Abstract
Vitamin A deficiency (VAD) has profound effects on immune responses in the gut, but its effect on other mucosal responses is less well understood. Sendai virus (SeV) is a candidate human parainfluenza virus type 1 (hPIV-1) vaccine and a candidate vaccine vector for other respiratory viruses. A single intranasal dose of SeV elicits a protective immune response against hPIV-1 within days after vaccination. To define the effect of VAD on acute responses toward SeV, we monitored both antibodies and CD8+ T cells in mice. On day 10 following SeV infection, there was a trend toward lower antibody activities in the nasal washes of VAD mice than in those of controls, while bronchoalveolar lavage (BAL) fluid and serum antibodies were not reduced. In contrast, there was a dramatic reduction of immunodominant CD8+ T cell frequencies in the lower respiratory tract (LRT) airways of VAD animals. These T cells also showed unusually high CD103 (the αE subunit of αEβ7) expression patterns. In both VAD and control mice, E-cadherin (the ligand for αEβ7) was better expressed among epithelial cells lining the upper respiratory tract (URT) than in LRT airways. The results support a working hypothesis that the high CD103 expression among T cell populations in VAD mice alters mechanisms of T cell cross talk with URT and LRT epithelial cells, thereby inhibiting T cell migration and egress into the lower airway. Our data emphasize that the consequences of VAD are not limited to gut-resident cells and characterize VAD influences on an immune response to a respiratory virus vaccine.
INTRODUCTION
Vitamin A deficiency (VAD) is a common dietary concern, particularly in developing countries. The deficiency is associated with increased susceptibility to disease, poor responses to vaccination, and increased mortality (28, 35, 37, 38). Vitamin A supplements can improve health in some circumstances, but there are also risks associated with excessive vitamin A supplementation (4, 6, 11, 30, 43, 48).
Vitamin A is usually acquired from the diet as all-trans-retinol, retinyl ester, or beta-carotene. All-trans-retinol and beta-carotene can be oxidized to all-trans-retinal by ubiquitous alcohol dehydrogenases, after which all-trans-retinal can be oxidized to all-trans-retinoic acid (RA) by retinal dehydrogenases (RALDHs), which are expressed primarily by intestinal epithelial cells and gut-associated dendritic cells (DCs) (6, 9, 20, 21, 23–25). RA is capable of activating nuclear receptors of the retinoic acid receptor (RAR) family and altering the transcription of many genes. Among its many functions, RA can increase the expression of metalloproteinases in DCs and migration to lymph nodes. RA can also enhance DC maturation and antigen presentation in the presence of inflammatory stimuli such as tumor necrosis factor (TNF) (3, 22, 31, 32).
Immune responses of the gut are particularly susceptible to VAD, in part due to RA influences on intestinal B and T cell activities (9, 20, 21, 23–25, 36, 46). For example, vitamin A and RA can support B cell activation, inhibit B cell apoptosis, augment IgA antibody-forming cells (AFCs) in the gut mucosa, and increase levels of total IgA in the intestinal lumen. Improvements to immune function in the gut are greatly influenced by the effects of vitamin A on lymphocyte homing. RA induces retinol-metabolizing activity in gut DCs, which can, in turn, “imprint” T cells to adopt specific tissue homing properties. As examples, T cells marked with high α4β7-integrin and CC-chemokine receptor 9 (CCR9) molecules exhibit enhanced T cell infiltration into the small intestine (6, 13, 23, 40), while cells marked with α4β7-integrin and low CCR9 levels may more readily traffic to other sites. Imprinting is most pronounced on Peyer's patch-induced effector and memory lymphocytes, and many T lymphocyte subsets can be influenced (e.g., CD8+, Th17, and CD4+ T-regulatory cells [16, 20, 21, 23–25, 45]). Blocking RAR can cause a significant decrease in T cell homing. Similarly, B cells can be educated to acquire or lose gut-homing potential when they are stimulated in the presence or absence, respectively, of RA (24, 25). There is some debate concerning the relative influence of vitamin A on nongut tissues, because cells in locations outside the intestine are relatively weak in vitamin A metabolizing capacity (17, 23, 35, 38).
Sendai virus (SeV; a murine parainfluenza virus type 1 [PIV-1]) is currently being studied as a candidate respiratory viral vaccine for pediatric croup caused by its cousin, human parainfluenza virus type 1 (hPIV-1). Due to its close sequence and structural similarities with hPIV-1, SeV stimulates cross-reactive responses and protection against hPIV-1 in cotton rats and in nonhuman primates (12, 34). SeV can also be used in a recombinant form to protect against other serious human pediatric viruses such as respiratory syncytial virus and human PIV-3 in animal models (14, 41, 42, 49, 50).
SeV is particularly attractive as a human pediatric vaccine and vaccine backbone because of the rapidity with which immune response can be generated. Both antibodies and CD8+ T cell effectors are induced within 10 days following a single intranasal (i.n.) inoculation with SeV (33, 34). When SeV is tested in a cotton rat model, a model susceptible to human respiratory virus infections, protection against human challenge viruses can also be detected within days after vaccination (34). This rapid induction of protective immunity may be essential for a respiratory virus vaccine that targets the human pediatric arena, because young infants require immediate protection against viral disease.
We sought to determine whether the acute murine response to an i.n. inoculation with SeV is influenced by VAD. We show that in the context of VAD, (i) there is a trend toward reduction of SeV-specific antibody in the upper respiratory tract (URT), (ii) there is a dramatic reduction of immunodominant SeV-specific CD8+ T cells in the lower respiratory tract (LRT) airway, (iii) this population exhibits an unusually high percentage of CD103-positive cells, and (iv) E-cadherin, the ligand for αEβ7, exhibits normal expression levels in VAD mice; there is much higher E-cadherin expression in URT than in LRT airway epithelial cells, a pattern that may influence the migration of SeV-specific CD103-positive T cells. Data emphasize that the impact of VAD is not exclusive to the gut. Rather, this dietary deficiency can significantly alter an acute immune response to a respiratory viral vaccine.
MATERIALS AND METHODS
Animals and housing.
Pregnant female C57BL/6 (H-2b) mice were purchased from Charles River (Wilmington, MA) for the VAD studies. SeV-seronegative animals were housed in filter-top cages in a biosafety level 2+ containment area with sentinel caged mice, following guidelines outlined by the Association for Assessment and Accreditation for Laboratory Animal Care (AAALAC). Studies were approved by the Institutional Animal Care and Use Committee (IACUC).
