Abstract
Despite the availability of measles vaccines, infants continue to die from measles. Measles vaccine responses vary between individuals, and poor immunogenicity is likely to preclude protection against measles. CD46 is a ubiquitously expressed specific receptor for vaccine strains of measles virus. CD46 polymorphisms have not been functionally investigated but may affect CD46 protein expression, which in turn may mediate primary measles antibody responses in infants. In a cohort of children aged 12 to 14 months from Perth, Australia (n = 137), after their first dose of measles-mumps-rubella (MMR) vaccine, CD46 polymorphisms were genotyped, and postvaccination measles IgG and CD46 protein expression before and after measles lysate stimulation of cells were measured. Three CD46 variants (rs7144, rs11118580, and rs2724384) were significantly associated with measles virus-specific IgG levels (P = 0.008, P = 0.026, and P = 0.018, respectively). There were significant differences between CD46 rs7144 genotypes and CD46 protein expression on T cells, as well as the downregulation of CD46 and T-cell frequency after measles lysate stimulation. We show that CD46 polymorphisms were associated with primary measles antibody responses in naive infants. We also report the first association of a measles virus receptor polymorphism with functional effects on the receptor, suggesting a possible mechanism through which antibody responses are altered. Elucidating all of the interconnecting genetic factors that alter primary measles vaccine responses may be important for identifying children at risk of poor immunogenicity or vaccine failure and for the future design of vaccine strategies to help these children.
INTRODUCTION
Measles virus (MV) is one of the most highly transmissible pathogens in humans, infecting 30 to 40 million individuals and causing up to 164,000 deaths globally each year (2, 32). Measles vaccine is administered as two doses of a combination measles-mumps-rubella (MMR) vaccine beginning at 12 months in industrialized countries. Despite the targeted increase in vaccine coverage worldwide, measles outbreaks continue to occur and measles has not yet been eradicated.
This may be due in part to vaccine failure, where an individual does not mount a specific antibody response despite vaccination (13). As many as 10% of children do not produce a sufficiently protective response following MMR vaccination at 12 months (1). The infant immune system is immature compared to adults and older children, with an impaired ability to produce immune responses against infection and vaccination (11, 29), so it is important to determine the factors influencing primary vaccine responses in the vulnerable infant population.
Host immunogenetics is likely to be a critical factor in the modulation of vaccine responses (22), with measles vaccine antibody responses in particular shown to have high heritability (18, 28). In previously primed school children and adults, associations have been identified with human leukocyte antigen (HLA) alleles (19), cytokine and cytokine receptor genes (6), MV receptor genes (7), and Toll-like receptors (8). However, the influence of genetic variants on measles vaccine responses in naive infants immediately following their first vaccination has not been previously elucidated.
The measles cellular receptor CD46 specifically recognizes and binds to vaccine strains of the measles virus to aid its entry into the host cell (17), as well as produce an antiviral response against MV (14, 23). CD46 genetic variants may affect the interaction between CD46 and MV, leading to differences in antibody response to vaccines and possible vaccine failure and susceptibility to measles infection (5). A number of single-nucleotide polymorphisms (SNPs) have been identified in the CD46 gene; however, it is not known whether these SNPs are functional.
We sought to investigate associations between CD46 polymorphisms and measles IgG levels in a population of naive infants from Perth, Australia after their first measles vaccination. Furthermore, we investigated the associations of the CD46 polymorphisms with functional effects on receptor protein expression to perhaps suggest a mechanistic link between genetic variants and measles antibody responses.
MATERIALS AND METHODS
Study population.
Healthy unselected 12- to 14-month-old children (n = 150) were recruited at Princess Margaret Hospital for Children in Perth, Western Australia (34). All subjects received a single dose of MMR (Priorix; GlaxoSmithKline, Belgium), with (n = 48) or without (n = 102) concomitant varicella vaccine (Varilrix; GlaxoSmithKline). Prevaccination and 42 to 56 days postvaccination blood samples were taken. The data from all groups were pooled (n = 137 with both DNA and antibody data).
Genotyping and antibody assays.
