Abstract
The performance of the QuantiFERON-cytomegalovirus (CMV) assay was compared to that of a flow cytometry intracellular cytokine staining (ICS) method for the detection of CMV-specific gamma interferon (IFN-γ)-producing CD8+ T-cell responses in allogeneic stem cell transplant (allo-SCT) recipients and for estimations of their magnitude and functionality. A total of 90 whole-blood specimens from 23 allo-SCT recipients was analyzed by both methods. Overall, the percentage of specimens that yielded concordant results by both methods was 68.8% (κ = 0.691; 95% confidence interval [CI], 0.548 to 0.835), and the sensitivity of the QuantiFERON-CMV assay for the detection of positive IFN-γ T-cell responses (>0.2 IU/ml), taking the ICS method as the reference, was 76.3%. The magnitude of IFN-γ-producing CD8+ T-cell responses to CMV-specific peptides measured with the QuantiFERON-CMV assay correlated significantly (σ = 0.695; P = <0.001) with that of the total IFN-γ-producing CD8+ T cells and dual-functional (IFN-γ/tumor necrosis factor alpha [TNF-α] [σ = 0.652; P = <0.001] and IFN-γ/CD107a [σ = 0.690; P = <0.001]) and trifunctional (IFN-γ/TNF-α/CD107a [σ = 0.679; P = >0.001]) CMV-specific CD8+ T-cell responses, as quantitated by ICS. In summary, the data indicated that the QuantiFERON-CMV assay is less sensitive than the ICS method for the detection of CMV-specific IFN-γ-producing CD8+ T-cell responses in the allo-SCT setting. Nevertheless, it allowed the estimation of the total and polyfunctional CMV-specific IFN-γ-producing CD8+ T-cell responses in specimens that tested positive by both methods.
INTRODUCTION
The assessment of the magnitude and the functionality of T-cell immunity against cytomegalovirus (CMV) is emerging as a clinically useful tool for the therapeutic management of CMV infection in the allogeneic stem cell transplantation (allo-SCT) setting (7, 13). The monitoring of CMV-specific CD8+ or CD4+ T-cell responses may allow for the optimization of preemptive antiviral therapy regimens on an individual basis and the identification of patients who may benefit from prophylactic antiviral or adoptive T-cell transfer therapeutic strategies (1, 7, 13). In recent years, several methods have been developed for the ex vivo quantitation and functional characterization of T-cell responses, among which flow cytometry for surface immunophenotyping and intracellular cytokine staining (ICS) are currently considered the “gold standard” (13). The QuantiFERON-CMV assay (Cellestis Ltd., Melbourne, Australia) is a commercially available test that allows the inference of the size of the CMV-specific T-cell response by quantitating the level of gamma interferon (IFN-γ), produced mostly by CMV-specific CD8+ T cells, upon the stimulation of whole blood with a number of immunogenic peptides mapped within IE-1, IE-2, pp65, pp50, and gB and restricted by several widespread HLA-I haplotypes (17). The performance of the QuantiFERON-CMV assay has been assessed mostly in the solid-organ transplant (SOT) setting (see reference 4 for a review). Recently reported data, although preliminary, lend support to the suitability of the QuantiFERON-CMV assay for the monitoring of the CMV-specific IFN-γ-producing CD8+ T-cell responses in allo-SCT recipients (2). Nevertheless, it is largely unknown how this method compares to ICS assays for such a purpose. In previous studies, we reported the development and clinical utility of an ICS assay for the quantitation of CMV-specific IFN-γ-producing CD8+ and CD4+ T cells (11, 12, 14–16). The assay was found to reliably predict protection from the development of CMV DNAemia in allo-SCT recipients, and it was recently used in the setting of a novel strategy for the guidance of preemptive antiviral therapy based on combined virological and immunological monitoring (12). In the current study, we compared the performance of the QuantiFERON-CMV assay with that of our ICS method for the detection and quantitation of CMV-specific IFN-γ-producing CD8+ T-cell responses in allo-SCT recipients.
MATERIALS AND METHODS
Patients and specimens.