To establish VAD mice, day 4 to 5 estrus C57BL/6 females were placed on characterized diets (Harlan Laboratories, Madison, WI) upon arrival in the animal facility at St. Jude Children's Research Hospital. The VAD diet (item number TD.10762) was formulated with casein, dl-methionine, sucrose, corn starch, cottonseed oil, cellulose, mineral mix AIN-76 (item number 170815), calcium carbonate, vitamin mix (lacking vitamin A) plus choline, and food coloring. The control diet included vitamin A palmitate at 15 IU/g (item number TD.10764). Animals were sustained on the diet throughout their pregnancies, and weaned pups were on the diet until the completion of experiments. This procedure typically renders serum retinol levels of ≤0.35 μmol/liter in VAD test animals (10). Infections of mice involved anesthesia with isoflurane or tribromoethanol (Avertin) followed by i.n. inoculations with 250 to 500 PFU of SeV. Immediately prior to sacrifice for cell collection, mice were anesthetized with tribromoethanol and exsanguinated. Following sacrifice and prior to tissue removal for digestions, mice were perfused with phosphate-buffered saline (PBS).
Nasal wash and BAL samples.
Nasal washes were collected after clipping the trachea, by flushing the upper airway with 0.2 ml of PBS. Bronchoalveolar lavage (BAL) samples were collected by inserting catheters into the trachea and washing three times with 1 ml of PBS each time (3 ml total). Wash samples were centrifuged to separate cellular material.
ELISA.
For the detection of SeV-specific antibodies, sucrose gradient-purified SeV was lysed in disruption buffer (0.05% Triton X-100, 60 mM KCl, 10 mM Tris [pH 7.8]) and diluted with PBS to yield 10 μg/ml for the coating of 96-well enzyme-linked immunosorbent assay (ELISA) plates. After overnight (ON) incubation with 50 μl of the diluted virus lysate per well at 4°C, plates were washed three times with PBS and blocked with 200 μl of PBS containing 3% bovine serum albumin (BSA; Sigma, St. Louis, MO). Samples from vaccinated and control animals were serially diluted in PBS containing 1% BSA and 0.1% Tween 20. Diluted samples (50 μl) were applied to the plates and incubated for 1 h at 37°C. Plates were then washed six times with PBS-Tween 20 (0.05%) and incubated with alkaline phosphatase-conjugated goat anti-mouse Ig(H+L) (SouthernBiotechnology Associates, Birmingham, AL; catalog number 1010-04) diluted 1:1,000 in PBS containing 1% BSA and 0.1% Tween 20, for 1 h at 37°C. Plates were washed six times with PBS-Tween 20 (0.05%) and developed by addition of p-nitrophenyl phosphate substrate (0.5 mg/ml) in diethanolamine buffer (0.1 M Tris, 0.1 M NaCl, 5% diethanolamine, 10 mM MgCl2 [pH 9.8]). Reactions were stopped after 20 min with 5 μl of 0.5 M EDTA (catalog number E-7889; Sigma). Readings for optical density (OD) at 405 nm (EMax precision microplate reader; Molecular Devices, Inc., Sunnyvale, CA) were analyzed with GraphPad Prism software.
Preparation and staining of tissue sections.
After euthanasia, mice were perfused with 10% buffered formalin (Thermo Scientific, Kalamazoo, MI) through the left cardiac ventricle. The tissues were postfixed by immersion in 10% buffered formalin for at least 24 h. URT samples were decalcified in formic acid (TBD-2 decalcifier; Thermo Scientific) for 2 days. Tissues were embedded in paraffin for sectioning. Slides of paraffin-sectioned tissues were submerged in xylene followed by submersion in 50 ml of acetone containing 25 μl of 30% H2O2. Slides were air dried, soaked in PBS, and then blocked with 3% BSA-PBS for 1 h at room temperature (RT) in a humidified chamber. Slides were next incubated with biotinylated goat anti-mouse E-cadherin (R&D Systems; catalog number BAF748) in 3% BSA-PBS overnight at 4°C. After a PBS wash, slides were incubated with avidin-horseradish peroxidase (avidin-HRP) (1:100; BD Pharmingen; catalog number 554058). Another PBS wash was followed by slide incubation with substrate (20 mg of 3-amino-9-ethyl carbazole [AEC; Sigma; catalog number A5754] dissolved in dimethyl sulfoxide [DMSO] and then mixed in 50 ml of 50 mM sodium acetate, pH 5, containing 25 μl of 30% H2O2, prefiltered) for 10 min at room temperature. Slides were washed with PBS and counterstained with hematoxylin solution (Sigma; catalog number GHS316). Following a water rinse, slides were layered with Clear-Mount (Electron Microscopy Sciences; catalog number 17985-12), allowed to dry at room temperature, layered with Permount (Fisher; catalog number SP15-100), and mounted with a coverslip.
Digestion of URT and LRT tissues for culture and FACS analyses.
URT tissues were harvested by removing skin, lower jaws, soft palates (including the attached organized nose-associated lymphoid tissue [o-NALT]), muscles, cheek bones, and incisors from the heads (2, 8). Remaining snouts were separated from the head and cut into small pieces. Lungs were removed and chopped into small pieces. To release cells, samples were treated with collagenase (200 U/ml) and dispase (50 U/ml) in PBS for 30 min at 37°C with shaking, 10 ml per gram tissue. DNase was then added to a concentration of 15 U/ml for an additional 30 min of incubation at 37°C. Complete tumor medium (CTM; a modified Eagle medium [Invitrogen, Grand Island, NY] supplemented with dextrose [500 μg/ml], glutamine [2 mM], 2-mercaptoethanol [3 × 10−5 M], essential and nonessential amino acids, sodium pyruvate, sodium bicarbonate, and antibiotics [15, 47]) with 10% heat-inactivated fetal calf serum (FCS) was added and cells were passed through a 70-μm BD Falcon filter. Cells were washed and used either for immediate fluorescence-activated cell sorter (FACS) analyses or for culture with or without SeV infection. In the latter case, cells were suspended in serum-free CTM and incubated for 1 h at 37°C with SeV at a multiplicity of infection (MOI) of 5. Infected cells and uninfected controls were centrifuged and resuspended in CTM (10% FCS) for ON incubation at 37°C. Cells were harvested the following day by scraping (Sarstedt, Inc.; catalog number 83.1830) for FACS analyses.
