Abstract
Rabbit immunogenicity studies on an experimental trivalent native outer membrane vesicle vaccine derived from three serogroup B strains were conducted to evaluate the effectiveness of this vaccine at inducing an antibody response with serum bactericidal activity against meningococcal strains of other serogroups in addition to serogroup B strains. The results showed that the vaccine was capable of inducing an effective broad-based bactericidal antibody response in rabbits against a small sample of Neisseria meningitidis strains of serogroups C, W135, and X and, to a lesser extent, serogroups A and Y. Analysis of antibody specificity using a bactericidal depletion assay revealed that antibodies to lipooligosaccharide (LOS), PorA, and NadA induced in rabbits by the experimental trivalent outer membrane vesicle vaccine were responsible for most of the bactericidal activity against strains of the other N. meningitidis serogroups. In the case of serogroup A N. meningitidis strains, the outer membrane antigen NadA was primarily responsible for protection. The outer membrane antigens fHbp and OpcA were also effective in removing some bactericidal activity from the sera.
INTRODUCTION
Except for serogroup B Neisseria meningitidis, capsular polysaccharides have been used successfully as human vaccines to prevent infections with N. meningitidis serogroups A, C, Y, and W135 (1, 5, 13, 27). These capsular vaccines are serogroup specific and do not induce cross protection. Unlike the other serogroups, the serogroup B capsular polysaccharide has the same structure as polysialic acid expressed in certain tissues in the human body (10), which makes it a poor immunogen and, if used as a vaccine, raises the possibility of inducing an autoimmune response. Therefore, efforts to develop a serogroup B vaccine have largely focused on the subcapsular antigens.
Currently at least five subcapsular vaccines are being developed for broad protection against group B N. meningitidis: (i) the PorA-based vaccines (Hexamen and Nonamen) (6, 28); (ii) a lipooligosaccharide (LOS)-based vesicle vaccine (30); (iii) a pentavalent vaccine containing Neisserial adhesin A (NadA), factor H-binding protein variant 1 (fHbp-1), neisserial heparin-binding antigen (NHBA), GNA 2091, GNA 1030, and outer membrane vesicles (OMVs) from the New Zealand epidemic strain (12); (iv) the bivalent fHbp subfamily A and subfamily B vaccine (25); and (v) the recently described native outer membrane vesicle (NOMV) vaccines (33). All of these serogroup B N. meningitidis vaccines, which are based on the subcapsular antigens, have the added benefit of generating a cross-reactive antibody response against some strains of the other serogroups because of the conserved nature of some of the antigens in the vaccines and the fact that the subcapsular antigens are expressed independently of the serogroup. Recently, factor H binding protein (fHbp), which is present in three of the vaccines, was shown to be protective against the serogroup A, C, W135, and X N. meningitidis strains (2). Such vaccines would be expected to greatly help in combating the disease in regions such as sub-Saharan Africa, where serogroups A, W135, and X appear to be a major problem. In a previous communication (33), we reported the development of a complex multivalent group B vaccine candidate that was shown to induce serum bactericidal antibody (SBA) responses effective against a broad range of serogroup B N. meningitidis strains. This vaccine did not rely on a single antigen but was designed to include multiple outer membrane antigens, such as PorAs, fHbp, NadA, OpC, and LOS, each with the capacity to induce SBA. This vaccine was based on the use of native outer membrane vesicles (NOMVs) that had not been exposed to detergent or denaturing solvents. The vesicles were obtained from three antigenically diverse vaccine strains that had been genetically modified to express three sets of antigens that both individually and collectively could potentially induce a broad-based protective antibody response. Furthermore, given the composition of this experimental vaccine, it could potentially be used as a multiserogroup N. meningitidis vaccine candidate. In a recent communication (26), we presented data from mouse immunogenicity studies that showed that the NOMV vaccine was indeed capable of inducing protective antibodies against clinical isolates of serogroups C, Y, W135, and X and NadA-expressing serogroup A N. meningitidis isolates, in addition to serogroup B isolates. However, we were not able to analyze the contribution of the individual outer membrane antigens in the vaccine in inducing a bactericidal response. To confirm our previous findings and determine the contribution of the individual vaccine components to the bactericidal response against non-serogroup B N. meningitidis strains, we studied the bactericidal response of rabbits to the trivalent NOMV vaccine. In this article, we confirm our previous findings using rabbit sera and show that the NOMV serogroup B vaccine is able to induce a positive bactericidal response in rabbits against other non-B N. meningitidis serogroups. Furthermore, analysis of the specificity of the antibody responses by the bactericidal depletion assay showed that the antigen primarily responsible for the induction of bactericidal antibodies appeared to be LOS, except for serogroup A strains, where NadA induced the most bactericidal antibodies, which is consistent with our previous findings in mice. The present study also shows that the other outer membrane antigens such as fHbp, OpcA, and PorA contributed significantly to the overall potency of the vaccine.
MATERIALS AND METHODS
Bacterial strains and genetic modifications of the strains used to make the vaccine.
The antigenic profiles of the N. meningitidis serogroup B strains used to make the vaccines are shown in Table 1. The genetic modifications that were made to them have been described by Zollinger et al. (33). Also shown in Table 1 are the phenotypes of the N. meningitidis target strains from serogroups A, C, W135, Y, and X. The bactericidal target strains used for serogroups C, Y, and W135 were mostly isolated from army personnel prior to the time that vaccination with the tetravalent polysaccharide vaccine was initiated (about 1982) in the U.S. Army. The four serogroup A target strains were obtained from other investigators over a period of years (1970 to 1989) and originated in Egypt, Africa, Finland, and Germany. The serogroup X strains were obtained from the Naval Medical Unit-3 (NAMRU-3) in 2009 and were isolates from the meningococcal meningitis outbreak in western Kenya (22).
