Abstract
Hepatitis C virus (HCV)-specific cell-mediated immunity (CMI) has been reported among exposed individuals without viremia or seroconversion. Limited data are available regarding CMI among at-risk, seronegative, aviremic Egyptian health care workers (HCW), where HCV genotype 4 predominates. We investigated CMI responses among HCW at the National Liver Institute, where over 85% of the patients are HCV infected. We quantified HCV-specific CMI in 52 seronegative aviremic Egyptian HCW using a gamma interferon (IFN-γ) enzyme-linked immunospot assay in response to 7 HCV genotype 4a overlapping 15-mer peptide pools covering most of the viral genome. A positive HCV-specific IFN-γ response was detected in 29 of 52 HCW (55.8%), where 21 (40.4%) had a positive response for two to seven HCV pools and 8 (15.4%) responded to only one pool. The average numbers of IFN-γ total spot-forming cells (SFC) per million peripheral blood mononuclear cells (PBMC) (± standard error of the mean [SEM]) in the 29 responding and 23 nonresponding HCW were 842 ± 141 and 64 ± 15, respectively (P < 0.001). Flow cytometry indicated that both CD4+ and CD4− T cells produced IFN-γ. In summary, more than half of Egyptian HCW demonstrated strong HCV multispecific CMI without viremia or seroconversion, suggesting possible clearance of low HCV exposure(s). These data suggest that detecting anti-HCV and viremia to determine past exposure to HCV can lead to an underestimation of the true disease exposure and that CMI response may contribute to the low degree of chronic HCV infection in these HCW. These findings could have strong implications for planning vaccine studies among populations with a high HCV exposure rate. Further studies are needed to determine whether these responses are protective.
INTRODUCTION
Hepatitis C virus (HCV) infection is a global health problem (12, 31), with the highest worldwide disease prevalence reported in Egypt: 15% of the general population (14, 45, 51). Primary HCV infections are usually asymptomatic (4), with most cases developing chronic infection (5) and only 15 to 50% undergoing spontaneous viral clearance (42). Natural protective immunity against HCV has been demonstrated in both humans (34, 38) and chimpanzees (8), with host-specific cell-mediated immunity (CMI) playing a pivotal role in controlling infection (9, 19, 44). While HCV spontaneous clearance remains poorly understood, natural resistance to infection exists and continues to spur researchers in their attempts to develop an effective vaccine (23, 48).
Recent reports have documented sporadic cases of detectable HCV-specific CMI in exposed uninfected persons (3, 17, 24, 29, 37, 39, 41, 52) without evidence of persistent infection, i.e., without viremia or seroconversion. In these HCV-seronegative aviremic subjects, HCV-specific CMI is the only biomarker of host exposure to the virus and may provide protection against chronic infection (38). In this regard, HCV-specific T-cell responses have been demonstrated without viremia or seroconversion in individuals exposed to HCV infection, such as family members living with two or more HCV-infected subjects (3), sexual contacts of acute HCV patients (24), intravenous drug users (37, 52), and health care workers (HCW) after needle stick injury (29). These data support the view that host immune responses may determine the course of the disease (3). Therefore, identification of host protective immune mechanisms in individuals who successfully clear infection is crucial for determining past exposure, understanding the natural history of HCV infection, and planning for its prevention and control.
HCW are at a high risk of HCV infection after occupational exposure (28, 30), with the risk of exposure from an HCV-contaminated needle stick injury estimated at 0 to 5%, or almost 10-fold the risk of acquiring HIV from a similar event (7, 22). Limited data are available about genotype 4 HCV-specific CMI in seronegative, aviremic HCW working in high-risk environments such as the National Liver Institute (NLI), where HCW provide care for 120,000 outpatients and 15,000 inpatients annually. Over 85% of these patients are seropositive for HCV antibodies, with 55 to 65% of them having HCV RNA and chronic liver disease (46, 49). The potential of exposure among persons providing care to these patients is more than 10-fold higher than that of medical personnel in the United States and Europe and is as great as that of many intravenous drug users.
Similarly to other at-risk groups (3, 17, 24, 29, 37, 39, 41, 52), HCW at the NLI may not develop anti-HCV antibodies or viremia despite repeated exposure to the virus, and this has not been examined yet among HCW in a community, such as Egypt, where HCV is highly endemic. We hypothesized that low levels of HCV exposures or even short-term viremia among Egyptian HCW may lead to HCV-specific CMI without seroconversion or measurable viremia. CMI to genotype 4 HCV, which is the most prevalent HCV genotype in Egypt (10), has been examined in HCV-infected subjects using either recombinant proteins derived from genotype 1 (24–26) or genotype 4a (13) or a restricted set of genotype 1 (24) or 4 (15) peptides, possibly underestimating the real frequency of responders due to suboptimal detecting reagents or methods. In the present study, the presence, magnitude, and breadth of CMI responses in seronegative, aviremic HCW from the NLI in the Nile Delta region were examined using a sensitive ex vivo enzyme-linked immunospot (ELISpot) assay and a large set of HCV genotype 4 peptides covering most of the viral genome.
MATERIALS AND METHODS
Study subjects.
