Abstract
In this study, we focused on evaluating the occurrence of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli in fecal samples of healthy ducks and environmental samples from a duck farm in South China. Duck cloacal swabs and pond water samples were cultivated on MacConkey agar plates supplemented with ceftiofur. Individual colonies were examined for ESBL production. Bacteria identified as E. coli were screened for the presence of ESBL and plasmid-borne AmpC genes. The genetic relatedness, plasmid replicon type, and genetic background were determined. Of 245 samples analyzed, 123 had E. coli isolates with ceftiofur MICs higher than 8 μg/ml (116 [50.4%] from 230 duck samples and 7 [46.7%] from 15 water samples). blaCTX-M, blaSHV-12, blaCMY-2, and blaDHA-1 were identified in 108, 5, 9, and 1 isolates, respectively. The most common blaCTX-M genes were blaCTX-M-27 (n = 34), blaCTX-M-55 (n = 27), blaCTX-M-24e (n = 22), and blaCTX-M-105 (n = 20), followed by blaCTX-M-14a, blaCTX-M-14b, blaCTX-M-24a, and blaCTX-M-24b. Although most of the CTX-M producers had distinct pulsotypes, clonal transmission between duck and water isolates was observed. blaCTX-M genes were carried by transferable IncN, IncF, and untypeable plasmids. The novel CTX-M gene blaCTX-M-105 was flanked by two hypothetical protein sequences, partial ISEcp1 upstream and truncated IS903D, iroN, orf1, and a Tn1721-like element downstream. It is suggested that the horizontal transfer of blaCTX-M genes mediated by mobile elements and the clonal spread of CTX-M-producing E. coli isolates contributed to the dissemination of blaCTX-M in the duck farm. Our findings highlight the importance of ducks for the dissemination of transferable antibiotic resistance genes into the environment.
INTRODUCTION
Escherichia coli is a Gram-negative, rod-shaped bacterium commonly found in the lower intestinal tract of warm-blooded organisms. It can be easily disseminated in different ecosystems through the food chain and water supply, and it has been widely used as an indicator of fecal contamination (16, 25, 27). While most strains are harmless, many E. coli strains are harmful pathogens in humans and animals. Broad-spectrum cephalosporins are frequently used to treat serious infections caused by this bacterium. Resistance to broad-spectrum cephalosporins in Enterobacteriaceae has posed considerable and serious challenges for effective medical treatment (23). The main mechanism for Enterobacteriaceae resistance to cephalosporins is the production of extended-spectrum β-lactamases (ESBLs) or plasmid-encoded AmpC β-lactamases (pAmpC) (28). Food animals colonized with ESBL-producing E. coli have been considered potential sources of resistant E. coli causing infection in the community. These ESBL-producing E. coli isolates have been increasingly detected in food animals in different countries since 2002 and have gained considerable attention worldwide (28).
Researchers have revealed growing concerns about the release of antibiotic-resistant bacteria and their resistance genes from animal production facilities to natural environments, including groundwater and agricultural soils (12). This release of resistance genes into natural environments may pose significant challenges to human health and the evolution of environmental microbial populations (22). The presence of ESBL genes has been reported in wastewater, surface water, sewage, and sediment samples (8, 21). Despite extensive studies surveying ESBLs in pig, chicken, and cattle (15, 20), the diversity of ESBLs in duck and its living environments has seldom been investigated. According to the data from the Food and Agricultural Organization of the United Nations in 2005, 72% of the world's duck population was fed in mainland China (32). This percentage continues to increase annually. In the People's Republic of China, domestic ducks raised in the traditional free-range system frequently share water with wild waterfowl and are often in close contact with poultry, livestock, and humans from the same farm or village. To prevent and control the spread of infectious diseases, antimicrobial prophylaxis at the flock level was commonly used on duck farms, which could facilitate the selection of antimicrobial-resistant bacteria. Therefore, domestic ducks can act as potential vessels for resistant bacteria and may play an important role in the dissemination of resistant genes (32).
This study was conducted to investigate the prevalence of ESBLs among commensal E. coli isolates from duck and environmental samples from a duck farm and to characterize the phenotype and genotype of ESBL genes, the replicon types of blaCTX-M-carrying plasmids, and the surrounding genetic background of blaCTX-M genes.
MATERIALS AND METHODS
Sampling and isolation of bacteria.