Cell staining.
All cells were pretreated with a 1:200 dilution of Fc block CD16/CD32 in PBS plus 1% FCS prior to membrane staining. For the staining of SeV-specific immunodominant CD8+ T cells, major histocompatibility complex (MHC) class I (Kb) tetramers were generated by folding Sendai virus NP324–332 (FAPGNYPAL) with heavy and light chains as described previously (7, 26). Tetramers were stored as aliquots at 4°C. BAL fluid lymphocytes were stained with phycoerythrin (PE)-conjugated tetrameric reagent for 1 h at RT, followed by staining with labeled anti-CD62L, anti-CD44, anti-CD11a, anti-CD69, anti-CD103, or anti-LPAM-1 (BD Pharmingen, San Diego, CA) on ice for 20 min. Samples were analyzed on a FACSCalibur. Lymphocytes were gated for analyses based on forward- and side-scatter parameters.
To examine digested URT and LRT tissues before or after culture, cells were stained with anti-CD11b (BD Pharmingen) and 7AAD (1 μg/ml; Invitrogen). Additional stains were anti-E-cadherin (R&D Systems) and anti-ICAM-1 (Biolegend). Dead cells, platelets, red blood cells (RBCs), small lymphocytes, and macrophages were excluded from analyses based on forward scatter and positivity for 7AAD and CD11b. Data were analyzed using FlowJo version 7.6.4 software (Tree Star Inc., Ashland, OR).
Virus measurements in infected animals.
The lungs and nasal turbinates were removed aseptically and washed in PBS. Suspensions were centrifuged at 2,000 × g for 10 min to clear cellular debris. Virus titers were measured as 50% tissue culture infectious doses (TCID50). The TCID50 measurements were performed by plating serial 10-fold dilutions of lung and nasal turbinate suspensions on LLC-MK2 cells with minimal essential medium containing 0.1% bovine serum albumin in the presence of 5 μg/ml of acetylated trypsin and 50 μg/ml of gentamicin. Cell supernatants were collected after 4 to 5 days of incubation and mixed 1:1 with chicken red blood cells (0.5%) in PBS for hemagglutination detection. TCID50 values were calculated using the Reed-Muench formula.
RESULTS
Robust virus-specific serum antibody responses in VAD animals on day 10 postinfection.
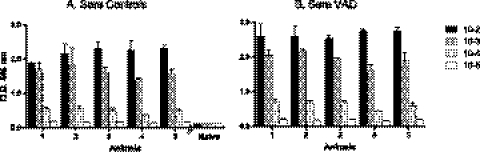
Previous work has shown that the antibody responses to SeV are rapidly induced and are long-lasting (14, 33, 34). We therefore questioned whether serum antibody responses to SeV might be reduced in the context of VAD. Sera from VAD and control animals were sampled on day 10 after infection for SeV-specific antibody levels. As demonstrated in Fig. 1, the serum antibody levels were not reduced in VAD compared to control animals.
Fig 1.
Robust virus-specific acute-phase serum antibody responses in VAD mice. Ten days after infection with 250 PFU of SeV, sera were serially diluted and tested for the presence of SeV-specific antibodies. ELISA results are shown for a representative of 4 individual experiments. Each group included 5 mice (x axis), and each dilution for each serum sample was tested in replicate. Means and standard deviations are shown. Naïve mouse serum samples served as negative controls. Antibody levels in mice deficient in vitamin A (B) were not reduced compared with antibody levels in mice on the control diet (A).
Virus-specific antibody responses in nasal washes and BAL fluid of VAD animals on day 10 postinfection.
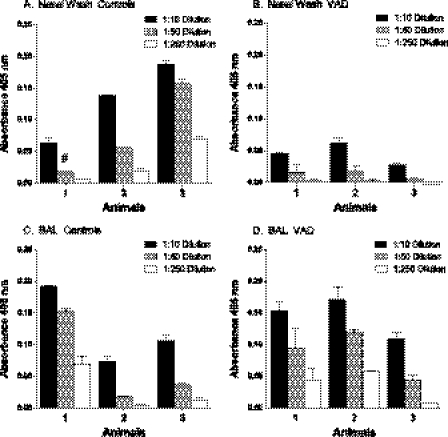
We also questioned whether SeV-specific immune responses were altered in the nasal washes or BAL fluid of vaccinated VAD animals compared to controls. As shown in Fig. 2A and B, there was a trend toward lower antibody levels in nasal washes of VAD animals (P = 0.085 for unpaired t test of mean 1:10 dilution values for 3 mice per group). The trend toward lower nasal wash antibodies in VAD mice was similar in a repeat experiment. In contrast, there was no indication of antibody reduction in the BAL fluid.
Fig 2.
Virus-specific acute-phase antibody responses in nasal wash and BAL fluid of VAD mice. Ten days after infection with 250 PFU of SeV, nasal wash (A and B) and BAL fluid (C and D) samples were collected from 3 individual control (A and C) and VAD mice (B and D) for assessment of antibody activity. ELISA results are shown for a representative of 2 independent experiments. Each dilution for each nasal or BAL sample was tested in replicate. Means and standard deviations are shown. The pound sign identifies one bar in which an unexpectedly high outlier value was associated with the 1:50 sample dilution. It was higher than all values associated with the 1:10 sample dilution and was therefore excluded from the calculation.
VAD animals exhibit reduced frequencies of SeV-specific CD8+ T cells in the BAL fluid.
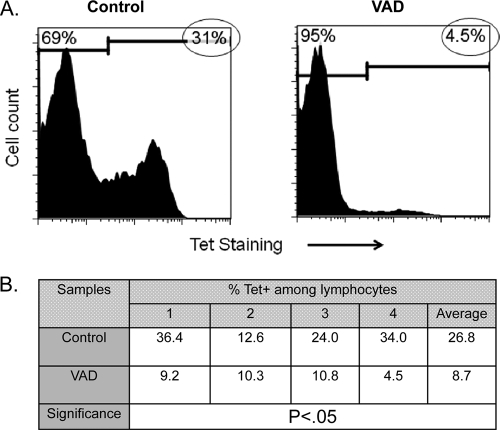
To examine CD8+ T cell responses in VAD animals, we used a tetramer that marks immunodominant SeV-specific CD8+ T cells in C57BL/6 mice (7). In normal animals, the CD8+ T cell response can typically be measured by sampling the BAL fluid 10 days after infection (very few cells can be harvested from the nasal wash). As expected, we found that Tet+ T cells were present at high frequency among lymphocytes in the BAL fluid of control animals by day 10 (Fig. 3A). However, in VAD animals, there was a reproducible and significant reduction of Tet+ T cell frequencies. The results from 4 individual mice in an independent experiment are shown in Fig. 3B, demonstrating statistical significance. Total lymphocyte counts in the BAL fluid were often reduced by as much as 50% in VAD mice compared to controls, indicating that Tet+ T cells in the LRT airways of VAD mice were reduced both by percentages among lymphocytes and by absolute numbers.