DNA was extracted from whole blood by salt precipitation (16). The polymorphisms chosen to study had a minor allele frequency >10% and were in regions with the potential to alter receptor expression (i.e., 3′ untranslated region [3′UTR]) or have shown previous associations with measles responses. No exonic SNPs of sufficient frequency are found in the MV-binding areas of the CD46 protein. Two CD46 SNPs (rs7144 and rs2724384) were genotyped using PCR-restriction fragment length polymorphism (RFLP). The primers used were as follows (with underlined bases denoting deliberate mismatches to incorporate RE recognition sites): rs7144 forward (ATGGTGCGAAGTGAACACTGTAGTCTTGA) and reverse (TCTTTATTTAA GGAGGGAGAGAAAAACACAT) and rs2724384 forward (GCAAGTCCCATTTCCTCCAG) and reverse (CCATGGACTGTGGTCTGGCA). A random 10% of the samples were sequenced for confirmation of genotyping using the ABI Prism BigDye Terminator v3.1 cycle sequencing kit (PE Biosystems, Foster City, CA). The remaining polymorphisms (rs11118580, rs14374, and rs2796270) were genotyped using an iPLEX assay on a MALDI-TOF MassARRAY platform (Sequenom, Inc., San Diego, CA). Haplotypes were inferred using PHASE (27). MV- and mumps virus-specific IgG titers were measured in plasma using Enzygnost immunoassay kits (Dade Behring, Marburg, Germany). Rubella virus IgG was measured using the AxSYM Rubella IgG kit (Abbott, New South Wales, Australia). Seronegative cutoffs were determined according to the manufacturer's instructions. The sensitivity and specificity of the measles immunoassay kit as reported by the manufacturers were 99.6 and 100%, respectively.
Cell culture and flow cytometry.
Peripheral blood mononuclear cells (PBMCs) were cryopreserved and cultured as previously described (24). PBMCs were resuspended in RPMI 1640 supplemented with 5% pooled human AB serum (Cambrex, East Rutherford, NJ) for measles lysate (ML) cultures or AIM-V serum-free medium supplemented with 4 × 10−5 mol of 2-mercaptoethanol (Sigma-Aldrich, New South Wales, Australia)/liter for cultures with phytohemagglutinin (PHA). The cells were incubated with UV-inactivated ML from MV Edmonston strain-infected rhesus monkey kidney (RMK) cells (4 × 105 PFU/ml, kindly provided by Steven Wesselingh, Macfarlane Burnet Institute for Medical Research, Melbourne, Australia [12]) or control RMK cell lysate alone, for 24 h as previously described (25), or with PHA (1 μg/ml; Murex Biotech, Kent, United Kingdom) for 48 h. To measure the CD46 expression (mean fluorescence intensity [MFI]) on seven cell types, the cells (0 h and harvested) were washed in phosphate-buffered saline supplemented with 0.1% sodium azide and 1% normal human serum and then incubated for 20 min on ice with one of three staining protocols containing the monoclonal antibodies anti-CD46-FITC (and isotype control mouse IgG2a), anti-CD3-PerCP (to identify T cells), anti-CD4-PE (for T-helper [Th] cells), anti-CD8-APC (for T-cytotoxic [Tc] cells), anti-CD14-PerCP (for monocytes) (BD Biosciences, NSW, Australia), anti-CD1c-PE (for myeloid dendritic cells [mDCs]), and anti-CD303-APC (for plasmacytoid DCs [pDCs]) (Miltenyi Biotec, North Ryde, New South Wales, Australia). The data were acquired using a FACSCalibur flow cytometer and analyzed by using CellQuest (BD Biosciences) and FlowJo software (Tree Star, Ashland, OR). The percent CD46 downregulation after ML or PHA stimulation was calculated using the method of Sakurai et al. (26).
Statistical analyses.
Chi-squared tests were used to determine the Hardy-Weinberg equilibrium. Antibody levels and receptor expression were analyzed by using Kruskal-Wallis tests since the levels were not normally distributed after log transformation. Wilcoxon signed-rank tests were used to compare receptor expression before and after stimulation. Analyses were performed using SPSS version 11.0 (SPSS Inc., Chicago, IL), and statistical significance was defined as P < 0.05.
RESULTS
Study demographics and genotyping.
Demographics are detailed in Table 1. Genotype frequencies for the five CD46 SNPs were in Hardy-Weinberg equilibrium, and minor allele frequencies were similar to those reported previously for Caucasian populations (Utah residents with European ancestry) by the HapMap project (http://www.hapmap.org).
Table 1.
Demographics of study population from Perth, Australia
| Demographic | Data |
|---|---|
| Median age at vaccination (range) | 1.05 yr (10 mo to 2 yr) |
| Gender (no. [%]) | |
| Males | 66 (44) |
| Females | 84 (56) |
| No. (%) of subjects seronegative for MV | 14 (10.2) |
Associations between CD46 polymorphisms and measles antibody response.
