Abstract
In this study, the effect of dexamethasone on the formation of pneumococcal antibodies during community-acquired pneumonia (CAP) was investigated. No differences between CAP patients receiving dexamethasone as additional therapy and patients receiving a placebo were found with respect to immune response rates and mean baseline and convalescent-phase antibody concentrations.
TEXT
Community-acquired pneumonia (CAP) is a very common disease causing considerable morbidity and mortality worldwide (4). Streptococcus pneumoniae is the most frequently identified causative agent of CAP (15). Natural defense against S. pneumoniae is mediated by serotype-specific anticapsular IgG (9). Recently, a study was conducted in which the addition of dexamethasone, a glucocorticosteroid, to antibiotic therapy in patients with CAP reduced the median length of hospitalization by 1 day (8). Beneficial effects of glucocorticosteroids in CAP are due primarily to damping of the systemic inflammatory response (10). Although these agents suppress mainly cell-mediated immunity, they can also affect humoral immunity (1, 3, 6, 7). In this study, we investigated the potential negative effect of dexamethasone on the formation of pneumococcal antibodies during CAP.
Patients participated in a double-blind, placebo-controlled trial investigating the effect of dexamethasone therapy on the length of hospitalization for CAP (8). All patients were above 18 years of age and nonimmunocompromised. Patients were randomized to receive 5 mg dexamethasone or a placebo once a day for the first 4 days after hospital admission. In the present study, only patients in whom S. pneumoniae was diagnosed as the causative agent were included. These were patients with a positive blood or sputum culture with S. pneumoniae or with a positive urine antigen test (BinaxNOW). Pneumococcal strains were serotyped by the Quellung reaction. Serum samples for antibody measurements were obtained from day 0 to day 3 (baseline samples) and from day 11 to day 100 (convalescent-phase samples) after hospital admission. Excluded were patients with a duration of symptoms of more than 10 days before admission, because in these cases, a possible immune response at the onset of disease would remain undetected. The concentrations of IgG against 14 pneumococcal serotypes were measured using the Luminex XMAP Pneumococcal Immunity Panel (Luminex Corporation, Austin, TX). The serotypes included in this panel are 1, 3, 4, 6B, 7F, 8, 9N, 9V, 12F, 14, 18C, 19A, 19F, and 23F (Danish nomenclature). A positive immune response was defined as at least a 2-fold antibody concentration increase between the baseline and convalescent-phase serum samples with an end concentration of at least 0.35 μg/ml (16). If the increase in antibody against a certain serotype was at least 2-fold higher than the increase in antibody against any other serotype, it was determined to be the infecting serotype (16). Statistical significance of the difference between the immune response rates of the dexamethasone- and placebo-treated groups was determined by using the χ2 test. In patients in whom the infecting serotype could be determined, the mean concentrations of antibody against the infecting serotype in both the baseline and convalescent-phase samples were compared between the treatment groups by the Student t test. A P value of <0.05 was considered to represent a statistically significant difference.