In December 2006, a total of 230 cloacal swab samples were randomly collected from ducks of different growth phases (1 week old, FW; 3 weeks old, TW; 7 weeks old, HD; 10 weeks old, RD; 22 weeks old, DD) on a large duck breeding farm located in south China (Table 1). In addition, 15 water samples (10 ml/sample) were collected in sterile bottles from selected sites in the pools inhabited by ducks. All of the tested samples were obtained at the same point in time. Different sheds and ponds were assigned to the various growth phases of the ducks. The distance between two ponds averaged 55 m. Samples were plated on MacConkey agar plates containing ceftiofur (8 μg/ml). Presumptive E. coli colonies were identified by classical biochemical methods (20). One colony per plate was selected. The production system of this farm was all in and all out, and all of the samples were independent. The antimicrobial usage records for this duck farm indicated that aside from the ceftiofur, which was used for the prophylactic injection of day-old ducklings in duck production, other antibiotics, such as amikacin, enrofloxacin, and florfenicol, are used commonly and extensively to treat illness of the ducks during production.
Table 1.
Distribution of ceftiofur-resistant E. coli isolates from duck and water samples
| Sample source | No. of samples | No. (%) of samples containing ceftiofur-resistant E. coli isolates | No. of ESBL producers |
|---|---|---|---|
| 1-wk-old duck | 60 | 32 (53.3) | 31 |
| 3-wk-old duck | 20 | 12 (60.0) | 12 |
| 7-wk-old duck | 70 | 40 (57.1) | 40 |
| 10-wk-old duck | 60 | 27 (45.0) | 24 |
| 22-wk-old duck | 20 | 5 (25.0) | 5 |
| All duck cloacal samples | 230 | 116 (50.4) | 112 |
| Pool water | 15 | 7 (46.7) | 7 |
| Total | 245 | 123 (50.2) | 119 |
Antimicrobial susceptibility testing and ESBL confirmation.
The antimicrobial susceptibilities of the E. coli isolates were determined by using the agar dilution and disk diffusion methods, and the results were interpreted according to Clinical and Laboratory Standards Institute (CLSI) recommendations (4, 5). Eight β-lactam antibiotics, including ampicillin, ceftiofur, ceftriaxone, cefotaxime, ceftazidime, cefoxitin, meropenem, and amoxicillin-clavulanic acid, as well as six non-β-lactam antibiotics, including gentamicin, amikacin, ciprofloxacin, chloramphenicol, florfenicol, and tetracycline, were tested. ESBL producers were identified by the phenotypic confirmatory test using both cefotaxime and ceftazidime, alone and in combination with clavulanic acid (5).
Detection of β-lactamase genes.
The PCR amplification of blaCTX-M, blaTEM, blaSHV, blaCMY, and blaDHA genes was carried out by PCR as previously described (29). Purified PCR products were directly sequenced from both ends or cloned into pMD18-T and then sequenced. The DNA sequences and deduced amino acid sequences were compared to genes in GenBank or at the Lahey Clinic (www.lahey.org/studies/) to confirm the subtypes of β-lactamase genes.
Conjugation experiments and plasmid analysis.
Conjugation experiments were carried out with the blaCTX-M-positive strains by the liquid mating-out assay using streptomycin-resistant E. coli C600 as the recipient strain as previously described (29). Transconjugants were selected on MacConkey agar containing cefotaxime (4 μg/ml) and streptomycin (2000 μg/ml). Selected transconjugants were further characterized for their antimicrobial susceptibility, ESBL phenotype, and the presence of blaCTX-M genes. Incompatibility (Inc) groups were assigned by the PCR-based replicon typing (PBRT) of plasmids (2) from transconjugants. The replicon sequence typing (RST) of IncF plasmids and plasmid multilocus sequence typing (pMLST) of IncN plasmids were determined according to the procedure described previously (10, 31), and alleles or sequence types (STs) were assigned by submitting the amplicon sequence to the plasmid MLST web site (www.pubmlst.org/plasmid).
Epidemiological typing.
The phylogenetic group of all ceftiofur-resistant E. coli isolates was determined by multiplex PCR assays, using a combination of three DNA markers (chuA, yjaA, and TspE4.C2) as described by Clermont et al. (3). Sixty randomly selected blaCTX-M-carrying E. coli isolates were also characterized by PFGE using the CHEF Mapper system (Bio-Rad Laboratories, Hercules, CA) as described by Gautom (11). The results were interpreted according to the criteria of Tenover et al. (30).
Genetic background of the blaCTX-M gene.
To characterize the genetic environment of the blaCTX-M gene, plasmid DNA was digested with EcoRI, ligated into pUC18 (TaKaRa Biotechnology, Dalian, China), and introduced into E. coli DH5α. Transformants were selected on LB agar plates containing 4 μg/ml cefotaxime. Sequencing was carried out by primer walking on both DNA strands. The sequences and the deduced amino acid sequences were analyzed and compared by using the NCBI BLAST program.