A total of 90 whole-blood samples, obtained from 23 nonconsecutive patients (median age, 53 years [range, 20 to 71 years]; 16 males and 7 females) who underwent peripheral blood (n = 20) or umbilical cord blood (n = 3) allo-SCT at the Hospital Clínico Universitario of Valencia, Spain, between February 2010 and June 2011, was analyzed in this study. The underlying diseases of the patients were acute myeloid leukemia (n = 10), non-Hodgkin's lymphoma (n = 7), chronic lymphocytic leukemia (n = 2), multiple myeloma (n = 2), or myelodysplastic syndrome (n = 1). The types of transplant were related/matched (n = 10), unrelated/matched (n = 9), unrelated/mismatched (n = 3), and related/mismatched (n = 1). The conditioning regimen was nonmyeloablative (n = 17) or myeloablative (n = 6), and the paired CMV serostatuses of the donors (D) and recipients (R) were D+/R+ (n = 9), D−/R+ (n = 9), D+/R− (n = 2), and D−/R− (n = 3). The study was approved by the Ethics Committees. All patients gave their informed consent to participate in the study.
CMV serology.
The CMV serological testing of the donors and recipients was performed by use of the DiaSorin Liaison CMV IgG assay (DiaSorin, Saluggia, Italy) according to the recommendations of the manufacturer.
CMV plasma DNAemia quantitation.
The CMV DNA load in the plasma was quantitated by real-time PCR (Abbott CMV PCR kit; produced by Qiagen GmbH, Hilde, Germany, for Abbott Diagnostics, Des Plaines, IL). The PCRs were performed by using the m2000RT system (Abbott Molecular, IL), as previously described (3). The DNA extractions were performed by using 500 μl of plasma on an m24 SP instrument (Abbott Diagnostics, IL).
Enumeration of CMV-specific IFN-γ-producing CD8+ T cells.
The quantitation of the total numbers of CMV-specific IFN-γ-producing CD8+ T cells and selectively bifunctional (IFN-γ/tumor necrosis factor alpha [TNF-α] and IFN-γ/CD107a) and trifunctional (IFN-γ/TNF-α/CD107a) CMV-specific IFN-γ-producing CD8+ T cells was performed by ICS. Whole blood (0.5 ml) was simultaneously stimulated with two sets of 15-mer overlapping peptides encompassing the sequence of the pp65 and IE-1 CMV proteins (1 μg/ml/peptide), obtained from JPT Peptide Technologies GmbH (Berlin, Germany), in the presence of 1 μg/ml of costimulatory monoclonal antibodies (MAbs) to CD28 and CD49d, an anti-CD107a MAb coupled with allophycocyanin (APC), brefeldin (5 μg/ml), and monensin (1 μM) for 6 h at 37°C. The cells were washed in phosphate-buffered saline (PBS)–2% fetal calf serum (FCS), lysed in BD FACS lysis solution, stained with surface markers (anti-CD8-peridinin chlorophyll protein [PerCP]–Cy5-5 and anti-CD3-APC-Cy7), permeabilized (BD FACS permeabilizing solution 2), washed, and finally stained for intracellular cytokines (anti-IFN-γ–fluorescein isothiocyanate [FITC] and anti-TNF-α–phycoerythrin [PE]). All antibodies and solutions were purchased from Becton Dickinson (San Jose, CA). The cells were stored at 4°C in PBS–1% formaldehyde, acquired within 4 h with a BD FCSCantoII flow cytometer (BD Biosciences Immunocytometry Systems, San Jose, CA), and analyzed with the software program Infinicyt (Cytognos, Salamanca, Spain). The negative controls (absence of peptide stimulation) were processed in parallel for all experiments. The total number of each CD8+ T-cell subpopulation was calculated by multiplying the corresponding percentage of CMV-specific T cells (after background subtraction) by the absolute number of CD8+ T cells. Specific responses were considered those that were >0.1% for each population. CMV-specific IFN-γ-producing CD8+ T cells were enumerated in parallel by using the BD Fastimmune kit (BD-Becton Dickinson and Company Biosciences, San Jose, CA) as previously described (11, 12, 14–16) but employing the BD FCSCantoII flow cytometer and the software program Infinicyt for the analyses. The levels of IFN-γ-producing CD8+ T cells (total number) measured by the two above-described procedures were comparable (data not shown).
QuantiFERON-CMV assay.
