Abstract
The toxicity of soft metals is of broad interest to microbiologists, both because such metals influence the community structures in natural environments and because several metals are used as antimicrobial agents. Their potency roughly parallels their thiophilicity, suggesting that their primary biological targets are likely to be enzymes that contain key sulfhydryl moieties. A recent study determined that copper poisons Escherichia coli in part by attacking the exposed [4Fe-4S] clusters of dehydratases. The present investigation sought to test whether other soft metals also target these enzymes. In vitro experiments revealed that low-micromolar concentrations of Ag(I) and Hg(II) directly inactivated purified fumarase A, a member of the dehydratase family. The enzyme was also poisoned by higher levels of Cd(II) and Zn(II), but it was unaffected by even millimolar concentrations of Mn(II), Co(II), Ni(II), and Pb(II). Electron paramagnetic resonance analysis and measurements of released iron confirmed that damage was associated with destruction of the [4Fe-4S] cluster, and indeed, the reconstruction of the cluster fully restored activity. Growth studies were then performed to test whether dehydratase damage might underlie toxicity in vivo. Barely toxic doses of Ag(I), Hg(II), Cd(II), and Zn(II) inactivated all tested members of the [4Fe-4S] dehydratase family. Again, activity was recovered when the clusters were rebuilt. The metals did not diminish the activities of other sampled enzymes, including NADH dehydrogenase I, an iron-sulfur protein whose clusters are shielded by polypeptide. Thus, the data indicate that dehydratases are damaged by the concentrations of metals that initiate bacteriostasis.
INTRODUCTION
Soft metals are functionally defined by their polarizability, which enables them to associate tightly with sulfhydryl groups. Broadly speaking, mercury(II), silver(I), copper(I), lead(II), cadmium(II), nickel(II), zinc(II), and cobalt(II) all have substantial affinities for protein thiols, and they are toxic to bacteria roughly in proportion to their affinities for sulfur (31). The use of silver and copper for drinking vessels of the classical period exploited the observation that these metals suppress spoilage. In the 20th century, silver and mercury in particular were applied as antibiotics to prevent or treat a variety of human infections. Today, mercury is still used in parts of the world as a preservative for vaccines and seeds (3), silver tips have been developed for catheters to resist biofilms (5), and both silver and copper coatings have been applied to frequently touched surfaces in hospitals in order to suppress nosocomial disease (14, 33).
Given this long history, it is somewhat surprising that the molecular basis of soft-metal toxicity toward microbes remains unclear (33, 39). Three general models have been considered. First, in vitro studies show that the divalent soft metals can compete for the metal-binding sites of proteins that normally contain divalent cations; the ensuing mismetallation may, for example, undermine the function of zinc-finger proteins (26, 32). However, such a mechanism seems less likely to pertain to the monovalent ions Ag(I) and Cu(I), which should bind poorly to divalent-metal sites but are nevertheless among the most toxic soft metals. Second, given their predilection for binding sulfur atoms, soft metals are potential inhibitors of enzymes that require active-site thiols for activity (7, 34). Finally, a variety of studies have linked soft-metal overloading with signs of oxidative stress (19, 40, 42, 43), including the accumulation of oxidized DNA and lipid hydroperoxides. Yet most of these metals are not redox active, so that an underlying mechanism of oxidative activity is not immediately apparent.
In a recent study, we investigated the mechanism by which copper poisons Escherichia coli. It was found that copper(I) exerted toxicity by destroying the iron-sulfur clusters of dehydratase-family enzymes (27). These [4Fe-4S] clusters are directly involved in catalysis, as they bind substrate and serve as Lewis acids to promote the dehydration reaction. To do so, the clusters must be openly exposed to solvent, a trait not shared by the more familiar clusters that operate in electron-transfer enzymes. A consequence is that copper(I) has access to the sulfur atoms of the cluster, and upon binding them it displaces the catalytic iron atoms. Activity is thereby abolished. Enzymes of this type are involved in key metabolic pathways, and their inactivation can arrest growth.
Thus, it is plausible that other soft metals might have the same destructive effect upon this unusual enzyme family. In this study, we first determined which soft metals can damage purified dehydratases in vitro. We then tested whether dehydratase activities are lost at the point when such metals poison E. coli. The data indicate that dehydratases are damaged by the concentrations of metals that initiate bacteriostasis.
MATERIALS AND METHODS
Reagents.
d-Glucose and glycerol were purchased from Fisher Scientific; mercury chloride, cadmium chloride, and lead chloride were purchased from Acros Organics; and EDTA disodium salt dehydrate was purchased from Fluka. Silver nitrate, zinc chloride, cobalt chloride, manganese sulfate, nickel sulfate hexahydrate, ferrous ammonium sulfate, l-(−)-malic acid disodium salt, 2,2′-dipyridyl, l-amino acids, acid-hydrolyzed Casamino Acids, d-gluconate, thiamine, chloramphenicol, kanamycin, o-nitrophenyl-β-d-galactopyranoside (ONPG), d,l-dithiothreitol, NADH, deamino-NADH, isopropyl-β-d-thiogalactopyranoside (IPTG), β-mercaptoethanol, ampicillin, citraconate, l-lactic dehydrogenase solution from rabbit muscle, catalase from bovine liver, potassium d-gluconate, 6-phosphogluconic acid tri(cyclohexylammonium), oxalacetic acid, and DEAE-Sepharose were obtained from Sigma. Metal stock solutions were prepared in water at 100 mM for Ag(I), at 50 mM for Cd(II), Zn(II), Mn(II), Ni(II), and Co(II) salts, and at 10 mM for Hg(II) and Pb(II) salts.