Nucleotide sequence accession numbers.
The sequence of blaCTX-M-105 and the genetic context of blaCTX-M-105 on pHNDD81-1 have been deposited in GenBank under the accession numbers HQ833651 and JN232518, respectively.
RESULTS
E. coli isolation and antimicrobial susceptibility.
A total of 116 and 7 ceftiofur-resistant E. coli strains were isolated from 230 duck samples and 15 environment samples, respectively, from the same duck farm (Table 1). Using the double-disk synergy test, ESBL production was confirmed in 119 out of the 123 ceftiofur-resistant E. coli isolates.
Antimicrobial susceptibility tests showed that all of the 123 E. coli isolates were resistant to ampicillin, cefotaxime, and ceftriaxone, while 74.8, 20.0, and 16.0% of isolates showed resistance to ceftazidime, cefoxitin, and amoxicillin-clavulanic acid, respectively. In addition, some of these isolates showed resistance to other classes of antibiotics, including tetracycline (96.2%), gentamicin (90.2%), ciprofloxacin (84.8%), chloramphenicol (80.2%), florfenicol (59.2%) (MIC > 16 μg/ml), and amikacin (52.0%). However, all of the isolates were susceptible to meropenem.
Identification of β-lactamase genes.
blaTEM, blaSHV, blaCTX-M, blaCMY, and blaDHA genes were found to be present in 68 (56.7%), 5 (4.1%), 108 (87.8%), 9 (7.5%), and 1 (0.8%) of the 123 ceftiofur-resistant E. coli isolates, respectively. blaCTX-M was shown to be dominant in these isolates. Sequence analysis revealed that the most common blaCTX-M type was blaCTX-M-27 (n = 34), followed by blaCTX-M-55 (n = 27), blaCTX-M-24e (n = 22), blaCTX-M-105 (n = 20), blaCTX-M-14a (n = 12), blaCTX-M-14b (n = 3), blaCTX-M-24a (n = 3), and blaCTX-M-24b (n = 1) (Table 2). blaCTX-M-105 was a novel variant of the CTX-M-9 group gene, specifying CTX-M-105 with two amino acids substitutions (A80V and A208E) compared to CTX-M-27. This new blaCTX-M-27-like gene, blaCTX-M-105, has been deposited in GenBank under the accession number HQ833651. Multiple types of blaCTX-M genes (blaCTX-M-55 in combination with the CTX-M-9 group gene) were identified in 14 isolates. Seven E. coli isolates carried both blaCTX-M and blaCMY-2 genes. All blaTEM genes were found to be blaTEM-1 by sequencing. In addition, blaSHV-12 was identified in 5 isolates, of which 4 were CTX-M producers. Genotypes of isolates of different origins are shown in Table 2.
Table 2.
Distribution of β-lactamase genes among ceftiofur-resistant E. coli isolatesa
| Sample source (n) | No. of isolates carrying: |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any blaCTX-M | blaCTX-M-27 | blaCTX-M-55 | blaCTX-M-24e | blaCTX-M-105 | blaCTX-M-14a | blaCTX-M-14b | blaCTX-M-24a | blaCTX-M-24b | blaSHV-12 | blaCMY-2 | blaDHA-1 | blaTEM-1 | |
| FW (32) | 26 | 8 | 7 | 10 | 2 | 3 | 0 | 0 | 1 | 2 | 1 | 21 | |
| TW (12) | 12 | 3 | 2 | 2 | 4 | 1 | 0 | 1 | 0 | 1 | 7 | ||
| HD (40) | 24 | 7 | 9 | 5 | 4 | 3 | 0 | 0 | 0 | 2 | 16 | ||
| RD (70) | 36 | 13 | 5 | 1 | 8 | 5 | 3 | 1 | 0 | 3 | 5 | 1 | 22 |
| DD (27) | 3 | 0 | 3 | 2 | 1 | 0 | 0 | 0 | 0 | 1 | |||
| EV (7) | 7 | 3 | 1 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | |||
| Total (123) | 108 | 34 | 27 | 22 | 20 | 12 | 3 | 3 | 1 | 5 | 9 | 1 | 67 |
FW, 1-week-old duck; TW, 3-week-old duck; HD, 7-week-old duck; RD, 10-week-old duck; DD, 22-week-old duck; EV, pool water.
Characterization of blaCTX-M plasmids.
