Abstract
The mycobacterial immunodominant ESAT-6 and CFP-10 antigens are strongly recognizable in tuberculosis-infected cattle, and they do not elicit a response in cattle without infection. In addition, they are absent in most environmental mycobacterial species, and therefore, their use can be an alternative to purified protein derivative (PPD) tuberculin in the development of a more specific skin diagnostic test in cattle. The aim of the current study was to assess the potential of an ESAT-6 and CFP-10 (E6-C10) protein cocktail in a skin test format in naturally tuberculosis-infected and paratuberculosis-infected cattle. We also included MPB83 as a third component in one of the protein cocktail preparations. The protein cocktail was tested at different dose concentrations (5, 10, and 15 μg per protein). The best skin response to the E6-C10 protein cocktail was obtained with 10 μg. Subsequently, this concentration was tested in 2 herds with high and low bovine tuberculosis prevalence, the latter with paratuberculosis coinfection. Our data show that the E6-C10 cocktail allows identification of an important proportion of animals that PPDB is not able to recognize, especially in low-prevalence herds. The protein cocktail did not induce reactions in tuberculosis-free cattle or in paratuberculosis-infected cattle. Addition of MPB83 to the protein cocktail did not make any difference in the skin reaction.
INTRODUCTION
Bovine tuberculosis (TB) control requires identification and removal of infected animals from the herd. The primary diagnostic test is the tuberculin skin test (TST) (25). TST is based on the measurement of the skin thickness increment 72 h after the intradermal injection of mycobacterial extracts called purified protein derivatives (PPD). The inflammatory reaction results in an infiltration of antigen-specific lymphocytes and the production of inflammatory cytokines (5). Despite the variability in specificity and sensitivity, TST has become a tool that assists in prevalence reduction and eradication of bovine tuberculosis in different countries (reviewed in references 12 and 23). TST may be applied in a single format, injecting bovine PPD (PPDB) only, or in a comparative format, using avian PPD (PPDA) and PPDB. The latter format is the most specific, and although it provides a good indication of environmental sensitization, the test does not always discriminate between tuberculosis-infected cattle and those exposed to nonpathogenic organisms (19). This is a serious problem in places where coinfection with Mycobacterium avium subsp. paratuberculosis (MAP) is common (6). Therefore, it is important to identify specific antigens that improve the diagnosis of the disease.
The mycobacterial antigens ESAT-6 and CFP-10 are low-molecular-weight proteins present in virulent Mycobacterium bovis culture filtrate; both antigens are strongly recognized in infected cattle, and they do not elicit a response in cattle without infection (9, 27). In addition, they are absent in most environmental mycobacterial species (14). Results from recent publications have shown the practical application of such antigens in the gamma interferon (IFN-γ) whole-blood test (1, 2). However, the IFN-γ assay is expensive compared to TST and requires a well-established laboratory, which is not always available; therefore, it would be more useful to integrate the use of these proteins in a TST format. The initial assessments of ESAT-6 in the skin test suggested that a very high dose of protein (22) or its use in combination with a synthetic bacterial lipopeptide (30) was necessary to induce skin thickness. Recently, Whelan et al. showed that a protein cocktail with low concentrations of recombinant proteins elicited skin test responses in naturally infected cattle (29).
The aim of this study was to assess the diagnostic potential of a protein cocktail of ESAT-6 plus CFP-10 (E6-C10), using a skin test format in M. bovis-infected cattle. In order to achieve our goal, we tested infected cattle in two settings: (i) a high-prevalence herd and (ii) a low-prevalence herd coinfected with MAP. Also, we assessed the specificity of the protein cocktail in two tuberculosis-free herds, one of them with MAP infection. Our results suggest that the E6-C10 protein cocktail improves the specificity of the skin test and allows better identification of M. bovis-infected cattle, especially in herds with MAP coinfection.
MATERIALS AND METHODS
Antigens.
Bovine and avian PPD were purchased from Pronabive (Register Sagarpa B-0653-035). The full-length and histidine-tagged recombinant proteins ESAT-6, CFP-10, and MPB83 were a kind gift from M. Singh (Lionex, Braunschweig, Germany).
Pilot study animal selection.
To investigate the optimal concentration of the protein cocktail to be used in the skin test, 10 naturally infected cattle from a farm with a confirmed history of bovine TB were selected. These animals were single intradermal comparative cervical test (SICCT) reactors in two previous tests, and they were positive in the IFN-γ whole-blood assay and PCR from nasal swabs. The SICCTs were performed 60 and 120 days before the start of the pilot study, whereas the IFN-γ assay and PCR took place 1 week before the beginning of the trial. The same concentrations of the protein cocktail were tested in 10 tuberculosis-free cattle that came from a TB-free herd. The IFN-γ whole-blood test and PCR from nasal swabs were used to confirm the TB-free status. The SICCT was performed and interpreted according to the Official Mexican Norm against Bovine Tuberculosis (NOM 031-Z00-1995). The scores against PPDB and PPDA were plotted in the official graphic and interpreted according to the standard analysis. Briefly, the criteria for classifying an animal as positive, negative, or suspect are as follows: positive if the difference between PPDB and PPDA is ≥5 mm; negative if the difference between PPDB and PPDA is ≤2 mm; suspect if the difference between PPDB and PPDA is >2 but <5 mm.