Fumarase A was overproduced and purified from a wild-type strain containing pFUMA, following the protocols described previously (9, 18). IscS protein was purified from an overproducer strain (17).
Strains and growth conditions.
The strains used in this study are derivatives of the wild-type K-12 strain MG1655. FX10 contains a ΔzntA724::kan allele that eliminates the zinc efflux system (37); the mutation was introduced into MG1655 by P1 transduction from JW3434-1 (E. coli Genetic Stock Center) with selection for kanamycin resistance. Inheritance of the mutation was confirmed by PCR analysis. SJ32 is a Δ(fumC1::cat)1 mutant of the MG1655 descendant BW25113 (6); the mutation eliminated the activity of the cluster-free fumarase C isozyme (24). Plasmid pFUMA overexpresses fumA from a tac promoter (18). The lac mutation and the promoter fusion were produced and integrated as described previously (17). DW3110 is a copA::kan mutant of wild-type strain W3110 lacking the proton motive force-driven copper efflux protein (36).
Luria broth (30) contained 10 g Bacto tryptone, 5 g yeast extract, and 10 g NaCl per liter. Standard minimal medium contained minimal A salts (30), 0.2% glucose, 1 mM MgSO4, and 5 mg/liter thiamine. Where indicated, minimal medium was supplemented with 0.2% Casamino Acids and 0.5 mM tryptophan. Histidine (0.5 mM) was added to anaerobic minimal medium cultures because MG1655 exhibits a histidine auxotrophy when oxygen is absent. In experiments that tested the abilities of metals to poison intracellular 6-phosphogluconate dehydratase or β-galactosidase, 0.2% gluconate or 0.2% lactose, respectively, was substituted for glucose. Media were supplemented with 150 μg/ml chloramphenicol or 100 μg/ml ampicillin when needed. Anaerobic cultures were grown at 37°C in an anaerobic chamber (Coy Laboratory Products). Aerobic cultures were grown at 37°C with vigorous shaking.
All growth studies were performed with cells that were first established in exponential phase. To do so, overnight cultures were diluted to an optical density at 600 nm (OD600) of 0.005 and then grown to an OD600 of ∼0.1. These cells were then diluted again into fresh medium containing the indicated concentration of toxic metal. Overnight cultures, precultures, and challenged cultures were all prepared in the same medium.
In vitro assays.
Purified fumarase A (2 μM) in anaerobic 50 mM Tris-Cl buffer (pH 7.65) was inactivated by the addition of freshly prepared silver, mercury, cadmium, zinc, nickel, cobalt, manganese, or lead at room temperature inside the anaerobic chamber. In tests of protection by substrate, the indicated concentrations of l-malic acid were also present. After exposure, the enzyme was diluted 200-fold and assayed by monitoring the production of fumarate at 250 nm (18).
Reactivation of the inactivated enzymes was initiated by 1:2 dilution and anaerobic incubation with 2.5 mM dithiothreitol (DTT) and 200 μM Fe(NH4)2(SO4)2 for 10 min at room temperature. By binding the soft metals, DTT blocked further damage, and the combination of DTT and ferrous iron allows the reactivation of clusters that have been degraded to their [3Fe-4S] form (17). Where indicated, 2.5 mM cysteine and purified IscS (0.16 mU) were included with the DTT-iron, and the mixture was incubated at room temperature for 30 min in order to rebuild damaged clusters that had lost bridging sulfur atoms. After these reactivation procedures, the enzyme was diluted 100-fold and immediately assayed.
Iron lost during enzyme damage was quantified by trapping with dipyridyl (ε, 8.65 mM−1 cm−1 for the iron/dipyridyl complex at A522) (18). The reaction mixture included 6.7 μM purified fumarase and 1 mM dipyridyl in 50 mM anaerobic HEPES buffer (pH 7.65). HEPES buffer was used in this experiment to avoid spectroscopic interference from silver complexes with components of Tris-Cl buffer. Standard curves were determined using Fe(NH4)2(SO4)2.
Electron paramagnetic resonance (EPR) analysis of cluster damage (17) was performed by incubating purified fumarase A (20 μM) with 100 μM H2O2, 300 μM Zn(II), or 60 μM Ag(I) in 50 mM anaerobic Tris-Cl, 10 mM Mg(II), and 10% glycerol for 5 min. Catalase was then added to remove the H2O2 (and was added to the metal-treated enzyme for the sake of consistency). The reaction mixture was immediately transferred to EPR tubes and frozen in dry ice. The EPR spectrum was obtained under the following conditions: microwave power, 1 mW; microwave frequency, 9.05 GHz; modulation amplitude, 8 G at 100 kHz; time constant, 0.032; temperature, 15 K.
Measurements of enzyme damage occurring in vivo.