Forty CTX-M-producing isolates were randomly selected for conjugation experiments to determine the transferability of cefotaxime resistance. Plasmids carrying blaCTX-M from 33 isolates were successfully transferred to recipients. All of the transconjugants showed resistance to cefotaxime but were susceptible to cefoxitin, amoxicillin-clavulanic acid, and the other 6 non-β-lactam antibiotics, except 16 transconjugants exhibited resistance to ceftazidime and two transconjugants exhibited resistance to amikacin and gentamicin (Table 3).
Table 3.
Characteristics of blaCTX-M-positive E. coli isolates and transconjugants
| Strain | Sample source | Phylogenetic group | PFGE typec | β-Lactamase gene type(s)b | MIC (μg/ml) of transconjugantse |
Non-β-lactam resistance phenotypea | Plasmid replicon type (pMLST or IncF allele)d | |
|---|---|---|---|---|---|---|---|---|
| CTX | CAZ | |||||||
| FW15 | 1-wk-old duck | A | 1 | blaCTX-M-105 | >128 | 32 | GEN, CIP, TET | UT |
| TW102 | 3-wk-old duck | B1 | 14 | blaCTX-M-105, blaTEM-1 | 64 | 16 | AMI, GEN, CIP, CHL, FFC, TET | IncI1, IncN (ST8) |
| DD81 | 22-wk-old duck | A | 15 | blaCTX-M-105, blaCTX-M-55, blaTEM-1 | 128 | 32 | CIP, CHL, FFC, TET | UT |
| HD99 | 7-wk-old duck | A | 3 | blaCTX-M-105 | 128 | 32 | AMI, GEN, CIP, CHL, FFC, TET | UT |
| RD125 | 10-wk-old duck | A | 4 | blaCTX-M-105 | 128 | 4 | GEN, CIP, CHL, FFC, TET | IncN |
| RD191 | 10-wk-old duck | A | 4 | blaCTX-M-105 | 128 | 8 | AMI, GEN, CIP, CHL, FFC, TET | UT |
| FW16 | 1-wk-old duck | A | 2 | blaCTX-M-105, blaCTX-M-55, blaTEM-1 | 128 | 8 | GEN, CIP, CHL, FFC, TET | IncN (ST8) |
| EV221 | Pool water | B1 | 5 | blaCTX-M-105, blaCTX-M-55, blaTEM-1 | 128 | 32 | GEN, CIP, CHL, FFC, TET | UT |
| HD96 | 7-wk-old duck | A | ND | blaCTX-M-24e, blaCTX-M-55, blaTEM-1 | 128 | 8 | AMI, GEN, CIP, CHL, TET | UT |
| HD101 | 7-wk-old duck | D | 16 | blaCTX-M-55 | 32 | 8 | UT | |
| HD111 | 7-wk-old duck | B1 | 17 | blaCTX-M-55 | 128 | 8 | CIP, CHL, FFC, TET | UT |
| FW122 | 1-wk-old duck | A | 18 | blaCTX-M-55, blaTEM-1 | >128 | 64 | AMI, GEN, CIP, CHL, FFC, TET | UT |
| FW51 | 1-wk-old duck | D | 19 | blaCTX-M-14, blaTEM-1 | 64 | 8 | AMI, CIP, CHL, FFC, TET | UT |
| HD109 | 7-wk-old duck | D | 6 | blaCTX-M-14 | >128 | 64 | GEN, CIP, CHL, FFC, TET | UT |
| RD187 | 10-wk-old duck | A | 7 | blaCTX-M-14 | 32 | 4 | GEN, CIP, CHL, FFC, TET | IncN |
| FW14 | 1-wk-old duck | D | 8 | blaCTX-M-24b | 32 | 4 | AMI, GEN, CIP, CHL, TET | UT |
| FW11 | 1-wk-old duck | D | 20 | blaCTX-M-24e | >128 | 16 | AMI, GEN, CIP, CHL, TET | IncFII (F43) |
| FW20 | 1-wk-old duck | D | 21 | blaCTX-M-24e, blaTEM-1 | 64 | 4 | AMI, GEN, CIP, CHL, FFC, TET | UT |
| RD189 | 10-wk-old duck | B1 | 22 | blaCTX-M-24e, blaTEM-1 | 128 | 2 | AMI, GEN, CIP, CHL, FFC, TET | IncI1 |
| FW23 | 1-wk-old duck | B1 | 27 | blaCTX-M-27, blaTEM-1 | 128 | 4 | TET | IncN |
| FW41 | 1-wk-old duck | A | 10 | blaCTX-M-27, blaTEM-1 | 128 | 64 | AMI, GEN, CIP, CHL, TET | UT |
| HD104 | 7-wk-old duck | A | ND | blaCTX-M-27, blaCTX-M-55, blaTEM-1 | 128 | 64 | AMI, GEN, CIP, CHL, TET | UT |