The QuantiFERON-CMV assay (Cellestis Ltd., Melbourne, Australia) was performed according to the manufacturer's instructions. Briefly, 1-ml aliquots of heparinized whole blood were collected into three QuantiFERON-CMV collection tubes, one containing a number of CMV immunogenic peptides (CMV antigen), another with no peptides (nil control), and the other containing a polyclonal stimulating antigen (phytohemagglutinin) (mitogen control). The tubes were shaken vigorously for 5 s and then incubated for 18 to 24 h at 37°C. The supernatants were then harvested, and the levels of IFN-γ were measured by an enzyme-linked immunosorbent assay (ELISA). A standard curve was generated for each run. The results of the assay were interpreted according to the criteria established by the manufacturer: (i) nonreactive at <0.2 IU/ml (CMV minus nil) and ≥0.5 IU/ml (mitogen minus nil), (ii) reactive at ≥0.2 IU/ml (CMV minus nil) and any value of mitogen minus nil, and (iii) indeterminate at <0.2 IU/ml (CMV minus nil) and <0.5 IU/ml (mitogen minus nil). According to the manufacturer, indeterminate results are not interpretable.
Statistical analysis.
The data were analyzed with the aid of the statistical package SPSS, version 17.0 (SPSS, North Chicago, IL). Differences between the medians were compared by using the Mann-Whitney U test. Spearman's rank test was used to analyze correlations between continuous variables. Two-sided exact P values are reported. A P value of <0.05 was considered statistically significant.
RESULTS
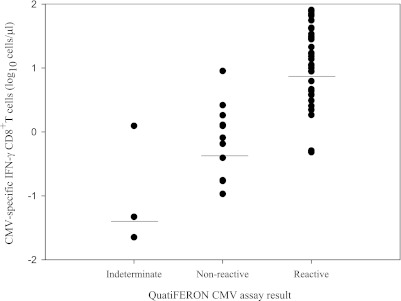
A total of 90 blood samples from 23 allo-SCT recipients was analyzed by ICS and by the QuantiFERON-CMV assay. The data are summarized in Table 1. Fifty-five samples (61.1%) from 17 patients had CMV-specific IFN-γ-producing CD8+ T-cell responses detectable by ICS; of these, 42 samples (76.3%) from 13 patients were reactive (>0.2 IU/ml), and 13 samples (from 4 patients) yielded negative (n = 10) or indeterminate (n = 3) results by the QuantiFERON-CMV assay. As shown in Fig. 1, specimens that tested positive by the QuantiFERON-CMV assay displayed significantly higher (P = 0.001) numbers of CMV-specific IFN-γ-producing CD8+ T cells, as measured by ICS, than those that tested negative or indeterminate. As shown in Table 1, 35 specimens from six patients lacked detectable CMV-specific IFN-γ-producing CD8+ T-cell responses, as determined by ICS. Of these, only one sample yielded a positive result by the QuantiFERON-CMV assay (2.5 IU/ml). All patients (and/or their donors) with negative results by the QuantiFERON-CMV assays throughout the study period displayed at least one HLA-I specificity able to present one of the immunogenic peptides included in the QuantiFERON-CMV assay pool (data not shown). A large number of specimens gave indeterminate results by the QuantiFERON-CMV assay (n = 17; 18.8%); most of these samples (n = 14) had no detectable CMV-specific IFN-γ-producing CD8+ T-cell responses, as measured by ICS. Overall, the percentage of specimens that yielded concordant results by both methods was 68.8% (κ = 0.691; 95% confidence interval [CI], 0.548 to 0.835), and the sensitivity of the QuantiFERON-CMV assay for the detection of positive IFN-γ T-cell responses, as determined by ICS, was 76.3%. Three specimens that tested positive by the ICS assay displayed IFN-γ levels within the range of >0.1 to <0.2 IU/ml in the QuantiFERON-CMV assay. Thus, a decrease of the cutoff level for a positive result to >0.1 IU/ml resulted in a slight increase in the sensitivity of the QuantiFERON-CMV assay (81.8%). Of note was the fact that 15 out of the 42 specimens that tested positive by the QuantiFERON-CMV assay gave optical density values above the upper limit of quantification of the assay and thus had to be further diluted in order to determine precisely the levels of IFN-γ.