In preparation for studies of fumarase, isopropylmalate isomerase (IPMI), and 6-phosphogluconate dehydratase, cells (250 ml) were cultured aerobically in glucose-Casamino Acids medium, standard minimal glucose medium, and gluconate-Casamino Acids medium, respectively, at 37°C to an OD600 of ∼0.2. Chloramphenicol (150 μg/ml) was then added to block further protein synthesis, and the tested metal was subsequently added for 30 min. Cells were then centrifuged, washed twice with minimal A salts, resuspended in 1 ml of the relevant anaerobic assay buffer, and lysed by 2 min sonication in the anaerobic chamber. Lysates were clarified by 10 min centrifugation at 15,000 × g. Assays of these enzymes were performed by established methods (18) either immediately or after cluster reconstitution as described above.
For tests of damage to β-galactosidase, malate dehydrogenase, and NADH dehydrogenase I, cells were cultured in aerobic lactose-Casamino Acids medium prior to the addition of chloramphenicol and metal, as described above. Extracts containing β-galactosidase and inverted vesicles containing NADH dehydrogenase I were subsequently prepared and assayed in 1 ml aerobic buffers, while malate dehydrogenase was extracted by sonication and assayed in anaerobic buffer in order to avoid NADH oxidation by the respiratory vesicles. For NADH dehydrogenase I assays, inverted membrane vesicles were prepared by French press in aerobic 50 mM morpholineethanesulfonic acid buffer (pH 6.0). Cell debris was removed by centrifugation, and the activity was then measured in 50 mM aerobic potassium phosphate buffer (pH 6.0) by monitoring the decrease of deamino-NADH (sodium salt) at 340 nm. NADH dehydrogenase II does not act on this NADH analogue (29).
RESULTS
Growth inhibition by soft metals.
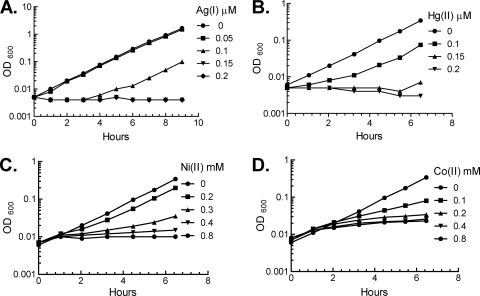
Exponentially growing E. coli cells were exposed to a variety of soft metals. Very low concentrations of Ag(I) and Hg(II) (0.15 μM) were sufficient to block the growth of E. coli in a glucose minimal medium, consistent with their known antibiotic effects (Fig. 1). In contrast, near-millimolar doses of Ni(II) and Co(II) were required to slow growth. No growth inhibition was seen when the medium was supplemented with even millimolar levels of Cd(II), Zn(II), Mn(II), or Pb(II).
Fig 1.
Soft metals inhibit E. coli growth. At time zero, MG1655 (wild-type) cultures growing exponentially in aerobic minimal glucose medium were subcultured into the same medium containing the indicated concentrations of metals.
Several parameters strongly affected the sensitivity of cells in these experiments. These were closely documented using Ag(I) as a representative metal. First, sensitivity was strongly suppressed in complex medium; for example, whereas 0.1 μM Ag(I) inhibited growth in defined medium (Fig. 1A), 20 μM was required for a similar effect in LB medium (data not shown). This protection is likely to be due to chelation of the metal by medium components, an effect that was documented previously in the studies of copper (27). Second, toxicity was strongly diminished when cells were at a higher density when metals were added. Thus, the minimal Ag(I) concentration required for prolonged stasis was 0.25 μM at an OD600 of 0.005 but 7 μM at an OD600 of 0.2. We believe that this phenomenon arises because some metals are sequestered through adventitious associations with the cell surface. Indeed, the dose-response curves manifested a tipping point: although 0.2-OD-unit cells tolerated 5 μM Ag(I) with little effect upon growth rate, 6.25 μM Ag(I) absolutely blocked growth, as if a sequestering component had been titrated. In any case, the effect is that toxic doses cannot easily be described as MIC values, and the toxicity of these metals can be strongly underestimated if experiments are performed in complex media and/or at high culture densities.
Like copper, Ag(I) inhibited anaerobic cells as effectively as it did aerobic cells (data not shown). This outcome indicates that Ag(I) toxicity is not mediated by reactive oxygen species.
Intermediate doses of Ag(I) created a short lag, which initially suggested an adaptive protective response (Fig. 1A). However, when the outgrowing cells were centrifuged and resuspended in fresh medium of the same Ag(I) content, growth abruptly stopped (data not shown). We infer that the cells conditioned the medium by absorbing a limited amount of Ag(I); preexposed cells lacked any further capacity to tolerate new Ag(I).
In sum, very low doses of Ag(I) and Hg(II) are sufficient to block E. coli growth, although the precise inhibitory concentration is affected by cell density and medium composition. These metals are most potent under the conditions that are most commonly found in nature: dilute media and low cell densities.
Several soft metals inactivate iron-sulfur-dependent dehydratases in vitro.
Copper can poison cells by directly destroying the catalytic iron-sulfur clusters of the dehydratase-family enzymes (27). This action stems from its ability to bind the sulfur ligands of the cluster, a trait which other soft metals might be expected to share. Purified fumarase A was exposed to Mn(II), Co(II), Ni(II), Zn(II), Ag(I), Cd(II), Hg(II), and Pb(II) in vitro. This experiment was performed under anaerobic conditions to preclude any involvement of oxygen species. Figure 2A demonstrates that low-micromolar concentrations of Ag(I) and Hg(II) quickly inactivated the enzyme. Substantially higher doses of Cd(II) and Zn(II) were required. Fumarase activity decreased by 90% after 5 min exposure to 3 μM Hg(II), 10 μM Ag(I), 80 μM Cd(II), and 200 μM Zn(II). This pattern correlates with the order of their thiophilicity. In contrast, Mn(II), Co(II), Ni(II), and Pb(II) had no effect even at millimolar concentrations. The negative result with Co(II) agreed with inferences that Ranquet et al. drew from studies with intact cells (35). The fact that Pb(II), a very soft metal, failed to damage fumarase indicates that softness per se is not a sufficient predictor of its effect (see Discussion).