| HD141 | 7-wk-old duck | A | 21 | blaCTX-M-27 | 32 | 4 | CIP, CHL, FFC, TET | UT |
| HD77 | 7-wk-old duck | A | 10 | blaCTX-M-27, blaTEM-1 | >128 | 64 | AMI, GEN, CIP, CHL, TET | UT |
| RD175 | 10-wk-old duck | B1 | 24 | blaCTX-M-27, blaTEM-1 | >128 | 64 | GEN, CIP, CHL, TET | UT |
| RD95 | 10-wk-old duck | A | 11 | blaCTX-M-27, blaTEM-1 | >128 | 32 | GEN, CIP, CHL, FFC, TET | IncN (ST8) |
| RD215 | 10-wk-old duck | A | 11 | blaCTX-M-27 | 64 | 4 | GEN, CIP, CHL, FFC, TET | IncN |
| EV224 | Pool water | A | 12 | blaCTX-M-27 | >128 | 64 | GEN, CIP, CHL, TET | IncN (ST8) |
| EV222 | Pool water | A | 1 | blaCTX-M-27 | >128 | 64 | GEN, CIP, TET | IncFII (F43) |
| EV220 | Pool water | A | 1 | blaCTX-M-27 | 128 | 4 | GEN, CIP, CHL, FFC, TET | IncN |
| TW144 | 3-wk-old duck | A | 1 | blaCTX-M-27, blaTEM-1 | 32 | 4 | AMI, GEN, CIP, CHL, FFC, TET | IncN |
| HD149 | 7-wk-old duck | A | 26 | blaCTX-M-27, blaTEM-1 | 128 | 16 | AMI, GEN, CHL, TET | IncI1 |
| HD163 | 7-wk-old duck | B1 | 25 | blaCTX-M-55 | 128 | 8 | CIP, CHL, TET | IncFII (F34) |
AMI, amikacin; GEN, gentamicin; CIP, ciprofloxacin; CHL, chloramphenicol; FFC, florfenicol; TET, tetracycline. Patterns transferred by conjugation are underlined. The criterion for florfenicol is >16 μg/ml.
Genes that were transferred by conjugation as determined by PCR are underlined.
PFGE types were assigned 1, 2, 3, etc., by the visual inspection of the macrorestriction profile. ND, not determined.
UT, untypeable; ST, sequence type.
CTX, cefotaxime; CAZ, ceftazidime.
The plasmid replicon typing of transconjugants revealed significant plasmid diversity. IncN, IncFII, and IncI1 replicons were detected in 10, 3, and 3 transconjugants, respectively (Table 3). Two plasmid replicons (IncN in combination with IncFII or IncI1) were present simultaneously in 2 transconjugants. Within FII replicons, F34 and F43 alleles were identified in 1 and 3 transconjugants, respectively. pMLST of the 10 IncN plasmids revealed that 4 of them were assigned to ST8. For the other 6 IncN plasmids, the repA allele was repA4, but these plasmids were negative for PCR amplifying traJ and korA.
Epidemiological typing.
Phylogenetic group analysis showed that group A (50.4%; n = 63) was dominant among the E. coli isolates producing blaCTX-M enzymes, followed by groups D (25.6%; 32) and B1 (21.6%; 27). Only one isolate belonged to the phylogenetic group B2.
Chromosomal DNA of 54 isolates was available for PFGE typing, and the 54 isolates displayed 43 different PFGE profiles. No PFGE fragment pattern was obtained from the other 6 isolates. A majority of isolates showed unique, unrelated PFGE profiles and were unlikely to be considered the cause of an epidemic. However, 11 E. coli isolates carrying different blaCTX-M type genes (7 blaCTX-M-27, 3 blaCTX-M-105, and 1 blaCTX-M-24) belonged to the same PFGE pattern (Table 3). Of the 11 isolates, 8 isolates were obtained from ducks and 3 were obtained from environmental samples, which suggested that the water of the duck swimming pool was an important vehicle for the clonal dissemination of resistant E. coli.
Genetic background of the blaCTX-M-105 gene.