Table 1.
Detection of cytomegalovirus-specific IFN-γ-producing CD8+ T cells by ICS and the QuantiFERON-CMV assay in whole-blood specimens from allogeneic stem cell transplant recipients
| Result of ICS method (no. of specimens) | No. of specimens with QuantiFERON-CMV assay result of: |
||
|---|---|---|---|
| Positive | Negative | Indeterminate | |
| Positive (55) | 42 | 10 | 3 |
| Negative (35) | 1 | 20 | 14 |
Fig 1.
Number of cytomegalovirus pp65/IE-1-specific IFN-γ-producing CD8+ T cells (total) enumerated by flow cytometry for intracellular cytokine staining (ICS) of specimens that tested positive, negative, or intermediate by the QuantiFERON-CMV assay. The data are given as log10 values; each dot represents a single measurement, and the bars represent median values.
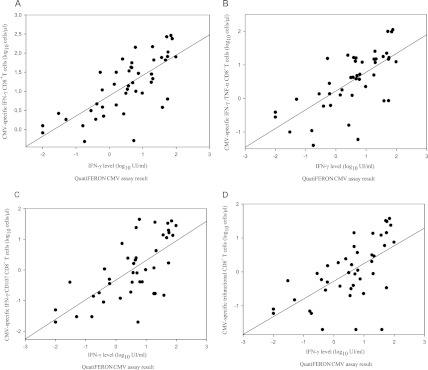
We next assessed to what extent the QuantiFERON-CMV assay allowed size and functional diversity estimations of CMV-specific IFN-γ-producing CD8+ T-cell responses, as determined by ICS. For this, we performed an analysis of the correlation of the levels of IFN-γ measured by the QuantiFERON-CMV assay with the number of different functional populations of IFN-γ-producing CD8+ T cells quantitated by the ICS assay for 42 specimens that showed detectable responses by both methods. Note that 20 out of these 42 specimens were obtained during episodes of nontreated (n = 11 from 3 patients) or ganciclovir-treated (n = 9 from 2 patients) CMV plasma DNAemia. In addition, the remaining 22 specimens were obtained from eight patients who had a prior episode of CMV DNAemia. As shown in Fig. 2, the magnitude of the IFN-γ responses to the CMV-specific peptides measured with the QuantiFERON-CMV assay was significantly correlated (σ = 0.695; P = <0.0001) with that quantitated by ICS for the total IFN-γ-producing CD8+ T cells. Furthermore, the IFN-γ levels determined by the QuantiFERON-CMV assay were also significantly correlated with the number of dual-functional (σ = 0.652 and P = <0.001 for IFN-γ/TNF-α; σ = 0.690 and P = <0.0001 IFN-γ/CD107a) and trifunctional (σ = 0.679 and P = <0.0001 for IFN-γ/TNF-α/CD107a) CMV-specific CD8+ T cells.
Fig 2.
Correlation between the number (log10) of pp65/IE-1-specific IFN-γ-producing CD8+ T cells enumerated by flow cytometry for intracellular cytokine staining (ICS) and IFN-γ levels (log10) measured by the QuantiFERON-CMV assay. The data for 42 whole-blood specimens (from 13 allogeneic stem cell transplant recipients) that tested positive by both methods are depicted. (A) Total number of IFN-γ-producing CD8+ T cells; (B) dual-IFN-γ/TNF-α CD8+ T cells; (C) dual-IFN-γ/CD107a CD8+ T cells; (D) trifunctional (IFN-γ/TNF-α/CD107a) CD8+ T cells.
