Abstract
Tuberculosis (TB) caused by Mycobacterium tuberculosis remains a major infectious disease worldwide. Moreover, latent M. tuberculosis infection is more likely to progress to active TB and eventually leads to death when HIV infection is involved. Thus, it is urgent to develop a novel TB vaccine with immunogenicity to both M. tuberculosis and HIV. In this study, four uncharacterized T cell epitopes from MPT64, Ag85A, Ag85B, and TB10.4 antigens of M. tuberculosis were predicted, and HIV-1-derived p24, an immunodominant protein that can induce protective responses to HIV-1, was used as an immunogenic backbone. M. tuberculosis epitopes were incorporated separately into the gene backbone of p24, forming a pP24-Mtb DNA vaccine. We demonstrated that pP24-Mtb immunization induced a strong M. tuberculosis-specific cellular response as evidenced by T cell proliferation, cytotoxicity, and elevated frequency of gamma interferon (IFN-γ)-secreting T cells. Interestingly, a p24-specific cellular response and high levels of p24-specific IgG were also induced by pP24-Mtb immunization. When the protective effect was assessed after mycobacterial challenge, pP24-Mtb vaccination significantly reduced tissue bacterial loads and profoundly attenuated the mycobacterial infection-related lung inflammation and injury. Our findings demonstrated that the pP24-Mtb tuberculosis vaccine confers effective protection against mycobacterial challenge with simultaneously elicited robust immune responses to HIV-1, which may provide clues for developing novel vaccines to prevent dual infections.
INTRODUCTION
Tuberculosis (TB) is a reemerging disease that remains one of the leading causes of mortality in humans (44). It accounts for 4 deaths every minute and 2 million deaths annually and severely threatens the health of humankind (26). In recent years, the prevalence of HIV infection among tuberculosis cases has increased dramatically (28). A latent Mycobacterium tuberculosis infection (LTBI) is 20 to 30 times more likely to progress to active TB when HIV infection is involved (14, 23). Similarly, at least one-third of HIV-infected individuals are already infected with TB, and one-quarter of the persons infected with HIV die from TB (16, 23). However, Mycobacterium bovis bacillus Calmette-Guérin (BCG), the only tuberculosis vaccine approved for human use, is effective only in children, and its protective efficacy wanes significantly over a period of 10 to 15 years (1, 37). To date, the development of an effective HIV vaccine is still under way (4, 24). Therefore, the serious situation of dual epidemics asks for a novel vaccine with immunogenicity to both Mycobacterium tuberculosis and HIV.
It is well established that T cell responses play an important role in the development of resistance to M. tuberculosis, primarily through production of gamma interferon (IFN-γ) and eradication of intracellular pathogens (32, 33). Therefore, antigens to predominantly induce T cell response are used more in the development of TB vaccine. To avoid unfavorable components for humans and for the humoral response induced by B cell epitopes (21, 30, 36, 39), it is better to apply T cell epitopes than to apply entire antigens in TB vaccine design. Furthermore, a strategy that incorporates multiple T cell epitopes into one vaccine has the potential to induce broad T cell immunity against several immunodominant antigens (10, 31, 34).
In this study, we predicted four T cell epitopes from four well-defined protective antigens of M. tuberculosis, MPT64 (M. tuberculosis protein 64), Ag85A (M. tuberculosis antigen 85A), Ag85B, and TB10.4 via epitope prediction software online. Epitopes are short peptides, and their immunogenicity is low unless introduced into a carrier protein (39). Here we used p24, an immunodominant protein of HIV-1 widely used in the development of HIV vaccines (15), as the protein backbone for incorporation of M. tuberculosis epitopes. The gene segments of these epitopes were grafted into various regions of the p24 gene scaffold, and a multiepitope DNA vaccine containing immunogens from M. tuberculosis and HIV-1 was obtained. The immunogenicity of the vaccine to both M. tuberculosis and HIV-1 was evaluated.
MATERIALS AND METHODS
Prediction of T cell epitopes.
Potential major histocompatibility complex class I (MHC-I)- or MHC-II-binding T cell epitopes were screened from MPT64, Ag85A, Ag85B, and TB10.4 proteins using epitope prediction software online (http://www.syfpeithi.de/, http://www.ddg-pharmfac.net/mhcpred/MHCPred/, and http://www.imtech.res.in/raghava/propred/). Similarity was scored using position-specific scoring matrixes derived from aligned peptides. Four epitopes, including MPT6476-84 (MPT64 from amino acids 76 to 84) (KFLSAATSS), Ag85A242-250 (KLIANNTRV), Ag85B184-192 (IYAGSLSAL), and TB10.474-82 (STHEANTMA), were sorted.
Construction of pP24-Mtb DNA vaccine.
Plasmids containing the gene for HIV-1 p24 were obtained from the laboratory of Robert Tycko. The four selected T cell epitopes were engineered into the p24 scaffold separately (Fig. 1D). The chimeric p24-Mtb gene was constructed by gene splicing through overlap extension of several synthetic nucleotide sequences and was then incorporated into pcDNA3.1 plasmid (Invitrogen, Carlsbad, CA) driven by a cytomegalovirus (CMV) promoter.
