOI] [PubMed] [Google Scholar]
- 40. Utaisincharoen P, Anantagool N, Limposuwan K, Chaisuriya P, Sirisinha S. 2003. Involvement of beta interferon in enhancing inducible nitric oxide synthase production and antimicrobial activity of Burkholderia pseudomallei-infected macrophages. Infect. Immun. 71:3053–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Utaisincharoen P, Tangthawornchaikul N, Kespichayawattana W, Chaisuriya P, Sirisinha S. 2001. Burkholderia pseudomallei interferes with inducible nitric oxide synthase (iNOS) production: a possible mechanism of evading macrophage killing. Microbiol. Immunol. 45:307–313 [DOI] [PubMed] [Google Scholar]
- 42. Weighardt H, et al. 2000. Increased resistance against acute polymicrobial sepsis in mice challenged with immunostimulatory CpG oligodeoxynucleotides is related to an enhanced innate effector cell response. J. Immunol. 165:4537–4543 [DOI] [PubMed] [Google Scholar]
- 43. Weiser JN, Gotschlich EC. 1991. Outer membrane protein A (OmpA) contributes to serum resistance and pathogenicity of Escherichia coli K-1. Infect. Immun. 59:2252–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. White NJ. 2003. Melioidosis. Lancet 361:1715–1722 [DOI] [PubMed] [Google Scholar]
- 45. Wiersinga WJ, van der Poll T. 2009. Immunity to Burkholderia pseudomallei. Curr. Opin. Infect. Dis. 22:102–108 [DOI] [PubMed] [Google Scholar]
- 46. Wiersinga WJ, Wieland CW, Roelofs JJ, van der Poll T. 2008. MyD88 dependent signaling contributes to protective host defense against Burkholderia pseudomallei. PLoS One 3:e3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wongratanacheewin S, et al. 2004. Immunostimulatory CpG oligodeoxynucleotide confers protection in a murine model of infection with Burkholderia pseudomallei. Infect. Immun. 72:4494–4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yamamoto S, et al. 1992. Unique palindromic sequences in synthetic oligonucleotides are required to induce IFN and augment IFN-mediated natural killer activity. J. Immunol. 148:4072–4076 [PubMed] [Google Scholar]