DISCUSSION
The QuantiFERON-CMV assay is the only commercially available method for the measurement of CMV-specific IFN-γ-producing CD8+ T-cell responses. This assay was previously evaluated with SOT and allo-SCT recipients (2, 4, 5, 8, 10, 17, 18). Preliminary data supported the clinical utility of this method for the assessment of the risk of late-onset CMV end-organ disease in SOT recipients (5) and for the prediction of the occurrence of CMV DNAemia in allo-SCT patients (2). Nevertheless, information on how the QuantiFERON-CMV assay correlates with ICS methods for estimations of the magnitude and assessments of the functionality of CMV-specific CD8+ T-cell responses in transplant recipients is still scarce. In the current study, we compared the performance of the QuantiFERON-CMV assay with that of an ICS method developed by our group that has been proven to be clinically useful in the management of active CMV infection in the allo-SCT setting (11, 12, 14–16). Overall, we found both methods to yield concordant qualitative results for around 70% of specimens. This finding was not entirely unexpected, as 18 out of the 21 peptides included in the CMV tube of the QuantiFERON-CMV assay map within pp65 (n = 15) and IE-1 (n = 3). When the results of the QuantiFERON-CMV assay were interpreted as indicated by the manufacturers (positive responses if IFN-γ levels were >0.2 IU/ml), the sensitivity of the assay, considering the ICS method as the reference assay, was 76.37%. The sensitivity of the assay slightly improved (81.8%) when the cutoff threshold for positive IFN-γ responses was lowered to 0.1 IU/ml, as previously suggested (5). All patients (and/or their donors) who tested negative by the QuantiFERON-CMV assay throughout the study period displayed at least one HLA-I variant included among the specificities covered by the test. The specificity of the QuantiFERON-CMV assay approached 100%. In fact, there was only one specimen that gave a positive result by the QuantiFERON-CMV assay (2.5 IU/ml) but that tested negative by the ICS method; this could have been a false-positive result, since a follow-up specimen obtained 3 days later from the same patient tested negative by both assays. The QuantiFERON-CMV assay gave a large number of indeterminate results. This observation is in keeping with previously reported data (2). Most of the specimens that yielded indeterminate results (14 out of 17) and those that tested negative had undetectable CMV-specific IFN-γ-producing CD8+ T-cell responses, as measured by ICS.
The levels of IFN-γ measured by the QuantiFERON-CMV assay were found to correlate significantly with the total number of CMV-specific IFN-γ-producing CD8+ T cells quantitated by the ICS assay. This observation is in accordance with the data reported in a recent study in which the QuantiFERON-CMV assay was compared to an ICS method using a library of CMV peptides mapped within pp65 as the stimulating antigen (2).
Recent data seem to favor the idea that polyfunctional rather than monofunctional CMV-specific CD8+ T cells, in particular those with the ability to produce cytokines with antiviral properties, such as IFN-γ and TNF-α, and to simultaneously display cytotoxic activity (cells expressing CD107a), are crucial for protection from and the resolution of episodes of active CMV infection in the allo-SCT setting (6, 10, 19). The QuantiFERON-CMV assay cannot distinguish between different functional CD8+ T-cell populations producing IFN-γ in response to CMV replication. In this context, we were interested to assess to what extent the QuantiFERON-CMV assay could allow the estimation of levels of CMV-specific polyfunctional IFN-γ-producing CD8+ T cells. We found that IFN-γ levels determined by the QuantiFERON-CMV assay significantly correlated with the number of CMV-specific dual-functional (IFN-γ/TNF-α and IFN-γ/CD107a) and trifunctional (IFN-γ/TNF-α/CD107a) CD8+ T cells. It should be stressed that the above-described correlations were observed in a very precise virological setting, i.e., in patients with an ongoing episode of active CMV infection or in patients who had recently resolved an episode of CMV DNAemia. In this context, we observed that the expansion and contraction of monofunctional and polyfunctional CMV-specific IFN-γ-producing CD8+ T-cell responses elicited by CMV replication followed different kinetic patterns and also showed wide variations on an individual basis (9). It is thus likely that the ability of the QuantiFERON-CMV assay to accurately estimate the size of CMV-specific polyfunctional IFN-γ-producing CD8+ T-cell responses may ultimately depend on the past and current status of CMV replication. The current study has two main limitations: first, both assays are not entirely comparable, as they are of distinct natures and, most importantly, employ different stimulating antigens (Table 2), and second, the ICS method taken as the reference standard for the measurement of CMV-specific CD8+ T-cell responses in the current study lacked extensive interlaboratory validation. Despite these limitations, we are convinced that studies to compare the performance of the QuantiFERON-CMV assay with that of ICS methods with proven reliability to assess and quantitate CMV-specific IFN-γ-producing CD8+ T-cell responses conferring protection against CMV infection are of major clinical interest.
Table 2.