Field study animal selection.
A total of 303 bovines from 4 herds in Mexico were included in this study, of which 214 animals were from 2 herds with a history of bovine TB. These 214 animals were grouped by prevalence (defined as the percentage of animals that had been positively diagnosed by a PPD skin test at the most recent herd skin test control) as high (>25%) and low (<1%) based on previous records (138 and 76 animals, respectively). The low-prevalence herd also had a paratuberculosis coinfection. The remaining 89 animals were considered negative controls; 59 animals were from a herd with no history of bovine TB during the previous 5 years, and 30 animals were also tuberculosis free, but in contrast to the other control herd, paratuberculosis infection was present. All animals were selected randomly, and all were tested by an IFN-γ whole-blood test. Confirmation of disease status was made by PCR from nasal swabs (Table 1).
Table 1.
Herds enrolled to assess the E6-C10 protein cocktail in the field study
| Location of farm (Mexico) | No. of animals tested | Expected prevalencea |
|---|---|---|
| Tizayuca, Hidalgo | 138 | High |
| Tequisquiapan, Querétarob | 76 | Low |
| Mtz. De la Torre, Veracruz | 59 | None |
| Topilejo, Federal Districtb | 30 | None |
| Total | 303 |
High, >25%; low, <1%; none, negative control herd free of bovine tuberculosis.
Herd infected by M. avium subsp. paratuberculosis.
Limulus assay.
The lipopolysaccharide (LPS) content of recombinant proteins was measured with a QCL-1000 kit (Lonza) based on the Limulus amebocyte lysate assay according to the manufacturer's instructions.
Skin test procedures.
In order to evaluate different concentrations of the E6-C10 protein cocktail (5, 10, and 15 μg of each protein), seven intradermal injection sites were used on each animal in the pilot study. A fourth treatment, including ESAT-6, CFP-10, and MPB83 (10 μg of each protein), was also applied. MPB83 was included based on the benefit shown in the guinea pig skin test (27). Bovine and avian PPD were always included in each test, as well as 1× sterile phosphate-buffered saline (PBS) as a negative control. The skin sites were clipped free of hair, and skin reactions were measured using calipers prior to the test and every 24 h for 4 days. In the field study, four intradermal injection sites were used for (i) the optimal concentration of the protein cocktail as determined by the pilot study, (ii) bovine PPD, (iii) avian PPD, and (iv) the negative control. Skin reactions were measured using calipers prior to and 72 h after the skin test.
In the pilot study, the antigens were inoculated into the neck (4 in the right side and 3 in the left side) in the middle third section according to the following design: two antigens in the upper site 10 cm below the crest of the neck and 13 cm apart from each other and two antigens in a downward fashion 13 cm below the upper-site inoculations and 13 cm apart from each other. On the other hand, in the field study, four antigens were applied to the right side of the neck following the previously described arrangement (7, 16, and 21). All antigens were administered in a 0.1-ml volume and were injected intradermally using 27-gauge hypodermic needles.
IFN-γ whole-blood test.
Blood samples were taken before the skin test from all the animals, except for one tuberculosis-free herd, where samples were taken 3 days later due to logistic problems. Whole-blood cultures were stimulated with either avian PPD (10 μg/ml), bovine PPD (10 μg/ml), the E6-C10 protein cocktail (4 μg/ml), pokeweed mitogen (1 μg/ml) (Sigma-Aldrich), or the no-antigen control for 20 h in a humidified 5% CO2 incubator at 37°C. The levels of IFN-γ in the culture supernatants were measured using the commercially available Bovigam enzyme-linked immunosorbent assay (Prionics, Schlieren-Zurich, Switzerland). The results were expressed using an optical density index (ODI), which is defined as the ratio of the OD of stimulated cultures to the OD of nonstimulated cultures. For all antigens tested, an ODI value of 2 was selected as the cutoff value to indicate a positive result (2).
Collection of nasal swabs.
Nasal swabs were collected using a sterile 30-cm wooden homemade swab with a compact cotton wool tip. The swabs were submerged in 2 ml of sterile phosphate-buffered saline in 15-ml centrifuge tubes, and the tubes were centrifuged at 20,817 × g for 10 min. The nasal sediments were frozen at −20°C until DNA extraction.
DNA extraction from nasal swabs.
