Abstract
Here, we suggest that natural streptomycin resistance of many sphingomonads resides within rpsL. We constructed a dominant, streptomycin-sensitive rpsL allele and demonstrated its use as a counterselection marker in several sphingomonads. An rpsL-based markerless gene deletion system was developed and validated by deleting four genes in Sphingomonas sp. strain Fr1.
TEXT
Bacteria of the genus Sphingomonas and the closely related genera Novosphingobium, Sphingopyxis, and Sphingobium (commonly referred to as sphingomonads) (1, 29) are well known for their potential in bioremediation and use in industrial applications (2–4, 16, 18, 22) and have recently also been described as potential agents for biocontrol (7). Genetic studies of sphingomonads have suffered from a limited number of genetic tools, e.g., the lack of a markerless gene deletion and allelic exchange system. Such a system is advantageous over existing methods for insertional gene inactivation or gene replacement in that it avoids polar effects on the expression of downstream genes and allows recycling of the antibiotic resistance marker. Markerless gene deletion systems follow a two-step homologous recombination strategy that involves successive selection and counterselection (28). Whereas selection markers are usually antibiotic resistance cassettes, most counterselectable markers render the host sensitive to a specific substance, such as sucrose (sacB) or p-chlorophenylalanine (pheS) (9, 10, 23). However, several wild-type sphingomonads are impaired in growth in the presence of p-chlorophenylalanine (see Table S1 in the supplemental material), making pheS an unsuitable counterselection marker. Moreover, although sacB has been reported as a counterselection marker in Sphingomonas sp. strain SYK-6 (15), we failed to reproducibly obtain mutants using existing sacB-based markerless gene deletion systems (13, 25) in Sphingomonas sp. strain Fr1 (data not shown), probably because of the high frequency of spontaneous sucrose resistance (see Results in the supplemental material), a common drawback of sacB-based counterselection systems (20, 25, 32). Thus, a generally applicable counterselectable marker is needed for sphingomonads.
Another commonly used counterselection marker is based on rpsL, which encodes the ribosomal protein S12, a target of streptomycin (19). In the classical approach based on rpsL, naturally streptomycin-sensitive bacteria are first selected for spontaneous streptomycin-resistant mutants with mutations usually found within rpsL and the dominant, streptomycin-sensitive wild-type rpsL allele (12) is subsequently used in the streptomycin-resistant background as a counterselectable marker (23, 24). One disadvantage of this method is that it requires prior manipulation of the wild type to make it streptomycin resistant. Most sphingomonads are naturally streptomycin resistant (31), and we therefore questioned (i) whether this property resided within the rpsL gene and, if so, (ii) whether it could be exploited to construct a dominant, streptomycin-sensitive allele for use as a counterselectable marker in wild-type sphingomonads. A comparison of rpsL alleles of several sphingomonads revealed that all encoded arginine at position 88 (Fig. 1), which is precisely the amino acid known to render naturally streptomycin-sensitive rpsL alleles resistant when replacing the original Lys-88 residue in diverse species (6, 17, 30). To test whether this residue was responsible for resistance, Arg-88, encoded by Sphingomonas sp. Fr1 rpsL, was replaced by lysine, and the mutant allele, rpsLR88K, was cloned in a multicopy plasmid, pCM62 (14), under the control of its native promoter. Whereas expression of wild-type rpsL from pCM62 did not affect growth on Luria broth (LB; Lennox) supplemented with 200 μg streptomycin/ml, expression of rpsLR88K from pCM62 abolished growth of Sphingomonas sp. Fr1 at a streptomycin concentration of 20 μg/ml or higher but did not impair growth on LB without streptomycin. Given that streptomycin-sensitive rpsL alleles are dominant over streptomycin-resistant rpsL alleles (12), our results suggest that Arg-88 in wild-type rpsL confers natural streptomycin resistance to Sphingomonas sp. Fr1 and show that the rpsLR88K allele is a suitable counterselection marker for the Sphingomonas sp. Fr1 wild-type strain.