Table 1.
MLST and phenotype of the vaccine and bactericidal target strainsa
| Strain | Group | ST | Clonal complex | PorA | fHbp no. | NadA allele no. | Presence/absence of OpcA | LOS | Antigen shared with vaccine strain |
|---|---|---|---|---|---|---|---|---|---|
| 5878 | A | 4 | ST-4 complex/subgroup IV | P1.7,13-1 | 1.5 | − | L9 | fHbp-1 | |
| 8991 | A | ST-5 complex/subgroup III | P1.20,9 | 1.5 | 3 | + | L8,9 | fHbp-1, NadA, OpC, and LOS | |
| 7891 | A | 5 | ST-5 complex/subgroup III | P1.20,9 | 1.5 | 3 | − | L11 | NadA and fHbp-1 |
| 8822 | A | 7776 | ST-1 complex/subgroup I/II | P1.5-1,2-2 | 1.4 | + | L10 | fHbp-1 | |
| 8837 | C | 6657 | ST-41/44 complex/lineage 3 | P1.19,15 | 2.72 | − | L3 | fHbp-2, LOS, and PorA | |
| 8241 | C | 2552 | ST-334 complex | P1.19-3,15 | 1.13 | − | L3 | fHbp-1, LOS, and PorA | |
| 5416 | C | 11 | ST-11 complex/ET-37 complex | P1.5,2-1 | 2.22 | − | L3 | fHbp-2, LOS, and PorA | |
| 5660 | C | 11 | ST-11 complex/ET-37 complex | P1.5,2-1 | 2.22 | 2 | − | L3 | fHbp-2, LOS, NadA, and PorA |
| 7510 | W135 | ST-178 complex | P1.19,15 | 1.34 | − | L3,7 | fHbp-1, LOS, and PorA | ||
| 6309 | W135 | 52 | ST-11 complex/ET-37 complex | P1.5,2 | 2.22 | 2 | − | L3,7 | fHbp-2, LOS, NadA, and PorA |
| 8122 | W135 | 11 | ST-11 complex/ET-37 complex | P1.5,2 | 2.22 | 2 | − | L3,7 | fHbp-2, LOS, NadA, and PorA |
| 8020 | Y | ST-23 complex/cluster A3 | P1.5-1,2-2 | 2.25 | − | L3 | fHbp-2, LOS, and PorA | ||
| 9463 | Y | 167 | ST-167 complex | P1.5-1,10-4 | 2.23 | − | L3-5,7-5 | fHbp-2, LOS, and PorA | |
| 6972 | Y | 23 | ST-23 complex/A3 | P1.5-1,2-2 | 2.25 | 2 | + | L3-5,7-5 | fHbp-2, LOS, NadA, and PorA |
| 9557 | X | 5403 | ST-5403 | P1.19,26 | 1.61 | + | L8 | fHbp-1, LOS, and PorA | |
| 9558 | X | 5403 | ST-5403 | P1.19,26 | 1.61 | − | L8 | fHbp-1, LOS, and PorA | |
| 9559 | X | 5403 | ST-5403 | P1.19,26 | 1.61 | − | L8 | fHbp-1, LOS, and PorA | |
| B1b | B | 32 | ST-32 complex/ET-5 complex | P1.7,16 and P1.7-1,1 | 1.1 | 3 | + | L8-3 | |
| B2b | B | 33 | ST-32 complex/ET-5 complex | P1.19,15 and P1.22,14 | 1.1 | + | L8-5 | ||
| B3b | B | 5069 | ST-11 complex/ET-37 complex | P1.5,2 and P1.22-1,4 | 2.22 and 2.16 | − | L8-2 |
The MLST and antigenic profiles of the vaccine strain and the bactericidal target strains are shown.
The NadA allele 3 was overexpressed, and a second PorA (P1.7-1,1) was inserted in the strain B1 (ΔsynX ΔLpxL1 ΔlgtA) parent strain, 44/76. The NadA allele was determined only for the strains that expressed NadA on the surface, as determined by colony blotting. A second copy of fHbp-1 allele 1 was inserted in the nspA locus for overexpression, and a second PorA (P1.22,14) was also inserted in the B2 (ΔsynX ΔLpxL1) parent strain, 8570. A second copy of fHbp-2 allele 16 was inserted in the nspA locus for overexpression, and a second PorA (P1.22-1,4) was also inserted in the B3 (ΔsynX ΔLpxL1 Δlgt) parent strain, B16B6. OpcA expression was stabilized in the B1 and B2 vaccine strains. A variant of the B2 vaccine strain that did not express lgtA was used in the study. Detailed analysis of the 3 vaccine strains (B1, B2, and B3) that together constitute the experimental trivalent NOMV vaccine can be found in the study by Zollinger et al. (33).
MLST and PorA, fHbp, and NadA determination.
Genomic DNA was isolated from 1.0 ml of overnight culture using the Wizard genomic DNA purification kit (Promega, part no. TM050) following the vendor's instruction manual. Specific gene products were amplified by PCR from the genomic DNA and sequenced using the following primers. The primers for multilocus sequence typing (MLST) (7 genes), PorA and FetA were those listed in Neisseria Sequence Typing website (http://pubmlst.org/neisseria/info). The primers for fHbp were GAAGCAAATCTGCCTCGACCG (forward) and CGACGGCCAGGACGGGACGG (reverse). The primers used for NadA were ACCGGCAGAATTGACATCAGC (forward) and TTACCACTCGTAATTGACGCCG (reverse). Allelic profiles, sequence types (STs), and clonal complexes were assigned according to the PubMLST database (http://pubmlst.org/perl/bigsdb/bigsdb.pl?db=pubmlst_neisseria_isolates) based on the results from Maiden et al. (16). Allelic profiles not found in the database were mapped to clonal complexes having at least 5/7 MLST alleles in common with the strain's allelic profile.