Fig 2.
Soft metals inactivated fumarase A in vitro. (A) Purified fumarase A (2 μM) was exposed to the indicated concentrations of soft metals for 5 min in anaerobic room-temperature buffer prior to dilution and assay. Metals included Ag(I) (closed circle), Hg(II) (closed square), Cd(II) (closed triangle), Zn(II) (closed diamond), Co(II) (open circle), Ni(II) (open square), Mn(II) (open triangle), or Pb(II) (open diamond). (B) The inactivation of fumarase A by Ag(I) was not continuous.
Rate constants could not be determined, as the rate of inactivation was not constant over time (Fig. 2B). This effect apparently stems from the progressive absorption of the metal upon protein surfaces, since the extent and duration of damage were greatest when the protein was most dilute (data not shown). Similar effects were apparent in analogous experiments with copper (27).
Dehydratase inactivation stems from destruction of the [4Fe-4S] cluster.
In principle, metals might damage proteins in a number of ways. The presence of malate protected the fumarase. Malate is a substrate/product of this reversible enzyme, and median protection was achieved by a concentration (ca. 1 mM) close to the Km of the enzyme (Fig. 3A). The implication is that the metal must enter the active site in order to create damage. Interestingly, damage by Ag(I) and Hg(II) was not quite fully blocked by saturating concentrations of malate, indicating that these metals still have some access to the target site even when the cluster forms an enzyme-substrate complex with malate.
Fig 3.
Soft metals specifically degraded the [4Fe-4S] cluster of fumarase A. (A) Substrate protected the enzyme from damage. The indicated concentrations of malate were present during the anaerobic exposure of 2 μM fumarase A for 5 min to 10 μM Ag(I), 5 μM Hg(II), 100 μM Cd(II), or 200 μM Zn(II). Enzyme was subsequently diluted and assayed. (B) The activity of inactivated fumarase was restored by reconstruction of its iron-sulfur cluster. Purified fumarase A (2 μM) was anaerobically damaged by 10 μM Ag(I), 5 μM Hg(II), 100 μM Cd(II), or 200 μM Zn(II) in anaerobic buffer. Aliquots then received no further treatment or were incubated with Fe(II)-DTT or Fe(II)-DTT-IscS-cysteine prior to assay. (C) EPR analysis of the effects of Ag(I) and Zn(II) upon the [4Fe-4S] cluster of fumarase A. Purified fumarase A (20 μM) was exposed to 300 μM Zn(II) and 60 μM Ag(I) for 5 min. Treatment with 100 μM H2O2, which generates a [3Fe-4S]+ cluster, was performed as a control. The reaction mixture was then frozen for EPR analysis. (D) Progressive iron release during exposure of 6.7 μM fumarase A to 50 μM Ag(I). Iron was quantified by chelation with 1 mM dipyridyl.
When dehydratase clusters are damaged by oxidants, the [4Fe-4S]+ cluster degrades to a [3Fe-4S]2+ form that can be reactivated by subsequent treatment with ferrous iron and dithiothreitol (18, 22). This procedure restored only minimal activity when fumarase had been damaged with Ag(I), moderate activity when damaged by Hg(II) and Cd(II), and almost full activity when damaged by Zn(II) (Fig. 3B). One possible explanation was that the Ag(I)-treated enzyme had lost more than one iron atom—and, with it, the bridging sulfur atoms—whereas degradation of the Zn(II)-treated enzyme had arrested in the [3Fe-4S] stage. To test this, the damaged enzymes were also incubated in a mixture that included the IscS desulfurylase and cysteine, its substrate. This enzyme is involved in de novo cluster synthesis in E. coli and can be employed to activate iron-sulfur enzymes that are in the apoprotein form (17). Indeed, this treatment restored nearly full activity to all the damaged proteins. Thus, metal treatment specifically destroys the iron-sulfur clusters, without irreversible damage to the polypeptide itself.
EPR analysis showed that our purified fumarase A was predominantly in the [4Fe-4S] (EPR-silent) form, with a minor subpopulation being in the oxidized (EPR-visible) [3Fe-4S]+ form (Fig. 3C). Treatment with Ag(I) destroyed both classes of clusters, with no residual signal. In contrast, although Zn(II) exposure inactivated the holoenzyme, the [3Fe-4S]+ subpopulation was increased rather than degraded. This outcome confirms that Ag(I) degrades clusters more extensively than does Zn(II). To track the kinetics of iron loss, the ferrous iron chelator dipyridyl was included during the period of Ag(I) exposure. Iron release was biphasic, with a relatively rapid release of the first iron atom followed by a slower release of remaining iron (Fig. 3D). Thus, iron release is a stepwise event, rather than an all-or-none destruction of the cluster.