Fig 1.
Alignment of wild-type ribosomal protein S12 sequences of selected sphingomonads and E. coli. DSM 12444, Novosphingobium aromaticivorans F199; DSM 6014, Sphingomonas wittichii RW1; DSM 13593, Sphingopyxis alaskensis RB2256; NBRC 101211, Sphingobium japonicum UT26; DSM 24958, Sphingomonas sp. Fr1; E. coli, Escherichia coli K-12. Amino acids that differ among the selected sphingomonads are highlighted in gray. Arg-88 is highlighted in black. Amino acid substitutions known to render naturally streptomycin-sensitive S12 proteins resistant are shown above the E. coli S12 sequence and are highlighted by a black dot on top.
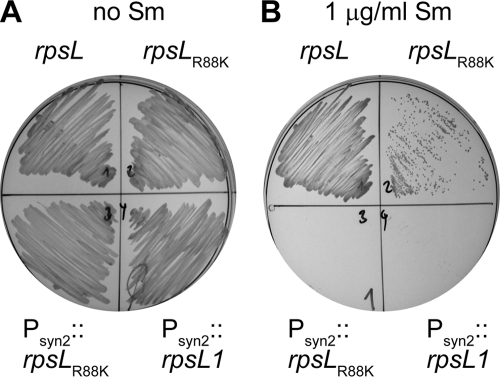
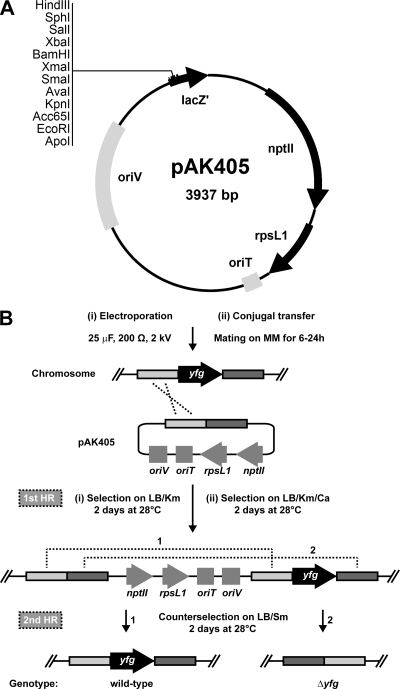
In order to use the rpsLR88K allele in a markerless gene deletion system, homologous recombination between plasmid-borne and chromosomally encoded rpsL alleles should be avoided. To this end, an rpsLR88K allele in which several codons were replaced by synonymous ones, so that no more than 41 successive nucleotides were identical to the wild-type sequence while maintaining the original GC bias, was synthesized (GeneArt; Invitrogen) (see Fig. S1 in the supplemental material). This allele, rpsL1, together with an optimized ribosome-binding site, was then cloned under the control of a strong synthetic promoter in pAK126a, a pCM62 derivative, to yield plasmid pAK126a-rpsL1. As a control, rpsLR88K was cloned in the same plasmid, resulting in pAK126a-rpsLR88K. Sphingomonas sp. Fr1 strains carrying pAK126a-rpsL1 or pAK126a-rpsLR88K were sensitive to as little as 1 μg streptomycin/ml, indicating expression and functionality of rpsL1 (Fig. 2). To construct a plasmid suitable for generating gene deletions, a fragment containing the synthetic promoter and rpsL1 was subcloned into a derivative of pK18mobsacB (25) lacking the sacB gene, yielding pAK405 (Fig. 3A). Cloning details are given in the supplemental material.
Fig 2.
Growth of Sphingomonas sp. Fr1 expressing different rpsL alleles on nutrient broth without (A) or with (B) 1 μg streptomycin/ml. rpsL, wild-type rpsL expressed from pCM62 under the control of its native promoter; rpsLR88K, rpsLR88K allele expressed from pCM62 under the control of its native promoter; Psyn2::rpsLR88K, rpsLR88K with an improved ribosome-binding site expressed from pAK126a under the control of the strong synthetic promoter Psyn2; Psyn2::rpsL1, rpsL1 allele with an improved ribosome-binding site expressed from pAK126a under the control of the strong synthetic promoter Psyn2.