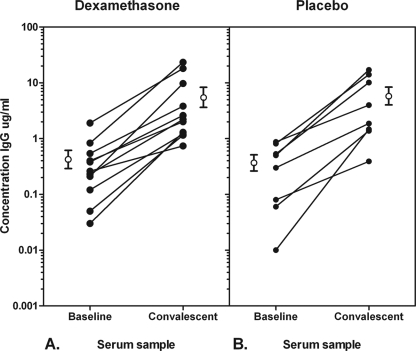
In the original trial, 304 patients were enrolled, of which 151 were randomized to receive dexamethasone and 153 were randomized to receive a placebo (Fig. 1). The baseline characteristics of the patients in the two treatment groups were comparable. S. pneumoniae was identified as the causative agent of CAP in 64 patients, 35 in the dexamethasone group and 29 in the placebo group. Three and two patients in both groups, respectively, were excluded due to a duration of symptoms of more than 10 days before admission. Representative baseline and convalescent-phase serum samples for antibody measurements were available for 48 patients, 25 patients in the dexamethasone group and 23 patients in the placebo group, the total number of patients included in this study. Pneumococcal strains isolated from 22 of the 48 pneumococcal pneumonia patients were serotyped; in 18 cases, the etiological diagnosis was based solely on a positive urine antigen test, which made serotyping impossible, and in 8 cases, the isolate was not available for serotyping. The most frequently identified serotype was serotype 1 (n = 6), followed by 7F (n = 3), 4, 8, 14, and 9V (all n = 2). A pneumococcal immune response was elicited in a total of 31 patients (2-fold increase in antibody concentrations in time and an end concentration of >0.35 μg/ml), 18 (72%) of 25 patients in the dexamethasone group compared to 13 (57%) of 23 patients in the placebo group (difference nonsignificant [NS]). In 19 of these patients (11 in the dexamethasone group and 8 in the placebo group), the infecting pneumococcal serotype could be determined because the increase in the concentrations of antibody against this serotype was at least 2-fold higher than the increase in the concentrations of antibody against any other serotype. All but one serotype-specific antibody responses corresponded to the type of the isolated strain identified with the Quellung reaction; in one patient infected with S. pneumoniae serotype 6A, serotype 6B was determined to be the infecting serotype by a positive immune response. The mean baseline concentration of antibody against the infecting serotype was 0.45 (range, 0.03 to 1.90; standard error [SE], 0.16) μg/ml in the dexamethasone group compared to 0.39 (range, 0.01 to 0.87; SE, 0.12) μg/ml in the placebo group (NS) (Fig. 2). The end concentrations were 6.00 (range, 0.74 to 23.30; SE, 2.34) μg/ml and 6.50 (range, 0.39 to 17.00; SE, 2.30) μg/ml, respectively (NS).
Fig 1.

Flow chart of the inclusion criteria and number of patients included in this study. Three hundred four CAP patients were randomized to receive either dexamethasone or placebo therapy. A diagnosis of S. pneumoniae as the causative agent was based on either a positive sputum or blood culture or a positive urine antigen test. A baseline serum sample drawn from day 0 to day 3 and a convalescent-phase sample drawn from day 11 to day 100 of hospitalization had to be available.
Fig 2.
Concentrations of IgG against the infecting pneumococcal serotype in CAP patients receiving dexamethasone (A, n = 11) or a placebo (B, n = 8). The baseline serum sample was obtained from day 0 to day 3 after hospital admission, and the convalescent-phase serum sample was obtained from day 11 to day 100 after hospital admission. The open circles are mean antibody levels ± SE.
In a recently published trial, the addition of dexamethasone to antibiotic therapy for patients with CAP reduced the median length of hospitalization by 1 day. Many of the possible effects of this therapy on natural immunity remain uncertain. In this study, it was shown that dexamethasone does not negatively affect the formation of serotype-specific antibodies in pneumococcal CAP patients. The immune response rate in the dexamethasone-treated group was even nonsignificantly higher than in the placebo treated group. In earlier studies, the relationship between the long-term use of corticosteroids and suppression of the humoral immune system was established (3, 6, 12). Several in vitro and in vivo studies provided evidence that even short-term use of corticosteroids can negatively affect IgG subclass concentrations (1, 7). Until now, no negative effect of corticosteroid use on the antibody response to pneumococcal vaccination has been described (2, 13). This study is the first to provide evidence that short-term corticosteroid use does not affect pneumococcal antibody formation during natural infection. The cutoff value of a positive immune response in this study was set at 0.35 μg/ml. This is the protective concentration of pneumococcal antibodies in children designated by the WHO (11). The protective concentration of pneumococcal antibodies for CAP in adults is not known, and therefore, it is not possible to interpret the immune responses observed in our patients in terms of function. It is, however, reasonable to assume that dexamethasone does not negatively influence the functionality of antibodies irrespective of their concentration. Patients participated in a double-blind, placebo-controlled trial which allows for an unbiased comparison between therapy groups (8). There were, however, differences between the two groups in the serotype distribution of the infecting strains, which may have had an impact on immunogenicity (5). An immune response against serotype 6B was elicited in one patient infected with S. pneumoniae serotype 6A. This type of cross-reactivity has been described after pneumococcal vaccination (14). Potential negative effects of corticosteroids on the immune system might limit the general applicability of dexamethasone in CAP. Our data indicate that dexamethasone therapy does not affect the formation of pneumococcal antibodies during CAP. No drawback to the incorporation of dexamethasone in the standard therapy scheme for CAP was found with respect to the pneumococcal immune response.