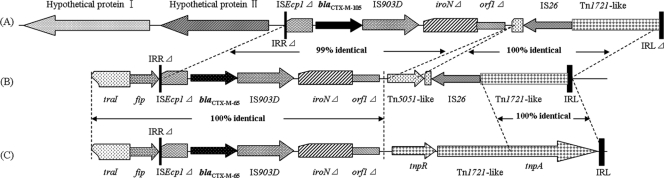
DNA of three plasmids carrying blaCTX-M-105 was digested with EcoRI and cloned into pUC18 by selection for cefotaxime resistance. Only an 11.5-kb EcoRI-digested fragment from plasmid pHNDD81 carrying blaCTX-M-105 was successfully cloned and then sequenced. In general, the genetic context of blaCTX-M-105 on pHNDD81 was highly similar to that of blaCTX-M-65 on pKC396 and pWCE307, with only minor variations (Fig. 1). As in the case of pKC396 and pWCE307, blaCTX-M-105 was associated with partial ISEcp1, including its right-hand inverted repeat (IRR). Downstream of blaCTX-M-105, IS903D and a truncated gene, iroN (a virulence gene for E. coli, encoding an outer membrane iron receptor protein), were identified. This ISEcp1-blaCTX-M-105-IS903D-iroNΔ structure was part of a Tn1721-like element and was located adjacent to a fragment of orfI, which potentially encodes a methyl-accepting chemotaxis protein.
Fig 1.
Genetic context of blaCTX-M-105 and similar structures. (A) Genetic context of blaCTX-M-105 on pHNDD81-1 (GenBank accession no. JN232518). (B) Genetic context of blaCTX-M-65 on pKC396 (GenBank accession no. HM138653). (C) Genetic context of blaCTX-M-65 on pWCE307 (GenBank accession no. HM440049).
DISCUSSION
More than 50% of the duck fecal samples obtained in 2006 from a duck farm revealed ESBL-producing E. coli isolates. This percentage is similar to that recently found in fecal samples of healthy humans in China (19) but is higher than the percentages (10 to ∼40%) of fecal carriage of ESBL-producing E. coli isolates detected in other countries (6, 13). Ceftiofur is approved for therapeutic parenteral use in swine, cattle, and poultry in China. In ducks, day-old poultry may be injected subcutaneously with ceftiofur to control colibacillosis, which may provide antibiotic selective pressure for the colonization of resistant bacteria in the gastrointestinal tract.
Similarly to findings of other studies from China (14, 19, 20, 29), blaCTX-M alleles were the predominant genes encoding the ESBL phenotype among these isolates. Eight CTX-M gene types were detected, indicating a high diversity of blaCTX-M genes in E. coli isolates from this duck farm. The main subtypes of blaCTX-M ESBLs were similar to those of our previous reports (20, 29). However, unlike other studies in China where blaCTX-M-14 was the most common blaCTX-M variant (14, 19, 20, 29), blaCTX-M-27 was found to be the most frequent type in this study. CTX-M-27 differs from CTX-M-14 by a single substitution of D240G that confers higher levels of resistance to ceftazidime than CTX-M-14 (1). It was only sporadically detected in some studies (14, 19). Previous reports showed that blaCTX-M-27 was usually associated with duck isolates in China (GenBank accession no. GQ896550) (20), although it has also been detected in isolates of other origins, including humans and the environment (19, 21). Interestingly, a recent study of the diversity of ESBL-producing organisms in a sediment sample taken from a river located in Guangdong province showed that blaCTX-M-27 was the most dominant of the ESBL genes (21). The basis for the successful dissemination of blaCTX-M-27, which has increased resistance to ceftazidime on this duck farm, is unknown, but the high prevalence of blaCTX-M-27 in commensal E. coli isolates from duck feces as well as duck pond water may pose a great threat to human medicine.
Bacterial contamination of surface water, particularly contaminated with feces-borne bacteria, has long been a water quality issue owing to the potential for disease transmission (9). As a waterfowl, ducks usually deposit fecal material into the pond water and sediment, which facilitates the release of ESBL-producing organisms into the environment. In this study, the prevalence of ceftiofur-resistant E. coli isolates and the distribution of ESBL gene types in the pond water samples were similar to those of duck fecal samples. In addition, the clonal spread of CTX-M producers and the horizontal transfer of similar plasmids (IncN ST8) carrying blaCTX-M genes between duck isolates and pond water isolates were identified, which indicated that the feces-contaminated water was an important vehicle for the dissemination of the resistance genes.