Fifty-two anti-HCV-negative and aviremic HCW were enrolled in this cross-sectional study to assess their HCV-specific CMI responses. These HCW were identified from an ongoing study investigating the incidence of HCV among 858 HCW at risk of exposure to, and infection with, HCV at the Menoufiya University's NLI in the Nile Delta. The study protocol was reviewed and approved by the institutional review board at the NLI, and all subjects gave informed consent prior to participation in the study. Demographic data, risk factors for exposure to blood-borne infections, medical history, current symptoms (including those related to liver disease), needle stick or other parenteral injuries, and history of hepatitis B (HBV) vaccination were collected using a questionnaire adapted from the Exposure Prevention Information Network (EPIN) (http://www.healthsystem.virginia.edu/internet/epinet/about_epinet.cfm). Blood samples for immunological studies and other laboratory testing were collected. Fifteen healthy individuals with no known exposure to HCV or HCV-infected patients, matched for age (mean age, 33 ± 8.6 years), served as a control group (unexposed subjects).
Detection of HCV and other biomarkers.
Serum samples were collected in plain Vacutainer tubes, and serum alanine aminotransferase (ALT) levels were measured using routine clinical test kits. Anti-HCV was tested by a third-generation enzyme immunoassay (EIA) (Murex anti-HCV, version 4.0) according to the manufacturer's instructions. Detection and quantification of HCV-RNA were performed on sera after extraction of RNA using a Qiagen viral RNA extraction kit (QIAgen, USA) and using a quantitative real-time reverse transcriptase PCR (RT-PCR) via a strand-specific AgPath-ID one-step assay according to the manufacturer's instructions (Applied Biosystems-Life Technologies Corporation, CA). The kit uses HCV-specific primers and probes as well as internal controls. HBV surface antigen (HBsAg) (Murex) and anti-HBs (Adaltis, Italy) EIAs were performed according to the manufacturer's instructions and as previously described (2, 35). Detection of Schistosoma mansoni-specific IgG was performed by EIA according to the manufacturer's instructions (Novatec Immunodiagnostica GmbH, Germany).
Synthetic HCV antigens and other control antigens.
HCV genotype 4a isolate ED43 peptide antigens composed of 15 (15-mer) and overlapping by 11 amino acids were used in this study. These synthetic peptides were obtained from the National Institute of Allergy and Infectious Diseases' Biodefense and Emerging Infections Research Resources Repository and were at least 80% pure. They were 585 peptides that were combined in seven pools and were labeled with alphabetical letters as follows: “E2,” representing the viral envelope protein E2 (92 peptides); “F,” comprising the N-terminal half of the NS3 protein (78 peptides); “G,” comprising the remaining half of NS3 (78 peptides); “H,” comprising the NS4a and NS4b proteins (79 peptides); “I,” comprising the NS5a protein (111 peptides); “L,” representing the first half of NS5b (75 peptides); and “M,” covering the remainder of the NS5b protein (72 peptides). Negative-control cultures included cells stimulated with culture medium alone but containing the solvent used for the preparation of the peptides (dimethyl sulfoxide [DMSO]). Cytomegalovirus lysate (Virusys Corporation, USA) and a CEF (human cytomegalovirus [C], Epstein-Barr virus [E], and influenza virus [F]) peptide pool (Pantecs GmbH, Germany) were used as positive controls for antigen-specific responses. Staphylococcal enterotoxin B (SEB) was used as a polyclonal positive control for ELISpot assays, and SEB and phorbol myristate acetate (PMA) plus ionomycin were used as positive controls for flow cytometry (all from Sigma, MO, USA).
IFN-γ ELISpot assay.
Approximately 15 ml of whole blood was collected into EDTA Vacutainer tubes (Becton Dickinson Biosciences, NJ, USA). Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque density gradient centrifugation, and viability was determined by the trypan blue exclusion method. The ELISpot assay is described elsewhere (18). Briefly, PBMC (200,000/well) were incubated in triplicate cultures in the ELISpot plates (Whatman Unifilter, USA) coated with anti-human gamma interferon (IFN-γ) antibody and incubated for ∼16 h with or without recombinant HCV antigens at 3 μg/ml of each single peptide in complete RMPI-1640 medium. Negative and positive controls included medium containing DMSO alone and 0.1 μg/ml of SEB or other polyclonal stimuli, respectively. At the end of the incubation period, the assay was developed until the appearance of spots, and then the wells were rinsed with tap water to stop the reaction. The number of spots per well was counted using an automated ELISpot reader (Cellular Technology Ltd., Cleveland, OH). Mean numbers of IFN-γ spot-forming cells (SFC) in control wells were subtracted from antigen-stimulated wells to correct for background cytokine production and are expressed as SFC/million PBMC. An HCV antigen-specific response was considered positive if the number of SFC in the presence of antigen was at least 3-fold the number of SFC in the medium control and if there were >55 SFC/million PBMC, as we previously reported (3).
Flow cytometric analysis of intracellular IFN-γ by HCV-specific T cells.
