Abstract
The glutamate decarboxylase (GAD) system is important for the acid resistance of Listeria monocytogenes. We previously showed that under acidic conditions, glutamate (Glt)/γ-aminobutyrate (GABA) antiport is impaired in minimal media but not in rich ones, like brain heart infusion. Here we demonstrate that this behavior is more complex and it is subject to strain and medium variation. Despite the impaired Glt/GABA antiport, cells accumulate intracellular GABA (GABAi) as a standard response against acid in any medium, and this occurs in all strains tested. Since these systems can occur independently of one another, we refer to them as the extracellular (GADe) and intracellular (GADi) systems. We show here that GADi contributes to acid resistance since in a ΔgadD1D2 mutant, reduced GABAi accumulation coincided with a 3.2-log-unit reduction in survival at pH 3.0 compared to that of wild-type strain LO28. Among 20 different strains, the GADi system was found to remove 23.11% ± 18.87% of the protons removed by the overall GAD system. Furthermore, the GADi system is activated at milder pH values (4.5 to 5.0) than the GADe system (pH 4.0 to 4.5), suggesting that GADi is the more responsive of the two and the first line of defense against acid. Through functional genomics, we found a major role for GadD2 in the function of GADi, while that of GadD1 was minor. Furthermore, the transcription of the gad genes in three common reference strains (10403S, LO28, and EGD-e) during an acid challenge correlated well with their relative acid sensitivity. No transcriptional upregulation of the gadT2D2 operon, which is the most important component of the GAD system, was observed, while gadD3 transcription was the highest among all gad genes in all strains. In this study, we present a revised model for the function of the GAD system and highlight the important role of GADi in the acid resistance of L. monocytogenes.
INTRODUCTION
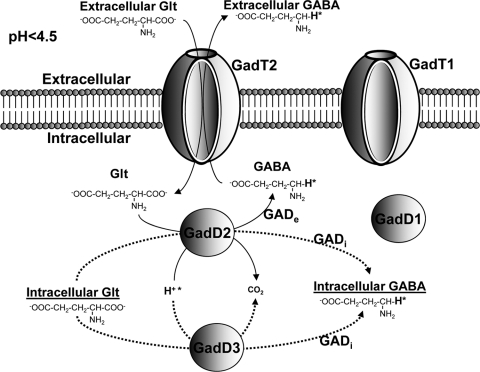
The glutamate decarboxylase (GAD) system is an important system of acid resistance in various Gram-negative and Gram-positive bacteria (8, 10). It is the most efficient system of acid resistance in Escherichia coli (4) and probably in Listeria monocytogenes (7). According to the current model for the function of the GAD system, an extracellular glutamate (Glte) is imported by an antiporter in exchange for an intracellular γ-aminobutyrate (GABAi). Each molecule of Glt is then decarboxylated by a decarboxylase to produce a molecule of GABAi in a process that consumes a proton which is incorporated in the GABAi molecule (Fig. 1). Subsequently, the GABAi is exported by the antiporter in exchange for another Glt molecule, which starts a new cycle, which will remove another proton from the intracellular milieu (22).
Fig 1.
Model for the function of the GAD system under severe acid conditions (pH < 4.5). The GadT2 antiporter imports extracellular Glt, which is decarboxylated by GadD2 to GABA with the concurrent consumption of a proton (H+*). GABA is then exported by the GadT2 with the simultaneous import of Glt. The above-described process is carried out by the GADe, which is depicted by bold lines. Intracellular Glt is decarboxylated by GadD3 and GadD2, resulting in the accumulation of GABAi. The latter process is carried out by GADi, which is depicted by dotted lines. The contribution of GadD1 and GadT1 in both the GADe and GADi processes is minor according to the results presented here. GadD3 has previously been suggested by various authors to be a Glt decarboxylase, but further work is required to prove this.
The GAD system plays an essential role in the acid resistance of the bacterial food-borne pathogen L. monocytogenes (6, 7). It promotes the growth of this bacterium under mild acidic conditions or survival under severe acidic conditions, which can occur in certain foods (9). Furthermore, it promotes passage through the stomach, enabling it to reach the intestine, where it can invade the intestinal epithelial cells and initiate a potentially fatal disease called listeriosis (7). A basic prerequisite for the function of the system is the presence of Glt, which is contained in all foods and living organisms. During the decarboxylation of Glt, one proton from the intracellular milieu is incorporated in the backbone of the Glt molecule in the place of the carboxyl group to form GABA (Fig. 1). This proton, which is attached with a highly stable bond, cannot be subject to ionization, and therefore, it cannot be released to the intracellular milieu. Subsequently, the GABA molecule that carries the removed proton is either exported by the antiporter as extracellular GABA (GABAe) or remains inside the cell (GABAi) as has been shown previously (12).