Measles antibody titers below 324 mIU/ml were considered seronegative. Fourteen subjects (10.2%) were seronegative for measles IgG postvaccination. Of these, three subjects were rs7144TT, nine were rs7144TC, and two were rs7144CC (P = 0.499). Seronegative children were also not clustered within a particular CD46 rs11118580 or rs2724384 genotype (P > 0.05). The median measles postvaccination IgG titer was 1071 mIU/ml (interquartile range [IQR], 417 to 1842). Three of the four CD46 polymorphisms investigated in the present study were significantly associated with measles IgG levels (Fig. 1). CD46 rs7144 CC and CT had significantly lower measles IgG levels compared to TT (P = 0.008) (it may also be suggested that the TT genotype is a “high responder,” i.e., those with TT had higher IgG levels compared to the other genotypes). CD46 rs11118580 and rs2724384 were also associated with measles antibody levels with the CC and GG genotypes, respectively, associated with lower measles IgG levels (P = 0.026 and P = 0.018, respectively) (Fig. 1). CD46 rs14374 was not associated with measles IgG (P = 0.801), although the CC frequency was low (n = 1). The CD46 SNPs that were associated with measles antibody response were not associated with mumps or rubella IgG levels (Fig. 2). Two CD46 haplotypes, ATTT and GCCT (in the order rs2724384, rs11118580, rs7144, and rs14374) were also significantly associated with measles IgG levels (Table 2).
Fig 1.
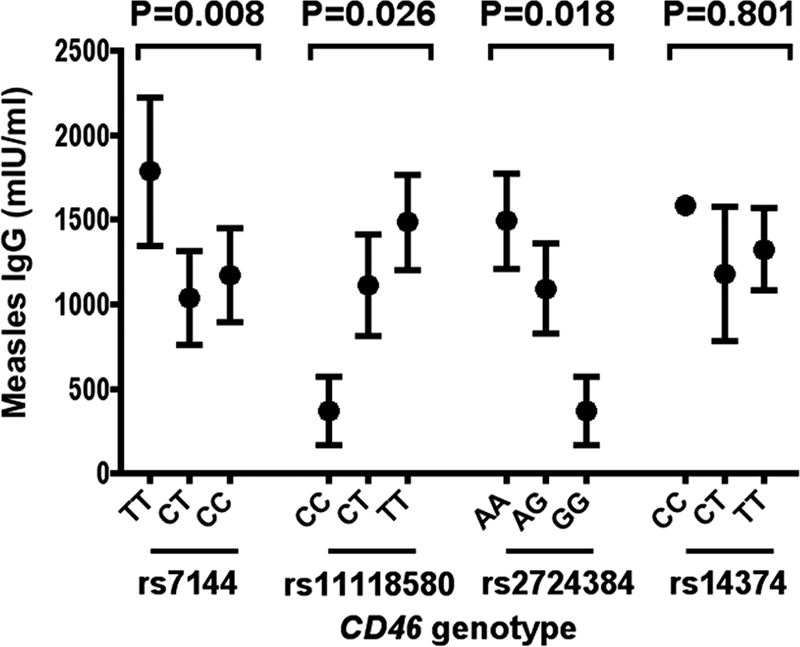
Associations between CD46 polymorphisms and measles IgG antibody levels in the Perth cohort. The data are presented as median values and interquartile ranges. P values were obtained by using Kruskal-Wallis tests.
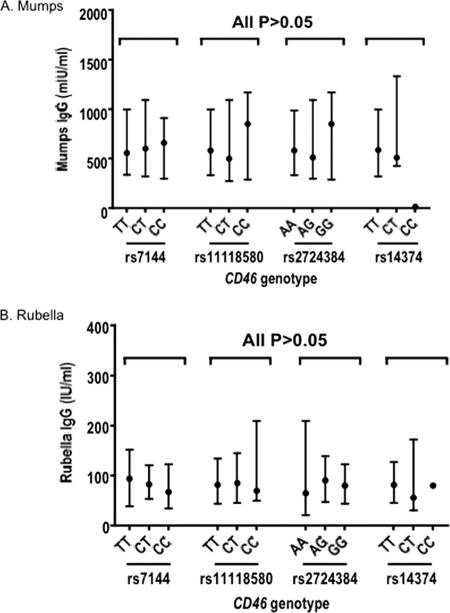
Fig 2.
CD46 polymorphisms and mumps and rubella IgG levels. (A and B) Mumps (A)- and rubella (B)-specific IgG levels. The data are presented as median values and interquartile ranges. P values are all >0.05 (obtained by using Kruskal-Wallis tests).
Table 2.
CD46 haplotypes and measles IgG levels in the Perth population
| CD46 haplotypea (% frequency in Perth cohort) | No. of copies of haplotype | No. of subjects | Median MV IgG levels in mIU/ml (IQR) | P |
|---|---|---|---|---|
| ATTT (54.4) | 0 | 31 | 1,096 (468–1,778) | 0.005 |
| 1 | 60 | 700 (324–1,613) | ||
| 2 | 44 | 1,531 (660–2,427) | ||
| GCCT (18.6) | 0 | 88 | 1,350 (432–2,066) | 0.019 |
| 1 | 41 | 1,023 (395–1,534) | ||
| 2 | 6 | 385 (221–550) |
Both haplotype sequences are indicated in the order: CD46 rs2724384, rs11118580, rs7144, and rs14374.