Fig 3.
Reduced frequencies of immunodominant virus specific (Tet+) T cells in the BAL fluid of infected VAD mice. Ten days after inoculation of C57BL/6 mice with 250 PFU of SeV, BAL fluid cells were collected for T cell phenotype analyses. (A) Cells pooled from 5 mice per group were gated on lymphocytes, and markers were set to discriminate between the Tet-positive (circled values) and Tet-negative phenotypes (naïve BAL fluid cells do not stain with the Tet reagent). There was a high frequency of Tet+ T cells in control animals (left) but not in VAD animals (right) in each of four separate experiments. (B) Values from 4 individually tested control and VAD mice from one experiment were tabulated and compared using an unpaired t test, demonstrating a significant difference (GraphPad Prism software).
Heightened frequencies of CD103-positive cells among Tet+ T cells in VAD animals.
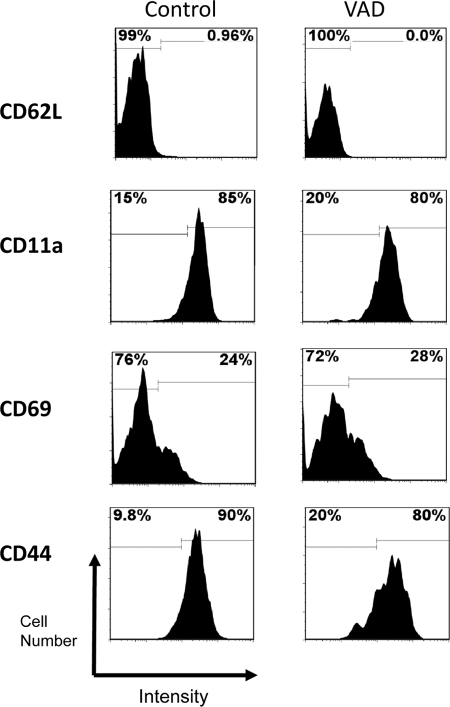
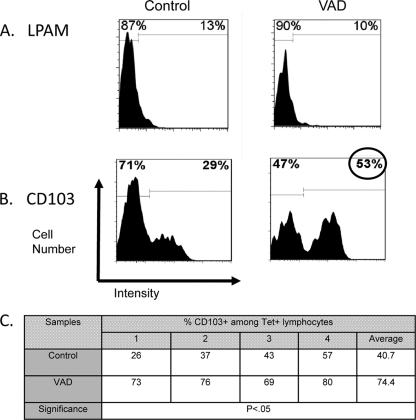
To help explain the reduced appearance of vaccine-induced Tet+ T cells in the BAL fluid of VAD animals, we examined the phenotype of these cells in test and control mice. Of particular interest were the patterns of membrane homing and activation antigens. As shown in Fig. 4, an array of markers were tested, including CD62L (L-selectin, a cell adhesion protein that binds the high endothelial venules of peripheral lymphoid tissues), CD11a (a subunit of integrin LFA-1, a receptor for ICAMs), CD69 (a marker of early activation, indicative of recent antigen encounter), and CD44 (an activation and adhesion membrane protein), but significant differences were not observed between VAD and control animals. In Fig. 5A and B are shown the results with antibodies specific for LPAM-1 (α4β7, an integrin prevalent on gut-associated T cells) and CD103 (the αE subunit of a second β7-integrin, αEβ7). We found that CD103 was upregulated in VAD animals compared to controls in multiple experiments. The results from the testing of individual mice in a representative experiment are shown in Fig. 5C, illustrating statistical significance.
Fig 4.
Levels of homing and activation antigens on Tet+ T cells in VAD animals. Markers CD62L, CD11a, CD69, and CD44 were examined among Tet+ cells in control (left) and VAD (right) mice 10 days after infection with 250 PFU of SeV. Cells from 5 mice per group were pooled. Representative histograms are shown. Results were similar for each of 4 separate experiments. Histogram markers were set to define differences between VAD and control mice, either in terms of positive or negative membrane protein expression (as for CD62L) or in terms of high or low protein expression (as for CD11a).
Fig 5.
Elevated CD103 expression in responding Tet+ T cells in VAD animals. Ten days after inoculation of C57BL/6 mice with 250 PFU of SeV, BAL fluid cells were examined by FACS analyses. Tet+ T cells were tested for LPAM (A) and CD103 (B) markers. Histograms are shown with a circle highlighting the high CD103 percentage among Tet+ T cells in VAD mice. Histogram markers were set to define differences between VAD and control mice for positive or negative membrane protein expression. Cells were from 5 mice per group (pooled), and results are representative of 4 separate experiments. (C) CD103 results from one experiment were averaged from 4 individually tested control and VAD mice. Differences between mouse groups were statistically significant as indicated by an unpaired t test (GraphPad Prism software). Absolute CD103 expression frequencies were variable among mice within and between experiments but were higher for VAD versus control Tet+ T cells.
E-cadherin, the ligand for αEβ7, is expressed similarly in the respiratory tracts of control and VAD mice, with increased levels in URT compared to LRT tissues.