The GAD system in most L. monocytogenes strains is encoded by a total of five genes. Two of these genes (gadT1, gadT2) encode antiporters, while gadD1 and gadD2 encode Glt decarboxylases. In general, the gadD1T1 operon is absent in serotype 4 strains (9). The fifth gene (gadD3) encodes a putative glutamate decarboxylase, but its role has not been demonstrated experimentally. No role for GadD3 in acid tolerance has been established, although a gadD3 insertion mutant has been shown to be defective for intracellular growth (11). Recently, the construction of a gadD3 deletion mutant has been reported by Begley et al. (2). However, in this work it was demonstrated that unlike GadD1, GadD3 does not play a role in nisin resistance, but no role in acid resistance was investigated. All five genes are organized in three separate genetic loci: gadD1T1, gadT2D2, and gadD3 (9). The gadT2D2 locus plays an important role in survival under extreme acidic conditions (7, 9), while the gadD1T1 locus is reported to enhance growth under mild acidic conditions (9).
We have shown previously that the GAD system can utilize intracellular Glt (Glti) to produce GABAi independently of the antiport (12). Due to the independent activity of these two processes, we propose for the first time the division of the GAD system into extracellular (GADe) and intracellular (GADi) components. We also investigated the significance of GADi in acid resistance and which genes contribute to it. Furthermore, we studied the activity of GADi over a range of pH values and the differences in the transcription of the gad genes of three reference strains during an acid challenge. The contribution of GADi in the overall GAD-dependent removal of protons was also investigated in a variety of food and clinical isolates.
The present study advances our understanding of the function of the GAD system in L. monocytogenes and its role in acid resistance. Furthermore, by refining the existing model for the GAD system, the data presented here also have implications for the understanding of the acid resistance of other important food-borne pathogens (e.g., E. coli and Shigella flexneri) or commensals (e.g., lactic acid bacteria) that also possess the GAD system.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
An array of strains including three reference strains and various food and clinical isolates was used in this study (Table 1). All strains were stored at −80°C in 15% (vol/vol) glycerol. Prior to experiments, stock cultures were streaked onto brain heart infusion (BHI) agar (LAB M, Lancashire, United Kingdom) and incubated at 37°C overnight. A single colony from this medium was transferred to 2 ml of sterile BHI (LAB M, Lancashire, United Kingdom), tryptone soy broth with 0.6% yeast extract (TSBY), or defined medium (DM) broth prepared according to the method of Amezaga et al. (1) with or without 10 mM Glt (Sigma-Aldrich, Steinheim, Germany) and incubated overnight at 37°C with shaking (160 rpm). Subsequently, a portion of these overnight cultures served as the inoculum (3% [vol/vol]; initial optical density at 600 nm [OD600], 0.06) to prepare the cultures that were used in the experiments. Cultures were prepared in 250-ml conical flasks containing 20 ml of the same medium as the one used for the inoculum and incubated overnight (∼18 h) at 37°C with shaking (160 rpm). Subsequently, these overnight cultures were used for all acid challenges and assays described below.
Table 1.
Strains used in this study
| Isolate | Source | Presence of gadD1T1a | Serotype | % of H+ removed by GADi compared to GAD in TSBY (pH 4) | Reference or source |
|---|---|---|---|---|---|
| 102 | Human clinical isolate | * | 1/2a | 15.31 | This study |
| 103 | Human clinical isolate | 4 | 15.66 | This study | |
| 104 | Human clinical isolate | * | 1/2c | 17.89 | This study |
| 294 | Fish | ND | 21.32 | This study | |
| 295 | Fish | ND | 18.62 | This study | |
| 299 | Fish | ND | 20.13 | This study | |
| 302 | Fish | ND | 18.90 | This study | |
| 437 | Chicken salad sandwich | 4b | 19.38 | This study | |
| 438 | Human clinical isolate | 4b | 19.84 | This study | |
| 439 | Chicken salad sandwich | 4b | 18.28 | This study | |
| 440 | Ham and coleslaw sandwich | 4b | 26.03 | This study | |
| 441 | Human clinical isolate | 4b | 25.87 | This study | |
| 442 | Human clinical isolate | 4b | 13.04 | This study | |
| 443 | Human clinical isolate | 4b | 22.54 | This study | |
| 444 | Swine | * | 1/2 | 11.79 | This study |
| 445 | Human clinical isolate | 4b | 25.92 | This study | |
| 446 | Human clinical isolate | 4b | 17.09 | This study | |
| 10403S | Lab strain, human clinical isolate | * | 1/2a | 6.21 | 12 |
| EGD-e | Lab strain, rabbit isolate | * | 1/2a | 100.00 | 20 |
| LO28 | Lab strain, clinical isolate | * | 1/2c | 28.98 | 7 |
| LO28 ΔgadD1 | Mutant | 1/2c | ND | 7 | |
| LO28 ΔgadD2 | Mutant | * | 1/2c | ND | 7 |
| LO28 ΔgadD1D2 | Mutant | 1/2c | ND | 7 |
* indicates the presence of gadD1T1.
Survival under acidic conditions.