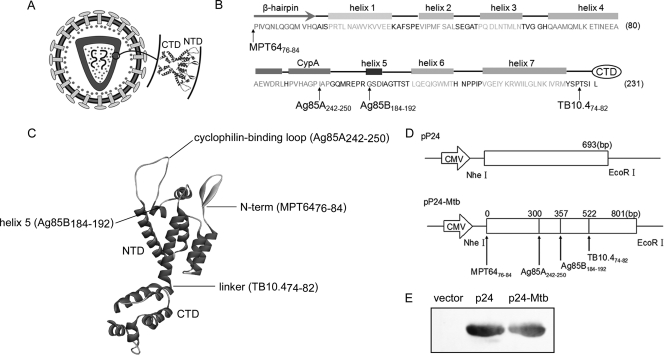
Fig 1.
Designation and construction of pP24-Mtb DNA vaccine. (A) Schematic model of the HIV-1 particle. p24 is composed of two domains, the N-terminal domain (NTD) and C-terminal domain (CTD). An enlargement of the two-domain structure is shown. (B) Secondary structure of the NTD. The β-hairpin, CypA binding loop, and helices 1 to 7 are included in the NTD. The positions indicated by the arrows are the insertion sites of the predicted epitopes. (C) Inserted epitopes are labeled on the three-dimensional structure of p24. (D) Schematic representations of the pP24 and pP24-Mtb plasmids. (E) p24 protein and p24-Mtb protein were expressed in the pET-32a E. coli expression system and analyzed by Western blotting with anti-p24 antibody. Individual experiments were conducted three times, with the results from one representative experiment shown for each group of mice.
Preparation of antigen peptides and proteins.
BCG was purchased from the Shanghai Institute of Biological Products. Polypeptides, MPT6476-84, Ag85A242-250, Ag85B184-192, and TB10.474-82 were commercially synthesized by GL Biochem Ltd. (Shanghai, People's Republic of China) with a purity of >95%. The polypeptides were then dissolved in phosphate-buffered saline (PBS) buffer and stored at −80°C until use. Recombinant p24 protein and p24-Mtb protein were expressed as histidine-tagged proteins in the pET-32a Escherichia coli expression system (Novagen, Madison, WI). The p24 gene and p24-Mtb gene were inserted into the pET-32a vector to construct two recombinant plasmids, pET-P24 and pET-P24-Mtb. The recombinant plasmids were then transformed into the competent Escherichia coli strain BL21(DE3). The cells were grown in a shaker at 37°C, isopropyl-β-d-thiogalactoside (IPTG) (Sigma, St. Louis, MO) was added to induce recombinant protein synthesis at an optical density at 600 nm (OD600) of 0.5, and incubation was continued for another 6 h. The cells were lysed, and the proteins were purified by Ni Sepharose 6 Fast Flow (GE Healthcare, Uppsala, Sweden). The concentration of the purified proteins was determined by bicinchoninic acid test using a microplate BCA (bicinchoninic acid) protein assay reagent kit (Pierce, Rockford, IL).
Western blot assay.
The E. coli strain BL21(DE3) was transformed with pET-P24 or pET-P24-Mtb and induced by IPTG. The cells were collected and disrupted by sonication. After centrifugation, the supernatants were collected and electrophoresed on SDS-polyacrylamide gels and transferred to polyvinylidene fluoride membranes. Each membrane was probed with anti-p24 polyclonal antibody (Abcam, Cambridge, United Kingdom), followed by horseradish peroxidase (HRP)-conjugated goat anti-mouse antibody (Southern Biotech, Birmingham, AL), and the signals were developed using chemiluminescence (Fig. 1E).
Animals and vaccination.
Six- to 8-week-old female BALB/c mice (H-2d) were purchased from the experimental animal center of the Chinese Academy of Science (Shanghai, People's Republic of China) and remained in pathogen-free conditions. All animal experiments were performed according to the guidelines for the care and use of laboratory animals (28a) and the guidelines of the Laboratory Animal Ethical Commission of Fudan University. Endotoxin-free plasmids were prepared using an EndoFree plasmid purification megaprep kit (Qiagen, Valencia, CA). Mice were immunized intramuscularly with 50 μg pP24-Mtb, pP24, or pcDNA3.1 (defined as vector) 4 times at biweekly intervals. Serum was collected every 2 weeks by retro-orbital bleeding and stored at −70°C for further analysis.
ELISA measurement of p24- or peptide-specific antibody.
The wells on 96-well enzyme-linked immunosorbent assay (ELISA) plates (Corning Costar, Cambridge, MA) were coated with p24 protein or the mixed M. tuberculosis peptides at a final concentration of 10 μg/ml at 4°C overnight and washed with PBS containing 0.05% Tween 20 (PBST). After blocking with PBS containing 5% nonfat milk at 37°C for 2 h, serum (1:100 dilution) was added in duplicate and incubated at 37°C for 1 h. After the wells were washed, HRP-conjugated goat anti-mouse IgG, IgG1, or IgG2a (Southern Biotech) was added, followed by the addition of HRP substrate. The absorbance at 450 nm was measured in a microplate reader (Bio-Rad Lab, Hercules, CA). To determine the antibody titers, 5-fold serially diluted sera were measured.
Avidity of p24-specific antibody.
The avidity of the p24-specific antibody was determined by ELISA with a urea elution step (5). Briefly, sera (1:100 dilution) were tested in duplicate plates. In one of the plates, 6 M urea (Sigma) was added after incubation with samples and incubated for 10 min. Results were expressed as avidity index calculated as follows: (endpoint titer in the presence of urea/endpoint titer in the absence of urea) × 100.
IFN-γ ELISPOT assays.