The failure of malate to fully block damage by Ag(I) and Hg(II) had raised the question of whether the residual damage involved the iron-sulfur cluster at all. However, we observed that the iron-DTT-IscS treatment reversed the inactivation of enzyme that occurred with saturating malate (data not shown). Aside from showing that these metals did no other damage to the protein, this result indicates that accumulated substrate can slow but not fully block enzyme damage.
Soft metals attack dehydratases in vivo.
Metabolite concentrations are high inside cells, and it seemed plausible that these various compounds might chelate soft metals so effectively that dehydratase damage would be minimal even in overloaded cells. We cultured cells in defined medium at the higher cell densities, to enable biochemical analysis of recovered fumarase in the lysate. Intracellular fumarase was inactivated when cells were exposed to doses of Ag(I) and Hg(II) that were just sufficient to inhibit growth (Fig. 4). Damage was also apparent in Cd(II)- and Zn(II)-treated cells. In all cases, most of the lost fumarase activity was recovered when lysates were incubated with iron-DTT-IscS. We conclude that dehydratases comprise one of the first targets that these metals damage.
Fig 4.
Ag(I), Hg(II), Cd(II), and Zn(II) also damaged the [4Fe-4S] clusters of fumarase A in vivo. Strain SJ32 (0.2 OD unit) was exposed to soft metals for 30 min in glucose medium in the presence of chloramphenicol to block protein synthesis. Lysates were then prepared and analyzed in anaerobic buffer. Lysates were assayed without further treatment, after incubation with Fe(II)-DTT, or after incubation with Fe(II)-DTT-IscS-cysteine.
Other iron-sulfur dehydratases were also damaged. Figure 5 demonstrates the loss of activity of isopropylmalate isomerase (Fig. 5A) and 6-phosphogluconate dehydratase (Fig. 5B) during Ag(I) stress. Notably, the concentration dependence showed the same tipping-point effect seen in the growth-curve experiments (Fig. 1).
Fig 5.
Silver also damaged other [4Fe-4S] dehydratases inside cells. Cells were grown aerobically to an OD600 of ∼0.2, chloramphenicol was added to block further protein synthesis, and Ag(I) was then added for 30 min. (A) MG1655 (wild type) was grown in glucose minimal medium, and isopropylmalate isomerase (IPMI) activity was monitored. (B) MG1655 was grown in gluconate Casamino Acids medium, and 6-phosphogluconate dehydratase (Edd) activity was monitored.
The effect was specific: none of the metals inactivated malate dehydrogenase or β-galactosidase, two control enzymes that do not employ iron-sulfur clusters (data not shown). Further, the metals also did not affect the activity of NADH dehydrogenase I, the complex I enzyme that contains at least nine clusters in its electron-transfer pathway. These clusters are buried in polypeptide and are presumably inaccessible to dissolved metals.
Of the most thiophilic metals, only Zn(II) is deliberately imported into the E. coli cytoplasm, where it serves as a cofactor for numerous enzymes. Its intracellular concentration is held in check by the Zn(II) export protein ZntA (37). Indeed, mutants that lack this export system were more sensitive to growth inhibition by zinc. The zntA mutants also exhibited a greater degree of damage to 6-phosphogluconate dehydratase: upon treatment with 0.6 mM zinc, activity in wild-type cells declined only to 24% ± 2% of the normal level, while in the ZntA-deficient strain, the activity fell to 5% ± 4%. In this experiment, zinc was added in the presence of chloramphenicol, so that the loss of activity represents damage to extant enzyme rather than interference with de novo cluster assembly. Thus, the [4Fe-4S] dehydratases are targets that the zinc homeostasis system substantially protects.
DISCUSSION
The microbicidal capacity of soft metals has long been recognized, but its molecular basis has been uncertain. It has been observed that the softest metals—that is, those that bind sulfur ligands most strongly—tend to be the most toxic to microorganisms. Two of these, silver and mercury, have traditionally been used as antiseptics, and much current work focuses on the use of copper for the same purpose (14). We observed that these metals are particularly effective at inhibiting the growth of E. coli.
These three metals quickly destroy the solvent-exposed iron-sulfur clusters of the aconitase-family dehydratases. Iron loss occurs in a stepwise fashion, presumably due to SN1-type displacement of iron as the soft metals bind either cysteinyl or bridging sulfur atoms. Zinc, the least thiophilic of the damaging metals, displaced only the unique substrate-coordinating iron atom, leaving behind a [3Fe-4S] product. We suspect that this difference arises because the residual iron atoms are more strongly coordinated by four sulfur ligands, including a polypeptide cysteine, whereas the labile iron atom initially has only three bridging sulfide ligands and a water molecule.
The avidity with which the displacing metals remain associated with the clusters is uncertain; the damaged enzymes can be immediately rebuilt in vitro, presumably indicating that the binding is only transient. A similar repair process occurs in vivo, so that the steady-state activity of these enzymes inside metal-stressed cells represents the balance between cluster damage and repair (11, 13).
Although dehydratase [4Fe-4S] clusters are also targeted by the oxidants superoxide and hydrogen peroxide (10, 18, 22), both the mechanism of damage and the nature of the damaged product differ from those of soft-metal damage. Oxidants must directly bind the catalytic iron atom; thus, substrate-bound enzyme is fully protected. Oxidation generates a [4Fe-4S]3+ form that quickly decays to a stable [3Fe-4S]+ species; oxidants cannot attack this form further, and so it is quickly repaired in vivo. Both of these traits dampen the potency of oxidative stress. In contrast, thiophilic metals can still attack enzyme-substrate complexes, since substrates do not coordinate the sulfur atoms that the metals target, and the softer metals degrade the cluster beyond the stage at which it can be reactivated by simple reduction/remetallation. Repair likely requires the function of the Isc system, which, others have shown, may also be inhibited by soft metals (4, 35).