Acid survival experiments were performed in DM and BHI. The use of different media and different strains resulted in great differences in acid resistance. Therefore, to achieve cell death at a measurable rate, different pH values needed to be applied in each medium. In these experiments, the pH of the overnight cultures was adjusted to 2.7 (BHI; see Fig. 2B), 3.2 (DM without Glt [DM (−Glt)]; see Fig. 3B), or pH 3.0 [DM (−Glt) or BHI; see Fig. 4], depending on the medium or the strain challenged with the addition of 3 M HCl. Samples were obtained prior to the pH adjustment and thereafter at regular time intervals, and 10-fold serial dilutions were prepared from those samples and plated onto BHI agar in triplicate. These plates were incubated at 37°C overnight, and subsequently, colonies were counted to assess survival under lethal acidic conditions.
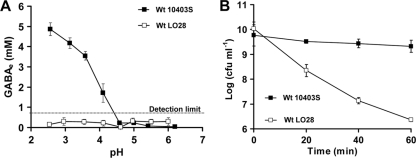
Fig 2.
Strain LO28 does not use the GADe system in BHI. (A) Concentration of GABAe measured at 80 min after acidification of the cultures to various pH values. The detection limit for GABAe quantification in BHI was 0.8 mM. (B) Acid resistance of stationary-phase cultures of wild-type (Wt) strains LO28 and 10403S following acid challenge at pH 2.7. All cultures were grown to stationary phase overnight at 37°C, and viability of cells was determined prior to acid challenge and every 20 min thereafter. Markers represent an average of measurements performed in triplicate, and error bars represent the standard deviation.
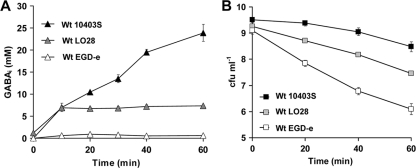
Fig 3.
Survival of strains 10403S, LO28, and EGD-e in DM in the absence of any extracellular Glt corresponds with the accumulation of GABAi. Cells were grown overnight in DM (−Glt) until stationary phase (∼18 h) at 37°C with shaking. Subsequently, cultures were acid challenged at pH 4, where GABAi was measured (A), or acid challenged at pH 3.2, where viability was determined at regular time intervals (B). Acidification of cultures was achieved with the addition of 3 M HCl. Markers represent an average of measurements performed in triplicate, and error bars represent the standard deviation.
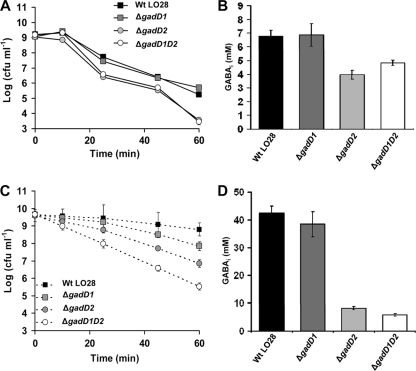
Fig 4.
The GADi system is able to confer increased acid tolerance in the absence of any extracellular Glt. The GAD system is able to function solely on intracellular Glt, and the strains that accumulate higher GABAi demonstrate the highest acid tolerance. (A) Acid resistance of wild-type LO28 and its ΔgadD1, ΔgadD2, and ΔgadD1D2 isogenic mutants in DM (−Glt) at pH 3. (B) Accumulation of GABAi in wild-type LO28 and its ΔgadD1, ΔgadD2, and ΔgadD1D2 isogenic mutants in DM (−Glt) at pH 4. (C and D) Acid resistance (C) and GABAi accumulation (D) of wild-type LO28 and its isogenic mutants were measured in BHI at pH 3 and pH 4, respectively. Markers represent an average of measurements performed in triplicate, and error bars represent the standard deviation.
GABase assay.
A commercial preparation known as GABase was used to determine the GABAi and GABAe concentrations. GABAi was quantified as described by O'Byrne et al. (21), while GABAe was quantified according to the method of Tsukatani et al. (24), as modified by Karatzas et al. (12). Values for GABAi are estimations of the concentration in the cell obtained following calculations taking into account the concentration of cells and their hypothetical volume, as shown previously (12, 21). Overnight cultures had their pH, optical density, and cell concentrations estimated. Subsequently, the pH of the cultures was adjusted to 4.0 to quantify the levels of GABAi and GABAe, with the exception of the experiments whose results are presented in Fig. 5 and Fig. 2A, where cultures were adjusted to various pH values to quantify the GABAi and/or GABAe. Although survival was tested under lethal conditions, GABAe and GABAi were assessed at the nonlethal pH of 4 to avoid any interference of cell death in the GABAi quantification. This value of pH 4 was specifically selected to create conditions as close to lethal as possible, without resulting in cell death, which could compromise the interpretation of the GABase results. The course of the GABase reaction was monitored by measurement of the absorbance at 340 nm every 1 min for 3 h at 37°C using a Sunrise spectrophotometer (Tecan, Männedorf, Switzerland) operated by Magellan software (Tecan, Männedorf, Switzerland). All reagents used for the GABase assay were obtained from Sigma-Aldrich (Steinheim, Germany).
Fig 5.