PBMC were stimulated with HCV antigens and the intracellular cytokine production was examined as described previously (32). Briefly, 1 million cells in complete RPMI 1640 medium were stimulated with the HCV antigens mentioned above (at 3 μg/ml) in a short-term assay of 16 h. Brefeldin A (10 μg/ml) was used during the last 15 h of the assay to inhibit cytokine secretion by the cells. Negative and positive controls were medium alone and 50 ng/ml PMA plus 1 μg/ml ionomycin or SEB at 5 μg/ml, respectively. After the stimulation period, PBMC were washed and stained with surface fluorescein isothiocyanate (FITC)-labeled anti-human CD4 (BD Biosciences), and PerCP anti-human CD3 (BD Biosciences) antibodies at 4°C for 30 min. The cells were then washed, fixed, and permeabilized for 10 min with FACS Perm 2 solution (BD Biosciences) and then washed and stained with APC-labeled anti-human IFN-γ monoclonal antibody (MAb) (BD Pharmingen) for 30 min at room temperature in a dark place. The cells were then washed, fixed, and stored until data acquisition on a FACSCalibur flow cytometer (BD Biosciences). A maximum of 100,000 total events were acquired and cytokine-producing cells were analyzed using the FlowJo software (Tree Star Inc., CA). The cells were gated on small lymphocytes using side and forward light scatter profiles and then on CD3+ T cells. IFN-γ synthesis was shown as a function of CD4 expression on gated CD3+ T cells. The percentage of CD4+ and CD4− T cells (mostly CD8 T cells) producing IFN-γ was determined. Negative controls and compensation tubes were used to verify the assay staining specificity.
Statistical analysis.
All data were entered into a Microsoft Access database (Redmond, WA). Duplicate data entry was performed to ensure quality control. Analysis was done on SPSS package version 17.0. (SPSS Inc., Chicago, IL). Chi-square tests were performed for categorical data, while Student's t test (or the Mann-Whitney U test when appropriate) was performed for comparison of continuous data.
RESULTS
Study population.
The demographic characteristics of the 52 HCV antibody- and RNA-negative HCW participating in this study show that they had a mean age (± standard deviation) of 31 ± 8.2 years, 17 (32.7%) were females, and 57.7% lived in rural areas (Table 1). These HCW had a mean job duration of 8.3 ± 0.9 years, and approximately one-third of them were nurses (34.6%). Almost two-thirds of the subjects were anti-HBs antibody positive (61.5%), and 38.5% had detectable Schistosoma mansoni antibodies, suggesting current or past exposure to this parasite. The great majority (84.6%) of individuals reported dealing with syringes while caring for their patients in the 3 months prior to participation in the study but had a very low average of needle stick injuries per HCW per year (0.27 ± 0.08). Notably, none of the 52 subjects reported any history of clinical symptoms of hepatitis and all had normal ALT levels at the time of sampling. There were no significant differences between HCV-responding and nonresponding subjects, as defined in Materials and Methods and detailed below, in all demographic, clinical, and laboratory data (P > 0.05).
Table 1.
Demographic and clinical history data of the study subjects (n = 52)
| Parameter | Total n (%) | Result fora: |
P value | |
|---|---|---|---|---|
| Responders (n = 29 [55.8%]) | Nonresponders (n = 23 [44.2%]) | |||
| Age (yr ± SD) | 31 ± 8.2 | 30.0 ± 7.3 | 32.3 ± 7.3 | 0.169 |
| No. (%) female | 17 (32.7) | 10 (34.5) | 7 (30.4) | 0.852b |
| No. (%) with rural residence | 30 (57.7) | 15 (51.7) | 15 (65.2) | 0.345b |
| Duration of current job (yr ± SD) | 8.3 ± 0.9 | 7.7 ± 1.1 | 9.0 ± 1.5 | 0.238 |
| No. (%) in occupational category | ||||
| Physician/medical student | 9 (17.3) | 3 (10.3) | 6 (26.1) | |
| Nursing staff/student | 18 (34.6) | 14 (48.3) | 4 (17.4) | |
| Lab technician | 3 (5.8) | 3 (10.3) | 0 | 0.900b |
| Housekeeping | 10 (19.2) | 2 (6.9) | 8 (34.8) | |
| Other | 11 (21.2) | 7 (24.1) | 4 (17.4) | |
| No. positive for HBsAg | 0 | 0 | 0 | |
| No. (%) with history of HBV vaccination | 32 (61.5) | 16 (55.2) | 16(69.6) | 0.103 |
| No. (%) anti-HBs positive | 32 (61.5) | 18 (62.1) | 14 (60.8) | 0.455 |
| No. (%) with Schistosoma mansoni antibodies | 20 (38.5) | 10 (34.5) | 10 (43.5) | 0.218 |
| No. (%) dealing with syringes 3 months earlier | 44 (84.6) | 24 (82.8) | 20 (87.0) | 0.206 |
| No. (%) with needle stick injuries in last year, by no. of sticks | ||||
| Mean | 0.27 ± 0.08 | 0.32 ± 0.13 | 0.19 ± 0.08 | |
| None | 42 (80.8) | 23 (79.3) | 19 (82.6) | |
| 1 | 8 (15.4) | 4 (13.8) | 4 (17.4) | 0.229 |
| 2 | 1 (1.9) | 1 (3.4) | 0 | |
| 3–5 | 1 (1.9) | 1 (3.4) | 0 | |
| >5 | 0 | 0 | 0 | |
| No. with history of clinical jaundice/hepatitis | 0 | 0 | 0 | |
| Mean ± SD ALT level (IU/liter−1) | 29.1 ± 2.3 | 29.7 ± 3.2 | 28.3 ± 3.9 | 0.39 |
Responders and nonresponders are those individuals who elicited or failed to elicit, respectively, a positive HCV-specific IFN-γ response by ELISpot assay to one to seven HCV genotype 4a overlapping 15-mer peptide pools.