Fig 3.
pAK405 plasmid map and schematic of the gene deletion strategy. (A) Plasmid pAK405 has the following features: a pBR322 oriV (suicide plasmid for Sphingomonas), the RP4 oriT (for conjugal transfer between E. coli and Sphingomonas), the pUC18 multiple cloning site in lacZ′ (allowing blue/white screening), nptII (for selection on kanamycin), and rpsL1 (for streptomycin counterselection). Unique restriction sites in the multiple cloning site are shown. (B) Outline of the gene deletion strategy with delivery of pAK405 through electroporation (i) or conjugal transfer (ii). The gene of interest (yfg, “your favorite gene”) to be deleted is shown as a black arrow, and up- and downstream regions are depicted in light and dark gray boxes, respectively. Homologous recombination (HR) events are represented by dashed lines. For simplicity, only one of two possibilities for the first HR event is shown (HR via the upstream region). For the second HR event, the two possibilities leading to the wild-type (1) and mutant (2) genotypes are depicted. Antibiotics are indicated as follows: Km, 50 μg kanamycin/ml; Ca, 50 μg carbenicillin/ml; Sm, 100 μg streptomycin/ml.
To test the pAK405-based gene deletion system, four genes—ecfG, phyR, crtI, and crtY—with easily traceable mutant phenotypes (see below) were deleted in Sphingomonas sp. Fr1 following the general methodology outlined in Fig. 3B (see the supplemental material for the detailed methods). Briefly, upstream and downstream regions of approximately 750 bp that flanked each gene were PCR amplified, joined by PCR overlap extension, and cloned into pAK405. pAK405 derivatives were subsequently transformed into Sphingomonas sp. Fr1 by electroporation or delivered via conjugal transfer from Escherichia coli S17-1(λpir) (27). For conjugal transfer, mating was performed on minimal medium (MM) without a carbon source (21) and bacteria were subsequently plated on LB supplemented with 50 μg kanamycin/ml (for selection of Sphingomonas sp. Fr1 merodiploids) and 50 μg carbenicillin/ml (for E. coli counterselection). Individual colonies were restreaked once on the same medium and then plated on LB supplemented with 100 μg streptomycin/ml to select for the second homologous recombination event. Resulting colonies were restreaked on both LB supplemented with 100 μg streptomycin/ml and LB containing 50 μg kanamycin/ml, and kanamycin-sensitive clones were analyzed by colony PCR using primers flanking the respective loci. Of 200 colonies tested, 8.5% (17/200) of streptomycin-resistant clones were kanamycin resistant, indicating that spontaneous streptomycin resistance is a minor concern in the counterselection step. Overall, 62.7% (94/150), 64.3% (30/84), 30.6% (22/72), and 42.7% (50/117) of kanamycin-sensitive, streptomycin-resistant clones carried the ecfG, phyR, crtI, and crtY deletions, respectively, while the remaining had reverted to the wild type. These values are close to the theoretical 1:1 ratio of the wild type to the mutant genotype and thus prove the efficiency of the system.
All mutants displayed the expected phenotypes. phyR and ecfG encode the response regulator PhyR and the alternative sigma factor σEcfG, respectively, two master regulators of the general stress response in Alphaproteobacteria (5, 8), and the mutants showed the osmotic stress-sensitive phenotypes (see Fig. S2A in the supplemental material) described previously (8). crtY encodes a putative lycopene cyclase and crtI encodes a putative phytoene desaturase/dehydrogenase that are predicted to be involved in carotenoid biosynthesis (26). The crtI mutant was white, which is in line with the predicted role of phytoene desaturase/dehydrogenase in converting colorless phytoene into lycopene (see Fig. S2B in the supplemental material). The crtY mutant was reddish, which is also consistent with a defect in carotenoid biosynthesis and the accumulation of the red intermediate lycopene due to the lack of lycopene cyclase (see Fig. S2B in the supplemental material).