Enzyme-linked immunospot (ELISPOT) assays were performed with ELISPOT assay kit (BD PharMingen, San Diego, CA). Briefly, wells on the plates were coated with the capture anti-IFN-γ monoclonal antibody (MAb) at 4°C overnight and then blocked with complete medium for 2 h at room temperature. Splenocytes from immunized mice were isolated, plated (1 × 106 cells/well), and cultured with the mixed M. tuberculosis peptides or p24 protein (each peptide or protein at a final concentration of 20 μg/ml) at 37°C for 36 h. After the plates were washed with deionized water and PBST, the biotinylated anti-IFN-γ MAb was added for 2 h at room temperature. Streptavidin-alkaline phosphatase (AP) was added to the plates and incubated for 1 h, and the color was developed by AP colorimetric substrate. An immunospot analyzer (Cellular Technology, Cleveland, OH) was used to enumerate the spots.
Intracellular IFN-γ staining.
Two weeks after the immunization, splenocytes were isolated and stimulated in vitro with the mixed M. tuberculosis peptides or p24 protein (each peptide or protein at a final concentration of 20 μg/ml) at 37°C for 6 h. The cells were stained with anti-CD4 antibody conjugated to peridinin chlorophyll protein (anti-CD4-PerCP) and anti-CD8 antibody conjugated to fluorescein isothiocyanate (anti-CD8-FITC) (BD PharMingen). After the cells were washed, they were treated with fixation/permeabilization solution (BD PharMingen) for 30 min at 4°C and then stained with anti-IFN-γ antibody conjugated to phycoerythrin (anti-IFN-γ-PE) (BD PharMingen). The percentages of CD4+ IFN-γ-positive (IFN-γ+) and CD8+ IFN-γ+ T cells were determined by flow cytometry using a fluorescence-activated cell sorter FACSCalibur instrument (BD Biosciences, San Jose, CA).
ELISA of cytokines.
Splenocytes from immunized mice were plated (5 × 106 cells/well) and cultured with the mixed M. tuberculosis peptides or p24 protein (10 μg/ml) for 72 h. The concentrations of IFN-γ, tumor necrosis factor alpha (TNF-α), interleukin-4 (IL-4), and IL-10 in the culture supernatants were measured by using an ELISA kit (eBioscience, San Diego, CA) following the manufacturer's procedures.
Lymphocyte proliferation assay.
Proliferation of splenocytes from immunized mice was measured 2 weeks after the last immunization. Viable splenocytes were adjusted to a concentration of 5 × 106 cells/ml and were added to 96-well flat-bottomed plates at 5 × 105 cells/well with 10 μg/ml of the mixed M. tuberculosis peptides or p24 protein. The plates were cultured at 37°C in a humidified incubator with 5% CO2 for 72 h. The reagent of the cell counting kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was then added to each well (10 μl/well), and the plates were incubated for an additional 6 h. After incubation, the absorbance of the samples at 450 nm was measured. Each sample was analyzed in triplicate. The proliferative responses of individual mice were expressed as stimulation index (SI) and calculated by the following formula: SI = mean OD value of the stimulated cells/mean OD value of the cells in medium alone.
Cytotoxic T lymphocyte measurement.
Two weeks after the final immunization, splenocytes were isolated and stimulated in vitro with the mixed M. tuberculosis peptides or p24 protein (each peptide or protein at a final concentration of 20 μg/ml) at 37°C in a humidified incubator with 5% CO2. The processed cells were washed and resuspended in RMPI 1640 at a concentration of 2.5 × 106/ml as effector cells. The mouse myeloma cell line SP2/0 cells were pulsed with or without the mixed M. tuberculosis peptides or p24 protein (10 μg/ml) for 8 h at 37°C, washed, and resuspended in RPMI 1640 at a concentration of 1 × 105/ml as target cells. The cytotoxic T lymphocyte (CTL) assay was then performed according to the instructions of the manufacturer of the CCK-8 kit. Briefly, effector cells and target cells were added in 96-well plates at an effector-to-target cell (E:T) ratio of 25:1 and incubated in an incubator for 4 h. CCK-8 reactant was added to each well and incubated for another 3 h. The absorbance of the samples at 450 nm was then measured. The cytotoxicity of CTLs was calculated by the following formula:
M. bovis BCG challenge and CFU determination.
M. bovis BCG (Denmark strain 1331) was grown in Middlebrook 7H9 broth supplemented with Middlebrook oleic acid-albumin-dextrose-catalase (OADC) enrichment (BD PharMingen), 0.002% glycerol, and 0.05% Tween 80 for 10 to 15 days and then stored frozen at −70°C. Before use, the bacteria were washed with PBS containing 0.05% Tween 80 twice and passed through a needle 10 times to disperse clumps. Immunized mice were challenged intranasally with 1 × 107 CFU of M. bovis BCG at 4 weeks postimmunization. The number of bacteria in the spleens and lungs was determined 4 weeks postchallenge by serial dilutions of individual whole-organ homogenates in duplicate on 7H11 medium. The plates were incubated for 4 weeks at 37°C, and then the colonies were counted and the calculations were made. Protective efficacy is expressed as log10 bacterial count in immunized mice compared with bacterial count in the controls.
Histopathology.
Pulmonary tissues were harvested for histological evaluation 4 weeks after M. bovis BCG challenge, preserved in 10% formalin, and embedded in paraffin. Sections 4 μm thick were stained with hematoxylin and eosin (HE). To score lung inflammation and damage, the entire lung section was analyzed with confluent inflammatory infiltration which was quantified and expressed as a percentage of the lung surface.