DNA was extracted from the sediments according to a method described for mycobacteria (8). Briefly, the pellets from the nasal swabs were resuspended in 400 μl of 1× Tris-EDTA buffer and heated to 80°C for 1 h to inactivate the bacteria. Then, 50 μl of 10-mg/ml lysozyme (Sigma-Aldrich) was added. The mixture was incubated overnight at 37°C. The next day, 75 μl of 10% SDS and 50 μl of 1-mg/ml proteinase K (Sigma-Aldrich) were added and incubated for 20 min at 65°C. One hundred microliters of 5 M NaCl and 100 μl of 5% N-cetyl-N,N,N,trimethlyl ammonium bromide/NaCl were added, and the solution was mixed thoroughly until it formed a white, milky suspension. It was then incubated for 10 min at 65°C, treated twice with 1,000 μl of chloroform-isoamylic alcohol (24:1 [vol/vol]), and centrifuged for 5 min at 15,000 × g. The supernatant was transferred to a new tube, and 0.7 volume of isopropanol was added, followed by overnight incubation at −20°C. The tube was centrifuged at 11,000 × g for 15 min, the supernatant was discarded, and 2 washes were performed in 70% ethanol. The DNA pellet was dried and resuspended in 20 μl of water, and 2 μl was used for PCR. The quality and concentration of DNA were assessed by spectrophotometry with a Nanodrop ND-1000 spectrophotometer.
PCR.
A nested PCR was performed as previously described to amplify a region of the mpb70 gene of the Mycobacterium tuberculosis complex. Reactions were carried out in a final volume of 25 μl. The PCR mixture contained a final concentration of 0.2 μM of each primer, 200 μM deoxynucleoside triphosphates (dNTPs), 1 U DNA polymerase, PCR buffer (75 mM Tris-HCl, pH 9.0, 50 mM KCl, 1.5 mM MgCl2), and 2.5 mM MgCl2. The PCR programs were run in a Hybaid PCR Express thermal cycler (Thermo-Hybaid), and the products were analyzed in 2% agarose gels containing ethidium bromide (0.5 μg/ml) and photographed on a UV transilluminator (Gel Logic 200 Imaging System; Kodak, United Kingdom). A single PCR was run by amplifying a 372-bp segment of the mpb70 gene using specific primers (M70F, 5′-GAACAATCCGGAGTTGACAA-3′, and M70R, 5′-AGCACGCTGTCAATCATGTA-3′) (10, 11) and the following protocol: first cycle, 5 min at 94°C; 30 cycles of 45 s at 94°C, 30 s at 60°C, 45 s at 72°C; and a final cycle of 5 min at 72°C. Then, a nested PCR was run using 1 μl of the previous reaction mixture to amplify a 208-bp fragment within the 372-bp region of the mpb70 gene with the primers M22F, 5′-GCTGACGGCTGCACTGTCGGGC-3′, and M22R, 5′-CGTTGGCCGGGCTGGTTTGGCC-3′ (13). In this case, a touchdown protocol was performed: 7 cycles of 30 s at 94°C and 30 s at 70°C; 10 cycles with the same temperatures, decreasing 1°C each cycle; and 8 cycles of 30 s at 94°C, 30 s at 60°C, and 30 s at 72°C.
Statistical analysis.
The data were analyzed using the nonparametric Kruskal-Wallis test with application of Dunn's multiple-comparison test. The Kruskal-Wallis test was performed by applying Prism 5 software (GraphPad Inc.) Receiver operator curve (ROC) analysis was carried out with the STATA 11 program (StataCorp LP).
RESULTS
A concentration of 20 μg of the protein cocktail (E6-C10) elicited the best skin inflammatory response in naturally M. bovis-infected cattle.
The LPS contents of the ESAT-6 and CFP-10 proteins were assessed before carrying out the in vivo studies. The LPS levels of both proteins were lower than 1.3 EU (endotoxin units)/mg, which is below the maximum limit (50 EU/mg) determined with the FDA guide (20).
The E6-C10 protein cocktail was tested at different dose concentrations (10, 20, and 30 μg of total protein). In addition, a protein cocktail containing E6-C10-M83 (10 μg each) was also tested. Ten naturally M. bovis-infected PPDB-, PCR-, and IFN-γ-positive cattle were selected for this purpose. Skin thicknesses were measured and compared for the different antigen concentrations up to 96 h postinjection.