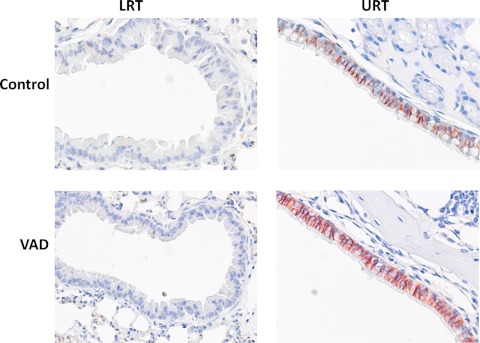
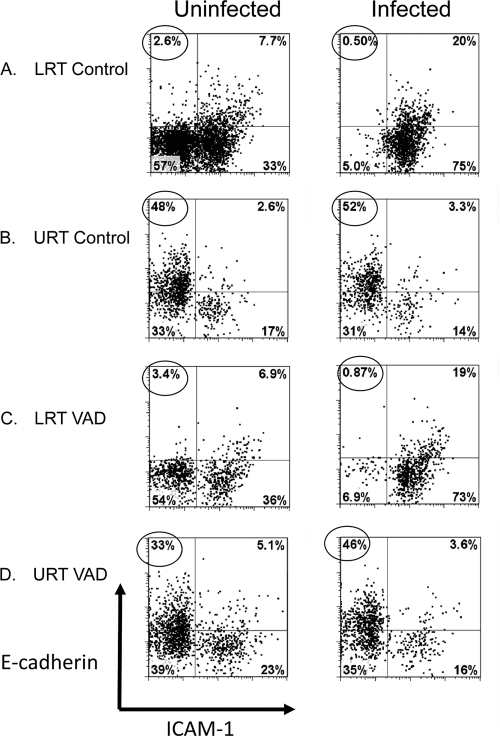
CD103 is the αE subunit of the αEβ7-integrin protein with affinity for a membrane ligand, E-cadherin, that marks respiratory tract epithelial cells. We questioned whether E-cadherin levels were altered in the context of VAD. In Fig. 6 are shown fixed tissue sections from the URT and LRT airways of uninfected control and VAD animals. As shown, there was relatively low expression in the bronchoalveolar epithelial tissue sections (LRT) compared to nasal epithelial tissue sections (URT), but there was no discernible difference between VAD and control animals. To better quantify E-cadherin levels in both control and VAD animals, we digested URT and LRT tissues with collagenase, dispase, and DNase to release matrix cells for analyses. Cells were placed in overnight culture followed by a 1-day culture with or without SeV. Cells were then costained with antibodies for E-cadherin and ICAM-1, the ligand for CD11a. Results in Fig. 7 support those in Fig. 6, in that cells from control and VAD mice were similar and there were higher levels of E-cadherin in URT than in LRT cells. The E-cadherinHIGH cells in the URT were generally ICAM-1LOW in cultures with or without SeV (circled values indicate percentages of E-cadherinHIGH ICAM-1LOW cells). E-cadherinLOW ICAM-1HIGH cells were more prevalent in the LRT cultures, particularly after SeV infection.
Fig 6.
Expression of E-cadherin is higher in URT than in LRT tissues. Fixed LRT and URT tissues from naïve mice were stained for E-cadherin. Photographs are shown for LRT (left) and URT (right) from control and VAD mice. The LRT sections show bronchiolar epithelium lining the bronchioles surrounded by alveoli. The URT nasal sections show the ciliated upper respiratory epithelium with underlying turbinate bone. Tissues were stained with hematoxylin (blue) and anti-E-cadherin antibody (red), the latter being most prominent in the URT.
Fig 7.
E-cadherinHIGH ICAM-1LOW cells in the URT. Cells from LRT and URT tissues from individual naïve mice were isolated by digestions with collagenase, dispase, and DNase and cultured overnight with and without SeV. Harvested cells were evaluated for E-cadherin and ICAM-1 staining. Small lymphocytes, platelets, RBC, dead cells, and macrophages were excluded from analyses based on low forward scatter or high 7AAD or CD11b staining. Results from cells incubated with (right) and without (left) SeV are shown for LRT (A and C) and URT (B and D) cells from control and VAD mice. Dot plot markers were set to discriminate E-cadherinHIGH ICAM-1LOW (top left quadrant) and E-cadherinLOW ICAM-1HIGH (bottom right quadrant) populations. Circled values identify E-cadherinHIGH ICAM-1LOW cells. Results were similar in each of 2 independent experiments.
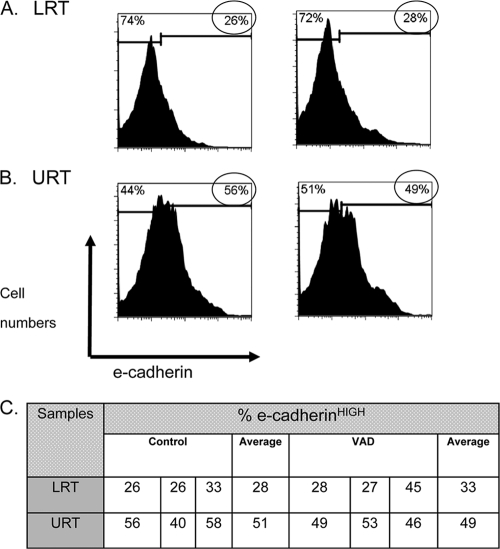
URT and LRT cells from control and VAD animals were also examined after a 10-day in vivo infection with SeV (Fig. 8). In this case, cells were not cultured prior to analyses. Again as expected, the percentages of total E-cadherinHIGH cells (as indicated by circled values) were similar between control and VAD mice and were significantly higher in URT than in LRT tissues. Figure 8A and B show representative profiles, while Fig. 8C shows results from individually tested mice. It is likely that the ultimate positioning of T cells in LRT versus URT tissues following an i.n. infection is dependent on these differential displays of matrix membrane molecules.
Fig 8.
E-cadherin expression levels are higher in URT than in LRT tissues in infected mice. Ten days following infection with 250 PFU of SeV, LRT and URT tissues were isolated from VAD and control mice by digestions with collagenase, dispase, and DNase for immediate FACS analyses. Cells were from 1 of 3 individual mice described in panel C. Small lymphocytes, platelets, RBCs, dead cells, and macrophages were excluded from analyses based on low forward scatter or high 7AAD or CD11b staining. Histograms of E-cadherin staining are shown for LRT (A) and URT (B) cells from control (left) and VAD (right) mice. Histogram markers and circled values identify E-cadherinHIGH cells in each population. Results were similar in each of 2 independent experiments. (C) Results from individual mice demonstrate statistically significant differences between E-cadherin expression levels in LRT and URT tissues for both VAD and control mice (unpaired t tests, P < 0.05, GraphPad Prism).
Weight loss and virus load in VAD mice.