Antigens used in the bactericidal depletion assay.
LOS used in the bactericidal depletion assay was purified according to the method of Westphal and Jann (29). The factor H binding protein variant 1 (fHbp-1) was cloned in the pT7-MAT-Tag-Flag-1 vector (Sigma), with the His tag at the N terminus of the protein. The fHbp-1 His-tagged protein was purified on a nickel column. Purified OpcA was obtained from Milan Blake (FDA), and the purified individual recombinant PorAs in liposomes were obtained from Lan Zhang (Merck). The purity of the antigens was determined on SDS-PAGE gels (see Fig. S1 in supplemental material).
Spot blotting/colony blotting.
The phenotypes of the vaccine strains and the bactericidal target strains for serogroup A, C, Y, W135, and X N. meningitidis were determined by spot blotting as described by Zollinger et al. (33). Spot blotting, a variation of colony blotting in which a suspension of the culture is spotted onto nitrocellulose rather than lifting colonies directly from an agar plate, was used to determine the LOS immunotype of target strains from the other groups. Colony blotting was performed as described by Moran et al. (19). The following monoclonal antibodies were used in the blotting procedures to determine the LOS immunotype of the target strains: 4C4 (L8,11, obtained from M. Apicella), 14-1-L10 (L10), 25-1-LC1 (L8-5), 2-1-L8 (L8), 9-2-L379 (L3,7,9), 17-1-L1 (L1), and 1B2-1B7 (lacto-N-neotetraose) (obtained from ATCC). The monoclonal antibodies Jar5 (fHbp-1) and Jar11 (fHbp-2) were obtained from Dan Granoff. Polyclonal mouse anti-NadA antiserum was used to test for the presence of NadA on the cell surface.
Vaccine preparation.
The current good manufacturing practice (cGMP) preparation of the vaccine has been described by Zollinger et al. (33) and Pinto et al. (26). Briefly, vaccine strains were grown in liquid culture using modified Catlin's medium in which the individual amino acids were replaced by 1% Casamino Acids (Becton Dickenson, Franklin Lakes, NJ) and iron (ferric sulfate) was reduced to 10% of the normal level (0.5 mg/liter) to induce expression of iron uptake proteins. No antibiotics were added to the growth medium. The vaccines were prepared according to current good manufacturing practice in a 400-liter fermentor using the same medium. Bacterial growth was allowed to continue for about 1 h into the stationary phase before inactivation of the cultures with phenol at a concentration of 0.5%. Mazu Df 204 antifoam was inhibitory above 0.005% and was therefore used at or below this concentration.
NOMVs were extracted from packed cells using a modification of previously described methods (11, 31). The procedure of isolating the vesicles from the high-speed supernatant by ultracentrifugation was replaced by treating the high-speed supernatant with Benzonase (Merck GKaA, Darmstadt, Germany) at 100 U/ml for 60 to 80 min at room temperature followed by ultrafiltration using a 750,000-molecular-weight (MW)-cutoff membrane cartridge (UFP-750-E-6A; A/G Technology Corp., Needham, MA) and 0.01 M Tris-HCl as the ultrafiltration buffer. During ultrafiltration, the retentate containing NOMVs was concentrated to approximately one-fourth the original volume and following ultrafiltration subjected to ultracentrifugation at 200,000 × g for 1 h. The pellets were suspended in 0.01 M Tris-HCl buffer (pH 7.5) and passed through a microfluidizer (model M-110Y; Microfluidics Corp., Newton, MA) to facilitate sterilization by passage through a 0.2-μm-pore membrane filter. The bulk vaccine from each strain was stored at −80°C until used.
Portions of the bulk vaccine from all three strains were pooled to make the trivalent NOMV vaccine. The purified NOMV products of the three vaccine strains were suspended in 0.01 M Tris-HCl (pH 7.5)-buffered 5% dextrose and mixed before bottling to give a final concentration of 300 μg/ml of protein in the final trivalent (B1, B2, and B3) product, which is made up of 100 μg/ml of each of the three NOMVs. The bottled vaccines were stored at −80°C before use in this study.
Immunization of animals.
Vaccines were thawed and formulated at the desired concentration prior to vaccination. New Zealand White female rabbits (3 to 8 months of age and weighing 1.5 to 1.8 kg) were vaccinated intramuscularly with the NOMV trivalent vaccine at a protein dose of 75 μg/rabbit along with alum as an adjuvant. The amount of aluminum hydroxide used was 0.17 mg/dose, which is equivalent to one-fifth of the proposed human dose of 0.85 mg/dose (0.3 mg of aluminum). In all experiments, three doses were given at 0, 4, and 8 weeks, and blood was taken at 0, 7, and 10 weeks following the initial vaccination. The data reported were obtained using the final (10-week) sera.
Serological assays.