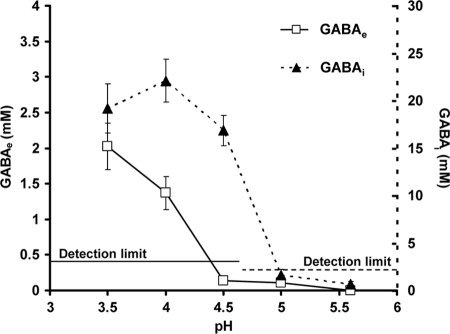
GABAe versus GABAi accumulation in L. monocytogenes 10403S. Utilization of intracellular Glt initiates at milder pH (4.5) than that of extracellular Glt (pH 4), suggesting that the decarboxylases are active at milder pH values than the antiporters. Cells were grown overnight in TSBY until stationary phase (∼18 h) at 37°C with shaking. Cultures were acid challenged at different pH values with the addition of 3 M HCl, and measurements of GABAe and GABAi took place following 2 h at the corresponding pH value. In parallel, viability was tested under these conditions, and it was confirmed that cell death did not occur for the length of time that the experiment took place. The detection limits for GABAe and GABAi were 0.4 and 2.2 mM, respectively. Markers represent an average of measurements performed in triplicate, and error bars represent the standard deviation.
Real-time RT-PCR determination of gad gene transcription.
Transcription of the gad genes in response to acidification was quantified as previously described by Karatzas et al. (12) following real-time reverse transcription-PCR (RT-PCR). The primers used previously (12) were designed as such to recognize the corresponding sequences in all three reference strains in which they were used. Efficiencies of the primer pairs 16SF-16SR, gadT1F-gadT1R, gadD1F-gadD1R, gadT2F-gadT2R, gadD2F-gadD2R, and gadD3F-gadD3R were 2.27, 1.94, 2.12, 2.07, 2.09, and 2.03, respectively, and these values, which were all close to 2, were used for efficiency correction in the quantification step. In all cases, overnight cultures grown for ∼18 h in DM were acidified at pH 4.0 with 3 M HCl to create conditions as close to lethal as possible without killing the cells. Samples were taken before acidification (0 min) and at 12 and 30 min postacidification. Relative expression was calculated as a ratio between expression of each of the target genes (gadD1, gadT1, gadD2, gadT2, and gadD3) and the expression of the 16S rRNA gene, which served as the reference gene in each cDNA sample. Calculations were carried out following the advanced relative quantification settings of the LightCycler 480 software program, with PCR efficiency correction as described previously (12). Relative expression of each gene was calculated by comparison of its expression relative to that of the 16S rRNA gene in each strain and under each condition and expressed as a percentage of the maximal level detected for that transcript in all strains and under all conditions (see Fig. 6). Furthermore, to allow comparisons between the expression of the different gad genes within the same strain, we present the expression of all genes per strain (see Fig. S1 in the supplemental material). To allow comparisons between strains, all transcription data are expressed in this case as a percentage of the maximal level detected for any gene in any strain.
Fig 6.
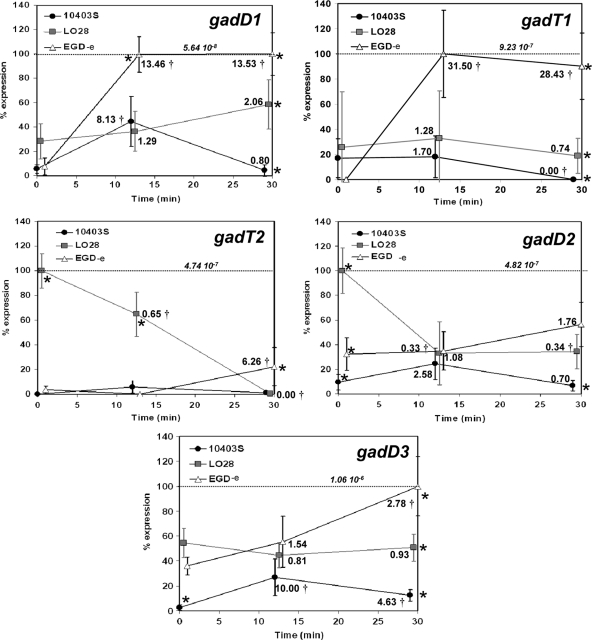
Transcription of each gad gene in different reference strains in response to acidification in DM at pH 4. Relative normalized (on the basis of the 16S rRNA gene) expression of all gad genes (gadD1, gadT1, gadT2, gadD2, and gadD3) in 10403S, LO28, and EGD-e in DM before (0 min) and after (12 and 30 min) acidification in pH 4 achieved with 3 M HCl. Expression of each gene was calculated following advanced relative quantification and normalization based on its relative transcription compared to that of the 16S rRNA gene in each strain and time point. For each comparison, the data are expressed as a percentage of the maximal level detected for that transcript in all strains and under all conditions. In order to allow comparisons between the expression of the different gad genes, the actual relative normalized (based on the 16S rRNA gene) expression level for each maximum value is also indicated on each graph. The fold change of the expression of each gene in each strain compared to the initial expression at time zero was calculated, and it is indicated close to each marker. A statistically significant change (P < 0.05), as estimated by Student's t test, is marked (†). A statistical analysis comparing the expression of each gene in each different strain was performed with Tukey's multiple-comparison test, and if P was <0.05, it was deemed statistically significant and indicated on the graphs (*). Error bars represent standard deviations.