The fact that Pb(II) did not damage fumarase is notable. Pb(II) is distinguished from the other soft metals in that it requires asymmetric coordination geometry, due to lone-pair electrons that the other metals lack. Indeed, the substitution of Pb(II) into divalent-metal enzymes can distort the local structure, as the polypeptide ligands are rearranged (12, 28). Such flexibility may be absent in the rigid iron-sulfur environment, possibly excluding Pb(II) from the site. The ionic radius of Pb(II) (133 pm) is also substantially larger than that of the other metals, including Zn(II) (88 pm) and even Hg(II) (116 pm).
Mn(II), Ni(II), and Co(II) did not directly damage dehydratases, a result that accords with the lesser affinity of these metals for sulfur ligands. Excessive intracellular levels of these metals are nevertheless toxic to E. coli, albeit by other mechanisms. Since manganese toxicity can be alleviated by mutations in Fur protein—which thereby triggers rapid iron import—it is generally inferred that manganese competes with iron for binding to iron-dependent proteins, although the specifics remain uncertain (16). A recent report demonstrates that nickel competes with zinc for fructose-1,6-bisphosphate aldolase; the nickel-loaded enzyme has poor activity so that glycolysis fails (Fig. 1C) (25). Further, while cobalt cannot directly inactivate iron-sulfur enzymes, it can inhibit the Isc system that assembles the clusters. Over time the effect is a gradual diminution in the activities of client proteins (35). This effect might explain the progressive slowing of growth over several hours that was observed in this study (Fig. 1D).
A key question, of course, is whether cluster destruction is a main element of the toxic actions of Ag(I), Hg(II), Cd(II), or Zn(II). In a previous study, we found that copper specifically inactivated the branched-chain biosynthetic pathway: at the lowest toxic doses of copper, growth was restored when the medium was supplemented with leucine, isoleucine, and valine (27). That pathway employs two iron-sulfur dehydratase enzymes, and their loss of activity correlated with the growth defect. In the current study, we again observed that dehydratase damage occurred at the same critical metal doses that blocked growth, which once more fits the notion that these enzymes are primary targets. However, the toxicity of silver and mercury could not be abated with branched-chain supplements. Dose-response experiments revealed that below a certain threshold, metals appeared to be innocuous; but when that threshold was exceeded, they abruptly poisoned cells. This threshold may correlate with the adsorbing capacity of cell surfaces. If so, at the point of toxicity, the intracellular metal levels may suddenly rise enough to simultaneously incapacitate a variety of targets, making it difficult to correlate growth problems with the progressive damage of a particular enzyme. Members of the dehydratase family belong to a variety of catabolic and anabolic pathways, so that metabolism might be disrupted at several levels. Of course, it is plausible that metal-independent thiolate enzymes are also affected. However, at marginal doses of soft metals, one might expect the iron-sulfur enzymes to be particularly vulnerable, since even the momentary binding of a soft metal will cause cluster damage that typically requires many minutes for repair in vivo, in contrast to thiolate enzymes, whose inhibition requires continuous association with the metal. Nonetheless, although the present data confirm that iron-sulfur dehydratases are among the first targets of silver and mercury, the data do not show that they are exclusive in this regard.
A variety of indirect observations have led workers to consider that some soft metals might also stimulate damage by reactive oxygen species (19, 40, 42, 43). The chemistry by which such injuries might arise has been unclear, since most soft metals cannot redox cycle at physiological redox poises. The fact that these metals can degrade iron-sulfur clusters might offer at least a partial explanation: destruction of these clusters can swell the cellular pools of unincorporated iron enough to substantially accelerate Fenton reactions, which can drive the oxidation of DNA and the peroxidation of lipids (20). Further, some organisms use the accumulation of cluster-free aconitase as a signal that the cell is iron deficient: the apoprotein stabilizes RNA messages that encode iron-import systems (1, 21). Disruption of that cluster by soft metals would mimic the effect of iron depletion and might be expected to trigger excessive iron accumulation. Thus, it is possible that oxidative stress is an indirect effect of soft-metal overloading.
The prevalence or essentiality of iron-sulfur dehydratases varies among organisms. Microbes that depend upon the Entner-Doudoroff or tricarboxylic acid cycle pathways for energy production will be vulnerable to soft metals, due to their reliance upon 6-phosphogluconate dehydratase (Edd) or aconitase/fumarase, respectively; glycolysis, in contrast, does not involve iron-sulfur dehydratases. Further, while the IPMI and dihydroxy-acid dehydratases are required for the synthesis of branched-chain amino acids and vitamins, some microbes occupy habitats in which these products are directly available and need not be synthesized. Streptococcus pneumoniae, for example, does not rely on any members of this enzyme class, and one expects it to be resistant to this mode of soft-metal toxicity. This consideration may be relevant to the impacts that these metals will have both in the environment and when applied as antiseptics.
Finally, it is interesting to consider how dehydratase-utilizing cells shield themselves from the toxic effects of these metals. The primordial earth was anaerobic, and soft metals were trapped as insoluble sulfide minerals; this circumstance allowed early organisms to employ iron-sulfur enzymes without the threat that they might be damaged by either reactive oxygen species or soft metals (2, 8).