Mann-Whitney U test was used for the analysis.
Frequency and magnitude of HCV-specific cell-mediated immune response in seronegative aviremic HCW in a high-risk environment.
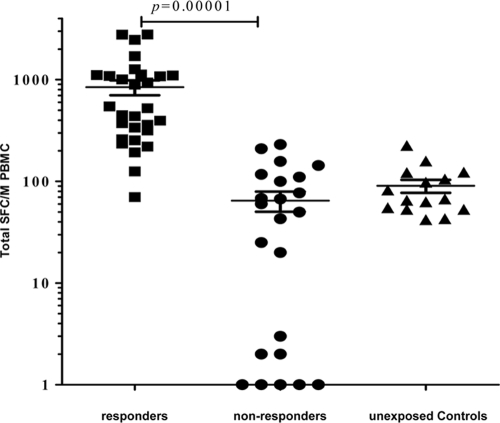
An ELISpot assay was used to quantify IFN-γ production by PBMC isolated from the study subjects in response to 7 HCV genotype 4a overlapping 15-mer peptide pools as described in Materials and Methods. A positive HCV-specific IFN-γ response to one to seven HCV genotype 4a overlapping 15-mer peptide pools was detected by ELISpot assay in 29 HCW (55.8%; responders), while 23 subjects (44.2%) did not respond to any of the pools tested (nonresponders). The average total IFN-γ SFC/million PBMC (± standard error of the mean [SEM]) was 842 ± 141 and 64 ± 15 in the responders and nonresponders, respectively (P < 0.001; Fig. 1). Of the 29 responders, 8 subjects (15.4% of the total) responded to only one HCV peptide pool with an average total of 313 ± 114 IFN-γ SFC/106 PBMC, which was still statistically significant (P = 0.0013) compared to results for nonresponders. If these 8 anti-HCV-negative aviremic subjects are excluded from the analysis, the percentage of subjects responding to two to seven HCV antigens without viremia or seroconversion is 40.4%, with an average of 1,044 ± 171 SFC/million PBMC, which is still significantly different from results for nonresponders (P < 0.001). The average total SFC/million PBMC among the 15 healthy control subjects (with no known exposure to HCV) was 90 ± 12.6, with no detectable CMI responses above the fixed cutoff for any of the HCV pools tested. These data show that almost half of HCW demonstrated strong HCV-specific T cell responses without seroconversion or viremia.
Fig 1.
Strong HCV-specific IFN-γ ELISpot responses in seronegative aviremic Egyptian health care workers. PBMC were stimulated in triplicate with seven sets of pooled overlapping 15-mer HCV genotype 4a peptide antigens for ∼16 h as described in Materials and Methods. The 52 enrolled subjects were classified as responders to between one and seven pools and nonresponders. Fifteen healthy control subjects with no known exposure to HCV (unexposed controls) were used as a control group. For each subject, the total cumulative number of HCV-specific IFN-γ spot-forming cells (SFC) per 106 PBMC, calculated by summing the reactivity to each of the seven antigenic pools after correcting for background, is shown. The crossed lines represent the mean and SEM.
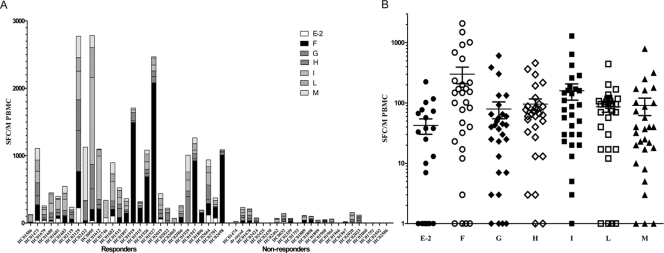
Breadth of HCV-specific CMI among seronegative aviremic HCW.
The IFN-γ ELISpot response of the 52 HCW to the seven HCV overlapping peptide pool antigens is shown in Fig. 2. The 29 subjects responding to at least one HCV antigen pool are shown on the left side of Fig. 2A, with an average response to 3.5 ± 0.4 antigen pools, indicating broad and multispecific responses to HCV. The nonresponders are shown on the right side of Fig. 2A. As shown in Fig. 2B, the mean IFN-γ SFC per HCV antigen pools E2 through M in the responding subjects were 42 ± 8.2, 300 ± 73.4, 79 ± 19.6, 96 ± 17.3, 159 ± 38.1, 86 ± 13.9, and 91 ± 23.0 IFN-γ SFC per million PBMC, respectively. The highest CMI responses were to pools F (the first half of NS3) and I (NS5a). Also, the responses were distributed among the pools tested (broad), with the lowest response in the E2 envelope region of the virus. Nonresponders had an average of fewer than 21 IFN-γ SFC/million PBMC per antigen pool (data not shown). None of the 15 healthy control subjects with no known exposure to HCV had detectable HCV-specific CMI responses in any of the pools tested.
Fig 2.
Breadth of HCV-specific CMI responses among seronegative aviremic Egyptian health care workers. (A) IFN-γ ELISpot response to individual HCV peptide pools among the 52 seronegative aviremic Egyptian HCW per 106 PBMC is shown, with responders grouped on the left side and nonresponders on the right side. (B) The average number of HCV-specific SFC for the individual peptide pools (E2 through M, ± SEM) in the responding subjects is shown in a grouped column scatter plot, with responses mainly in the F and I pools (NS3 and NS5a). The responses shown are after subtraction of the DMSO control.