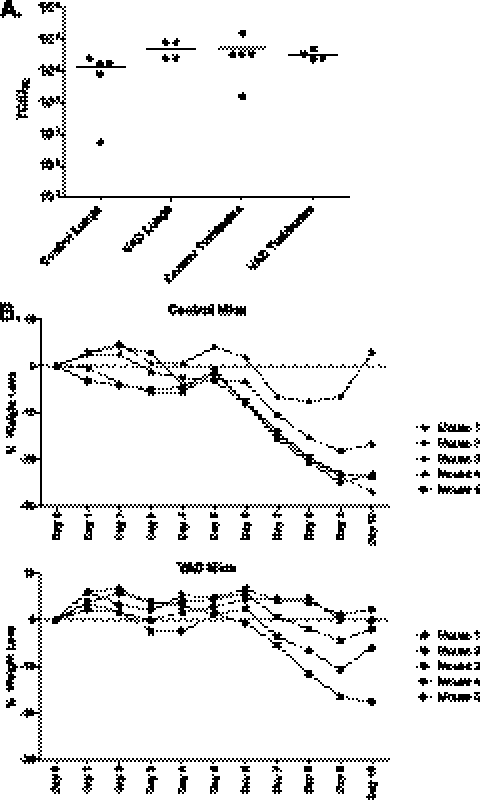
To determine if VAD impacted SeV infectivity, virus loads were compared between VAD and test mice on day 7 after SeV exposure. As demonstrated in Fig. 9A, the lung and nasal turbinate SeV titers in both VAD and control mice averaged within the same order of magnitude. In a separate set of experiments, animal weights were measured every day for 10 days after SeV infection. As demonstrated in Fig. 9B, both control and VAD mice lost weight approximately 1 week after SeV infection and then reached a plateau or showed signs of improvement by day 10. Data encourage future experimentation to determine if the aberrant immune responses elicited by this primary infection in VAD mice will protect against infection and weight loss upon secondary challenge with SeV.
Fig 9.
Weight loss and virus growth in SeV-infected VAD animals. (A) Lungs and nasal turbinates were removed on day 7 after infection with 500 PFU of SeV. Sample titers were determined, and each dot indicates a result from a different animal (4 or 5 mice per group). Horizontal bars indicate averages per group. (B) In a separate experiment, mice were weighed every day for 10 days after infection with 500 PFU of SeV. Results were similar in each of 2 independent experiments.
DISCUSSION
VAD alters the SeV-specific immune response.
The work described here was conducted to examine the effect of VAD on vaccine-induced immune responses in the respiratory tract. SeV is well known for its rapid induction of protective immunity. Both antibodies and CD8+ T cell responses are induced within 10 days of a single i.n. inoculation (33). To define the acute effect of VAD on SeV-specific immune activities, we first examined serum antibody levels and found that these were not reduced compared to those in control mice. BAL fluid antibody levels were similar among groups, but there was a trend toward lower antibody levels in nasal washes of VAD animals. When the immunodominant SeV-specific CD8+ T cell population was analyzed, significant changes were evident. These cells were diminished in the lower airway of VAD animals, and they exhibited unusually high CD103 membrane expression patterns.
When E-cadherin, the ligand for αEβ7, was examined, no differences were noted between VAD and control animals. However, differences between URT and LRT expression patterns were noted in both VAD and control animals, infected or uninfected. Whereas E-cadherin was relatively weakly expressed in the LRT, URT cells were strongly positive. E-cadherinHIGH cells in the URT were usually found to be ICAM-1LOW, as demonstrated by in vitro culture of dispase-, collagenase-, and DNase-digested cells with or without SeV.
We have previously shown that T cells capable of migration to URT or LRT tissues are clonally related (39), and others have shown that adherence of T cells to epithelial cell membrane markers (e.g., ICAM-1 and ICAM-2) affect cell migration and airway egress (27, 29). The increases in ICAM-1, a ligand for CD11a, among LRT cells following SeV infection (Fig. 7) may promote, at least in part, T cell residence in the LRT. It is also likely that the differing E-cadherin and ICAM-1 phenotypes between LRT and URT epithelial cells influence patterns of T cell migration, particularly when CD103 is overexpressed on responding T cells, as in VAD animals. Results support a working hypothesis that the high CD103 expression among T cell populations in VAD mice alters T cell interactions with URT and LRT epithelial cells, thus inhibiting T cell migration and egress into the lower airway. A further analysis of kinetics and migration patterns of Tet+ T cells in VAD mice is now warranted to test our hypothesis. Intravital imaging experiments may be conducted in concert with in vitro migration and adherence studies to facilitate a comprehensive examination of CD8+ T cell migration potentials in vaccinated VAD and control animals. Studies are also warranted to define the mechanism for upregulation of CD103 on membranes of respiratory tract Tet+ T cells, and the specific influences of various DC populations and cytokines (e.g., transforming growth factor β [TGF-β]) on this process. Expectations are that respiratory tract DCs, like gut DCs, are altered by VAD in terms of CD103 expression, migration, and functional capacities. The VAD influence on DCs may then be relayed to T cells during the processes of antigen presentation and imprinting.
VAD and altered T cell imprinting in gut mucosal tissues.
Our work supplements the more extensive studies of VAD effects on gut immunity. Respiratory and intestinal tracts share numerous features in that each is lined by layers of epithelial cells which are constantly bombarded by foreign antigens. Associated with the epithelial cells are DCs which sample foreign antigens, migrate to lymph nodes, and activate naïve B and T cell populations. The activated lymphocytes may then leave lymph nodes, enter the bloodstream, and traffic back to the site of original antigen exposure.
There have already been detailed analyses of mechanisms by which intestinal DCs imprint gut B and T cell populations. As described above, DC-mediated imprinting of cells with CCR9 and α4β7-integrin directs homing of lymphocytes to the small intestine (9, 20). Obstruction of vitamin A functions (e.g., by inhibition of RAR with drug [LE540] [21, 23, 25]) alters lymphocyte marking and migration patterns, similar to the situation defined in our studies of the respiratory tract.