It was not until 2 billion years later that oxygen levels surged, in response to global photosynthesis. At that point these enzymes were confronted with reactive oxygen species, which are generated by adventitious redox reactions, and with the soft metals, which were solubilized by the oxidation of sulfide minerals. Evolving microorganisms countered the reactive oxygen species by inventing scavenging enzymes. Ultimately, they shielded themselves from the softest metals by excluding them from the cell interior (41), where the vulnerable iron-sulfur dehydratase and thiolate enzymes are located. To this end, bacteria commonly express dedicated silver, copper, and mercury export systems that pump these metals out of the cytoplasm (31, 38, 39). The cellular organization ensures that there is no need to allow these metals to mix with dehydratases: the few soluble copper-dependent enzymes that require access to this metal reside exclusively in the periplasm, the peripheral compartment that lacks iron-sulfur enzymes (27). In eukaryotes, the cytoplasmic compartment does contain both copper and iron-sulfur enzymes, but the copper is carefully chaperoned during its delivery to copper-requiring apoproteins (23), a system that presumably arose to ensure that copper could not associate with enzymes that it could poison.
Both eukaryotic and prokaryotic organisms employ zinc as a common cofactor of cytoplasmic enzymes. This arrangement may be tolerable because zinc reacts much more sluggishly with iron-sulfur clusters than does silver or copper, and the primary product is a [3Fe-4S] cluster that can be readily fixed by simple remetallation without resort to the slower Isc desulfurase system. Further, the counterbalancing Zur and ZntR regulatory systems (15) ensure that the intracellular zinc concentration is restricted within narrow limits that are too low to affect iron-sulfur enzymes. Thus, it is plausible that the threat of soft metals has had a substantial influence upon the identities, compartmentalization, and trafficking of biological metals.
ACKNOWLEDGMENTS
This study was funded by NIH grants GM49640 and GM101012 to J.A.I. and by a China Scholarship Council award (File No. 2009663034) to F.F.X.
We thank Mark Nilges of the Illinois EPR Research Center for his help with EPR experiments and Soojin Jang for both technical assistance and strains used in this study. Chris Rensing and the E. coli Genetic Center also generously provided strains. We also thank Liang Chen and Jianshe Liu for their support of this cooperative program.
Footnotes
Published ahead of print 17 February 2012
REFERENCES
- 1. Alén C, Sonenshein AL. 1999. Bacillus subtilis aconitase is an RNA-binding protein. Proc. Natl. Acad. Sci. U. S. A. 96:10412–10417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anbar AD. 2008. Elements and evolution. Science 322:1481–1483 [DOI] [PubMed] [Google Scholar]
- 3. Aschner M, Onishchenko N, Ceccatelli S. 2010. Toxicology of alkylmercury compounds. Met. Ions Life Sci. 7:403–434 [DOI] [PubMed] [Google Scholar]
- 4. Barras F, Fontecave M. 2011. Cobalt stress in Escherichia coli and Salmonella enterica: molecular bases for toxicity and resistance. Metallomics 3:1130–1134 [DOI] [PubMed] [Google Scholar]
- 5. Beattie M, Taylor J. 2011. Silver alloy vs. uncoated urinary catheters: a systematic review of the literature. J. Clin. Nurs. 20:2098–2108 [DOI] [PubMed] [Google Scholar]
- 6. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dieguez-Acuna FJ, Woods JS. 2000. Inhibition of NF-kB-DNA binding by mercuric ion: utility of the non-thio reductant, Tris(2-carboxyethyl)phosphoine hydrochloride (TCEP), on detection of impaired NF-kB-DNA binding by thiol-directed agents. Toxicol. In Vitro 14:7–16 [DOI] [PubMed] [Google Scholar]
- 8. Dupont CL, Grass G, Rensing C. 2011. Copper toxicity and the origin of bacterial resistance—new insights and applications. Metallomics 3:1109–1118 [DOI] [PubMed] [Google Scholar]
- 9. Flint DH, Emptage MH, Guest JR. 1992. Fumarase A from Escherichia coli: purification and characterization as an iron-sulfur cluster containing enzyme. Biochemistry 31:10331–10337 [DOI] [PubMed] [Google Scholar]
- 10. Flint DH, Tuminello JF, Emptage MH. 1993. The inactivation of Fe-S cluster containing hydro-lyases by superoxide. J. Biol. Chem. 268:22369–22376 [PubMed] [Google Scholar]
- 11. Gardner PR, Fridovich I. 1992. Inactivation-reactivation of aconitase in Escherichia coli. A sensitive measure of superoxide radical. J. Biol. Chem. 267:8757–8763 [PubMed] [Google Scholar]
- 12. Garza A, Vega R, Soto E. 2006. Cellular mechanisms of lead neurotoxicity. Med. Sci. Monit. 12:RA57–RA65 [PubMed] [Google Scholar]
- 13. Gort AS, Imlay JA. 1998. Balance between endogenous superoxide stress and antioxidant defenses. J. Bacteriol. 180:1402–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grass G, Rensing C, Soliuz M. 2011. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 77:1541–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hantke K. 2001. Bacterial zinc transporters and regulators. Biometals 14:239–249 [DOI] [PubMed] [Google Scholar]