Statistical analysis.
All data were given as means ± standard deviations (SD). Statistical analysis of the data was performed by two-tailed independent Student's t test using SPSS 12.0. The level of statistical significance was set at P of <0.05.
RESULTS
Prediction of T cell epitopes from M. tuberculosis antigens and construction of pP24-Mtb DNA vaccine.
To design a novel multivalent DNA vaccine against M. tuberculosis and HIV-1, four M. tuberculosis T cell epitopes were screened from MPT64, Ag85A, Ag85B, and TB10.4 protein sequences and HIV-1-derived p24 was utilized as the protein backbone for incorporation of the four M. tuberculosis epitopes. As shown in Fig. 1A, p24 is composed of a N-terminal domain (NTD) and a C-terminal domain (CTD). Four T cell epitopes were incorporated into the NTD so as to preserve the functional domains and three-dimensional structure of the p24 protein. The MPT6476-84 epitope was inserted before the NTD. Ag85A242-250 was inserted between Ile91 and Ala92. Ag85B184-192 was inserted between Gly101 and Ser102. TB10.474-82 was placed between Pro147 and Thr148 (Fig. 1B and C). The p24-Mtb chimeric gene was then placed into pcDNA3.1 to construct a plasmid designated pP24-Mtb (Fig. 1D). The pP24 and pP24-Mtb plasmids were efficiently expressed in vitro as evidenced by the p24-specific bands (Fig. 1E).
T cell immune responses induced by pP24-Mtb immunization to both M. tuberculosis and HIV-1.
T cells play a critical role in protective immunity against mycobacterial and HIV-1 infection (32, 33, 43). To measure lymphocyte proliferation, splenocytes from vaccinated mice were stimulated with the mixed M. tuberculosis peptides or p24 protein in vitro. As shown in Fig. 2A, strong splenic T cell proliferation to M. tuberculosis peptides was observed in pP24-Mtb-vaccinated mice compared to that in pP24-treated mice (P < 0.05). In addition, splenocytes from pP24-Mtb-immunized mice also showed considerable proliferation in response to p24.
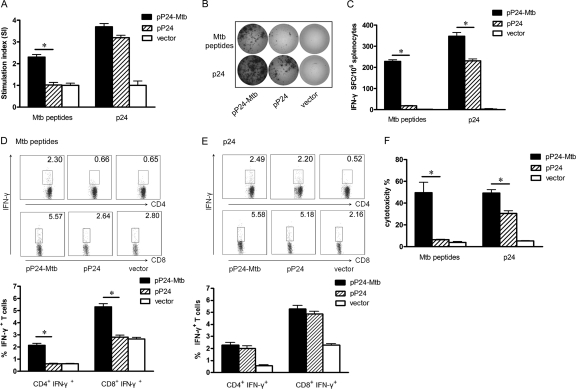
Fig 2.
M. tuberculosis- and p24-specific cellular responses induced by pP24-Mtb immunization. Two weeks after the last immunization, splenocytes were harvested and stimulated with M. tuberculosis peptides or p24 protein in vitro. (A) Lymphocyte proliferation was measured and expressed as stimulation index (SI). (B) IFN-γ-secreting lymphocytes were quantified by ELISPOT assay. Images represent splenic ELISPOT responses in the immunized mice. (C) Frequency of IFN-γ-secreting cells in the spleens of mice measured by ELISPOT assay. (D and E) The percentages of CD4+ IFN-γ+ or CD8+ IFN-γ+ T cells in the spleens were analyzed by flow cytometry following M. tuberculosis peptide stimulation (D) or following p24 stimulation (E). (F) M. tuberculosis- and p24-specific CTL activity was expressed as the percent cytotoxicity. Data in the graph are from one representative experiment of three independent experiments performed. Error bars represent the means plus standard deviations (SD) (n = 6). Values that are statistically significantly different (P < 0.05) are indicated by a short horizontal line and an asterisk.
IFN-γ ELISPOT assays and intracellular cytokine staining assays were performed to assess the functional T cell response. As shown in Fig. 2B and C, pP24-Mtb vaccination significantly increased the number of IFN-γ-secreting T cells to both M. tuberculosis peptides and p24 protein compared to pP24 vaccination (P < 0.05), suggesting that pP24-Mtb immunization induced potent M. tuberculosis- and p24-specific T cell responses. Similar data were achieved when CD4+ IFN-γ+ and CD8+ IFN-γ+ T cells were detected by flow cytometry (Fig. 2D and E), suggesting that pP24-Mtb vaccination effectively produced M. tuberculosis-specific CD4+ and CD8+ T cell responses and potent responses to HIV-1 p24 concurrently.
Specific CTL activity was assessed by using splenocytes stimulated with M. tuberculosis peptides or p24 protein as effector cells and pulsed SP2/0 cells as target cells. As shown in Fig. 2F, powerful M. tuberculosis-specific CTL activity was observed in pP24-Mtb-immunized mice. Moreover, splenocytes from pP24-Mtb-immunized mice also showed significantly increased p24-specific CTL activity compared with those from pP24-immunized mice (P < 0.05). These results demonstrated that pP24-Mtb vaccination induced robust specific T cell responses against both M. tuberculosis and HIV-1.
Humoral responses specific to p24 elicited by pP24-Mtb immunization.