The serum bactericidal assay was performed as previously described (32) using normal human serum prescreened for lack of bactericidal activity against the test strain as a source of complement. Serum from each rabbit was tested in the serum bactericidal assay. The rabbit sera were heat inactivated and tested using a starting dilution of 1:2. The reciprocal of the highest dilution of serum that killed ≥50% of the bacteria was taken as the endpoint titer of the serum. If there was less than 50% killing at a dilution of 1:2, the serum was assigned a titer of 1:1. The highest dilution tested was 1:512. All of the prevaccination sera from the rabbits lacked bactericidal antibodies against any of the test strains and were assigned a titer of 1:1. The bactericidal depletion assay was performed as described previously (32) by coating wells of a 96-well microplate with serial dilutions of antigens to be tested and then blocking and washing the wells. The sera to be tested were diluted to the 50% kill endpoint and incubated in the coated wells for 4 h, after which the sera were transferred to a fresh plate and tested for residual bactericidal activity.
RESULTS
Bactericidal activity against serogroup A N. meningitidis strains.
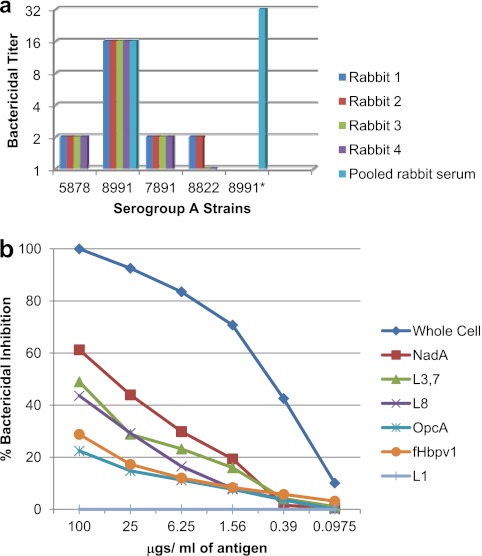
A limited number of strains from serogroup A were chosen from the WRAIR collection and tested against available lots of human complement. Strains that were not killed by at least one of the available complement lots were used as target strains in the bactericidal assay to test the effectiveness of the vaccine in inducing serum bactericidal antibodies cross-reactive with group A strains. The MLST and phenotype of the serogroup A strains are shown in Table 1. The antigenic epitopes shared between the bactericidal target strains and the vaccine strains are also shown in Table 1. As shown in Fig. 1a, the serogroup A N. meningitidis strains were mostly resistant to killing by the rabbit sera in the bactericidal assay. All rabbits showed a greater than 4-fold increase in titer to only one of the four strains (strain 8991). Strain 8991 was one of two strains to express NadA, and when a high-NadA-expressing subclone of 8991, designated 8991*, was isolated and used in the bactericidal assay, a 2-fold increase in titer was observed compared to the titer of the parent strain (Fig. 1a). Furthermore, the bactericidal depletion assay (Fig. 1b) showed that of the isolated antigens tested, NadA was the most effective in removing bactericidal antibodies from the pooled serum, but purified L8 and L3,7 LOS were also able to remove some of the bactericidal activity. This is consistent with the expression of L8 by strain 8991. L9, L10, and L11 LOS have a different core structure from any of the LOS immunotypes in the vaccine. Both antibodies to NadA and those to LOS may have been necessary for killing of strain 8991 since strain 7891 also expressed NadA, but not L8, and was not killed by the rabbit sera. The results with serogroup A strains are consistent with our earlier observations in mouse studies that anti-NadA antibodies played an important role in the bactericidal activity against serogroup A strains (26). Besides NadA, the target strain also expressed other protein antigens, such as fHbp-1 and OpcA, on its cell surface. The bactericidal depletion assay also showed that besides NadA, LOS, fHbp-1, and OpcA removed a small amount bactericidal activity from the tested serum, but the antibodies reactive with these antigens were apparently not sufficiently potent to kill serogroup A strains in the absence of antibodies to NadA and LOS. It is quite likely that either not enough of those antigens are expressed on the target cell surface or, in the case of fHbp-1, because of allelic differences (26) cross-reactive antibodies induced by the vaccine did not have sufficiently high avidity to be bactericidal.
Fig 1.
Sera from the four rabbits immunized with the experimental trivalent vesicle vaccine were tested in the bactericidal assay against 4 serogroup A target strains (a). Pooled rabbit serum was analyzed for bactericidal antibody specificity using N. meningitidis serogroup A strain 8991 as the target strain in the bactericidal depletion assay (b).
Bactericidal activity against serogroup C N. meningitidis strains.
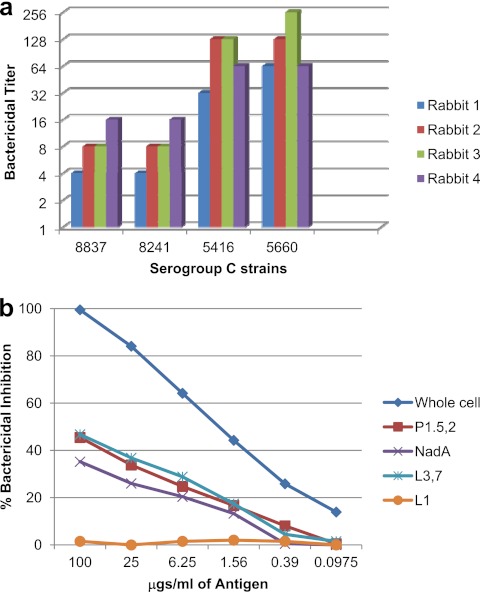
There were 4 serogroup C target strains for which we had nonreactive human complement that could be used in the bactericidal assay. Table 1 shows the MLSTs and phenotypes of the serogroup C bactericidal target strains, along with the antigens shared by these target strains with the vaccine strains. The serogroup C strains appeared to share more antigens with the vaccine strains than was the case in the serogroup A strains. Thus, it was not surprising that all of the rabbits showed at least a 4-fold increase in titer against all four serogroup C strains tested in the bactericidal assay (Fig. 2a). This confirms our earlier findings on the experimental trivalent vaccine in our mouse immunogenicity studies (24). Near 50% depletion of bactericidal antibody was observed when purified L3,7 LOS or PorA P1.5,2 was used in the bactericidal depletion assay with serogroup C strain 5660 as the target (Fig. 2b). While serogroup C strain 5660 expresses NadA allele 2, purified allele 3 NadA protein was also able to remove about one-third of the bactericidal antibody, which is not surprising since the three NadA alleles are known to induce cross-bactericidal antibodies (7). Thus, LOS, PorA, and NadA all appear to be involved in the induction of bactericidal antibodies to serogroup C target strains.