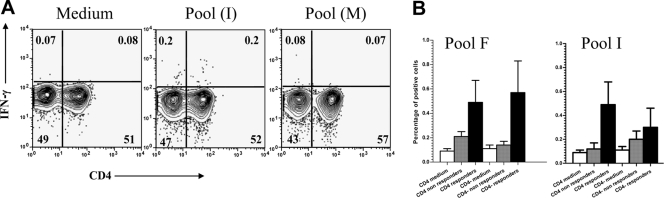
Flow cytometric analysis of cells responding to HCV peptide antigens demonstrates that both CD4+ and CD4− T cells produced IFN-γ.
The phenotype of the HCV-specific IFN-γ-producing cells for the responses shown above was determined by flow cytometry. Subjects for whom sufficient PBMC were available (seven responders and six nonresponders) were tested by stimulating their PBMC with the HCV peptide pools as described in Materials and Methods. Figure 3A shows a representative example of IFN-γ synthesis by both CD4+ and CD4− gated T cells in the presence of the medium control and a responding and a nonresponding HCV peptide pool. In the example shown, approximately 0.2% of both CD4+ and CD4− gated T cells were producing IFN-γ upon stimulation with a responding HCV peptide pool while 0.07% of these cells did so in the nonresponder pool. In general, a responder or a nonresponder was recorded as such in both the IFN-γ ELISpot assay and flow cytometry, indicating agreement between the two assays (not shown). The cumulative percentages of IFN-γ-producing CD4+ and CD4− T cells for the HCV antigenic peptide pools F and I (the two pools with the highest ELISpot response) in seven responders and six nonresponders as classified by ELISpot are shown in Fig. 3B. The data shown confirm that both cell types are able to produce IFN-γ.
Fig 3.
Flow cytometric analysis of cells responding to HCV peptide antigens demonstrates that both CD4+ and CD4− T cells produced IFN-γ in seronegative aviremic health care workers. PBMC were stimulated with 15-mer HCV peptide antigens, and the cells were harvested for flow cytometric analysis by intracellular staining for IFN-γ as described in Materials and Methods. Data were analyzed using FlowJo software (Tree Star Inc., CA). The cells were gated on small lymphocytes using side and forward light scatter profiles and then on CD3+ T cells (not shown). (A) Example of the synthesis of IFN-γ by CD4+ and CD4− T cells in the presence of medium (DMSO control), a responding HCV antigen pool (I), and a nonresponding pool (M) as shown in the panel from left to right, respectively. (B) Cumulative percentage of CD4+ and CD4− T cells producing IFN-γ in response to HCV antigen pools F (left) and I (right) among responding (n = 7) and nonresponding (n = 6) health care workers, respectively. Error bars represent SEM.
DISCUSSION
The main objective of this study was to investigate whether HCV-specific CMI responses existed among seronegative aviremic Egyptian HCW at high risk of infection and, if present, to determine their magnitude and breadth. We demonstrated a strong and broad HCV-specific IFN-γ response without detectable viremia or seroconversion in more than half (55.8%) of Egyptian HCW, with both CD4+ and CD4− T cells producing IFN-γ. We did not find a significant correlation between the strength and breadth of the HCV-specific CMI in these seronegative aviremic subjects and their demographic, clinical, or laboratory data. However, we have previously (2) shown that risk factors associated with HCV infection in these HCW were similar to those in the nearby rural communities (1, 6, 16, 20, 33) and that most HCV infections plausibly occurred in the community and may not be occupationally related to the NLI environment (2).
While several studies demonstrated HCV-specific T-cell responses in exposed seronegative aviremic individuals (3, 17, 24, 27, 37, 39, 41, 52), to our knowledge, only one other study (29) and ours demonstrated this important observation among HCW. In the report from Kubitschke and colleagues (29), CMI was prospectively followed in only 10 HCW who experienced an injury with an HCV-contaminated needle using a set of HLA-A2-restricted peptides. In our study, and for the first time, we monitored the T-cell response in Egyptian HCW using a large set of overlapping peptides derived from a genotype 4a sequence, the most prevalent genotype in Egypt (10). While more than half of our seronegative aviremic HCW had strong HCV-specific CMI responses, these responses may still be an underestimate given the lack of peptide pools matching regions of the HCV genome such as core, E1, and NS2. There are three other studies demonstrating HCV-specific T-cell responses without viremia or seroconversion among Egyptian individuals who are not HCW. One study reported a positive response in 18.3% of 71 household members at high risk of exposure (by living with two or more HCV-infected subjects) compared to only one (2.9%) of 35 subjects at low risk of exposure (no HCV-infected subjects living in the household) using a set of peptides spanning the core and the NS3 to NS5B region of the HCV BK strain (3). Compared to this report (3), our current data show a much higher percentage of seronegative aviremic responders in the NLI high-risk environment, thereby suggesting a higher rate of exposure than in the community. The second study included sexual contacts of acute HCV patients using a restricted set of 18 CD8-restricted peptides and recombinant proteins from genotype 1 (24). In the third study, our group demonstrated that one-third of seronegative aviremic children living with HCV-infected siblings had HCV-specific CMI responses (21).