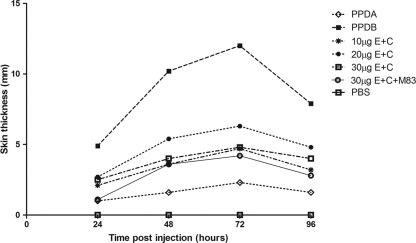
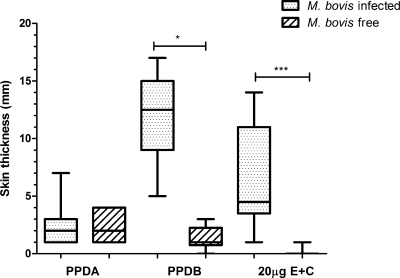
Responses to PPDB and the protein cocktail peaked at 72 h postinjection as classically determined for SICCT (Fig. 1). Skin responses to all the antigens intradermally inoculated were observed; however, the strongest response observed was to PPDB, followed by the different concentrations of the protein cocktail. Contrary to what was expected, skin response to the three-protein cocktail composed of E6-C10-M83 (10 μg each) was very low (4.0 mm); addition of the MPB83 protein did not improve the skin response, and skin induration was less defined to palpation. The best response to the E6-C10 protein cocktail was observed with 20 μg of total protein. The mean skin response at 72 h was 6.3 mm, compared to 12.5 mm seen in reactions to PPDB (Fig. 1). The same dose concentrations were assessed in 10 TB-free cattle. These animals responded mainly to PPDA, with a mean of 2.0 mm, unlike the mean skin response of 1.0 mm to PPDB, suggesting some sensitization to environmental mycobacteria. In some cases, shaving induced a minor inflammatory reaction that did not correspond to a real response to the protein cocktail because no palpable increase in skin thickness was observed, suggesting for the first time an improvement in specificity (Fig. 2). With these data, we performed a ROC analysis; a skin response of 1 mm to the E6-C10 protein cocktail was selected as the cutoff value to indicate a positive result (Table 2).
Fig 1.
Kinetics of skin responses to PPDB; PPDA; 10, 20, and 30 μg of E6-C10 protein cocktail; and 30 μg of E6-C10-MPB83 protein cocktail. Skin responses were measured every 24 h for 4 days. The results are expressed as the difference in skin thicknesses between post- and pre-skin test readings.
Fig 2.
Comparison of skin responses at 72 h postinjection in M. bovis-infected cattle and M. bovis-free cattle. The results are presented as medians and ranges and expressed as the difference in skin thicknesses between post- and pre-skin test readings. The statistical differences between responses were determined by using the Kruskal-Wallis test (*, P < 0.05; ***, P < 0.001).
Table 2.
Sensitivity and specificity ROC estimates for 20 μg of the E6-C10 protein cocktail in the skin testa
| Cutoff point (mm) | Sensitivity (%)b | Specificity (%)c |
|---|---|---|
| ≥1 | 100 | 90 |
| ≥2 | 90 | 100 |
| ≥4 | 80 | 100 |
| ≥5 | 30 | 100 |
| ≥10 | 20 | 100 |
Area under the ROC curve = 0.9950; 95% CI = 0.98114 to 1.00000.
Sensitivity was determined by using naturally infected cattle with disease confirmed by SICCT, IFN-γ test, and PCR (n = 10).
Specificity was determined by using TB-free cattle (n = 10).
The E6-C10 cocktail identifies a higher number of reactors than PPDB.
In order to evaluate the 20-μg E6-C10 protein cocktail, a field study including 303 bovines under different bovine tuberculosis prevalences and in the presence or absence of paratuberculosis coinfection was performed. Skin responses to the protein cocktail and both avian and bovine PPD were evaluated and compared to IFN-γ responses using the same antigens.
Under high-prevalence herd conditions, 45.6% (63/138) of the cattle were positive by SICCT using both PPDB and E6-C10 as antigens; in 76% of the cases, both antigens identified the same positive cattle.
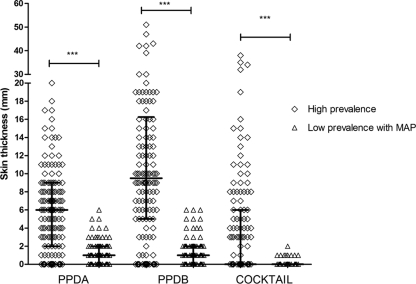
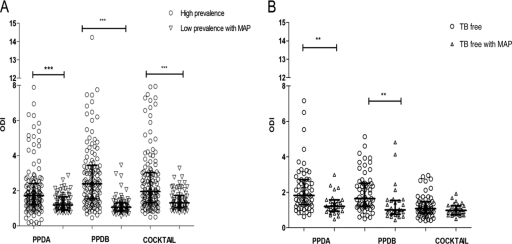
PPDB classified 33.3% (46/138) of the cattle as suspect and 21% (29/138) as negative, whereas E6-C10 identified 14 PPDB-suspect and 1 PPDB-negative cattle as positive in the whole population. This means that the protein cocktail identified 11% (15/138) more animals than PPDB (Tables 3 and 4). When we consider the PCR-positive population, the results were similar to what we described above: 60.3% (38/63) of reactors to PPDB in the skin test were confirmed by PCR. A similar proportion of animals positive to the protein cocktail 63.4% (40/63) were corroborated by PCR. Ten animals were not correctly identified by PPDB (9 suspect and 1 negative). Skin responses to PPDB, PPDA, and E6-C10 were higher under high-prevalence herd conditions, where the greatest responses were to PPDB (median [Me] = 9.5 mm). E6-C10 induced highly variable responses ranging from 1 to 40 mm (Fig. 3). The median skin response to PPDA was 6.0 mm; in consequence, 33.3% (46/138) of the animals were classified as suspect by SICCT (Fig. 3 and Tables 3 and 4). The IFN-γ test identified 65.2% (90/138) PPDB-positive cattle, of which 62.2% (56/90) were PPDB reactors in SICCT, whereas E6-C10 identified 52.9% (73/138) of the cattle as positive, of which 60.2% (44/73) were PPDB reactors in SICCT (Fig. 4; see Table 6). Under low-prevalence herd conditions, only one animal was a PPDB reactor in the SICCT; 13.1% (10/76) of the cattle were suspect by PPDB, and 85.5% (65/76) of the cattle were negative. Meanwhile, the E6-C10 protein cocktail identified 8 cattle as positive, and 87.5% (7/8) of them were confirmed by PCR (Table 5). The skin responses to PPDB and PPDA were similar (Me = 1.0 mm), probably because of the MAP coinfection. Unlike the skin responses in high-prevalence herds, the protein cocktail induced weak skin responses (up to 1 and 2 mm) in low-prevalence herds (Fig. 3). The IFN-γ test identified 5 cattle, including the PPDB reactor, as positive, whereas the protein cocktail identified 15 positive cattle (Table 6).