Characteristics, advantages, and limitations of the ICS method used in the current study and the QuantiFERON-CMV assay for evaluation of CMV-specific T-cell immunity
| Method | Specimen/vol (ml) | Time (h) to available results | CMV antigens tested | Knowledge of HLA genotype | Differentiation between CD8+ and CD4+ T cells | Functional analysis of T cells | Phenotypic analysis of T cells | Major advantages | Major limitations |
|---|---|---|---|---|---|---|---|---|---|
| ICS | Whole blood/1 | 8–10 | Two peptide pools consisting of 15-mer overlapping peptides spanning the entire sequences of pp65 and IE-1 | Not necessary | Yes | Yes | Yes | High sensitivity, precise enumeration of CMV-specific T cells, proven clinical utility | Lack of standardization, need for access to a flow cytometer |
| QuantiFERON-cytomegalovirus (CMV) assay | Whole blood/3 | 24–48 | 21 peptides mapped within IE-1, IE-2, pp65, pp50, and gB and restricted by several widespread HLA-I variants (HLA-A1, -A2, -A3, and -A24 and HLA-B7, -B8, -B27, -B35, -B44, and -B52) | Not necessary | No (detects mostly CD8+ T cells) | Yes (IFN-γ only) | No | Highly standardized, can be performed in any center, simple to perform | Suboptimal sensitivity (76%, taking ICS as the reference procedure and a threshold of 0.2 IU/ml, as recommended by the manufacturer), high percentage (∼20%) of indeterminate specimens, need for dilution of ∼30% of positive samples for precise quantitation, lack of studies demonstrating clinical utility in the allo-SCT setting |
Table 2 summarizes the characteristics, advantages, and limitations of the QuantiFERON-CMV assay and the ICS method developed by our group. In summary, the data presented here indicated that the QuantiFERON-CMV assay, although less sensitive than our ICS method, allowed the estimation of total and polyfunctional CMV-specific IFN-γ-producing CD8+ T-cell responses in specimens that tested positive by both methods. We wonder whether the use of overlapping pp65 and IE-1 peptide pools in the QuantiFERON-CMV assay instead of a mix of immunogenic CMV peptides would increase the sensitivity of the method. Further studies should be undertaken to confirm our observations and, most importantly, to assess the clinical utility of the QuantiFERON-CMV assay in this setting. In this context, it seems to be essential to establish threshold levels of IFN-γ associated with protection from or the resolution of episodes of active CMV infection and CMV end-organ disease.
ACKNOWLEDGMENTS
We thank Matilde Pastor and Amanda Mataix for their technical assistance. The QuantiFERON-CMV assay reagents were kindly provided by Alere (Barcelona, Spain).
This research study was supported by a grant (09/1117) from the FIS (Fondo de Investigaciones Sanitarias, Ministerio de Sanidad y Consumo, Spain).
Footnotes
Published ahead of print 29 February 2012
REFERENCES
- 1. Avetisyan G, Aschan J, Hägglund H, Ringdén O, Ljungman P. 2007. Evaluation of intervention strategy based on CMV-specific immune responses after allogeneic SCT. Bone Marrow Transplant. 40:865–869 [DOI] [PubMed] [Google Scholar]
- 2. Fleming T, Dunne J, Crowley B. 2010. Ex vivo monitoring of human cytomegalovirus-specific CD8(+) T-cell responses using the QuantiFERON-CMV assay in allogeneic hematopoietic stem cell transplant recipients attending an Irish hospital. J. Med. Virol. 82:433–440 [DOI] [PubMed] [Google Scholar]
- 3. Gimeno C, et al. 2008. Quantification of DNA in plasma by an automated real-time PCR assay (cytomegalovirus PCR kit) for surveillance of active cytomegalovirus infection and guidance of preemptive therapy for allogeneic hematopoietic stem cell transplant recipients. J. Clin. Microbiol. 46:3311–3318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Giulieri S, Manuel O. 2011. QuantiFERON-CMV assay for the assessment of cytomegalovirus cell-mediated immunity. Expert Rev. Mol. Diagn. 11:17–25 [DOI] [PubMed] [Google Scholar]