The data presented in this study suggest that a substantial percentage of Egyptian HCW had strong and broad CMI responses against HCV that possibly led to clearance of past HCV infection without seroconversion. These responses quite likely resulted from transient infection(s) with low levels of HCV that do not necessarily induce a humoral immune response against HCV as previously shown in chimpanzees (43, 47) and humans (36, 39). In this regard and during the conduct of our epidemiological study (2), we actually documented transient low-level viremia in one HCW, who remained seronegative and aviremic for at least 12 months of follow-up after this transient viremia (not shown), suggesting exposure and clearance of HCV without seroconversion. Although we cannot definitely conclude that these responses are protective in nature, we speculate that these seronegative aviremic HCW are repeatedly exposed to HCV and may be protected by these CMI responses. A similar observation has been reported among intravenous drug users who initially cleared a primary HCV infection and were protected against subsequent exposure to the virus; this protection was attributable mainly to T cells (38). These data suggest that depending only on anti-HCV antibody testing to identify prior exposure to HCV can result in a significant underestimation of past exposure, particularly in areas of endemicity or with high-risk subjects. Furthermore, there may be subpopulations of high-risk individuals repeatedly exposed to low viral doses that induce protective CMI and prevent detectable chronic infection (29) without detectable antibodies. If these CMI responses are indeed protective, then these data could have strong implications for vaccine development and for determining the burden of the disease in different communities.
Although it is possible that some of the identified HCV peptide-specific responses could be due to T-cell cross-reactivity to other common pathogens or unrelated antigens (50), we do not believe this to be the case in our study. While it is not possible to distinguish between primary HCV-primed and cross-reactive T cells among the 15% of our study subjects who responded to only one peptide pool using the current set of data, the fact that another 40% of our subjects had HCV-specific T cells that were multispecific in nature makes it unlikely that the peptide-specific responses could be attributed to cross-reactivity alone, and, therefore, they must have resulted from a true exposure to HCV. In addition, none of the 15 healthy control subjects with no known exposure to HCV had any detectable HCV-specific CMI responses, further confirming that the responses detected in our study were unlikely to be due to cross-reactivity. The broad specificity of HCV-specific T cells shown in our HCW is characteristic of subjects who clear HCV infection, whereas individuals with chronic infection typically have few to undetectable responses (11, 18, 32, 40). It should be noted that flow cytometric analysis of the phenotype of cells producing IFN-γ indicated that both CD4+ and CD4− T-cell subsets are able to produce this cytokine.
This study had several strengths, including the large number of enrolled subjects, their contact with a high-risk HCV-infected patient population, and the use of a large panel of genotype 4a peptide pools in a sensitive ex vivo ELISpot assay. While a limitation of this study was that our peptide pools did not span the entire length of the HCV genome, the responses we detected were robust, with a strong correlation between ELISpot and flow cytometry data (not shown). Another limitation of this study is the cross-sectional design, where responses in HCW enrolled in a currently ongoing prospective study were measured at only one time point. However, this study was intended to examine whether HCV-specific CMI responses existed in seronegative, aviremic HCW in an occupationally high-risk environment. The currently ongoing study (2) will continue to examine the longevity of these responses and determine the dominant epitopes in the responding peptide pools.
In conclusion, the induction of strong HCV-multispecific T-cell responses was demonstrated in a high proportion of seronegative aviremic Egyptian HCW occupationally exposed to the virus, which may be protective against future exposures. Our data suggest that the actual past exposure to HCV in Egyptian HCW is underestimated and that the presence of T-cell responses may contribute to the rather low seroconversion rate after occupational exposure to HCV (S. F. Abdelwahab, M. Hashem, I. Galal, Z. Zakaria, N. Mikhail, T. S. Abdel-Ghaffar, G. Galal, S. S. El-Kamary, I. Waked, and G. T. Strickland, unpublished data). Further studies are needed to determine the protective nature of these responses and whether they can be simulated by an HCV vaccine. These findings could have strong implications for planning vaccine studies among populations with a high HCV exposure rate, given that these individuals may already have preexisting CMI responses and may give a false impression about the vaccine-induced response.
ACKNOWLEDGMENTS
This work was supported by the 6th Framework Programme, contract number 0374435, to the HEPACIVAC Consortium, and by the Egyptian Company for Blood Transfusion Services (Egyblood). HCV genotype 4a peptides were provided by an NIH grant, number 1 U01 AI 070802.
We thank G. Thomas Strickland (University of Maryland) for his extremely helpful guidance and comments on this study, Enas S. Aziz (Egyblood) for her technical help with the data, and Iman Fathy (Egyblood) for her help with flow cytometry. We thank Howayda M. Ahmed, Rehab M. El-Sayed, Khaled Bedawey, Mohamed El-Tabakh, and Tamer Abdel-Ghaffar (all from the NLI) for their help with sample collection and enrollment of the HCW. In addition, we are deeply appreciative to Mohamed K. Mohamed and Nabeil Khoury (former Egyblood CEOs) for their immense managerial support throughout the conduct of the study and the dedicated assistance of their professional and technical staff. We also express our gratitude to the HEPACIVAC consortium members, led by R. Cortese, for their helpful comments during the conduct of the study.
None of the authors have any commercial or other association that might pose a conflict of interest.