The new variant of the blaCTX-M-9 group gene, blaCTX-M-105, was identified in 20 E. coli isolates of different origins (including pool water), which indicated that this gene has been circulating among E. coli isolates in the duck farm for a long time. Because most of the blaCTX-M-105 producers were clonally unrelated and the blaCTX-M-105 genes were located on different plasmids, we analyzed the genetic background surrounding it. blaCTX-M-105 was located in a Tn1721-like element on pHNDD81-1 and is associated with an ISEcp1-blaCTX-M-IS903D-iroNΔ structure which has been seen in several cases associated with blaCTX-M-65 (7, 33), blaCTX-M-24 (26), blaCTX-M-19 (24), and blaCTX-M-14 (17). ISEcp1 has been proved to be able to recognize part of the iroN gene sequence as its alternative IRR and then mobilize blaCTX-M, IS903, and the partial iroN gene (iroNΔ) into Tn1721. Since this ISEcp1-mediated mobilization into the exact same position of Tn1722 might have occurred only once, Zong et al. (33) speculated that blaCTX-M-14 diverged to generate variants such as blaCTX-M-19, blaCTX-M-24, and blaCTX-M-65 in this element. However, blaCTX-M-105 differs from blaCTX-M-14 by three nucleotide differences. In addition, there were two hypothetical protein sequences located upstream of the truncated ISEcp1. In GenBank, there was no sequence similar to the two hypothetical protein sequences, except that a 507-bp sequence specific to avian pathogenic E. coli has 90% similarity to the first hypothetical protein nucleotide sequence (GenBank accession no. DQ643394) (18).
In conclusion, both the clonal transfer of ESBL producers and the horizontal transfer of IncN, IncF, or untypeable plasmids are contributing to the rapid dissemination of the blaCTX-M gene in the duck farm. The commensal E. coli isolates from ducks are an important reservoir of ESBLs genes. Just as critical is the pond water of the duck farm, which serves as an important vehicle for the spread of the resistant bacteria. These resistant bacteria may be transmitted to human beings through the food chain and strengthen human clinical resistance. Therefore, the hygiene of the animal ecosystem and prudent use of cephalosporin is necessary for the control of the persistence and the spread of resistant bacteria.
ACKNOWLEDGMENT
This work was supported by a grant from the National Natural Science Foundation of China (no. U1031004).
Footnotes
Published ahead of print 9 March 2012
REFERENCES
- 1. Bonnet R, et al. 2003. Effect of D240G substitution in a novel ESBL CTX-M-27. J. Antimicrob. Chemother. 52:29–35 [DOI] [PubMed] [Google Scholar]
- 2. Carattoli A, et al. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219–228 [DOI] [PubMed] [Google Scholar]
- 3. Clermont O, Bonacorsi S, Bingen E. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555–4558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clinical and Laboratory Standards Institute 2008. Performance standards for antimicrobial disk susceptibility tests for bacteria isolated from animals. Approved standard, 3rd ed Document M31-A3 CLSI, Wayne, PA [Google Scholar]
- 5. Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing, 20th informational supplement. M100-S20 CLSI, Wayne, PA [Google Scholar]
- 6. Costa D, et al. 2009. Prevalence of extended-spectrum β-lactamase-producing Escherichia coli isolates in faecal samples of broilers. Vet. Microbiol. 138:339–344 [DOI] [PubMed] [Google Scholar]
- 7. Cullik A, Pfeifer Y, Prager R, von Baum H, Witte W. 2010. A novel IS26 structure surrounds blaCTX-M genes in different plasmids from German clinical Escherichia coli isolates. J. Med. Microbiol. 59:580–587 [DOI] [PubMed] [Google Scholar]
- 8. Dhanji H, et al. 2011. Isolation of fluoroquinolone-resistant O25b:H4-ST131 Escherichia coli with CTX-M-14 extended-spectrum β-lactamase from UK river water. J. Antimicrob. Chemother. 66:512–516 [DOI] [PubMed] [Google Scholar]
- 9. Dolejská M, Bierosová B, Kohoutová L, Literák I, Cízek A. 2009. Antibiotic-resistant Salmonella and Escherichia coli isolates with integrons and extended-spectrum β-lactamases in surface water and sympatric black-headed gulls. J. Appl. Microbiol. 106:1941–1950 [DOI] [PubMed] [Google Scholar]
- 10. García-Fernández A, et al. 2011. Multilocus sequence typing of IncN plasmids. J. Antimicrob. Chemother. 66:1987–1991 [DOI] [PubMed] [Google Scholar]
- 11. Gautom RK. 1997. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in 1 day. J. Clin. Microbiol. 35:2977–2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ghosh S, LaPara TM. 2007. The effects of subtherapeutic antibiotic use in farm animals on the proliferation and persistence of antibiotic resistance among soil bacteria. ISME J. 1:191–203 [DOI] [PubMed] [Google Scholar]