The above-described results show that rpsL1 is an efficient tool for counterselection and targeted gene deletion in Sphingomonas sp. Fr1. To evaluate whether rpsL1 could also be used for other sphingomonads, rpsL1 was expressed from pAK126a in several phylogenetically diverse representatives of this class and the resulting strains were assessed for their capacity to grow on streptomycin. pAK126a-rpsL1 carries a tetracycline resistance marker and thus could be selected for and maintained in all tested sphingomonads due to their sensitivity to tetracycline (Table 1). Whereas wild-type strains grew at streptomycin concentrations of 50 μg/ml or higher, expression of rpsL1 rendered all tested species streptomycin sensitive (Table 1). In addition, most strains were naturally sensitive to kanamycin and resistant to carbenicillin (Table 1), indicating that pAK405 and the gene deletion strategy via conjugal transfer as outlined above can also be used for these organisms. Finally, pAK405 or the general strategy of generating streptomycin-sensitive rpsL alleles as described here might also be applicable to closely related bacteria not belonging to the sphingomonads, such as Erythrobacter, Citromicrobium, or Zymomonas species, which are naturally streptomycin resistant and whose rpsL alleles encode Arg-88 (11, 33, 34).
Table 1.
Antibiotic susceptibility of selected sphingomonads and effect of rpsL1
| Strain | Growth with each antibiotica |
||||
|---|---|---|---|---|---|
| Wild type |
+ pAK126a-rpsL1 |
||||
| Tc10 | Km50 | Ca50 | Sm200 | Sm50 and Tc10b | |
| Sphingomonas sp. Fr1 (DSM 24958) | − | − | + | + | − |
| Sphingomonas sp. C3 (DSM 24957) | − | − | + | + | − |
| Novosphingobium rosa (DSM 7285) | − | − | + | + | − |
| Sphingomonas mali (DSM 10565) | − | − | + | + | − |
| Sphingomonas aerolata (DSM 14746) | − | − | − | +c | − |
| Sphingobium indicum (DSM 16412) | − | − | + | +c | − |
| Sphingobium rhizovicinum (DSM 19845) | − | − | + | + | − |
| Sphingomonas roseiflava (CIP 106847) | − | − | + | + | − |
| Sphingomonas wittichii (DSM 6014) | − | − | + | + | − |
Growth of wild-type strains (wild type) or otherwise-wild-type strains expressing rpsL1 from pAK126a (+ pAK126a-rpsL1) was assessed on nutrient broth (NB) supplemented with different antibiotics. Tc10, 10 μg tetracycline/ml; Km50, 50 μg kanamycin/ml; Ca50, 50 μg carbenicillin/ml; Sm200, 200 μg streptomycin/ml. +, growth after 2 days of incubation; −, no growth after 5 days of incubation.
A total of 10 μg tetracycline/ml was added for maintenance of pAK126a-rpsL1.
Growth was observed at a concentration of 50 μg streptomycin/ml but not at 200 μg streptomycin/ml.
In conclusion, we showed that a mutant rpsL allele encoding the R88K substitution (rpsL1) behaves as a dominant, streptomycin-sensitive allele in the wild-type background of several sphingomonads, suggesting that Arg-88, encoded by the endogenous rpsL allele, confers natural streptomycin resistance to these species. A plasmid based on rpsL1, pAK405, that can be used for markerless gene deletions and allelic exchange in Sphingomonas sp. Fr1 and most likely in a number of other, naturally streptomycin-resistant sphingomonads was constructed. The simple and efficient method presented in this work expands the genetic toolbox for sphingomonads.
Nucleotide sequence accession number.
The sequence of pAK405 is available in GenBank (http://www.ncbi.nlm.nih.gov/) under accession number JQ432562.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the Swiss National Science Foundation (SNF) through research grant 31003A-135623.