Footnotes
Published ahead of print 21 March 2012
REFERENCES
- 1. Abdel-Aziz F, et al. 2000. Hepatitis C virus (HCV) infection in a community in the Nile Delta: population description and HCV prevalence. Hepatology 32:111–115 [DOI] [PubMed] [Google Scholar]
- 2. Abdelwahab S, et al. 2012. Risk factors for hepatitis C virus infection among Egyptian healthcare workers in a national liver diseases referral centre. Trans. R. Soc. Trop. Med. Hyg. 106:98–103 [DOI] [PubMed] [Google Scholar]
- 3. Al-Sherbiny M, et al. 2005. Exposure to hepatitis C virus induces cellular immune responses without detectable viremia or seroconversion. Am. J. Trop. Med. Hyg. 73:44–49 [PubMed] [Google Scholar]
- 4. Alter H. 1999. Discovery of non-A, non-B hepatitis and identification of its etiology. Am. J. Med. 107:16S–20S [DOI] [PubMed] [Google Scholar]
- 5. Alter HJ, Bradley DW. 1995. Non-A, non-B hepatitis unrelated to the hepatitis C virus (non-ABC). Semin. Liver Dis. 15:110–120 [DOI] [PubMed] [Google Scholar]
- 6. Arafa N, et al. 2005. Changing pattern of hepatitis C virus spread in rural areas of Egypt. J. Hepatol. 43:418–424 [DOI] [PubMed] [Google Scholar]
- 7. Baldo V, et al. 2002. Occupational risk of blood-borne viruses in healthcare workers: a 5-year surveillance program. Infect. Control Hosp. Epidemiol. 23:325–327 [DOI] [PubMed] [Google Scholar]
- 8. Bassett SE, et al. 2001. Protective immune response to hepatitis C virus in chimpanzees rechallenged following clearance of primary infection. Hepatology 33:1479–1487 [DOI] [PubMed] [Google Scholar]
- 9. Bowen DG, Walker CM. 2005. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature 436:946–952 [DOI] [PubMed] [Google Scholar]
- 10. Chamberlain RW, Adams N, Saeed AA, Simmonds P, Elliott RM. 1997. Complete nucleotide sequence of a type 4 hepatitis C virus variant, the predominant genotype in the Middle East. J. Gen. Virol. 78(Pt 6):1341–1347 [DOI] [PubMed] [Google Scholar]
- 11. Chang KM, et al. 2001. Differential CD4(+) and CD8(+) T-cell responsiveness in hepatitis C virus infection. Hepatology 33:267–276 [DOI] [PubMed] [Google Scholar]
- 12. Di Bisceglie, A M. 1997. Hepatitis C and hepatocellular carcinoma. Hepatology 26:34S–38S [DOI] [PubMed] [Google Scholar]
- 13. Elrefaei M, El-sheikh N, Kamal K, Cao H. 2004. Analysis of T cell responses against hepatitis C virus genotype 4 in Egypt. J. Hepatol. 40:313–318 [DOI] [PubMed] [Google Scholar]
- 14. El-Zanaty F, Way A. 2009. Egypt Demographic and Health Survey, 2008. Ministry of Health, El-Zanaty and Associates, and Macro International, Cairo, Egypt: http://www.measuredhs.com [Google Scholar]
- 15. Farid A, et al. 2005. Schistosoma infection inhibits cellular immune responses to core HCV peptides. Parasite Immunol. 27:189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frank C, et al. 2000. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet 355:887–891 [DOI] [PubMed] [Google Scholar]
- 17. Freeman AJ, et al. 2004. Prevalence of production of virus-specific interferon-gamma among seronegative hepatitis C-resistant subjects reporting injection drug use. J. Infect. Dis. 190:1093–1097 [DOI] [PubMed] [Google Scholar]
- 18. Graham CS, et al. 2004. Comparison of HCV-specific intrahepatic CD4+ T cells in HIV/HCV versus HCV. Hepatology 40:125–132 [DOI] [PubMed] [Google Scholar]
- 19. Grakoui A, et al. 2003. HCV persistence and immune evasion in the absence of memory T cell help. Science 302:659–662 [DOI] [PubMed] [Google Scholar]
- 20. Habib M, et al. 2001. Hepatitis C virus infection in a community in the Nile Delta: risk factors for seropositivity. Hepatology 33:248–253 [DOI] [PubMed] [Google Scholar]
- 21. Hashem M, et al. 2011. Strong hepatitis C virus (HCV)-specific cell-mediated immune responses in the absence of viremia or antibodies among uninfected siblings of HCV chronically infected children. J. Infect. Dis. 203:854–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hofmann F, Michaelis M, Rieger MA, Hasselhorn HM, Berthold H. 1997. Occupational medicine significance of hepatitis C in health care employees. Gesundheitswesen 59:452–460 (In German.) [PubMed] [Google Scholar]
- 23. Houghton M. Prospects for prophylactic and therapeutic vaccines against the hepatitis C viruses. Immunol. Rev. 239:99–108 [DOI] [PubMed] [Google Scholar]
- 24. Kamal SM, et al. 2004. Cellular immune responses in seronegative sexual contacts of acute hepatitis C patients. J. Virol. 78:12252–12258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kamal SM, et al. 2001. Specific cellular immune response and cytokine patterns in patients coinfected with hepatitis C virus and Schistosoma mansoni. J. Infect. Dis. 184:972–982 [DOI] [PubMed] [Google Scholar]
- 26. Kamal SM, et al. 2004. Kinetics of intrahepatic hepatitis C virus (HCV)-specific CD4+ T cell responses in HCV and Schistosoma mansoni coinfection: relation to progression of liver fibrosis. J. Infect. Dis. 189:1140–1150 [DOI] [PubMed] [Google Scholar]