CD103 has previously been described as influencing lymphocyte homing potential in the gut. However, the influences on T cell migration and residence are complex. For example, early studies suggested that lymphocyte homing to the gut was more dependent on αEβ7 than on α4β7-integrin. The conclusion was based on a finding that β7-integrin-deficient mice, but not α4-integrin-deficient mice, exhibited reduced lymphocyte numbers in the gut's epithelial cell lining (1, 16, 44). A different conclusion was reached when mixed ovalbumin-specific transgenic CD8+ T cells (OT-1) from αE+ and αE− sources, or from β7+ and β7− sources, were transferred to wild-type host animals subsequently infected with a recombinant vesicular stomatitis virus (VSV) encoding ovalbumin. In this case, the transfer of mixed αE+ and αE− donor T cell populations resulted in the appearance of both cell types among intraepithelial lymphocytes (IEL) and in the lamina propria, but the transfer of mixed β7+ and β7− donor cells resulted in the preferential migration of β7+ cells. Researchers then proposed that T cell homing was more dependent on α4β7 than on αEβ7 lymphocyte markers (16). It is perhaps expected that the two studies support different conclusions. When considering the results from the direct study of knockout mice, one may consider that the lack of gene expression can affect multiple cell types in the animals and that there may be compensatory events during animal development. In the case of mixed, transferred T cell populations, one might also consider that one T cell population may exert positive or negative influences on the migration patterns of another. Our results demonstrate additional complexity in that the Tet+ T cells in LRT airways were not homogeneous with regard to membrane marker expression in either VAD or control animals. Clearly, cell-cell interactions facilitated by αEβ7-integrins are not autonomous but integrate with a variety of additional adherence mechanisms (e.g., CD11a interactions with ICAM-1) to define the overall outcome of T cell migration.
VAD, infectious disease, and vaccination.
VAD continues to pose a health risk to children and adults, as multiple organ systems depend upon dietary vitamin A. Immune defects associated with VAD include increased susceptibility to infectious disease and weakened responses to vaccination. VAD deficiencies can in some cases be corrected by vitamin A supplements, but in other cases vitamin A supplements cause additional harm (4, 11, 18, 19). A fine vitamin balance must be achieved to avoid a spiral of events that may upset cell differentiation and homing marker expression, upset T cell migration, and thereby impair the natural course of an adaptive immune response.
This report begins to dissect the influences of VAD on vaccine-induced immune responses in the respiratory tract. There are multiple additional respiratory tract-resident cells other than CD8+ T cells that may be influenced by vitamin A (e.g., Th2, Th17, and CD4+ Foxp3+ T-regulatory cells [5, 45]) and there are multiple cytokines (e.g., interleukin-2 [IL-2], IL-5, IL-6, and TGF-β [20, 21, 23–25, 36]) which may alter vitamin A effects. As stated above, the changes described here may be secondary to VAD-induced modifications of the innate immune system and/or the stromal cells of the respiratory tract. A comprehensive investigation of lymphocytes, dendritic cells, matrix cells, and soluble factors may be necessary to define the full influence of VAD on vaccines that are administered by the i.n. route.
ACKNOWLEDGMENTS
This work was supported in part by NIH NIAID grants P01 AI054955, R01 AI088729, and R01 AI076499; NIH NCI grant P30-CA21765; and the American-Lebanese Syrian Associated Charities (ALSAC).
We thank P. Vogel and the Veterinary Pathology Core for histology services.
Footnotes
Published ahead of print 8 March 2012
REFERENCES
- 1. Arroyo AG, Yang JT, Rayburn H, Hynes RO. 1996. Differential requirements for alpha4 integrins during fetal and adult hematopoiesis. Cell 85:997–1008 [DOI] [PubMed] [Google Scholar]
- 2. Asanuma H, et al. 1997. Isolation and characterization of mouse nasal-associated lymphoid tissue. J. Immunol. Methods 202:123–131 [DOI] [PubMed] [Google Scholar]
- 3. Balmer JE, Blomhoff R. 2002. Gene expression regulation by retinoic acid. J. Lipid Res. 43:1773–1808 [DOI] [PubMed] [Google Scholar]
- 4. Benn CS, Fisker AB, Diness BR, Aaby P. 2006. Neonatal vitamin A supplementation: sex-differential effects on mortality? J. Infect. Dis. 194:719. [DOI] [PubMed] [Google Scholar]
- 5. Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. 2007. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 204:1765–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen K, Cerutti A. 2010. Vaccination strategies to promote mucosal antibody responses. Immunity 33:479–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cole GA, Hogg TL, Woodland DL. 1994. The MHC class I-restricted T cell response to Sendai virus infection in C57BL/6 mice: a single immunodominant epitope elicits an extremely diverse repertoire of T cells. Int. Immunol. 6:1767–1775 [DOI] [PubMed] [Google Scholar]
- 8. Davis SS. 2001. Nasal vaccines. Adv. Drug Deliv. Rev. 51:21–42 [DOI] [PubMed] [Google Scholar]
- 9. Eksteen B, et al. 2009. Gut homing receptors on CD8 T cells are retinoic acid dependent and not maintained by liver dendritic or stellate cells. Gastroenterology 137:320–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gangopadhyay NN, Moldoveanu Z, Stephensen CB. 1996. Vitamin A deficiency has different effects on immunoglobulin A production and transport during influenza A infection in BALB/c mice. J. Nutr. 126:2960–2967 [DOI] [PubMed] [Google Scholar]
- 11. Humphrey JH, et al. 2006. Effects of a single large dose of vitamin A, given during the postpartum period to HIV-positive women and their infants, on child HIV infection, HIV-free survival, and mortality. J. Infect. Dis. 193:860–871 [DOI] [PubMed] [Google Scholar]
- 12. Hurwitz JL, et al. 1997. Intranasal Sendai virus vaccine protects African green monkeys from infection with human parainfluenza virus-type one. Vaccine 15:533–540 [DOI] [PubMed] [Google Scholar]