Statistical analysis of results.
In all sets of experiments except RT-PCR, mean values were calculated from experiments performed in triplicate, and standard deviations were also determined and are depicted with error bars on the graphs. In addition, in each separate experiment, values were calculated as an average from three technical replicates.
For each RT-PCR experiment, measurements were performed on three independent biological samples, with three technical replicates performed on each. The data were normalized to those for the 16S rRNA using the advanced relative quantification with PCR efficiency correction feature of the Roche LightCycler 480 software. Tukey's multiple-comparison test was then performed to define if the expression of each gene from a specific strain was statistically significantly different from that of the other strains at each time point. Tukey's multiple-comparison test was used because multiple comparisons between three different values were required for each gene and time point (see Fig. 6). P values were calculated and deemed statistically significant (*) when P was <0.05. Furthermore, to assess the change in the expression of each gene in each strain, the expression at each time point was compared to that at time zero and is shown as fold change. To estimate the statistical significance of this change, Student's t test comparing the expression at a certain time and that at time zero was performed, and if P was <0.05, it was deemed statistically significant and indicated (†).
Tukey's multiple-comparison test was also used to identify a significantly different expression of a specific gene in a strain at a specific time point, and if a statistically significant difference was found (P < 0.05), it was marked (*) (see Fig. S1 in the supplemental material). The fold change in the expression of each gene in each strain compared to that at time zero is also depicted only if it was >2.00 or <0.50 and is indicated (†) if it was statistically significant, as estimated by Student's t test (P < 0.05).
Presence of gad genes in strains.
The presence of all five gad genes in all strains used in this study was assessed by the use of PCR. The sets of primers used for each of the gad genes were the ones used for the RT-PCR previously described by Karatzas et al. (12).
RESULTS
GABAe export is strain and medium dependent.
We showed previously that under acidic conditions, Glt/GABA antiport is impaired in minimal media but not in rich ones, like BHI. The ability of L. monocytogenes LO28 and 10403S to export GABAe in response to acidification (pH 4) was measured in BHI. Both strains were cultured to stationary phase in BHI at 37°C, and then the medium pH was adjusted to a range of pH values from 6.3 to 2.5. GABAe levels in the culture supernatant were recorded following 80 min of acidification to allow sufficient GABAe export. Over the whole range of pH values, LO28 exported undetectable levels of GABAe (below the 0.8 mM GABA detection limit; Fig. 2A). In contrast, 10403S exported GABAe once the medium pH dropped below 4.5, with the highest level recorded when the pH was dropped to 2.5 (Fig. 2A). To assess the acid survival of these cultures, we treated them at pH 2.7. We selected a value between pH 2.5 and 3.0 because the first was too severe, causing rapid death, and the last was too mild. At pH 2.7, 10403S was found to survive well, whereas LO28 lost viability rapidly (Fig. 2B). Interestingly, we found that GABAe export in response to acidification was absent from LO28 only when the cells were cultured in some growth media but not in others. For example, we confirmed that when we cultured LO28 to stationary phase in TSBY, it was able to produce GABAe in response to acidification, as it has been shown previously (7). These data highlight that the Glt/GABA antiport is strain and medium dependent. Furthermore, as explained in more detail in the Discussion, the results presented above support the idea of a division of the GAD system into the intracellular decarboxylase system (GADi) transforming Glti to GABAi and the extracellular- or antiport-dependent decarboxylase system (GADe) transforming Glte to GABAe through the Glt/GABA antiport (Fig. 1).
Accumulation of GABAi in different L. monocytogenes reference strains correlates with acid tolerance.
In a recent study, we have shown that although L. monocytogenes 10403S was not able to produce any GABAe through GADe in DM with Glt, it was able to accumulate substantial pools of GABAi through GADi following acidification (12). This suggests that GABAi accumulation could be a response against acid and it might play a role in survival under these conditions. Therefore, we investigated whether GABAi accumulation could correlate with acid survival under conditions that do not permit Glt/GABA antiport [DM (−Glt)]. The capacity of three different reference strains to produce GABAi in response to acidification of the medium was determined. Cultures of 10403S, LO28, and EGD-e were grown to stationary phase (∼18 h) in DM and then adjusted to pH 4.0, after which the GABAi levels were measured at regular time intervals for a period of 1 h. Large differences in GABAi production were observed between the three strains. After 1 h at pH 4.0, L. monocytogenes 10403S had produced >3-fold more GABAi than LO28 and >10-fold more GABAi than EGD-e (Fig. 3A). We confirmed that under these conditions no GABAe was detected for any of the three strains. When acid survival rates were measured at pH 3.2 following growth under identical conditions, 10403S was found to be the most acid tolerant, while EGD-e was the least tolerant (Fig. 3B). Thus, the rates of viability loss at a lethal pH were inversely related to the capacity of these strains to produce GABAi in response to acidification at a nonlethal pH (Fig. 3), suggesting that higher GABAi accumulation coincided with higher acid resistance.