In summary, significant associations were found between CD46 SNPs and haplotypes and measles IgG antibody levels.
CD46 receptor protein expression.
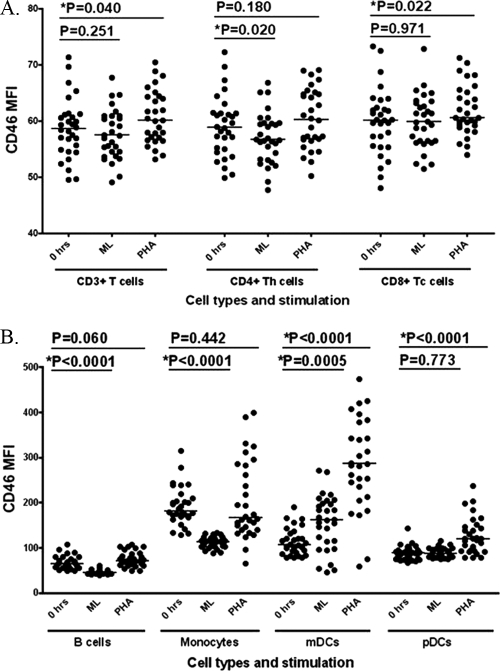
When assessing CD46 expression on PBMCs, all cells studied expressed CD46 as expected (14), with the highest expression at 0 h found on monocytes (Fig. 3). CD46 expression in antigen-presenting cells (APCs) was influenced by ML stimulation, with large decreases in expression in B cells (P < 0.0001) and monocytes (P < 0.0001) and a large upregulation of CD46 expression in mDCs (P = 0.0005) compared to expression at 0 h (and expression in mock-treated controls was comparable to expression at 0 h [P > 0.05]). CD46 expression on T cells was less affected by ML stimulation, with only a slight downregulation of expression in CD4+ T cells (compared to 0 h, P = 0.020) (Fig. 3). CD46 expression was increased after PHA stimulation in most cell types, particularly mDCs (P < 0.0001) (Fig. 3). The change in CD46 expression after ML stimulation compared to after PHA stimulation was significantly different in all cell types (P < 0.05 for T-cell subsets and P < 0.0001 for B cells and DCs).
Fig 3.
Cell surface protein expression of CD46 before and after measles lysate and PHA stimulation in all cell types. CD46 expression (i.e., the mean fluorescence intensity [MFI]) after stimulation with ML or PHA compared to at 0 h in T cells (A) and APCs (B) is depicted. Horizontal lines represent median values.
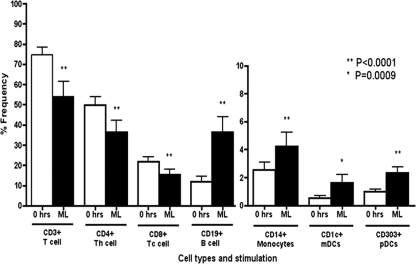
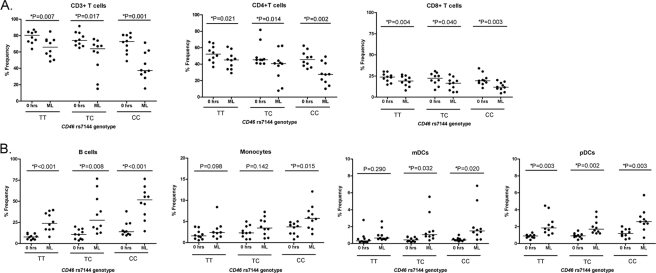
We then assessed the proportion of different cell types within the PBMC population before and after ML stimulation. The proportion of subpopulations of T cells, B cells, monocytes, mDCs, and pDCs was significantly altered after measles lysate stimulation compared to 0 h. The percentages of CD3+, CD4+, and CD8+ T cells were decreased after ML stimulation (all P < 0.0001), whereas the opposite was found in APCs (due to the ratio) (Fig. 4).
Fig 4.
Proportion of cells before and after ML stimulation in each cell type. The proportions of cells (% of total cells) at 0 h (□) and after ML stimulation (■) are shown. Monocytes and DCs have a smaller y axis compared to other cell types. **, P < 0.0001; *, P = 0.0009.