Fig 2.
Sera from the four rabbits immunized with the experimental trivalent vesicle vaccine were tested in the bactericidal assay (a) against 4 serogroup C target strains. Pooled rabbit serum was analyzed for bactericidal antibody specificity using N. meningitidis serogroup C strain 5660 as the target strain in the bactericidal depletion assay (b).
Bactericidal activity against serogroup W135 N. meningitidis strains.
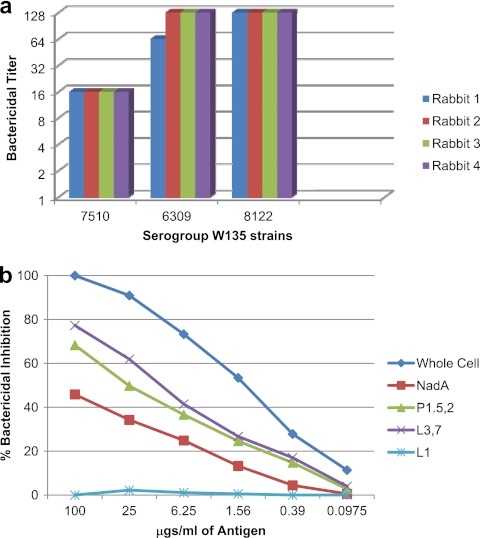
Three serogroup W135 N. meningitidis clinical isolates were used as target strains in the bactericidal assay. The MLSTs and phenotypes of the serogroup W135 bactericidal target strains, along with the antigens shared by these target strains with the vaccine strains, are shown in Table 1. The LOS and PorA antigens expressed by these strains were the same as or similar to those in the vaccine, and two of the W135 strains also expressed NadA. Strain 7510 expressed fHbp-2, and the other two strains expressed fHbp-1. As seen in Fig. 3a, all of the rabbits showed a greater than 4-fold increase in titers to all three tested strains in the bactericidal assay. The titers were lower against strain 7510, which did not express NadA, than against the other two strains. Bactericidal depletion assays using 8122 as the target strain showed that purified L3,7 LOS (with the same core structure as L8 in the vaccine) and PorA P1.5,2 were most active in removing bactericidal antibodies from the serum. While serogroup W135 strain 8122 expresses NadA allele 2, purified allele 3 NadA protein was also able to remove about more than a third of the bactericidal antibody (Fig. 3b).
Fig 3.
Sera from the four rabbits immunized with the experimental trivalent vesicle vaccine were tested in the bactericidal assay (a) against three different serogroup W135 target strains. Pooled rabbit serum was analyzed for bactericidal antibody specificity using N. meningitidis serogroup W135 strain 8122 as the target strain in the bactericidal depletion assay (b).
Bactericidal activity against serogroup Y N. meningitidis strains.
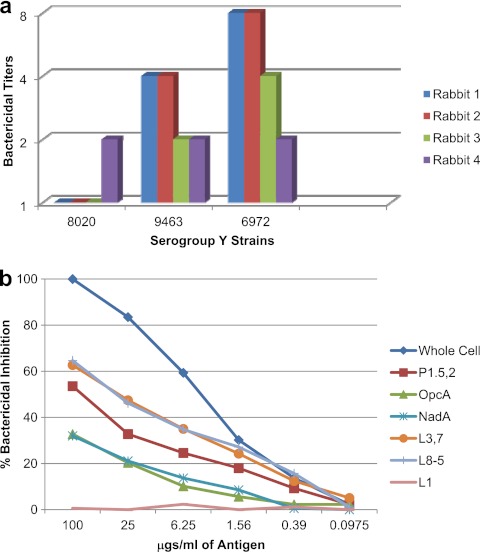
Three strains of serogroup Y were tested for their susceptibility to killing in the bactericidal assay with the immunized rabbit sera. The MLST and phenotype of the serogroup Y bactericidal target strains, along with the antigens shared by these target strains with the vaccine strains, are shown in Table 1. The LOSs expressed by these strains were immunotypes with core structures homologous to those in the vaccine (L8 and L8-5), and the PorA (P1.5-1,2-2) in two of the strains was similar but not identical to one of the PorAs (P1.5,2) present in the vaccine. As shown in Fig. 4a, the serogroup Y strain 8020 was resistant to killing with the immunized rabbit sera, and one rabbit did not mount a significant response to any of the three strains tested in the bactericidal assay. A 4-fold increase in titer was observed against serogroup Y strain 9463 in only two of the four rabbit sera tested, and a 4-fold or greater increase in titer was observed against strain 6972 in three of the four rabbit sera tested. Serogroup Y strain 6972 expressed NadA and OpcA, which were not detected in the other two strains. On the whole, the serogroup Y strains appeared to be much more resistant to killing (the highest titer observed was only 1:8) compared to strains of the other serogroups, despite sharing some of the major antigens with the vaccine strains. Bactericidal depletion assays using serogroup Y strain 6972 as the target strain showed that purified LOS (L8-5 or L3,7) and PorA P1.5,2 were most effective at removing bactericidal antibodies from the serum (Fig. 4b). The NadA and OpcA proteins were able to remove a smaller amount of antibody. Thus, based on the small sample of serogroup Y strains tested, it appears that the vaccine may not be quite as effective against strains of this serogroup, which is contrary to our findings in the mouse immunogenicity studies and could be attributed to species differences (26).