GADi contributes to survival under acidic conditions.
L. monocytogenes wild-type (LO28) cells and isogenic mutant derivatives lacking either the GadD1 or GadD2 decarboxylase, or both, were grown in DM (−Glt) or in BHI to stationary phase. In both media, LO28 is not able to perform Glt/GABA antiport and uses only Glti. When cultures were challenged under lethal acidic conditions in DM (−Glt) at pH 3.0, the mutants lacking the GadD2 decarboxylase were more sensitive, resulting in a 1.75-log-cycle difference in log reduction compared to the wild type (Fig. 4A). Under these conditions, no detectable GABAe but only GABAi was produced by LO28. The mutants lacking GadD2 were found to accumulate reduced levels of GABAi (which decreased by ∼40%), whereas mutants lacking the GadD1 system produced levels similar to those observed in the wild type (Fig. 4B). Thus, a good correlation between the capacity of GADi to accumulate GABAi and the ability to survive an acid challenge existed. When cells were acid challenged in BHI at pH 3.0, ΔgadD1, ΔgadD2, and ΔgadD1D2 cells showed 0.95- and 1.95-log-cycle differences and a significant 3.26-log-cycle difference in log reduction compared to the wild type, respectively, after 60 min (Fig. 4C). In this case, a good correlation between GABAi accumulation at pH 4 (Fig. 4D) and survival existed. These results show that the GAD system utilizing solely intracellular pools of Glt (GADi) contributes to the acid resistance of L. monocytogenes.
GADi contributes significantly to the overall GAD activity of various food and clinical isolates.
The GADi-dependent production of GABAi seems to contribute to the acid resistance, and therefore, it should contribute to the proton removal process. According to the current model for the GAD system, each mole of GABA produced carries 1 mole of protons (H+) which has been removed by the Glt decarboxylation process. Therefore, by measuring the levels of GABAi and GABAe, we could identify how many protons are removed by the GADi and GADe, respectively (Fig. 1).
Twenty different strains grown until stationary phase in TSBY were surveyed for their GABAi and GABAe production after acid challenge at pH 4 for 60 min. Strains included reference strains, as well as clinical and food isolates (Table 1). Based on the hypothesis that each mole of GABA removes a mole of protons, we calculated the number of millimoles of protons removed by the use of intracellular (GADi) or extracellular (GADe) Glt, and then we divided these values by the OD600 of each strain's culture at the moment of the GABA measurements to allow comparisons. Subsequently, the number of millimoles of protons removed by GADi was divided by the number of millimoles of protons removed by the overall GAD system (GADi and GADe). Overall, an average 23.11% ± 18.87% of the total number of millimoles of protons removed by the GAD system was removed by the GADi. All strains removed protons through the GADi, while in a unique fashion, the EGD-e strain did not remove any protons through the GADe. In EGD-e, which is the most-studied reference strain of L. monocytogenes, all protons removed by the GAD system were removed through the GADi. This and all previous work in DM suggest that a standard level of GADi activity always exists in all strains under acidic conditions, while the activity of GADe might be variable, depending on the environment and the strain (Table 1). Furthermore, the absence of gadD1T1 (all other genes were present in all strains) did not affect the proportion of protons removed by GADi compared to the number of protons removed by the overall GAD system.
In L. monocytogenes 10403S, GABAi accumulation occurs before GABAe is detected.
If GABAi production is critical to acid survival, then it might be expected to occur as a primary response to acidification of the culture medium. Alternatively, if the export of GABA is more important in acid survival, then this might be expected to be the primary response. Therefore, we investigated the levels of both GABAi and GABAe over a range of culture pH values (pH 5.6 to 3.5) in a growth medium that supported both GABAi and GABAe production (TSBY). The results revealed that the GADi began to accumulate GABAi 0.5 pH unit before GABAe was detected in the culture medium; significant GABAi levels were recorded at a medium pH of 4.5, whereas no GABAe was detected at this pH (Fig. 5). It was clear that GADi activity occurs as the primary response to acidification of the culture medium. Only when the pH had reached 4.0 was GABAe detected in the medium. Thus, the data show that GABAi accumulation occurs in advance of Glt/GABA antiport, which suggests that GADi is likely to be a critical factor in determining the ability of cells to survive during a transition into an acidic environment. The data further suggest that GADe exports GABA via the antiporters only when a critical pH value is reached.
Strain-to-strain differences in gad gene transcription.