- 5. Kumar D, et al. 2009. Cell-mediated immunity to predict cytomegalovirus disease in high-risk solid organ transplant recipients. Am. J. Transplant. 9:1214–1222 [DOI] [PubMed] [Google Scholar]
- 6. Lacey S, et al. 2006. Functional comparison of T cells recognizing cytomegalovirus pp65 and immediate-early antigen polypeptides in hematopoietic stem-cell transplant and solid organ transplant recipients. J. Infect. Dis. 194:1410–1421 [DOI] [PubMed] [Google Scholar]
- 7. Ljungman P. 2006. Would monitoring CMV immune responses allow improved control of CMV in stem cell transplant patients. J. Clin. Virol. 35:493–495 [DOI] [PubMed] [Google Scholar]
- 8. Lochmanova A, et al. 2010. Quantiferon-CMV test in prediction of cytomegalovirus infection after kidney transplantation. Transplant. Proc. 42:3574–3577 [DOI] [PubMed] [Google Scholar]
- 9. Muñoz-Cobo B, et al. 2012. Functional profile of cytomegalovirus (CMV)-specific CD8+ T cells and kinetics of NKG2C+ NK cells associated with the resolution of CMV DNAemia in allogeneic stem cell transplant recipients. J. Med. Virol. 84:259–267 [DOI] [PubMed] [Google Scholar]
- 10. Nebbia G, et al. 2008. Polyfunctional cytomegalovirus-specific CD4+ and pp65 CD8+ T cells protect against high-level replication after liver transplantation. Am. J. Transplant. 8:2590–2599 [DOI] [PubMed] [Google Scholar]
- 11. Solano C, et al. 2008. Enumeration of cytomegalovirus-specific interferongamma CD8+ and CD4+ T cells early after allogeneic stem cell transplantation may identify patients at risk of active cytomegalovirus infection. Haematologica 93:1434–1436 [DOI] [PubMed] [Google Scholar]
- 12. Solano C, et al. 2011. Immunological monitoring for guidance of preemptive antiviral therapy for active cytomegalovirus infection in allogeneic stem-cell transplant recipients: a pilot experience. Transplantation 92:e17–e20 [DOI] [PubMed] [Google Scholar]
- 13. Solano C, Navarro D. 2010. Clinical virology of cytomegalovirus infection following hematopoietic transplantation. Future Virol. 5:111–124 [Google Scholar]
- 14. Tormo N, et al. 17 January 2011, posting date Reconstitution of CMV pp65 and IE-1-specific IFN-γ CD8(+) and CD4(+) T-cell responses affording protection from CMV DNAemia following allogeneic hematopoietic SCT. Bone Marrow Transplant. [Epub ahead of print.] doi: 10.1038/bmt.2010.330 [DOI] [PubMed] [Google Scholar]
- 15. Tormo N, et al. 2010. Lack of prompt expansion of cytomegalovirus pp65 and IE-1-specific IFNgamma CD8+ and CD4+ T cells is associated with rising levels of pp65 antigenemia and DNAemia during pre-emptive therapy in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 45:543–549 [DOI] [PubMed] [Google Scholar]
- 16. Tormo N, et al. 2010. Kinetics of cytomegalovirus (CMV) pp65 and IE-1-specific IFNgamma CD8+ and CD4+ T cells during episodes of viral DNAemia in allogeneic stem cell transplant recipients: potential implications for the management of active CMV infection. J. Med. Virol. 82:1208–1215 [DOI] [PubMed] [Google Scholar]
- 17. Walker S, et al. 2007. Ex vivo monitoring of human cytomegalovirus-specific CD8+ T-cell responses using QuantiFERON-CMV. Transpl. Infect. Dis. 9:165–170 [DOI] [PubMed] [Google Scholar]
- 18. Westall GP, Mifsud NA, Kotsimbos T. 2008. Linking CMV serostatus to episodes of CMV reactivation following lung transplantation by measuring CMV-specific CD8+ T-cell immunity. Am. J. Transplant. 8:1749–1754 [DOI] [PubMed] [Google Scholar]
- 19. Zhou W, et al. 2009. Impact of donor CMV status on viral infection and reconstitution of multifunction CMV-specific T cells in CMV-positive transplant recipients. Blood 113:6465–6476 [DOI] [PMC free article] [PubMed] [Google Scholar]