Table 3.
PCR-positive cattle identified by SICCT and skin test with the E6-C10 protein cocktail in herds with a high prevalence of bovine tuberculosis
| Skin test (E6-C10) result | No. for SICCT result |
|||
|---|---|---|---|---|
| Positive | Negative | Suspect | Total | |
| Positive | 30 | 1 | 9 | 40 |
| Negative | 8 | 21 | 16 | 45 |
| Total | 38 | 22 | 25 | 85 |
Table 4.
PCR-negative cattle identified by SICCT and skin test with the E6-C10 protein cocktail in herds with a high prevalence of bovine tuberculosis
| Skin test (E6-C10) result | No. for SICCT result |
|||
|---|---|---|---|---|
| Positive | Negative | Suspect | Total | |
| Positive | 18 | 0 | 5 | 23 |
| Negative | 7 | 7 | 16 | 30 |
| Total | 25 | 7 | 21 | 53 |
Fig 3.
Skin responses to PPDB, PPDA, and protein cocktail in herds with high and low prevalences of bovine tuberculosis. The results are presented as medians and interquartile ranges. The statistical differences between responses were determined by using the Kruskal-Wallis test (***, P < 0.001).
Fig 4.
IFN-γ responses to PPDB, PPDA, and 20 μg of the E6-C10 protein cocktail in herds with high and low prevalences of bovine tuberculosis (A) and in TB-free herds (B). The results are presented as medians and interquartile ranges. The statistical differences between responses were determined by using the Kruskal-Wallis test (**, P < 0.01; ***, P < 0.001).
Table 5.
Cattle identified by SICCT, skin test with the E6-C10 protein cocktail, and PCR from nasal swabs in herds with a low prevalence of bovine tuberculosis and coinfected with M. avium. subsp. paratuberculosis
| PCR result | No. with result |
||||
|---|---|---|---|---|---|
| SICCT |
Skin test (E6-C10) |
||||
| Positive | Negative | Suspect | Positive | Negative | |
| Positive | 0 | 24 | 5 | 7 | 22 |
| Negative | 1 | 41 | 5 | 1 | 46 |
| Total | 1 | 65 | 10 | 8 | 68 |
Table 6.
Cattle identified by SICCT and by IFN-γ assay using PPDB and the E6-C10 protein cocktail in herds with high and low prevalences of bovine tuberculosis
| Herd | SICCT result | No. with IFN-γ test result |
|||
|---|---|---|---|---|---|
| PPDB |
E6-C10 cocktail |
||||
| Positive | Negative | Positive | Negative | ||
| High prevalence | Reactor | 56 | 7 | 44 | 19 |
| Suspect | 10 | 19 | 9 | 20 | |
| Negative | 24 | 22 | 20 | 26 | |
| Low prevalencea | Reactor | 1 | 0 | 0 | 1 |
| Suspect | 4 | 61 | 11 | 54 | |
| Negative | 0 | 10 | 4 | 6 | |
Herd coinfected with M. avium subsp. paratuberculosis.
The E6-C10 cocktail did not induce intradermal nonspecific reactions.