In order to understand the basis for strain-to-strain differences in GABAi production, real-time RT-PCR was used to measure the transcription of all five gad genes following acidification to pH 4.0 in three different strains of L. monocytogenes: 10403S, LO28, and EGD-e (Fig. 6). Cells were grown in DM (−Glt), conditions that support GABAi but not GABAe production. Furthermore, we have previously demonstrated that in this medium the GAD system showed the most rapid and prominent response compared to the response in BHI, where transcription remained mainly unchanged or even reduced (12). The data revealed that there was considerable strain-to-strain variation in the transcriptional responses of the gad genes to acidic pH. To allow a better overview of these differences, we have plotted the data per gene (Fig. 6) and per strain (see Fig. S1 in the supplemental material). In general, transcription was higher the more acid sensitive that the strain was (Fig. 6; see Fig. S1 in the supplemental material). More specifically, following 30 min at pH 4, EGD-e had the highest levels of transcription for all gad genes, 10403S had the lowest, and LO28 maintained levels intermediate between those of the other two strains (Fig. 6). Furthermore, following acid treatment for 30 min, transcription of gadD3 was the highest in all strains (see Fig. S1 in the supplemental material). In EGD-e there was a statistically significant increase in the transcription of gadD1 by 13.53-fold and gadT1 by 31.50-fold after 30 and 12 min, respectively, at pH 4, the most prevalent detectable upregulation after 30 min of acid treatment (Fig. 6). In this strain, acid treatment at pH 4 for 30 min caused a minor 2.78-fold increase for gadD3, reaching the highest level of transcription for any gad gene in any strain during the experiment. In contrast, transcription of the gad genes remained unaffected in LO28, with the exception of the gadT2D2 operon, which was downregulated during the acid challenge. Transcription of gadT2D2 and gadD3 in LO28 prior to acid challenge was higher than that in the other strains (see Fig. S1 in the supplemental material). Although transcription of gadD2 in LO28 was the second highest among all genes in all strains under basal conditions, no transcript could be detected for this gene after acid treatment for 30 min. In 10403S, there was a statistically significant upregulation only of gadD1 and gadD3, by 8.13- and 10-fold, respectively (Fig. 6). Even though both gadD1 and gadT1 belong to the same operon, in all strains the transcription of gadT1 was significantly higher than that of gadD1 (see Fig. S1 in the supplemental material). Another common feature in all strains was the inability to upregulate the gadT2D2 operon, which is the most important gad operon under severe acid challenges. Despite that, there was a 6.26-fold increase of gadT2 in EGD-e only after 30 min at pH 4 (Fig. 6).
DISCUSSION
The GAD system is the most important mechanism of acid resistance in Listeria monocytogenes (7). It utilizes the freely available Glte in the environment through Glt/GABA antiport and decarboxylation, removing protons in the process (22). Until recently, the only known prerequisites for the function of the GAD system in L. monocytogenes have been the entrance of cells in stationary phase, acidic conditions, and the presence of Glte. Recently, we have shown that despite the presence of all the above-described prerequisites, the Glt/GABA antiport of strain 10403S is impaired in DM but not in rich media like BHI, where the presence of unknown components is indispensable for its use, enhancing acid resistance (12). In this study, we have confirmed that 20 randomly selected clinical and food isolates (Table 1) were unable to use the Glt/GABA antiport in DM (with Glt; data not shown). The Glt/GABA antiport seems to be under complex regulation which is subject to strain variation, as LO28, in contrast to 10403S, is unable to export GABA in BHI (Fig. 2A), resulting in an acid-sensitive phenotype compared to 10403S (Fig. 2B). Interestingly, LO28 is able to use the Glt/GABA antiport in TSBY (Table 1). This strain- and medium-dependent variability of the Glt/GABA antiport might be associated with diverse activation signals present in different niches that each strain explores. Similar to the behavior of 10403S in DM (12), in BHI LO28 cannot use the Glt/GABA antiport but it is able to accumulate GABAi by the decarboxylation of Glti (Fig. 4D).
Since the above-described processes of GABAi accumulation function independently of the Glt/GABA antiport, we propose the concept of dividing the GAD system into two semi-independent systems, the intracellular (GADi) and the extracellular (GADe) GAD systems utilizing Glti and Glte through the Glt/GABA antiport, respectively (Fig. 1). Furthermore, this distinction is necessary since, stoichiometrically, GABAi and GABAe derive from equivalent amounts of Glti and Glte, respectively. The Glte imported by GADe does not contribute to the GABAi pools because it is rapidly decarboxylated and exported (Fig. 1).
Despite the variability in GADe activity, our work (12) and the results presented here (Fig. 3A and 4B and D) suggest that accumulation of the GABAi produced by the GADi is a standard cellular response against acidic conditions. Although GADi activity has previously been suggested to occur in E. coli (5) and S. flexneri (25), there is no report showing its role in acid resistance. To investigate that, we conducted experiments in DM (−Glt) where the absence of Glte in L. monocytogenes restricted the use of the GADe and limited the GAD system to utilize only Glti. Three common reference strains were tested for their ability to accumulate GABAi at pH 4 and ranked from the highest to the lowest as 10403S > LO28 > EGD-e (Fig. 3A), which corresponded well with their acid resistance at pH 3 (Fig. 3B).