Fig 4.
Sera from the four rabbits immunized with the experimental trivalent vesicle vaccine were tested in the bactericidal assay against three serogroup Y target strains (a). Pooled rabbit serum was analyzed for bactericidal antibody specificity using N. meningitidis serogroup Y strain 6972 as the target strain in the bactericidal depletion assay (b).
Bactericidal activity against serogroup X N. meningitidis strains.
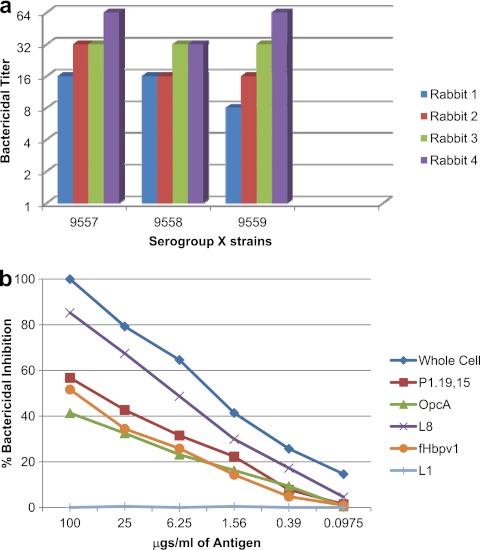
The immune sera from rabbits were tested on three N. meningitidis strains that were part of the serogroup X meningococcal meningitis outbreak in western Kenya (22). Since all three strains were part of a single outbreak and belong to sequence type 5403 (ST-5403), they are the same clone. The phenotypes of the serogroup X bactericidal target strains, along with the antigens shared by these target strains with the vaccine strains, are shown in Table 1. They expressed the same L8 LOS that was present on the B1 vaccine strain, the P1.19 PorA epitope that was present on the B2 vaccine strain, and fHbp-1. As shown in Fig. 5a, a greater than 4-fold increase in bactericidal antibody response was observed against the three strains in each of the immunized rabbit's sera. The bactericidal depletion assay using strain 9557 revealed that the key antigens responsible for the bactericidal activity were the L8 LOS, PorA (P1.19 epitope), OpcA, and fHbp-1. While the strains assayed in this study have ST-5403, the majority of strains responsible for the serogroup X N. meningitidis epidemic and carriage in Africa have either ST-181 or ST-751 (3, 8, 21). The serogroup X N. meningitidis strains that have ST-181 or ST-751 differ in their PorAs from the ST-5403 N. meningitidis strains: their OpcA and LOS immunotype is not known, but they do express fHbp-1, like the ST-5403 N. meningitidis strains. Based on the expression of fHbp-1 (2), it is possible that the strains would have been killed in the bactericidal assay when incubated with the sera from rabbits immunized with the experimental trivalent NOMV vaccine and complement. Thus, the rabbit immunogenicity studies show that the experimental trivalent vaccine was effective against serogroup X N. meningitidis strains (ST-5403) and would be expected to be effective against the other serogroup X N. meningitidis strains that were responsible for the African epidemic. These results are consistent with our findings in the mouse immunogenicity studies with the experimental trivalent vesicle vaccine (26).
Fig 5.
Sera from the four rabbits immunized with the experimental trivalent vesicle vaccine were tested in the bactericidal assay against three serogroup X test strains (a). Pooled rabbit serum was analyzed for bactericidal antibody specificity using N. meningitidis serogroup X strain 9557 as the target strain in the bactericidal depletion assay (b).
DISCUSSION
The trivalent experimental serogroup B N. meningitidis NOMV vaccine was designed to induce broad cross protection against serogroup B strains (33). The vaccine has multiple surface antigens, thereby avoiding dependency on a single antigen or antigen type and taking advantage of the potential for synergistic function of antibodies to different antigens in cases where the expression level of a particular antigen is low. Since the NOMVs contained surface antigens from three group B strains, it was not unreasonable to expect the vaccine to be protective against strains of other serogroups, given the distinct possibility that they may share a number of surface antigens. Pinto et al. (26) showed that the experimental trivalent vesicle vaccine induced bactericidal antibodies in mice against strains of serogroups C, Y, W135, and X and NadA-expressing strains of serogroup A N. meningitidis. However, the surface antigens present in the experimental trivalent NOMV vaccine that were responsible for inducing protective antibodies against the non-B serogroups were not thoroughly investigated in the earlier report (26). In this report, we show that LOS, PorA, and NadA were the most important outer membrane antigens responsible for inducing the protective bactericidal antibody responses against serogroup C, W135, and X N. meningitidis strains. Based on our observations, LOS-based vaccines would be expected to provide protection against strains of the same serogroups since they appear to express the core L8 LOS on their cell surface. While the LOS-based vaccine of Weyants et al. did induce bactericidal antibodies in mice against serogroup B N. meningitidis (30), it was poorly immunogenic for humans (4), thereby calling into question its ability to protect not only against serogroup B N. meningitidis, but also against other N. meningitidis serogroups. PorA, the other major outer membrane component, also did provide protection against certain strains in serogroups C, W135, and X. However, since protective antibodies to PorA tend to be specific to either variable region 1 or 2, PorA-based vaccines would be expected to provide protection only against strains that express the identical PorA on their surface. The minor outer membrane antigens such as fHbp and NadA have been found in other serogroups (20, 24), and the trivalent vaccine did induce protective antibodies to these antigens. In the case of serogroup A, anti-NadA antibodies induced by the experimental trivalent NOMV vaccine were primarily responsible for induction of the bactericidal antibodies, but anti-LOS antibodies were also important since strain 7891 (which also expressed NadA but no homologous LOS) was not killed. Since serogroup A N. meningitidis infections have been known to induce anti-NadA antibodies (24), serogroup B vaccines containing NadA such as the pentavalent vaccine (containing NadA, fHbp-1, NHBA, GNA 2091, GNA 1030, and OMVs from the New Zealand epidemic strain) may provide protection against serogroup A N. meningitidis infections. However, since NadA is also known to be phase variable (17, 23), vaccines containing other subcapsular antigens such as PorAs of representative serogroup A strains or fHbp may prove to be more effective in conferring protection. Given the effectiveness of the conjugated serogroup A capsular vaccine, it is quite likely that none of the subcapsular antigens may measure up to it.