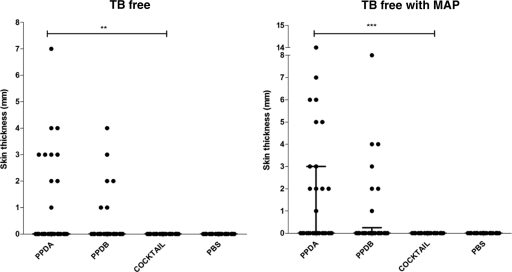
As expected in TB-free herds, the main skin responses observed were to PPDA, but some cattle also responded to PPDB. In the MAP-free herd, 2 animals were classified as suspect by SICCT when PPDB was used. The strongest response to PPDA was 4.0 mm in the MAP-free herd, unlike the 14.0 mm seen in the herd with MAP infection. Despite the strong reaction to PPDA in the MAP-infected herd, only one animal was classified as suspect by PPDB in SICCT (Fig. 5). All these suspects were negative in the IFN-γ test with PPDB and the protein cocktail (data not shown). Unlike PPD, the protein cocktail did not induce any skin response despite MAP infection and sensitization to environmental mycobacteria (Fig. 5).
Fig 5.
Skin test specificities of PPDB, PPDA, and the protein cocktail in TB-free herds. Two groups of animals from farms with no history of tuberculosis were tested. One farm had M. avium subsp. paratuberculosis infection. The responses for individual animals are represented by black dots, and the lines show the means and interquartile ranges, with the results expressed as the difference in skin thicknesses between post- and pre-skin test readings. The statistical differences between responses were determined by using the Kruskal-Wallis test (**, P < 0.01; ***, P < 0.001).
Sample collection for the IFN-γ test was not carried out as established by our protocol due to technical problems; therefore, it is possible that the antigen intradermal inoculation may interfere with IFN-γ (24). Our results showed that this is indeed the case, because we found 2 or 3 more nonspecific results in cattle where IFN-γ samples were taken 3 days after antigen intradermal inoculation. When the sample was taken the same day as the skin test, no animals were positive to the protein cocktail (Fig. 4).
DISCUSSION
Skin response to PPDB in SICCT is considered a marker for tuberculosis in cattle, but in order to perform a more specific diagnosis, a second test should be carried out. The IFN-γ test has been well analyzed, and its use has been approved in many countries (23). In this test, responses to PPDA and PPDB are compared in order to classify an animal as positive or negative. However, sensitization to environmental mycobacteria or coinfection with MAP affects the specificity in both tests (6). For this reason, the use of specific antigens, such as ESAT-6 and CFP-10, has been proposed (1, 2). The use of these antigens in the IFN-γ test has shown higher specificity; however, its applicability is limited, due to its high cost and the logistical constraints on its development. In this study, responses to the protein cocktail (E6-C10) and PPDB were compared with the aim of evaluating the cocktail in herds with high and low bovine tuberculosis prevalences using SICCT and IFN-γ formats.
The E6-C10 protein cocktail was tested intradermally using different dose concentrations (10, 20, and 30 μg of total protein) and four reading time points (24, 48, 72, and 96 h) in 10 naturally infected and 10 tuberculosis-free cattle. Previous studies pointed out the use of high concentrations of ESAT-6 to induce skin reactions in cattle (≥400 μg) and a longer time to read the skin reaction (96 h instead of 72 h) (15). In the current study, we demonstrated specific skin reactions starting from 10 μg of the E6-C10 protein cocktail; however, the best results were obtained using 20 μg of the cocktail with 72 h as the time selected for reading. Our results are consistent with those reported by Whelan et al. (29); they identified skin reactions with 10 μg each of the E6, C10, MPB83, and MPB70 proteins 72 h postinoculation. In our hands, addition of MPB83 did not improve the skin reaction at all; moreover, inclusion of MPB83 resulted in reactions that were less defined to palpation. Otherwise, inoculation of the protein cocktail did not induce a skin response in any noninfected cattle. Our results suggested that low concentrations of the protein cocktail are enough to induce a skin response comparable to that stimulated by PPDB; moreover, the reading time in both cases was 72 h, a clear advantage when using both antigens together. Our observations are in agreement with data presented by Lyashchenko et al. (17), who aimed to improve the accuracy of TST in experiments on sensitized guinea pigs. The results from this group demonstrated a higher potency of the recombinant-protein-based combination than of the single proteins and hence suggested the potential for lower protein concentrations when used in cocktails of two or more purified antigens. Based on the pilot study results, we decided to compare SICCT and the IFN-γ test in herds with different disease prevalences. In the high-prevalence herd, SICCT and the IFN-γ test identified 63/138 and 90/138 positive cattle using PPDB and 63/138 and 73/138 using the E6-C10 protein cocktail, respectively. Under low-prevalence herd conditions, the results were more discrete; however, the tendency is the same as in the high-prevalence herd (Tables 3 to 6). The results in both herds suggest that the IFN-γ test tends to improve sensitivity independently of the antigen used. In addition, it is easy to observe that the protein cocktail performs quite similarly to PPDB; however, the protein cocktail identified positive cattle in the PPDB-suspect and -negative populations, suggesting that E6-C10 increases the number of true-positive results; this was more evident in the low-prevalence herd, where the PPDA cross-reaction was stronger. Most of the E6-C10-positive cattle were confirmed by means of PCR. The decrease in specificity and sensitivity due to MAP coinfection has been previously reported (4, 15, 28). Experimental evidence showed that animals with dual infection were more likely to produce false-negative responses to the IFN-γ test (3).