- 13. Gonçalves A, et al. 2010. Genetic characterization of extended-spectrum β-lactamases in Escherichia coli isolates of pigs from a Portuguese intensive swine farm. Foodborne Pathog. Dis. 7:1569–1573 [DOI] [PubMed] [Google Scholar]
- 14. Ho PL, et al. 2011. Extensive dissemination of CTX-M-producing Escherichia coli with multidrug resistance to ‘critically important’ antibiotics among food animals in Hong Kong, 2008–10. J. Antimicrob. Chemother. 66:765–768 [DOI] [PubMed] [Google Scholar]
- 15. Horton RA, et al. 2011. Fecal carriage and shedding density of CTX-M extended-spectrum β-lactamase-producing Escherichia coli in cattle, chickens, and pigs: implications for environmental contamination and food production. Appl. Environ. Microbiol. 77:3715–3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ishii S, Sadowsky MJ. 2008. Escherichia coli in the environment: implications for water quality and human health. Microbes Environ. 23:101–108 [DOI] [PubMed] [Google Scholar]
- 17. Izumiya H, et al. 2005. Identification of CTX-M-14 β-lactamase in a Salmonella enterica serovar enteritidis isolate from Japan. Antimicrob. Agents Chemother. 49:2568–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kariyawasam S, Johnson TJ, Nolan LK. 2006. Unique DNA sequences of avian pathogenic Escherichia coli isolates as determined by genomic suppression subtractive hybridization. FEMS Microbiol. Lett. 262:193–200 [DOI] [PubMed] [Google Scholar]
- 19. Li B, et al. 2011. High prevalence of CTX-M β-lactamases in faecal Escherichia coli strains from healthy humans in Fuzhou, China. Scand. J. Infect. Dis. 43:170–174 [DOI] [PubMed] [Google Scholar]
- 20. Liu JH, et al. 2007. Detection and characterisation of CTX-M and CMY-2 β-lactamases among Escherichia coli isolates from farm animals in Guangdong Province of China. Int. J. Antimicrob. Agents 29:576–581 [DOI] [PubMed] [Google Scholar]
- 21. Lu SY, et al. 2010. High diversity of extended-spectrum β-lactamase-producing bacteria in an urban river sediment habitat. Appl. Environ. Microbiol. 76:5972–5976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martinez JL. 2009. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ. Pollut. 157:2893–2902 [DOI] [PubMed] [Google Scholar]
- 23. Pitout JD, Laupland KB. 2008. Extended-spectrum β-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect. Dis. 8:159–166 [DOI] [PubMed] [Google Scholar]
- 24. Poirel L, Decousser JW, Nordmann P. 2003. Insertion sequence ISEcp1B is involved in expression and mobilization of a blaCTX-M β-lactamase gene. Antimicrob. Agents Chemother. 47:2938–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scott TM, Rose JB, Jenkins TM, Farrah SR, Lukasik J. 2002. Microbial source tracking: current methodology and future directions. Appl. Environ. Microbiol. 68:5796–5803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shen P, et al. 2008. Complete nucleotide sequence of pKP96, a 67,850 bp multiresistance plasmid encoding qnrA1, aac(6′)-Ib-cr and blaCTX-M-24 from Klebsiella pneumoniae. J. Antimicrob. Chemother. 62:1252–1256 [DOI] [PubMed] [Google Scholar]
- 27. Skurnik D, et al. 2006. Effect of human vicinity on antimicrobial resistance and integrons in animal faecal Escherichia coli. J. Antimicrob. Chemother. 57:1215–1219 [DOI] [PubMed] [Google Scholar]
- 28. Smet A, et al. 2010. Broad-spectrum beta-lactamases among Enterobacteriaceae of animal origin: molecular aspects, mobility and impact on public health. FEMS Microbiol. Rev. 34:295–316 [DOI] [PubMed] [Google Scholar]
- 29. Sun Y, et al. 2010. High prevalence of blaCTX-M extended-spectrum β-lactamase genes in Escherichia coli isolates from pets and emergence of CTX-M-64 in China. Clin. Microbiol. Infect. 16:1475–1481 [DOI] [PubMed] [Google Scholar]
- 30. Tenover FC, et al. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Villa L, GarcíA-Fernández A, Fortini D, Carattoli A. 2010. Replicon sequence typing of IncF plasmids carrying virulence and resistance determinants. J. Antimicrob. Chemother. 65:2518–2529 [DOI] [PubMed] [Google Scholar]
- 32. Zhong CY, et al. 2009. Antibiotic susceptibility of Riemerella anatipestifer field isolates. Avian Dis. 53:601–607 [DOI] [PubMed] [Google Scholar]
- 33. Zong Z, Yu R, Wang X, Lü X. 2011. blaCTX-M-65 is carried by a Tn1722-like element on an IncN conjugative plasmid of ST131 Escherichia coli. J. Med. Microbiol. 60:435–441 [DOI] [PubMed] [Google Scholar]