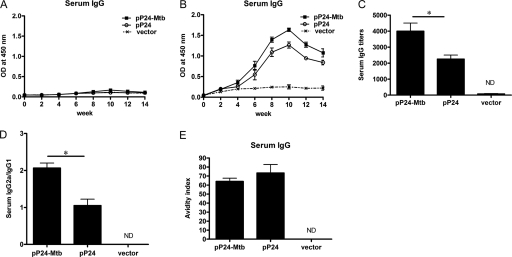
The potential of the vaccine to induce serum IgG was also evaluated. As shown in Fig. 3B, p24-specific serum IgG was induced, gradually increased after week 4, and peaked at week 10 in pP24-Mtb- or pP24-immunized mice with the IgG titer reaching 1:4,000 and 1:2,250, respectively (Fig. 3C). In contrast, there was no detectable IgG against M. tuberculosis peptides (Fig. 3A), which was not surprising because they are T cell epitopes. In addition, an elevated IgG2a/IgG1 ratio was observed following pP24-Mtb immunization (Fig. 3D) (P < 0.05), indicating a more Th1-polarized response. However, the avidity indices of serum IgG elicited by pP24-Mtb- and pP24-immunized mice were not statistically different (Fig. 3E). These data indicated that vigorous humoral responses to the HIV-1 protein were successfully elicited by pP24-Mtb immunization.
Fig 3.
p24-specific serum IgG elicited by pP24-Mtb immunization. Mice were immunized 4 times with pP24-Mtb, pP24, or pcDNA3.1 (vector) at 2-week intervals. (A and B) Serum M. tuberculosis-specific IgG (A) and p24-specific IgG (B) were detected by ELISA at the indicated time points. (C to E) p24-specific antibody titers (C), serum IgG subclasses (D), and avidity (E) were determined 2 weeks after the last immunization. Each bar represents the average value plus SD (error bar) for 6 mice, measured in duplicate experiments. Values that are statistically significantly different (P < 0.05) are indicated by a short horizontal line and an asterisk. ND, not detected.
Th1 predominant response generated by pP24-Mtb immunization.
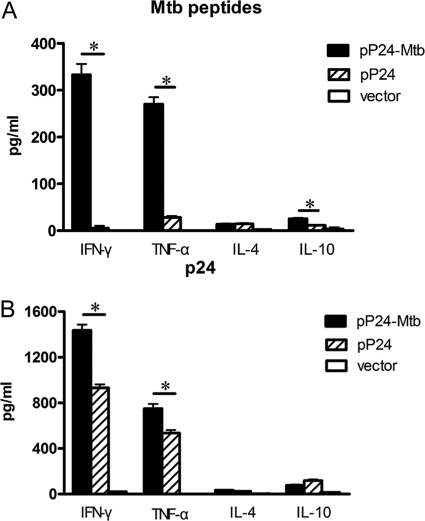
As enhanced serum IgG2a and IFN-γ+ T cell response induced by pP24-Mtb immunization indicated a Th1 immune response, the cytokine profiles produced by in vitro-primed lymphocytes were then analyzed. After stimulation with the pooled M. tuberculosis peptides, splenocytes from pP24-Mtb-vaccinated mice produced significantly higher levels of IFN-γ and TNF-α (P < 0.05) (Fig. 4A). Furthermore, pP24-Mtb vaccination also showed higher levels of p24-specific IFN-γ and TNF-α compared to pP24- or vector-treated mice (P < 0.05) (Fig. 4B). In comparison, only negligible levels of IL-4 and IL-10 were generated, indicating that vaccination with pP24-Mtb skewed the immune response toward the Th1 profile.
Fig 4.
Th1 immune response induced by pP24-Mtb immunization. The concentrations of cytokines in the splenocyte culture supernatant were determined by ELISAs. (A) Cytokine production by the splenocytes stimulated with M. tuberculosis peptides. (B) Cytokine production by the splenocytes stimulated with p24 protein. The data in this figure are from one representative experiment of three experiments performed and presented as the mean plus SD (n = 6). Values that are statistically significantly different (P < 0.05) are indicated by a short horizontal line and an asterisk.
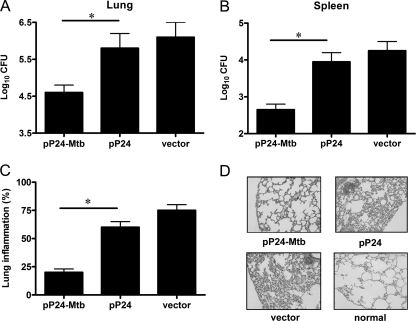
Protective effect of the pP24-Mtb vaccine against Mycobacterium bovis BCG challenge.
M. bovis BCG, which has been demonstrated to multiply well in vivo and induce obvious tuberculosis pathology, has been widely used for challenge experiments (8, 25). Here, mice were challenged intranasally with a high dose of 1 × 107 CFU BCG 4 weeks after the final immunization to determine the protective effect of the pP24-Mtb vaccination. Four weeks after challenge, the bacterial burden in the lungs and spleens of mice and pathological pulmonary injury were examined. As shown in Fig. 5A and B, pP24-Mtb vaccination led to an efficient protection both in the lung (log10 CFU = 4.6) and spleen (log10 CFU = 2.65) compared to pP24 or vector vaccination. These data demonstrated that the M. tuberculosis-specific immune responses elicited by pP24-Mtb led to an efficient host defense at local and systemic tissue compartments.
Fig 5.