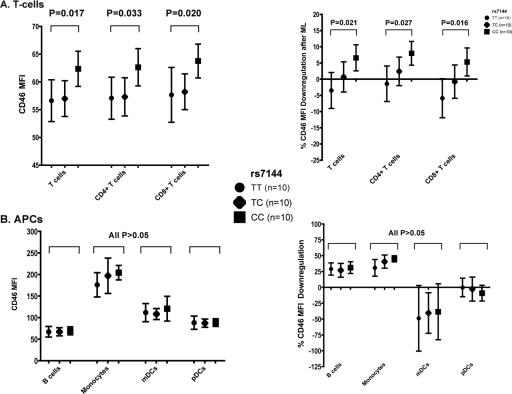
CD46 rs7144 was significantly associated with CD46 expression at 0 h, with those with the C allele having a higher level of expression in CD3+ (P = 0.017), CD4+ (P = 0.033), and CD8+ (P = 0.020) T cells (Fig. 5A). CD46 rs7144 genotypes were significantly associated with the downregulation of CD46 expression after ML stimulation in T cells, with those with the CC genotype having more downregulation (i.e., less expression after stimulation) compared to CT and TT (P = 0.021, P = 0.027, and P = 0.016 for CD3+, CD4+, and CD8+ T cells, respectively) (Fig. 5A). In contrast, CD46 expression in the APC subsets (B cells, monocytes, and DCs) were not significantly different between the CD46 rs7144 genotypes at 0 h or after ML stimulation (Fig. 5B). The other CD46 SNPs were not associated with CD46 expression.
Fig 5.
Associations between CD46 rs7144 genotypes and CD46 receptor protein expression at baseline and CD46 downregulation after ML stimulation in T cells and APCs. CD46 expression (i.e., the mean fluorescence intensity [MFI]) at 0 h (left) and the percent CD46 downregulation after ML stimulation (right) in T cells (A) and APCs (B) is depicted. The data are presented as median values and interquartile ranges. The CD46 rs7144 genotypes included TT (circles), CT (diamonds), and TT (squares). The percent CD46 downregulation after ML stimulation was calculated according to the method by Sakurai et al. (26).
After ML stimulation, the percentage of CD3+ T cells was significantly lower in cells of the rs7144CC genotype compared to the other genotypes (P = 0.035), with a similar trend for CD4+ T cells (P = 0.061) and CD8+ T cells (P = 0.078) (and opposite proportions in APCs). Within each genotype, the change in cell percentages before and after ML stimulation was consistently significantly different, decreased in T cells and increased in APCs (due to the ratio) (Fig. 6).
Fig 6.
Proportions of cells (percent frequency) before and after ML stimulation between the genotypes of CD46 rs7144 polymorphism. The cell frequencies (percentage of total cells) in T cells (A) and APCs (B) at 0 h and after ML stimulation are given. Horizontal lines represent means.
In summary, CD46 receptor expression was altered after stimulation with MV, particularly in B cells and monocytes. The percentage of T cells (but not APCs) was decreased after MV stimulation. CD46 rs7144CC had higher levels of CD46 expression in T cells at baseline and, after MV stimulation, greater downregulation of CD46 expression and a lower percentage of T cells compared to other genotypes.
DISCUSSION
Poor immunogenicity and vaccine failure in young children contribute to the ongoing burden of measles. The immunogenetic determinants of measles responses and vaccine efficacy in children are not yet well characterized. We aimed to identify whether genetic variation in the MV receptor gene CD46 contributed toward measles-specific antibody responses in an Australian cohort of infants immediately following their first contact with measles vaccine. We also aimed to investigate whether CD46 polymorphisms were associated with functional effects on the CD46 receptor in order to determine a possible mechanism through which the genetic variant acts on antibody responses. We demonstrated for the first time that CD46 polymorphisms are associated with cell-specific differences in receptor expression and with primary measles IgG antibody responses.
Three CD46 polymorphisms were associated with measles IgG levels (rs7144, rs11118580, and rs2724384), supporting the hypothesis that CD46 plays an important role in how a child responds to measles vaccine. Associations between CD46 and measles antibody levels have been identified in previously vaccinated American school-aged children and adults (7). The associations with rs11118580 and rs2724384 in our naive infants agree with those found in the older children and adults (7), with the same alleles (C and G, respectively) associated with the lower measles antibody levels. However, in contrast to the results of Dhiman et al. (7), we have identified, for the first time, a significant association between CD46 rs7144 and measles antibody levels. Genetic differences may have a different impact upon the naive immature immune system of infants than they would have on older children and adults once their immune systems have matured. Our results showed that CD46 variants may be involved in determining primary measles antibody responses in naive young children.
CD46 genotypes were significantly associated with measles antibody levels; however, they did not seem to determine whether or not a child was protected against measles (however, with only 10% seronegative children in this cohort, there was insufficient power to assess this properly). Regardless, measles antibody levels are known to wane over time (20), so children with a lower primary antibody response (even above the protective level) are more likely to fall below the protective level and become vulnerable to measles in a shorter period of time. These children may also produce lower levels of memory B cells (which would be interesting in future studies) which may affect their responses to their second dose of measles vaccine. Although our results showed associations with altered antibody levels in certain CD46 genotypes, this has unclear clinical relevance at this stage, and therefore further studies would need to determine whether lower antibody levels translate to poor immunogenicity and/or vaccine failure.