The outer membrane antigen fHbp is present in two serogroup B vaccines that are at an advanced stage of development (12, 25). In both the bivalent fHbp vaccine and the pentavalent vaccine containing recombinant protein plus OMV, fHbp was primarily responsible for inducing a protective response, although PorA in the OMV-containing vaccine did contribute to the protective response against strains that express that particular PorA. Unlike studies in animals (18), induction of cross-reactive anti-fHbp antibodies by the pentavalent recombinant protein-OMV vaccine was poor in infants (9). In our studies, fHbp appeared to offer protection only against serogroup X ST-5403 strains from the Kenyan outbreak. Although the other serogroups do express fHbp on their cells surface, as determined by spot blotting, the inability of anti-fHbp antibodies to provide protection may be attributed to either allelic differences or insufficient levels of fHbp in the cell surface. Thus, experimental serogroup B vaccines that solely rely on fHbp for induction of protection may not offer protection against other serogroups, although, it is quite possible that higher concentrations of fHbp may indeed induce cross-reactive antibodies, as suggested by Koeberling et al. (15). However, based on the available data, it appears that vaccines such as the NOMV vaccine with both LOS and other outer membrane antigens would possibly offer multiple levels of protection across a number of N. meningitidis serogroups.
The data in this study show that the bactericidal titers in rabbits are relatively low compared to those in mice for both the homologous serogroup B strains and the non-serogroup B strains (see Table S1 in supplemental material). Furthermore, the data also show that, unlike mice, sera from immunized rabbits were not particularly effective at killing N. meningitidis serogroup Y strains in the serum bactericidal assay. This difference in bactericidal titers could be attributed to species differences, which possibly suggests that the titers in humans may be different from those in both mice and rabbits. However, based on the data obtained from the human trial for the B2 component (14), it is quite likely that the human titers to the trivalent NOMV vaccine may be closer to the rabbit titers than the mouse titers. The target strains used in this study are only a small sample of strains from each serogroup, and while they do include current circulating strains, they do represent sampling of strains in each serogroup. A study involving a much larger panel of strains from each serogroup may offer a better glimpse of the potential of the experimental serogroup B trivalent NOMV vaccine to induce a protective antibody response to all N. meningitidis strains. fHbp has been shown to induce protective antibodies against not only serogroup B N. meningitidis but also serogroups A, W135, and X (2). Thus, studies like this one may help in designing a single vaccine formulation that may be sufficient to combat all N. meningitidis infections regardless of their serogroups.
Supplementary Material
ACKNOWLEDGMENTS
We express our gratitude to the late Milan Blake from the FDA for kindly providing us with purified OpcA and Dan Granoff (CHORI) for the Jar5 and Jar 11 monoclonal antibodies.
Funding for the work described in this paper was provided by the Military Infectious Disease Research Program Office of USAMRMC, U.S. Army.
This material has been reviewed by the Walter Reed Army Institute of Research, and there is no objection to its publication. The opinions or assertions contained herein are the private views of the author and are not to be construed as official or as reflecting true views of the Department of the Army or the Department of Defense.
This research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals (22a).