Protection against mycobacterial infection initiated by pP24-Mtb immunization. Four weeks following the last immunization, mice were intranasally challenged with 1 × 107 CFU of M. bovis BCG. (A and B) Four weeks postchallenge, the bacterial loads in the lungs (A) and spleens (B) were measured. The data are presented as the means plus SD (n = 6) and are from one representative experiment of three separate experiments. Values that are statistically significantly different (P < 0.05) are indicated by a short horizontal line and an asterisk. (C) Paraffin sections from lung tissues were stained with hematoxylin and eosin (HE) and evaluated for the level of lung inflammation. (D) Representative histology sections depict lung tissue of immunized mice, with a lung section of a healthy (normal) mouse as a negative control. Individual experiments were conducted three times, with the results from one representative experiment shown. Magnification, ×200.
When tissue pathology was observed, it was seen that lung tissues from vector- or pP24-immunized mice showed widespread and severe interstitial pneumonia, intense inflammation, and diffuse granuloma responses after M. bovis BCG infection, displaying excessive lymphocyte and macrophage infiltration (Fig. 5C and D). However, pP24-Mtb-immunized mice exhibited the pulmonary alveolar wall structure of healthy mice with very mild lung inflammation, indicating dramatically attenuated tuberculosis pathology. The histological evidence and the bacterial burden reduction confirmed the protective efficacy achieved by pP24-Mtb DNA immunization.
DISCUSSION
TB remains one of the leading causes of death worldwide (44), and HIV infection contributes to the number of deaths caused by TB (23). On the other hand, TB is the largest single cause of death in the AIDS setting, accounting for about 25% of AIDS-related deaths (16). Therefore, a novel and effective tuberculosis vaccine with immunogenicity to both M. tuberculosis and HIV-1 is needed. Im et al. (19) developed a live attenuated vaccine by constructing a recombinant M. bovis BCG expressing an HIV-1-derived immunogen. It is well established that DNA vaccines are safe and easy to prepare, as well as good at generating T cell responses, including cytotoxic T lymphocytes and type 1 helper T cells which are especially required for protection against M. tuberculosis and HIV-1 (11, 18). In the present study, we generated a novel DNA vaccine pP24-Mtb by incorporating four M. tuberculosis epitopes into the HIV-1 p24 backbone. We demonstrated that vaccination with pP24-Mtb inhibited the replication of M. bovis BCG and mitigated the pathology of tuberculosis and simultaneously elicited robust cellular and humoral responses to HIV-1. However, though M. tuberculosis is a global health problem with 2 billion people infected, most are in a latent state where mycobacteria are thought to persist for decades (6, 7, 12). HIV infection is the strongest risk factor for the progression of LTBI to active TB (14, 28). Thus, determining whether pP24-Mtb can also protect against LTBI or prevent LTBI from progressing to active TB would be meaningful.
As T cell epitopes are crucial for defending against intracellular pathogens (17, 40), such as M. tuberculosis, searching for epitopes from immunodominant M. tuberculosis antigens is critical. Studies show that Ag85A and Ag85B stimulate strong humoral and cell-mediated immune responses and produce significant protection against Mycobacterium tuberculosis H37Rv challenge (18, 20, 22, 38). In addition, MPT64, a member of the culture filtrate protein (CFP) family, can induce a strong T cell response (42). TB10.4, a member of a subfamily of the ESAT6 family, is even more vigorously recognized than ESAT-6 (6-kDa early secreted antigen target of M. tuberculosis) in TB patients (35). Therefore, in this study, we generated a vaccine harboring four uncharacterized T cell epitopes from the four antigens. We demonstrated that the pP24-Mtb vaccination efficiently induced M. tuberculosis-specific T cell immunity and protection against mycobacterial challenge which was evidenced by the greatly reduced bacterial burden and lung pathology. Moreover, we also examined the immunogenicity of individual epitopes and found that all four epitopes contributed to M. tuberculosis-specific T cell responses. Of the four epitopes, the Ag85B184-192 and Ag85A242-250 epitopes are more immunogenic than the TB10.474-82 and MPT6476-84 epitopes (data not shown).
Many epitopes are short peptides, with poor immunogenicity unless they are introduced into a carrier protein (39). Using a natural protein as a scaffold is ideal for the presentation of random sequences (13). Ofek et al. (29) grafted neutralizing antibody epitopes into protein scaffolds and elegantly demonstrated that epitope-specific humoral immune responses were successfully induced by this strategy. In the current study, we extended previous studies by grafting M. tuberculosis T cell epitopes into a p24 scaffold protein to form a pP24-Mtb vaccine. Strong epitope-specific T cell proliferation, cytotoxicity, and elevated frequency of IFN-γ+ T cells clearly demonstrated that a robust cellular response to these T cell epitopes was successfully induced by this strategy.
As the pP24-Mtb vaccine was designed to possess immunogenicity to both M. tuberculosis and HIV-1, it is crucial to select a suitable immunodominant antigen to induce protective responses to HIV-1. Studies show that HIV-1 p24 can stimulate robust antigen-specific humoral and cellular responses (3, 27, 41). Therefore, in the pP24-Mtb vaccine, the scaffold protein p24 for incorporation of M. tuberculosis epitopes was also used as an immunogen to induce an immune response to HIV-1. When achieving M. tuberculosis-specific T immune responses, the vaccine also mounted strong humoral and cellular responses specific to p24 protein as evidenced by high levels of specific antibody, Th1-dominated immune responses, and CTL activity, indicating the potential of this vaccine to induce protective immunity against HIV-1. Moreover, a stronger immune response to p24 was observed for pP24-Mtb vaccination than for pP24 vaccination. We presumed that the insertion of the epitopes affected the conformation of p24 or produced new epitopes in p24. These changes may increase its recognition and presentation to better activate the immune response, which is similar with previous work (2, 9). However, the underlying mechanisms need further research.