The main benefit of the application of the E6-C10 protein cocktail was observed in the TB-free herds, because E6-C10 did not induce a single positive skin reaction in cattle tested in this study under this category. Its performance was comparable to that of the negative control (PBS). In the same category of animals, PPDB stimulated several false-positive reactors. In addition, the presence of MAP coinfection did not affect the protein cocktail's performance, unlike when PPDB was used as an antigen.
Taken together, our results suggest that the E6-C10 protein cocktail provides an improvement in bovine tuberculosis diagnosis. E6-C10 refines sensitivity but mainly improves specificity. Moreover, the protein cocktail may be included in SICCT and IFN-γ formats, providing the same effect in terms of greater identification of true-positive cattle.
Unfortunately, it was not possible to sacrifice all the animals used in this study, but the PCR of nasal swabs helped us to corroborate some results achieved with the protein cocktail in the skin test. In the high-prevalence herd, almost the same proportion of animals positive to PPDB and the protein cocktail were corroborated by PCR (60.3 and 63.4%, respectively), while in the low-prevalence herd, 87.5% of the E6-C10-positive animals were confirmed by PCR. These results suggest that the skin responses to the protein cocktail are highly specific. Nonetheless, a negative result in PCR does not mean that the animal is not infected, only that it is in a nonshedding state. The shedding pattern is intermittent and varies from one animal to another due to the state of infection or a variation in susceptibility and resistance to the infection (18).
Another factor that supports the high specificity of the protein cocktail is that in the tuberculosis-free herd we observed no skin responses despite the MAP infection status of the herd. Many reports have shown that animals sensitized to environmental mycobacteria, infected with MAP, or vaccinated with M. bovis BCG do not respond to the protein cocktail (9, 26, 28, 29).
In conclusion, the use of the E6-C10 cocktail allows identification of an important proportion of animals that PPDB is not able to recognize, especially in a low-prevalence herd. The protein cocktail improves the specificity and sensitivity of the IFN-γ test and skin test mainly when it is used in addition to the SICCT. Our results are very encouraging; however, they require further validation using a gold standard method.
ACKNOWLEDGMENTS
This work was supported by CONACYT CB-2005-24794, PAPIIT 21-4009-3, and Macroproyecto línea 2 Tuberculosis grants from the Universidad Nacional Autónoma de México.
We gratefully acknowledge the excellent technical assistance of Cristina Parada Colin. We also acknowledge revision of the English language usage performed by Diego Gutiérrez Fernández.
Footnotes
Published ahead of print 14 March 2012
REFERENCES
- 1. Aagaard C, et al. 2010. Detection of bovine tuberculosis in herds with different disease prevalence and influence of paratuberculosis infection on PPDB and ESAT-6/CFP10 specificity. Prev. Vet. Med. 96:161–169 [DOI] [PubMed] [Google Scholar]
- 2. Aagaard C, et al. 2006. Optimizing antigen cocktails for detection of Mycobacterium bovis in herds with different prevalences of bovine tuberculosis: ESAT6-CFP10 mixture shows optimal sensitivity and specificity. J. Clin. Microbiol. 44:4326–4335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Álvarez J, et al. 2009. Effect of paratuberculosis on the diagnosis of bovine tuberculosis in a cattle herd with a mixed infection using interferon-gamma detection assay. Vet. Microbiol. 135:389–393 [DOI] [PubMed] [Google Scholar]
- 4. Amadori M, Lyashchenko KP, Gennaro ML, Pollock JM, Zerbini I. 2002. Use of recombinant proteins in antibody test for bovine tuberculosis. Vet. Microbiol. 85:379–389 [DOI] [PubMed] [Google Scholar]
- 5. Andersen P, Munk ME, Pollock JM, Doherty TM. 2000. Specific immune-based diagnosis of tuberculosis. Lancet 356:1099–1104 [DOI] [PubMed] [Google Scholar]
- 6. Aranaz A, et al. 2006. Assessment of diagnostic tools for eradication of bovine tuberculosis in cattle co-infected with Mycobacterium bovis and M. avium subsp. paratuberculosis. Vet. Res. 37:593–606 [DOI] [PubMed] [Google Scholar]
- 7. Baisden LA, Larsen AB, Vardaman TH. 1951. Relative sensitivity of different skin areas of cattle to intradermal test. Am. J. Vet. Res. 12:273–275 [PubMed] [Google Scholar]
- 8. Belisle J, Sonnenberg G. 1998. Isolation of genomic DNA from mycobacteria. Methods Mol. Biol. 101:31–44 [DOI] [PubMed] [Google Scholar]