Footnotes
Published ahead of print 29 March 2012
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1. Armand J, Arminjon F, Mynard MC, Lafaix C. 1982. Tetravalent meningococcal polysaccharide vaccine groups A, C, Y, W 135: clinical and serological evaluation. J. Biol. Stand. 10:335–339 [DOI] [PubMed] [Google Scholar]
- 2. Beernink PT, Caugant DA, Welsch JA, Koeberling O, Granoff DM. 2009. Meningococcal factor H–binding protein variants expressed by epidemic capsular group A, W-135, and X strains from Africa. J. Infect. Dis. 199:1361–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boisier P, et al. 2007. Meningococcal meningitis: unprecedented incidence of serogroup X-related cases in 2006 in Niger. Clin. Infect. Dis. 44:657–663 [DOI] [PubMed] [Google Scholar]
- 4. Bonvehi P, et al. 2010. Three doses of an experimental detoxified L3-derived lipooligosaccharide meningococcal vaccine offer good safety but low immunogenicity in healthy young adults. Clin. Vaccine Immunol. 17:1460–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bröker M, Dull PM, Rappuoli R, Costantino P. 2009. Chemistry of a new investigational quadrivalent meningococcal conjugate vaccine that is immunogenic at all ages. Vaccine 27:5574–5580 [DOI] [PubMed] [Google Scholar]
- 6. Claassen I, et al. 1996. Production, characterization and control of a Neisseria meningitidis hexavalent class 1 outer membrane protein containing vesicle vaccine. Vaccine 14:1001–1008 [DOI] [PubMed] [Google Scholar]
- 7. Comanducci M, et al. 2002. NadA, a novel vaccine candidate of Neisseria meningitidis. J. Exp. Med. 195:1445–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Delrieu I, et al. 2011. Emergence of epidemic Neisseria meningitidis serogroup X meningitis in Togo and Burkina Faso. PloS One 6:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Findlow J, et al. 2010. Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin. Infect. Dis. 51:1127–1137 [DOI] [PubMed] [Google Scholar]
- 10. Finne J, Leinonen M, Makela PH. 1983. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet ii:355–357 [DOI] [PubMed] [Google Scholar]
- 11. Fisseha M, et al. 2005. Characterization of native outer membrane vesicles from lpxL mutant strains of Neisseria meningitidis for use in parenteral vaccination. Infect. Immun. 73:4070–4080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Giuliani MM, et al. 2006. A universal vaccine for serogroup B meningococcus. Proc. Natl. Acad. Sci. U. S. A. 103:10834–10839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gotschlich EC, Liu TY, Artenstein MS. 1969. Human immunity to the meningococcus. 3. Preparation and immunochemical properties of the group A, group B, and group C meningococcal polysaccharides. J. Exp. Med. 129:1349–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Keiser, et al. 2011. A phase 1 study of a meningococcal native outer membrane vesicle vaccine made from a group B strain with deleted lpxL1 and synX, over-expressed factor H binding protein, two PorAs and stabilized OpcA expression. Vaccine 29:1413–1420 [DOI] [PubMed] [Google Scholar]
- 15. Koeberling O, Delany I, Granoff DM. 2011. A critical threshold of meningococcal factor H binding protein expression is required for increased breadth of protective antibodies elicited by native outer membrane vesicles vaccines. Clin. Vaccine Immunol. 18:736–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maiden MC, et al. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. U. S. A. 95:3140–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martin P, Makepeace K, Hill SA, Hood DW, Moxon ER. 2005. Microsatellite instability regulates transcription factor binding and gene expression. Proc. Natl. Acad. Sci. U. S. A. 102:3800–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Masignani V, et al. 2003. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J. Exp. Med. 197:789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moran EE, Brandt BL, Zollinger WD. 1994. Expression of the L8 lipopolysaccharide determinant increases the sensitivity of Neisseria meningitidis to serum bactericidal activity. Infect. Immun. 62:5290–5295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mothibeli KM, et al. 2011. Distribution of factor H binding protein beyond serogroup B: variation among five serogroups of invasive Neisseria meningitidis in South Africa. Vaccine 29:2187–2192 [DOI] [PubMed] [Google Scholar]
- 21. Mueller JE, et al. 2007. Molecular characteristics and epidemiology of meningococcal carriage, Burkina Faso, 2003. Emerg. Infect. Dis. 13:847–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mutonga DM, et al. 2009. Epidemiology and risk factors for serogroup X meningococcal meningitis during an outbreak in western Kenya, 2005–2006. Am. J. Trop. Med. Hyg. 80:619–624 [PubMed] [Google Scholar]
- 22a. National Research Council Guide for the care and use of laboratory animals. National Academy Press, Washington, DC [Google Scholar]
- 23. Norheim G, et al. 2006. Characterization of Neisseria meningitidis isolates from recent outbreaks in Ethiopia and comparison with those recovered during the epidemic of 1988 to 1989. J. Clin. Microbiol. 44:861–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Norheim G, et al. 2008. Specificity of the sub-capsular antibody responses following serogroup A meningococcal disease in Ethiopian patients. Clin. Vaccine Immunol. 15:863–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pillai S, et al. 2005. Outer membrane protein (OMP) based vaccine for Neisseria meningitidis serogroup B. Vaccine 23:2206–2209 [DOI] [PubMed] [Google Scholar]
- 26. Pinto VB, et al. 2011. An experimental outer membrane vesicle vaccine from N. meningitidis serogroup B strains that induces serum bactericidal activity to multiple serogroups. Vaccine 29:7752–7758 [DOI] [PubMed] [Google Scholar]
- 27. Reisinger KS, et al. 2009. Quadrivalent meningococcal vaccination of adults: phase III comparison of an investigational conjugate vaccine, MenACWY-CRM, with the licensed vaccine, Menactra. Clin. Vaccine Immunol. 16:1810–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Van Den Dobbelsteen G, et al. 2004. From HexaMen to NonaMen: expanding a multivalent PorA-based meningococcal outer membrane vesicle vaccine, p 153 Abstr. 14th Int. Pathogenic Neisseria Conf., Milwaukee WI, 5 to 10 September 2004 [Google Scholar]
- 29. Westphal O, Jann K. 1965. Bacterial lipopolysaccharides. Extraction with phenol-water and further application of the procedure. Methods Carbohydr. Chem. 5:83–91 [Google Scholar]
- 30. Weynants VE, et al. 2009. Genetically modified L3,7 and L2 lipooligosaccharides from Neisseria meningitidis serogroup B confer a broad cross-bactericidal response. Infect. Immun. 77:2084–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zollinger WD, Shoemaker D, Saunders AG, Brandt BL. May 2003. Vaccine against Gram negative bacteria. US patent 6,558,677 B2
- 32. Zollinger WD, Moran EE, Schmiel DH. 2009. Characterization of an antibody depletion assay for analysis of bactericidal antibody specificity. Clin. Vaccine Immunol. 16:1789–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zollinger WD, et al. 2010. Design and evaluation in mice of a broadly protective meningococcal group B native outer membrane vesicle vaccine. Vaccine 28:5057–5067 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.