- 13. Jaensson-Gyllenbäck E, et al. 2 February 2011. Bile retinoids imprint intestinal CD103+ dendritic cells with the ability to generate gut-tropic T cells. Mucosal Immunol. doi:10.1038/mi.2010.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jones B, et al. 2009. Human PIV-2 recombinant Sendai virus (rSeV) elicits durable immunity and combines with two additional rSeVs to protect against hPIV-1, hPIV-2, hPIV-3, and RSV. Vaccine 27:1848–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kappler JW, Skidmore B, White J, Marrack P. 1981. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J. Exp. Med. 153:1198–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lefrançois L, et al. 1999. The role of beta7 integrins in CD8 T cell trafficking during an antiviral immune response. J. Exp. Med. 189:1631–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Linderholm AL, et al. 2010. All-trans retinoic acid mediates DUOX2 expression and function in respiratory tract epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 299:L215–L221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Long KZ, et al. 2011. Vitamin A supplementation modifies the association between mucosal innate and adaptive immune responses and resolution of enteric pathogen infections. Am. J. Clin. Nutr. 93:578–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malaba LC, et al. 2005. Effect of postpartum maternal or neonatal vitamin A supplementation on infant mortality among infants born to HIV-negative mothers in Zimbabwe. Am. J. Clin. Nutr. 81:454–460 [DOI] [PubMed] [Google Scholar]
- 20. Mora JR, et al. 2003. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature 424:88–93 [DOI] [PubMed] [Google Scholar]
- 21. Mora JR, et al. 2006. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 314:1157–1160 [DOI] [PubMed] [Google Scholar]
- 22. Mora JR, Iwata M, Von Andrian UH. 2008. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat. Rev. Immunol. 8:685–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mora JR, Von Andrian UH. 2004. Retinoic acid: an educational “vitamin elixir” for gut-seeking T cells. Immunity 21:458–460 [DOI] [PubMed] [Google Scholar]
- 24. Mora JR, Von Andrian UH. 2008. Differentiation and homing of IgA-secreting cells. Mucosal Immunol. 1:96–109 [DOI] [PubMed] [Google Scholar]
- 25. Mora JR, Von Andrian UH. 2009. Role of retinoic acid in the imprinting of gut-homing IgA-secreting cells. Semin. Immunol. 21:28–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murali-Krishna K, et al. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177–187 [DOI] [PubMed] [Google Scholar]
- 27. Nawijn MC, Hackett TL, Postma DS, van Oosterhout AJ, Heijink IH. 2011. E-cadherin: gatekeeper of airway mucosa and allergic sensitization. Trends Immunol. 32:248–255 [DOI] [PubMed] [Google Scholar]
- 28. Pino-Lagos K, Benson MJ, Noelle RJ. 2008. Retinoic acid in the immune system. Ann. N. Y. Acad. Sci. 1143:170–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Porter JC, Hall A. 2009. Epithelial ICAM-1 and ICAM-2 regulate the egression of human T cells across the bronchial epithelium. FASEB J. 23:492–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prentice AM. 2010. Vitamin A supplements and survival in children. BMJ 340:c977. [DOI] [PubMed] [Google Scholar]
- 31. Privalsky ML. 2004. The role of corepressors in transcriptional regulation by nuclear hormone receptors. Annu. Rev. Physiol. 66:315–360 [DOI] [PubMed] [Google Scholar]
- 32. Ross AC, Ambalavanan N, Zolfaghari R, Li NQ. 2006. Vitamin A combined with retinoic acid increases retinol uptake and lung retinyl ester formation in a synergistic manner in neonatal rats. J. Lipid Res. 47:1844–1851 [DOI] [PubMed] [Google Scholar]
- 33. Rudraraju R, et al. 2011. Phenotypes and functions of persistent Sendai virus-induced antibody forming cells and CD8+ T cells in diffuse nasal-associated lymphoid tissue typify lymphocyte responses of the gut. Virology 410:429–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sealy R, Jones BG, Surman SL, Hurwitz JL. 2010. Robust IgA and IgG-producing antibody forming cells in the diffuse-NALT and lungs of Sendai virus-vaccinated cotton rats associate with rapid protection against human parainfluenza virus-type 1. Vaccine 28:6749–6756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Semba RD, Muhilal Scott AL, Natadisastra G, West KP, Jr, Sommer A. 1994. Effect of vitamin A supplementation on immunoglobulin G subclass responses to tetanus toxoid in children. Clin. Diagn. Lab. Immunol. 1:172–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sirisinha S, Darip MD, Moongkarndi P, Ongsakul M, Lamb AJ. 1980. Impaired local immune response in vitamin A-deficient rats. Clin. Exp. Immunol. 40:127–135 [PMC free article] [PubMed] [Google Scholar]
- 37. Sommer A, et al. 1986. Impact of vitamin A supplementation on childhood mortality. A randomised controlled community trial. Lancet i:1169–1173 [DOI] [PubMed] [Google Scholar]
- 38. Sommer A, Tarwotjo I, Hussaini G, Susanto D. 1983. Increased mortality in children with mild vitamin A deficiency. Lancet ii:585–588 [DOI] [PubMed] [Google Scholar]
- 39. Surman SL, et al. 2011. Clonally related CD8+ T cells responsible for rapid population of both diffuse nasal-associated lymphoid tissue and lung after respiratory virus infection. J. Immunol. 187:835–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Svensson M, et al. 2008. Retinoic acid receptor signaling levels and antigen dose regulate gut homing receptor expression on CD8+ T cells. Mucosal Immunol. 1:38–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Takimoto T, et al. 2004. Recombinant Sendai virus expressing the G glycoprotein of respiratory syncytial virus (RSV) elicits immune protection against RSV. J. Virol. 78:6043–6047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Takimoto T, et al. 2005. Recombinant Sendai virus as a novel vaccine candidate for respiratory syncytial virus. Viral Immunol. 18:255–266 [DOI] [PubMed] [Google Scholar]
- 43. Villamor E, Fawzi WW. 2005. Effects of vitamin A supplementation on immune responses and correlation with clinical outcomes. Clin. Microbiol. Rev. 18:446–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wagner N, et al. 1996. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature 382:366–370 [DOI] [PubMed] [Google Scholar]
- 45. Wang C, Kang SG, HogenEsch H, Love PE, Kim CH. 2010. Retinoic acid determines the precise tissue tropism of inflammatory Th17 cells in the intestine. J. Immunol. 184:5519–5526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wiedermann U, Hanson LA, Kahu H, Dahlgren UI. 1993. Aberrant T-cell function in vitro and impaired T-cell dependent antibody response in vivo in vitamin A-deficient rats. Immunology 80:581–586 [PMC free article] [PubMed] [Google Scholar]
- 47. Woodland DL, Happ MP, Bill J, Palmer E. 1990. Requirement for cotolerogenic gene products in the clonal deletion of I-E reactive cells. Science 247:964–967 [DOI] [PubMed] [Google Scholar]
- 48. Yassai MB, Malek F. 1989. Newborns vitamin A in relation to sex and birth weight. J. Trop. Pediatr. 35:247–249 [DOI] [PubMed] [Google Scholar]
- 49. Zhan X, et al. 2007. Respiratory syncytial virus (RSV) fusion protein expressed by recombinant Sendai virus elicits B-cell and T-cell responses in cotton rats and confers protection against RSV subtypes A and B. Vaccine 25:8782–8793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhan X, et al. 2008. Sendai virus recombinant vaccine expressing hPIV-3 HN or F elicits protective immunity and combines with a second recombinant to prevent hPIV-1, hPIV-3 and RSV infections. Vaccine 26:3480–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]