In conclusion, here we assessed the feasibility and immunological efficacy of the tuberculosis DNA vaccine in an HIV-1 p24 protein backbone. Immunization of pP24-Mtb predominantly produced M. tuberculosis-specific Th1 immune responses and CTL activity which conferred protection against mycobacterial infection. Meanwhile, an efficient humoral and cellular immune response to HIV-1 p24 was also elicited. This strategy may provide a new way to design future DNA vaccines against coinfection.
ACKNOWLEDGMENTS
We thank Robert Tycko (Laboratory of Chemical Physics, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health) for donation of plasmid HIV-1 p24 and Shuo Li for technical assistance.
This work was funded by the National Science & Technology Key Projects during the Eleventh Five-Year Plan Period of China (2012ZX10003-006), China NSFC grant (81072409), Jiangsu High Level “Shuang-Chuang” Project, Program for Outstanding Medical Academic Leader (LJ06011), and grant (10JC1400900) from the Science and Technology Commission of Shanghai Municipality.
Footnotes
Published ahead of print 29 March 2012
REFERENCES
- 1. Al-Attiyah R, Mustafa AS. 2008. Characterization of human cellular immune responses to novel Mycobacterium tuberculosis antigens encoded by genomic regions absent in Mycobacterium bovis BCG. Infect. Immun. 76:4190–4198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ametani A, et al. 2003. Amino acid residue substitution at T-cell determinant-flanking sites in beta-lactoglobulin modulates antigen presentation to T cells through subtle conformational change. Biosci. Biotechnol. Biochem. 67:1507–1514 [DOI] [PubMed] [Google Scholar]
- 3. Ammaranond P, et al. 2011. HIV immune escape at an immunodominant epitope in HLA-B*27-positive individuals predicts viral load outcome. J. Immunol. 186:479–488 [DOI] [PubMed] [Google Scholar]
- 4. Barouch DH. 2008. Challenges in the development of an HIV-1 vaccine. Nature 455:613–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Capozzo AV, et al. 2006. Neonatal immunization with a Sindbis virus-DNA measles vaccine induces adult-like neutralizing antibodies and cell-mediated immunity in the presence of maternal antibodies. J. Immunol. 176:5671–5681 [DOI] [PubMed] [Google Scholar]
- 6. Chan J, Flynn J. 2004. The immunological aspects of latency in tuberculosis. Clin. Immunol. 110:2–12 [DOI] [PubMed] [Google Scholar]
- 7. Chapman AL. 2011. New tests will improve detection of latent TB. Practitioner 255(1745):23–26 [PubMed] [Google Scholar]
- 8. Chen L, Wang J, Zganiacz A, Xing Z. 2004. Single intranasal mucosal Mycobacterium bovis BCG vaccination confers improved protection compared to subcutaneous vaccination against pulmonary tuberculosis. Infect. Immun. 72:238–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Culshaw A, Dong T, Rowland-Jones SL. 2012. A two amino acid shift in position leads to a substantial difference in the pattern of processing of two HIV-1 epitopes. J. Acquir. Immune Defic. Syndr. 59:335–339 [DOI] [PubMed] [Google Scholar]
- 10. Depla E, et al. 2008. Rational design of a multiepitope vaccine encoding T-lymphocyte epitopes for treatment of chronic hepatitis B virus infections. J. Virol. 82:435–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. D'Souza S, et al. 2003. Mapping of murine Th1 helper T-cell epitopes of mycolyl transferases Ag85A, Ag85B, and Ag85C from Mycobacterium tuberculosis. Infect. Immun. 71:483–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fallahi-Sichani M, Flynn JL, Linderman JJ, Kirschner DE. 2012. Differential risk of tuberculosis reactivation among anti-TNF therapies is due to drug binding kinetics and permeability. J. Immunol. 188:3169–3178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fernandez-Carneado J, et al. 2000. Surface grafting onto template-assembled synthetic protein scaffolds in molecular recognition. Biopolymers 55:451–458 [DOI] [PubMed] [Google Scholar]
- 14. Gao L, Zhou F, Li X, Jin Q. 2010. HIV/TB co-infection in mainland China: a meta-analysis. PLoS One 5:e10736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gong X, Gai W, Xu J, Zhou W, Tien P. 2009. Glycoprotein 96-mediated presentation of human immunodeficiency virus type 1 (HIV-1)-specific human leukocyte antigen class I-restricted peptide and humoral immune responses to HIV-1 p24. Clin. Vaccine Immunol. 16:1595–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harrington M. 2010. From HIV to tuberculosis and back again: a tale of activism in 2 pandemics. Clin. Infect. Dis. 50(Suppl. 3):S260–S266 [DOI] [PubMed] [Google Scholar]
- 17. Hart MK, et al. 1990. Synthetic peptides containing T and B cell epitopes from human immunodeficiency virus envelope gp120 induce anti-HIV proliferative responses and high titers of neutralizing antibodies in rhesus monkeys. J. Immunol. 145:2677–2685 [PubMed] [Google Scholar]
- 18. Huygen K, et al. 1996. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat. Med. 2:893–898 [DOI] [PubMed] [Google Scholar]