We have shown that genetic variation in the CD46 gene was associated with measles antibody responses, but it is not known what the mechanistic link is between these two variables. No known functional effects of CD46 polymorphisms have been published. We hypothesized that the CD46 SNPs that associate with measles IgG would functionally alter the expression of the CD46 receptor. We have reported, to our knowledge, the first data on the associations of a polymorphism in a measles receptor gene and receptor protein expression. A variant allele of a CD46 3′UTR polymorphism (rs7144) was associated with lower measles antibody levels in our Perth cohort. The 3′UTRs of genes can contribute to the regulation of mRNA translation and stability and thus protein expression (31). This polymorphism therefore has the potential to alter the expression of CD46 on the cell surface, thereby modulating the amount of receptor able to bind to MV, leading to a decrease in the immune response against measles.
We found that the highest CD46 expression was in monocytes, which follows previous in vivo studies that demonstrated that monocytes are the cell type predominantly infected by MV (10). After interaction with MV hemagglutinin, CD46 is internalized and downregulated from the cell surface (4, 17). We found CD46 expression was downregulated after measles lysate (ML) stimulation of PBMCs, dramatically in B cells and monocytes, with a smaller change in CD4+ T cells. Expression in mDCs, however, increased 3-fold after ML stimulation. It appears that the T-cell expression of CD46 is not activation induced, but the expression in APCs is considerably altered after contact with measles.
CD46 expression in T cells was not overly induced by ML stimulation, but it was significantly associated with the CD46 rs7144 SNP. Children with the rs7144CC genotype had significantly more CD46 expression at baseline in T cells but not in APCs. There was also a distinct difference between the genotypes in the regulation of CD46 expression in T cells; those with the CC genotype showed higher downregulation of the receptor after ML stimulation. This downregulation may affect CD46 signaling and antigen presentation (30, 33), as well as increase the probability of cell lysis (15). Children with rs7144CC also showed a decreased frequency of T cells after ML stimulation, perhaps reflecting this T-cell lysis.
Our results showing this CD46 rs7144 SNP associated with receptor expression and T-cell frequency suggest a possible mechanism that links genetic variation and altered measles antibody response (Table 3). Those with rs7144CC had a higher density of receptor on the T-cell surface, higher downregulation of receptor from the surface after measles (higher rate of MV binding and hence infection of the cell), and possible increased T-cell lysis (which is supported by our results that showed a decrease in T-cell frequency). This may lead to decreased T-cell help for measles-specific B cells and hence impaired measles antibody responses and, in fact, we showed that those with this same genotype had lower levels of measles IgG. It would be important to test this hypothesis of the mechanisms underlying how receptor expression affects antibody responses in additional functional studies.
Table 3.
Summary of results regarding CD46 rs7144 polymorphism
| Outcome | CD46 rs7144 polymorphisma |
|---|---|
| Baseline CD46 receptor expression | C allele ↑ expression on T cells |
| CD46 receptor downregulation after ML stimulation | CC ↑ downregulation (i.e., ↓ expression after ML) |
| Cell proportion after ML stimulation | CC ↓ T-cell proportion |
| Postvaccination measles IgG | C allele ↓ IgG |
Information is indicated compared to the alternative genotype/allele; ML, measles lysate stimulation of PBMCs.
CD46 rs11118580 and rs2724384, although showing significant associations with measles IgG levels, did not show any relationships with CD46 protein expression. Unlike 3′UTR SNPs such as rs7144 and polymorphisms in the promoter region, the intronic CD46 rs11118580 and rs2724384 variants are likely to functionally affect CD46 protein in an alternative way, perhaps affecting structure, trafficking, or localization of the protein, rather than the amount of expression. It would be important to investigate the functionality of these SNPs in future studies.