- 16. Hantke K. 1987. Selection procedure for deregulated iron transport mutants (fur) in Escherichia coli K 12: fur not only affects iron metabolism. Mol. Gen. Genet. 210:135–139 [DOI] [PubMed] [Google Scholar]
- 17. Jang S, Imlay JA. 2010. Hydrogen peroxide inactivates the Escherichia coli Isc iron-sulphur assembly system, and OxyR induces the Suf system to compensate. Mol. Microbiol. 78:1448–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jang S, Imlay JA. 2007. Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. J. Biol. Chem. 282:929–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jomova K, Valko M. 2011. Advances in metal-induced oxidative stress and human disease. Toxicology 283:65–87 [DOI] [PubMed] [Google Scholar]
- 20. Keyer K, Imlay JA. 1996. Superoxide accelerates DNA damage by elevating free-iron levels. Proc. Natl. Acad. Sci. U. S. A. 93:13635–13640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klausner RD, Rouault TA, Harford JB. 1993. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell 72:19–28 [DOI] [PubMed] [Google Scholar]
- 22. Kuo CF, Mashino T, Fridovich I. 1987. α,β-Dihydroxyisovalerate dehydratase: a superoxide-sensitive enzyme. J. Biol. Chem. 262:4724–4727 [PubMed] [Google Scholar]
- 23. Leitch JM, Yick PJ, Culotta VC. 2009. The right to choose: multiple pathways for activating copper,zinc superoxide dismutase. J. Biol. Chem. 284:24679–24683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liochev SI, Fridovich I. 1992. Fumarase C, the stable fumarase of Escherichia coli, is controlled by the soxRS regulon. Proc. Natl. Acad. Sci. U. S. A. 89:5892–5896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Macomber L, Elsey SP, Hausinger RP. 2011. Fructose-1,6-bisphosphate aldolase (class II) is the primary site of nickel toxicity in Escherichia coli. Mol. Microbiol. 82:1291–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Macomber L, Hausinger RP. 2011. Mechanisms of nickel toxicity in microorganisms. Metallomics 3:1153–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Macomber L, Imlay JA. 2009. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. U. S. A. 106:8344–8349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Magyar JS, et al. 2005. Reexamination of lead(II) coordination preferences in sulfur-rich sites: implications for a critical mechanism of lead poisoning. J. Am. Chem. Soc. 127:9495–9505 [DOI] [PubMed] [Google Scholar]
- 29. Matsushita K, Ohnishi T, Kaback HR. 1987. NADH-ubiquinone oxidoreductases of the Escherichia coli aerobic respiratory chain. Biochemistry 26:7732–7737 [DOI] [PubMed] [Google Scholar]
- 30. Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 31. Nies DH. 2003. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev. 27:313–339 [DOI] [PubMed] [Google Scholar]
- 32. Quintal SM, dePaula QA, Farrell NP. 2011. Zinc finger proteins as templates for metal ion exchange and ligand reactivity. Metallomics 3:121–139 [DOI] [PubMed] [Google Scholar]
- 33. Rai M, Yadav A, Gade A. 2008. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 27:76–83 [DOI] [PubMed] [Google Scholar]
- 34. Rajanna B, Hobson M, Harris L, Ware L, Chetty CS. 1990. Effects of cadmium and mercury on Na+-K+ ATPase and uptake of 3H-dopamine in rate brain synaptosomes. Arch. Int. Physiol. Biochim. 98:291–296 [DOI] [PubMed] [Google Scholar]
- 35. Ranquet C, Ollagnier-de-Choudens S, Loiseau L, Barras F, Fontecave M. 2007. Cobalt stress in Escherichia coli. The effect on the iron-sulfur proteins. J. Biol. Chem. 282:30442–30451 [DOI] [PubMed] [Google Scholar]
- 36. Rensing C, Fan B, Sharma R, Mitra B, Rosen BP. 2000. CopA: an Escherichia coli Cu(I)-translocating P-type ATPase. Proc. Natl. Acad. Sci. U. S. A. 97:652–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rensing C, Mitra B, Rosen BP. 1997. The zntA gene of Escherichia coli encodes a Zn(II)-translocating P-type ATPase. Proc. Natl. Acad. Sci. U. S. A. 94:14326–14331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Silver S. 2003. Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiol. Rev. 27:341–353 [DOI] [PubMed] [Google Scholar]
- 39. Summers AO. 2009. Damage control: regulating defenses against toxic metals and metalloids. Curr. Opin. Microbiol. 12:138–144 [DOI] [PubMed] [Google Scholar]
- 40. Valko M, Morris H, Cronin MTD. 2005. Metals, toxicity, and oxidative stress. Curr. Med. Chem. 12:1161–1208 [DOI] [PubMed] [Google Scholar]
- 41. Waldron KJ, Robinson NJ. 2009. How do bacterial cells ensure that metalloproteins get the correct metal? Nat. Rev. Microbiol. 6:25–35 [DOI] [PubMed] [Google Scholar]
- 42. Wysocki R, Tamas MJ. 2010. How Saccharomyces cerevisiae copes with toxic metals and metalloids. FEMS Microbiol. Rev. 34:925–951 [DOI] [PubMed] [Google Scholar]
- 43. Xu H, et al. 2012. Role of reactive oxygen species in the antibacterial mechanism of silver nanoparticles on Escherichia coli O157:H7. Biometals 25:45–53 [DOI] [PubMed] [Google Scholar]