- 19. Im EJ, et al. 2007. Vaccine platform for prevention of tuberculosis and mother-to-child transmission of human immunodeficiency virus type 1 through breastfeeding. J. Virol. 81:9408–9418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kamath AT, Feng CG, Macdonald M, Briscoe H, Britton WJ. 1999. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect. Immun. 67:1702–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Korber B, LaBute M, Yusim K. 2006. Immunoinformatics comes of age. PLoS Comput. Biol. 2:e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Launois P, et al. 1994. T-cell-epitope mapping of the major secreted mycobacterial antigen Ag85A in tuberculosis and leprosy. Infect. Immun. 62:3679–3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lawn SD, Churchyard G. 2009. Epidemiology of HIV-associated tuberculosis. Curr. Opin. HIV AIDS 4:325–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Letvin NL. 2007. Correlates of immune protection and the development of a human immunodeficiency virus vaccine. Immunity 27:366–369 [DOI] [PubMed] [Google Scholar]
- 25. Leversen NA, Sviland L, Wiker HG, Mustafa T. 12 January 2012. Long-term persistence of BCG Pasteur in lungs of C57BL/6 mice following intranasal infection. Scand. J. Immunol. doi:10.1111/j.1365–3083.2012.02683.x. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 26. Maartens G, Wilkinson RJ. 2007. Tuberculosis. Lancet 370:2030–2043 [DOI] [PubMed] [Google Scholar]
- 27. Mateu MG. 2009. The capsid protein of human immunodeficiency virus: intersubunit interactions during virus assembly. FEBS J. 276:6098–6109 [DOI] [PubMed] [Google Scholar]
- 28. Meya DB, McAdam KP. 2007. The TB pandemic: an old problem seeking new solutions. J. Intern. Med. 261:309–329. [DOI] [PubMed] [Google Scholar]
- 28a. Ministry of Health 1998. Care and use of laboratory animals, no. 55. Ministry of Health, Beijing, People's Republic of China [Google Scholar]
- 29. Ofek G, et al. 2010. Elicitation of structure-specific antibodies by epitope scaffolds. Proc. Natl. Acad. Sci. U. S. A. 107:17880–17887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Okazaki T, et al. 2006. Epitope enhancement of a CD4 HIV epitope toward the development of the next generation HIV vaccine. J. Immunol. 176:3753–3759 [DOI] [PubMed] [Google Scholar]
- 31. Parvanova I, Rettig L, Knuth A, Pascolo S. 2011. The form of NY-ESO-1 antigen has an impact on the clinical efficacy of anti-tumor vaccination. Vaccine 29:3832–3836 [DOI] [PubMed] [Google Scholar]
- 32. Prabhu Anand S, Selvaraj P, Narayanan PR. 2009. Effect of 1,25 dihydroxyvitamin D3 on intracellular IFN-gamma and TNF-alpha positive T cell subsets in pulmonary tuberculosis. Cytokine 45:105–110 [DOI] [PubMed] [Google Scholar]
- 33. Raby E, et al. 2008. The effects of HIV on the sensitivity of a whole blood IFN-gamma release assay in Zambian adults with active tuberculosis. PLoS One 3:e2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosa DS, et al. 2011. A DNA vaccine encoding multiple HIV CD4 epitopes elicits vigorous polyfunctional, long-lived CD4+ and CD8+ T cell responses. PLoS One 6:e16921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Skjot RL, et al. 2002. Epitope mapping of the immunodominant antigen TB10.4 and the two homologous proteins TB10.3 and TB12.9, which constitute a subfamily of the esat-6 gene family. Infect. Immun. 70:5446–5453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Song S, et al. 2007. Evaluation of antitumor immunity efficacy of epitope-based vaccine with B16 cell line coexpressing HLA-A2/H-2kb and CTL multiepitope in HLA transgenic mice. Vaccine 25:4853–4860 [DOI] [PubMed] [Google Scholar]
- 37. Sterne JA, Rodrigues LC, Guedes IN. 1998. Does the efficacy of BCG decline with time since vaccination. Int. J. Tuberc. Lung Dis. 2:200–207 [PubMed] [Google Scholar]
- 38. Tanghe A, et al. 1999. Immunogenicity and protective efficacy of tuberculosis DNA vaccines encoding putative phosphate transport receptors. J. Immunol. 162:1113–1119 [PubMed] [Google Scholar]
- 39. Tissot AC, et al. 2010. Versatile virus-like particle carrier for epitope based vaccines. PLoS One 5:e9809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Townsend AR, et al. 1986. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell 44:959–968 [DOI] [PubMed] [Google Scholar]
- 41. van Bockel DJ, et al. 2011. Persistent survival of prevalent clonotypes within an immunodominant HIV gag-specific CD8+ T cell response. J. Immunol. 186:359–371 [DOI] [PubMed] [Google Scholar]
- 42. Wang C, et al. 2011. A DNA vaccine expressing CFP21 and MPT64 fusion protein enhances BCG-induced protective immunity against Mycobacterium tuberculosis infection in mice. Med. Microbiol. Immunol. 200:165–175 [DOI] [PubMed] [Google Scholar]
- 43. Watkins DI, Burton DR, Kallas EG, Moore JP, Koff WC. 2008. Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nat. Med. 14:617–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. WHO 2010. Global tuberculosis control. WHO, Geneva, Switzerland [Google Scholar]