The limitations of the present study should be noted. First, the size of the Perth cohort was small, and therefore the study was not powered to detect the effect of the SNPs on the proportion of seronegative children. However, our sample size is comparable to other published studies in this area (7). Although it would be interesting to compare these initial responses with second dose responses, this cohort was not followed up at 4 years, and therefore comparisons could not be made. Corrections for multiple testing were not performed in the present study, since only a limited number of tests were performed, with the analyses based on specifically defined a priori hypotheses, and all of the tests that were performed were described (21). Although we showed a decrease in T-cell frequency following ML stimulation, we have not specifically quantified cell lysis; therefore, this is only a possible theory and should be followed up in future studies. It is possible these SNPs are in linkage disequilibrium with another functional polymorphism in CD46 or in another gene responsible for the association with measles antibody response. Alternative host genetic factors may also have an effect, such as genes of the HLA system (19) and cytokine and cytokine receptor genes (6) that have been shown to influence measles vaccine responses in adults. In addition, receptors such as the other measles-specific receptor, signaling lymphocyte activation molecule (SLAM), are likely to have effects on measles responses (7, 9). We have, however, recently shown that SLAM polymorphisms are not associated with measles antibody responses in this cohort (3). Regardless, it is biologically plausible and likely that CD46 plays an important role in specific responses to measles virus and vaccine.
In conclusion, we report significant associations with genetic variants in measles receptor CD46 and primary IgG antibody responses to measles vaccine in a population of Australian infants after their first vaccine dose. We also demonstrate the first findings of an association with an MV receptor polymorphism and functional effects on receptor protein expression, a possible explanation for the link between genetic variation and altered measles vaccine responses. In the future, the combination of genetic factors that reliably predict poor immunogenicity or vaccine failure need to be elucidated. Knowledge in this area may help in the identification of children at risk of poor immunogenicity and vaccine failure, which may lead to the development of different strategies for vaccinating these children and contribute to the control of MV infections worldwide.
ACKNOWLEDGMENTS
We thank the children and their families who were involved in these studies. We thank Jenny Langlands, Kate White, Angela Caskey, and the nurses of the Vaccine Trials Group at Princess Margaret Hospital for Children for the clinical conduct of the Perth study. We thank Barbara Holt and Jenny Tizard for assistance with sample processing and Ian Sampson and David Smith of PathWest for the measurement of MMR antibody responses. We also thank Jack Goldblatt and Andrew Currie for their reviews of the manuscript.
This study was funded by the National Health and Medical Research Council of Australia.
Footnotes
Published ahead of print 22 February 2012
REFERENCES
- 1. Brunell PA, Weigle K, Murphy MD, Shehab Z, Cobb E. 1983. Antibody response following measles-mumps-rubella vaccine under conditions of customary use. JAMA 250:1409–1412 [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention 2009. Global measles mortality, 2000–2008. Wkly. Epidemiol. Rec. 58:1321–1326 [Google Scholar]
- 3. Clifford HD, et al. 2011. SLAM and DC-SIGN measles receptor polymorphisms and their impact on antibody and cytokine responses to measles vaccine. Vaccine 29:5407–5413 [DOI] [PubMed] [Google Scholar]
- 4. Crimeen-Irwin B, et al. 2003. Ligand binding determines whether CD46 is internalized by clathrin-coated pits or macropinocytosis. J. Biol. Chem. 278:46927–46937 [DOI] [PubMed] [Google Scholar]
- 5. Dhiman N, Jacobson RM, Poland GA. 2004. Measles virus receptors: SLAM and CD46. Rev. Med. Virol. 14:217–229 [DOI] [PubMed] [Google Scholar]
- 6. Dhiman N, et al. 2007. Associations between measles vaccine immunity and single-nucleotide polymorphisms in cytokine and cytokine receptor genes. J. Infect. Dis. 195:21–29 [DOI] [PubMed] [Google Scholar]
- 7. Dhiman N, et al. 2007. Variations in measles vaccine-specific humoral immunity by polymorphisms in SLAM and CD46 measles virus receptors. J. Allergy Clin. Immunol. 120:666–672 [DOI] [PubMed] [Google Scholar]
- 8. Dhiman N, et al. 2008. Associations between SNPs in Toll-like receptors and related intracellular signaling molecules and immune responses to measles vaccine: preliminary results. Vaccine 26:1731–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Erlenhofer C, Duprex WP, Rima BK, Ter Meulen V, Schneider-Schaulies J. 2002. Analysis of receptor (CD46, CD150) usage by measles virus. J. Gen. Virol. 83:1431–1436 [DOI] [PubMed] [Google Scholar]