Footnotes
Published ahead of print 16 March 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Balkwill DL, Fredrickson JK, Romine MF. 2006. Sphingomonas and related genera, p 605–629 In Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E. (ed), The prokaryotes: a handbook on the biology of bacteria, vol 7 Proteobacteria, delta and epsilon subclasses. Deeply rooting bacteria Springer-SBM, New York, NY [Google Scholar]
- 2. Corvini PF, Schaffer A, Schlosser D. 2006. Microbial degradation of nonylphenol and other alkylphenols—our evolving view. Appl. Microbiol. Biotechnol. 72:223–243 [DOI] [PubMed] [Google Scholar]
- 3. Fialho AM, et al. 2008. Occurrence, production, and applications of gellan: current state and perspectives. Appl. Microbiol. Biotechnol. 79:889–900 [DOI] [PubMed] [Google Scholar]
- 4. Field JA, Sierra-Alvarez R. 2008. Microbial degradation of chlorinated dioxins. Chemosphere 71:1005–1018 [DOI] [PubMed] [Google Scholar]
- 5. Francez-Charlot A, et al. 2009. Sigma factor mimicry involved in regulation of general stress response. Proc. Natl. Acad. Sci. U. S. A. 106:3467–3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Funatsu G, Wittmann HG. 1972. Ribosomal proteins. 33. Location of amino-acid replacements in protein S12 isolated from Escherichia coli mutants resistant to streptomycin. J. Mol. Biol. 68:547–550 [DOI] [PubMed] [Google Scholar]
- 7. Innerebner G, Knief C, Vorholt JA. 2011. Protection of Arabidopsis thaliana against leaf-pathogenic Pseudomonas syringae by Sphingomonas strains in a controlled model system. Appl. Environ. Microbiol. 77:3202–3210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaczmarczyk A, et al. 2011. Role of Sphingomonas sp. strain Fr1 PhyR-NepR-σEcfG cascade in general stress response and identification of a negative regulator of PhyR. J. Bacteriol. 193:6629–6638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kast P. 1994. pKSS—a second-generation general purpose cloning vector for efficient positive selection of recombinant clones. Gene 138:109–114 [DOI] [PubMed] [Google Scholar]
- 10. Kast P, Hennecke H. 1991. Amino acid substrate specificity of Escherichia coli phenylalanyl-tRNA synthetase altered by distinct mutations. J. Mol. Biol. 222:99–124 [DOI] [PubMed] [Google Scholar]
- 11. Koblizek M, et al. 2003. Isolation and characterization of Erythrobacter sp. strains from the upper ocean. Arch. Microbiol. 180:327–338 [DOI] [PubMed] [Google Scholar]
- 12. Lederberg J. 1951. Streptomycin resistance: a genetically recessive mutation. J. Bacteriol. 61:549–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marx CJ. 2008. Development of a broad-host-range sacB-based vector for unmarked allelic exchange. BMC Res. Notes 1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marx CJ, Lidstrom ME. 2001. Development of improved versatile broad-host-range vectors for use in methylotrophs and other Gram-negative bacteria. Microbiology 147:2065–2075 [DOI] [PubMed] [Google Scholar]
- 15. Masai E, et al. 1999. Genetic and biochemical characterization of a 2-pyrone-4,6-dicarboxylic acid hydrolase involved in the protocatechuate 4,5-cleavage pathway of Sphingomonas paucimobilis SYK-6. J. Bacteriol. 181:55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mohn WW, Mertens B, Neufeld JD, Verstraete W, de Lorenzo V. 2006. Distribution and phylogeny of hexachlorocyclohexane-degrading bacteria in soils from Spain. Environ. Microbiol. 8:60–68 [DOI] [PubMed] [Google Scholar]