- 27. Kennedy PT, et al. 2006. The influence of T cell cross-reactivity on HCV-peptide specific human T cell response. Hepatology 43:602–611 [DOI] [PubMed] [Google Scholar]
- 28. Kosgeroglu N, Ayranci U, Vardareli E, Dincer S. 2004. Occupational exposure to hepatitis infection among Turkish nurses: frequency of needle exposure, sharps injuries and vaccination. Epidemiol. Infect. 132:27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kubitschke A, et al. 2007. Induction of hepatitis C virus (HCV)-specific T cells by needle stick injury in the absence of HCV-viraemia. Eur. J. Clin. Invest. 37:54–64 [DOI] [PubMed] [Google Scholar]
- 30. Lanphear BP, et al. 1994. Hepatitis C virus infection in healthcare workers: risk of exposure and infection. Infect. Control Hosp. Epidemiol. 15:745–750 [DOI] [PubMed] [Google Scholar]
- 31. Lauer GM, Walker BD. 2001. Hepatitis C virus infection. N. Engl. J. Med. 345:41–52 [DOI] [PubMed] [Google Scholar]
- 32. Lauer GM, et al. 2002. Comprehensive analysis of CD8+-T-cell responses against hepatitis C virus reveals multiple unpredicted specificities. J. Virol. 76:6104–6113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Medhat A, et al. 2002. Hepatitis c in a community in Upper Egypt: risk factors for infection. Am. J. Trop. Med. Hyg. 66:633–638 [DOI] [PubMed] [Google Scholar]
- 34. Mehta SH, et al. 2002. Protection against persistence of hepatitis C. Lancet 359:1478–1483 [DOI] [PubMed] [Google Scholar]
- 35. Meky FA, et al. 2006. Active surveillance for acute viral hepatitis in rural villages in the Nile Delta. Clin. Infect. Dis. 42:628–633 [DOI] [PubMed] [Google Scholar]
- 36. Meyer MF, et al. 2007. Prevalence of hepatitis C in a German prison for young men in relation to country of birth. Epidemiol. Infect. 135:274–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mizukoshi E, et al. 2008. Hepatitis C virus (HCV)-specific immune responses of long-term injection drug users frequently exposed to HCV. J. Infect. Dis. 198:203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Osburn WO, et al. 2010. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology 138:315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Post JJ, et al. 2004. Clearance of hepatitis C viremia associated with cellular immunity in the absence of seroconversion in the hepatitis C incidence and transmission in prisons study cohort. J. Infect. Dis. 189:1846–1855 [DOI] [PubMed] [Google Scholar]
- 40. Rosen HR, et al. 2002. Frequencies of HCV-specific effector CD4+ T cells by flow cytometry: correlation with clinical disease stages. Hepatology 35:190–198 [DOI] [PubMed] [Google Scholar]
- 41. Scognamiglio P, et al. 1999. Presence of effector CD8+ T cells in hepatitis C virus-exposed healthy seronegative donors. J. Immunol. 162:6681–6689 [PubMed] [Google Scholar]
- 42. Seeff LB. 2002. Natural history of chronic hepatitis C. Hepatology 36:S35–46 [DOI] [PubMed] [Google Scholar]
- 43. Shata MT, et al. 2003. Exposure to low infective doses of HCV induces cellular immune responses without consistently detectable viremia or seroconversion in chimpanzees. Virology 314:601–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shoukry NH, et al. 2003. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J. Exp. Med. 197:1645–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Strickland GT. 2006. Liver disease in Egypt: hepatitis C superseded schistosomiasis as a result of iatrogenic and biological factors. Hepatology 43:915–922 [DOI] [PubMed] [Google Scholar]
- 46. Strickland GT, et al. 2002. Role of hepatitis C infection in chronic liver disease in Egypt. Am. J. Trop. Med. Hyg. 67:436–442 [DOI] [PubMed] [Google Scholar]
- 47. Thomson M, et al. 2003. The clearance of hepatitis C virus infection in chimpanzees may not necessarily correlate with the appearance of acquired immunity. J. Virol. 77:862–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Torresi J, Johnson D, Wedemeyer H. 2011. Progress in the development of preventive and therapeutic vaccines for hepatitis C virus. J. Hepatol. 54:1273–1285 [DOI] [PubMed] [Google Scholar]
- 49. Waked IA, et al. 1995. High prevalence of hepatitis C in Egyptian patients with chronic liver disease. Gut 37:105–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wedemeyer H, Mizukoshi E, Davis AR, Bennink JR, Rehermann B. 2001. Cross-reactivity between hepatitis C virus and influenza A virus determinant-specific cytotoxic T cells. J. Virol. 75:11392–11400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Williams R. 2006. Global challenges in liver disease. Hepatology 44:521–526 [DOI] [PubMed] [Google Scholar]
- 52. Zeremski M, et al. 2009. Hepatitis C virus-specific T-cell immune responses in seronegative injection drug users. J. Viral Hepat. 16:10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]