- 10. Esolen LM, Ward BJ, Moench TR, Griffin DE. 1993. Infection of monocytes during measles. J. Infect. Dis. 168:47–52 [DOI] [PubMed] [Google Scholar]
- 11. Gans HA, et al. 1999. IL-12, IFN-gamma, and T cell proliferation to measles in immunized infants. J. Immunol. 162:5569–5575 [PubMed] [Google Scholar]
- 12. Huang Z, Dry I, Webster D, Strugnell R, Wesselingh S. 2001. Plant-derived measles virus hemagglutinin protein induces neutralizing antibodies in mice. Vaccine 19(15–16):2163–2171 [DOI] [PubMed] [Google Scholar]
- 13. Jacobson RM, Poland GA. 2004. The genetic basis for measles vaccine failure. Acta Paediatr. Suppl. 93:43–46 [DOI] [PubMed] [Google Scholar]
- 14. Katayama Y, Hirano A, Wong TC. 2000. Human receptor for measles virus (CD46) enhances nitric oxide production and restricts virus replication in mouse macrophages by modulating production of alpha/beta interferon. J. Virol. 74:1252–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liszewski MK, Post TW, Atkinson JP. 1991. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu. Rev. Immunol. 9:431–455 [DOI] [PubMed] [Google Scholar]
- 16. Miller SA, Dykes DD, Polesky HF. 1988. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 16:1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Naniche D, et al. 1993. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 67:6025–6032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Newport MJ, et al. 2004. Genetic regulation of immune responses to vaccines in early life. Genes Immun. 5:122–129 [DOI] [PubMed] [Google Scholar]
- 19. Ovsyannikova IG, Pankratz VS, Vierkant RA, Jacobson RM, Poland GA. 2006. Human leukocyte antigen haplotypes in the genetic control of immune response to measles-mumps-rubella vaccine. J. Infect. Dis. 193:655–663 [DOI] [PubMed] [Google Scholar]
- 20. Pebody RG, et al. 2002. Immunogenicity of second dose measles-mumps-rubella (MMR) vaccine and implications for serosurveillance. Vaccine 20(7–8):1134–1140 [DOI] [PubMed] [Google Scholar]
- 21. Perneger TV. 1998. What's wrong with Bonferroni adjustments. BMJ 316:1236–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Poland GA, Jacobson RM. 1998. The genetic basis for variation in antibody response to vaccines. Curr. Opin. Pediatr. 10:208–215 [DOI] [PubMed] [Google Scholar]
- 23. Rivailler P, Trescol-Biemont MC, Gimenez C, Rabourdin-Combe C, Horvat B. 1998. Enhanced MHC class II-restricted presentation of measles virus (MV) hemagglutinin in transgenic mice expressing human MV receptor CD46. Eur. J. Immunol. 28:1301–1314 [DOI] [PubMed] [Google Scholar]
- 24. Rowe J, et al. 2001. Heterogeneity in diphtheria-tetanus-acellular pertussis vaccine-specific cellular immunity during infancy: relationship to variations in the kinetics of postnatal maturation of systemic Th1 function. J. Infect. Dis. 184:80–88 [DOI] [PubMed] [Google Scholar]
- 25. Rowe J, et al. 2005. Th2-associated local reactions to the acellular diphtheria-tetanus-pertussis vaccine in 4- to 6-year-old children. Infect. Immun. 73:8130–8135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sakurai F, Akitomo K, Kawabata K, Hayakawa T, Mizuguchi H. 2007. Downregulation of human CD46 by adenovirus serotype 35 vectors. Gene Ther. 14:912–919 [DOI] [PubMed] [Google Scholar]
- 27. Stephens M, Smith NJ, Donnelly P. 2001. A new statistical method for haplotype reconstruction from population data. Am. J. Hum. Genet. 68:978–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tan PL, Jacobson RM, Poland GA, Jacobsen SJ, Pankratz VS. 2001. Twin studies of immunogenicity–determining the genetic contribution to vaccine failure. Vaccine 19:2434–2439 [DOI] [PubMed] [Google Scholar]
- 29. Upham JW, et al. 2002. Development of interleukin-12-producing capacity throughout childhood. Infect. Immun. 70:6583–6588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Varior-Krishnan G, Trescol-Biemont MC, Naniche D, Rabourdin-Combe C, Gerlier D. 1994. Glycosyl-phosphatidylinositol-anchored and transmembrane forms of CD46 display similar measles virus receptor properties: virus binding, fusion, and replication; down-regulation by hemagglutinin; and virus uptake and endocytosis for antigen presentation by major histocompatibility complex class II molecules. J. Virol. 68:7891–7899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wickens M, Anderson P, Jackson RJ. 1997. Life and death in the cytoplasm: messages from the 3′ end. Curr. Opin. Genet. Dev. 7:220–232 [DOI] [PubMed] [Google Scholar]
- 32. World Health Organization 2002. The World Health Report: reducing risks, promoting healthy life. World Health Organization, Geneva, Switzerland [Google Scholar]
- 33. Yant S, Hirano A, Wong TC. 1997. Identification of a cytoplasmic Tyr-X-X-Leu motif essential for down regulation of the human cell receptor CD46 in persistent measles virus infection. J. Virol. 71:766–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yerkovich ST, et al. 2007. Assessment of the potency and potential immunomodulatory effects of the measles mumps rubella and varicella vaccine in infants. Vaccine 25:1764–1770 [DOI] [PubMed] [Google Scholar]