- 17. Musser JM. 1995. Antimicrobial agent resistance in mycobacteria: molecular genetic insights. Clin. Microbiol. Rev. 8:496–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Neufeld JD, Mohn WW, de Lorenzo V. 2006. Composition of microbial communities in hexachlorocyclohexane (HCH) contaminated soils from Spain revealed with a habitat-specific microarray. Environ. Microbiol. 8:126–140 [DOI] [PubMed] [Google Scholar]
- 19. Ozaki M, Mizushima S, Nomura M. 1969. Identification and functional characterization of the protein controlled by the streptomycin-resistant locus in E. coli. Nature 222:333–339 [DOI] [PubMed] [Google Scholar]
- 20. Pavelka MS, Jr, Jacobs WR., Jr 1999. Comparison of the construction of unmarked deletion mutations in Mycobacterium smegmatis, Mycobacterium bovis bacillus Calmette-Guerin, and Mycobacterium tuberculosis H37Rv by allelic exchange. J. Bacteriol. 181:4780–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peyraud R, et al. 2009. Demonstration of the ethylmalonyl-CoA pathway by using 13C metabolomics. Proc. Natl. Acad. Sci. U. S. A. 106:4846–4851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raina V, et al. 2008. Enhanced biodegradation of hexachlorocyclohexane (HCH) in contaminated soils via inoculation with Sphingobium indicum B90A. Biodegradation 19:27–40 [DOI] [PubMed] [Google Scholar]
- 23. Reyrat JM, Pelicic V, Gicquel B, Rappuoli R. 1998. Counterselectable markers: untapped tools for bacterial genetics and pathogenesis. Infect. Immun. 66:4011–4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Russell CB, Dahlquist FW. 1989. Exchange of chromosomal and plasmid alleles in Escherichia coli by selection for loss of a dominant antibiotic sensitivity marker. J. Bacteriol. 171:2614–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schäfer A, Tauch A, Jäger Kalinowski WJ, Thierbach G, Pühler A. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73 [DOI] [PubMed] [Google Scholar]
- 26. Sieiro C, Poza M, de Miguel T, Villa TG. 2003. Genetic basis of microbial carotenogenesis. Int. Microbiol. 6:11–16 [DOI] [PubMed] [Google Scholar]
- 27. Simon R, Priefer U, Pühler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Nat. Biotechnol. 1:784–791 [Google Scholar]
- 28. Stibitz S. 1994. Use of conditionally counterselectable suicide vectors for allelic exchange. Methods Enzymol. 235:458–465 [DOI] [PubMed] [Google Scholar]
- 29. Takeuchi M, Hamana K, Hiraishi A. 2001. Proposal of the genus Sphingomonas sensu stricto and three new genera, Sphingobium, Novosphingobium and Sphingopyxis, on the basis of phylogenetic and chemotaxonomic analyses. Int. J. Syst. Evol. Microbiol. 51:1405–1417 [DOI] [PubMed] [Google Scholar]
- 30. Torii N, et al. 2003. Spontaneous mutations in the Helicobacter pylori rpsL gene. Mutat. Res. 535:141–145 [DOI] [PubMed] [Google Scholar]
- 31. Vanbroekhoven K, et al. 2004. Streptomycin as a selective agent to facilitate recovery and isolation of introduced and indigenous Sphingomonas from environmental samples. Environ. Microbiol. 6:1123–1136 [DOI] [PubMed] [Google Scholar]
- 32. Wu SS, Kaiser D. 1996. Markerless deletions of pil genes in Myxococcus xanthus generated by counterselection with the Bacillus subtilis sacB gene. J. Bacteriol. 178:5817–5821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang S, Pelletier DA, Lu TY, Brown SD. 2010. The Zymomonas mobilis regulator hfq contributes to tolerance against multiple lignocellulosic pretreatment inhibitors. BMC Microbiol. 10:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yurkov VV, Krieger S, Stackebrandt E, Beatty JT. 1999. Citromicrobium bathyomarinum, a novel aerobic bacterium isolated from deep-sea hydrothermal vent plume waters that contains photosynthetic pigment-protein complexes. J. Bacteriol. 181:4517–4525 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.