- 9. Buddle BM, et al. 1999. Differentiation between Mycobacterium bovis BCG-vaccinated and M. bovis-infected cattle by using recombinant mycobacterial antigens. Clin. Diagn. Lab. Immunol. 6:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cousins DV, Wilton SD, Francis BR. 1991. Use of DNA amplification for the rapid identification of Mycobacterium bovis. Vet. Microbiol. 27:187–195 [DOI] [PubMed] [Google Scholar]
- 11. Cousins DV, Wilton SD, Francis BR, Gow BL. 1992. Use of polymerase chain reaction for rapid diagnosis of tuberculosis. J. Clin. Microbiol. 30:255–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de la Rua-Domenech R, et al. 2006. Ante mortem diagnosis of tuberculosis in cattle: a review of the tuberculin tests, γ-interferon assay and other ancillary diagnostic techniques. Res. Vet. Sci. 81:190–210 [DOI] [PubMed] [Google Scholar]
- 13. Estrada-Chávez C, et al. 2004. Concordancia de PCR y métodos rutinarios para el diagnóstico de tuberculosis bovina. Vet. Méx. 35:225–236 [Google Scholar]
- 14. Harboe M, Oettinger T, Wiker HG, Rosenkrands I, Andersen P. 1996. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect. Immun. 64:16–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hope JC, et al. 2005. Exposure to Mycobacterium avium induces low-level protection from Mycobacterium bovis infection but compromises diagnosis of disease in cattle. Clin. Exp. Immunol. 141:432–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Larsen AB, Groth AH, Johnson HW. 1950. Allergic response to johnin and tuberculin of various skin regions of cattle. Am. J. Vet. Res. 11:301–303 [PubMed] [Google Scholar]
- 17. Lyashchenko K, et al. 1998. Use of Mycobacterium tuberculosis complex-specific antigen cocktails for a skin test specific for tuberculosis. Infect. Immun. 66:3606–3610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kao RR, et al. 2007. Mycobacterium bovis shedding patterns from experimentally infected calves and the effect of current infection with bovine viral diarrhoea virus. J. R. Soc. Interface 4:545–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Monaghan ML, Doherty ML, Collins JD, Kazda JF, Quinn PJ. 1994. The tuberculin test. Vet. Microbiol. 40:114–124 [DOI] [PubMed] [Google Scholar]
- 20. Munson TE. 1985. Guideline on validation of the Limulus amebocyte lysate test as an end-product endotoxin test for human and biological drug products. 189:211–220 [PubMed] [Google Scholar]
- 21. Paterson AB. 1959. Tuberculosis, p 716–744 In Stableforth AW, Galloway IA. (ed), Diseases due to bacteria, vol 2 Butterworth's, London, United Kingdom [Google Scholar]
- 22. Pollock JM, et al. 2003. Specific delayed-type hypersensitivity responses to ESAT-6 identify tuberculosis-infected cattle. J. Clin. Microbiol. 41:1856–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schiller I, et al. 2010. Bovine tuberculosis: a review of current and emerging diagnostic techniques in view of their relevance for disease control and eradication. Transbound. Emerg. Dis. 57:205–220 [DOI] [PubMed] [Google Scholar]
- 24. Schiller I, et al. 2010. Bovine tuberculosis: effect of the tuberculin skin test on in vitro interferon gamma responses. Vet. Immunol. Immunopathol. 136:1–11 [DOI] [PubMed] [Google Scholar]
- 25. Thoen OC, Ebel ED. 2006. Diagnostic tests for bovine tuberculosis, p 49–53 In Thoen OC, Steele HJ, Gilsdorf JM. (ed). Mycobacterium bovis infection in animals and humans. Blackwells, Oxford, United Kingdom [Google Scholar]
- 26. van Pinxteren LA, Ravn P, Agger EM, Pollock J, Andersen P. 2000. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin. Diagn. Lab. Immunol. 7:155–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vordermeier HM, et al. 1999. Development of diagnostic reagents to differentiate between Mycobacterium bovis BCG vaccination and M. bovis infection in cattle. Clin. Diagn. Lab. Immunol. 6:675–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Waters WR, et al. 2004. Use of recombinant ESAT-6:CFP-10 fusion protein for differentiation of infections of cattle by Mycobacterium bovis and by M. avium subsp. avium and M. avium subsp. paratuberculosis. Clin. Diagn. Lab. Immunol. 11:729–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Whelan AO, et al. 2010. Development of a skin test for bovine tuberculosis for differentiating infected from vaccinated animals. J. Clin. Microbiol. 48:3176–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Whelan AO, et al. 2003. Modulation of the bovine delayed type-hypersensitivity responses to defined mycobacterial antigens by a synthetic bacterial lipopeptide. Infect. Immun. 71:6420–6425 [DOI] [PMC free article] [PubMed] [Google Scholar]