Footnotes
Published ahead of print 29 February 2012
REFERENCES
- 1. Butler WT, Rossen RD. 1973. Effects of corticosteroids on immunity in man. I. Decreased serum IgG concentration caused by 3 or 5 days of high doses of methylprednisolone. J. Clin. Invest. 52:2629–2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Roux A, et al. 2004. Immunogenicity of the pneumococcal polysaccharide vaccine in COPD patients. The effect of systemic steroids. Respir. Med. 98:1187–1194 [DOI] [PubMed] [Google Scholar]
- 3. Fedor ME, Rubinstein A. 2006. Effects of long-term low-dose corticosteroid therapy on humoral immunity. Ann. Allergy Asthma Immunol. 97:113–116 [DOI] [PubMed] [Google Scholar]
- 4. File TM. 2003. Community-acquired pneumonia. Lancet 362:1991–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Go ES, Ballas ZK. 1996. Anti-pneumococcal antibody response in normal subjects: a meta-analysis. J. Allergy Clin. Immunol. 98:205–215 [DOI] [PubMed] [Google Scholar]
- 6. Klaustermeyer WB, Gianos ME, Kurohara ML, Dao HT, Heiner DC. 1992. IgG subclass deficiency associated with corticosteroids in obstructive lung disease. Chest 102:1137–1142 [DOI] [PubMed] [Google Scholar]
- 7. Klebl FH, Weber G, Kalden JR, Nusslein HG. 1994. In vitro and in vivo effect of glucocorticoids on IgE and IgG subclass secretion. Clin. Exp. Allergy 24:1022–1029 [DOI] [PubMed] [Google Scholar]
- 8. Meijvis SC, et al. 2011. Dexamethasone and length of hospital stay in patients with community-acquired pneumonia: a randomised, double-blind, placebo-controlled trial. Lancet 377:2023–2030 [DOI] [PubMed] [Google Scholar]
- 9. Musher DM, et al. 1993. Antibody to capsular polysaccharides of Streptococcus pneumoniae: prevalence, persistence, and response to revaccination. Clin. Infect. Dis. 17:66–73 [DOI] [PubMed] [Google Scholar]
- 10. Rhen T, Cidlowski JA. 2005. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N. Engl. J. Med. 353:1711–1723 [DOI] [PubMed] [Google Scholar]
- 11. Siber GR, et al. 2007. Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies. Vaccine 25:3816–3826 [DOI] [PubMed] [Google Scholar]
- 12. Tanizaki Y, et al. 1993. Effects of glucocorticoids on humoral and cellular immunity and on airway inflammation in patients with steroid-dependent intractable asthma. J. Asthma 30:485–492 [DOI] [PubMed] [Google Scholar]
- 13. Ulinski T, Leroy S, Dubrel M, Danon S, Bensman A. 2008. High serological response to pneumococcal vaccine in nephrotic children at disease onset on high-dose prednisone. Pediatr. Nephrol. 23:1107–1113 [DOI] [PubMed] [Google Scholar]
- 14. Väkeväinen M, Eklund C, Eskola J, Kayhty H. 2001. Cross-reactivity of antibodies to type 6B and 6A polysaccharides of Streptococcus pneumoniae, evoked by pneumococcal conjugate vaccines, in infants. J. Infect. Dis. 184:789–793 [DOI] [PubMed] [Google Scholar]
- 15. van der Poll T, Opal SM. 2009. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet 374:1543–1556 [DOI] [PubMed] [Google Scholar]
- 16. van Mens SP, et al. 2011. Longitudinal analysis of pneumococcal antibodies during community-acquired pneumonia reveals a much higher involvement of Streptococcus pneumoniae than estimated by conventional methods alone. Clin. Vaccine Immunol. 18:796–801 [DOI] [PMC free article] [PubMed] [Google Scholar]