In further experiments, to assess the contribution of the gad genes in GADi activity and acid resistance, we used mutants with mutations in gadD1 and gadD2 and their isogenic wild type, LO28. In TSBY, where GADe is functional, GadD1T1 promotes growth under mild acidic conditions, while GadT2D2 promotes survival under severe acid challenge (9). In DM (−Glt), LO28 ΔgadD1 and wild type showed similar GADi activities (Fig. 4B) and acid survival (Fig. 4A), suggesting an insignificant contribution of GadD1 in GADi activity and survival under severe acid challenge. However, in BHI, which also restricts GADe activity in LO28, the ΔgadD1 mutant was more sensitive than the wild type by 1 log cycle of viable counts (Fig. 4C), coinciding with lower levels of GABAi, without statistical significance, though, suggesting a minor role for GadD1 in acid resistance under severe acidic conditions. The absence of GadD2 under GADe restriction [DM (−Glt)] reduced GADi activity (Fig. 4B), which corresponded well with a significant decrease of ∼1.75 log cycles in acid resistance (Fig. 4A). In BHI and under GADe restriction, the reduction in GADi activity was even higher than that in DM (−Glt) (Fig. 4D), resulting in a greater decrease in acid resistance (3.26 log cycles; Fig. 4C). The increased effect in BHI compared to DM (−Glt) could be due to the higher GADi activity in this medium, resulting in higher differences in GABAi between the mutants and the wild type. Therefore, GadD2 and, to a lesser extent, GadD1 contribute to the GADi activity which affects intracellular pH homeostasis and acid resistance.
We previously thought that due to the limited levels of Glti in comparison to the vast levels of Glte, the contribution of GADi in acid resistance would be insignificant. However, the results presented above suggest the opposite. Our revised model (Fig. 1) proposes that GABAi and GABAe remove stoichiometrically intracellular protons through GADi and GADe activity, respectively. Through measurements of GABAi and GABAe in 20 different strains (TSBY; pH 4) and simple calculations presented in Results, we found that the GADi removes an average of 23.11% ± 18.87% of the protons removed by the overall GAD system (Table 1). This contribution of GADi to proton removal might be even higher, if we took into account a possible catabolism of GABAi in L. monocytogenes. In silico analysis did not reveal any GABA transporters in L. monocytogenes, while the reference strains failed to utilize known concentrations of GABAe preadded in the growth medium (data not shown). However, the possibility of a pathway metabolizing GABAi cannot be excluded, as GABAi is one of the most abundant metabolites (∼113 mM) under acidic conditions (12). Interestingly, in EGD-e—the most widely studied L. monocytogenes strain—the GADe system is nonfunctional in all media that we tested and the GADi system accounts for all the activity of the GAD system (Table 1). This unique characteristic of EGD-e, together with other peculiarities described previously, supports the idea that its usefulness as a model strain is debatable (23).
Since no Glt is present in DM (−Glt), at least a part of the Glti derives from the glutamine present in the medium. It has previously been suggested that E. coli might import glutamine via the antiporter GadC (25), but this seems unlikely for L. monocytogenes, as no GABAe was detected in acidified DM (−Glt). Glutamine is probably imported by Lmo0847, a putative glutamine transporter (19), and subsequently converted to Glti. Pools of Glti could be used instantly by the GADi when required. The instant utilization of Glti is suggested by the fact that production of GABAi initiates at milder pH values (4.5 to 5.0) than production of GABAe (4 to 4.5; Fig. 5). This could be attributed to a different optimal functional pH between the decarboxylases and the antiporters or possible differences in their transcription.
We investigated these possible differences in transcription of the gad genes through RT-PCR in three reference strains with high (10403S), medium (LO28), and low (EGD-e) acid resistance during an acid treatment in DM (Fig. 6). DM was the medium of choice, as the gad genes in 10403S previously showed a noticeable transcriptional response, unlike in rich media like BHI, where no response was observed (12). Our results show that after 30 min at pH 4, the transcription of every gene was higher the more acid sensitive that the strain was (Fig. 6). This suggests an attempt of the more sensitive strains to recover or maintain a level of GAD activity that would help them survive. However, its effect is questionable since no transcriptional upregulation of gadT2D2 occurred in any of the strains during the acid challenge (Fig. 6; see Fig. S1 in the supplemental material). Therefore, this behavior that we previously observed in 10403S (12) is common between strains, and it is puzzling because GadT2D2 is the main contributor of acid resistance in this bacterium and yet the cells have to rely on preexisting levels of these proteins produced prior to the acid challenge. Maybe this is related to an attempt to strike a balance between stress resistance and virulence, as it is known that upregulation of acid or stress resistance mechanisms can impair virulence (3, 13–17). Furthermore, no transcriptional upregulation of the gad genes occurred in LO28, which could be due to the high transcription of gadT2D2 and gadD3 at basal levels (see Fig. S1 in the supplemental material).
The transcription of lmo2434 or gadD3, as it is called in most publications, was the highest among all gad genes in all strains during the whole experiment (see Fig. S1 in the supplemental material), and it showed a transcriptional upregulation in response to acid treatment in EGD-e and 10403S, which suggests a role in acid stress. This gene has previously been suggested to encode a third Glt decarboxylase (9) regulated by σB (7, 18, 26), but the inability of ΔgadD1D2 to export any GABAe through GADe raised doubts about this (7, 9). In this study, we were able to clear these doubts by detecting a significant GADi activity in this mutant (Fig. 4B) and thus confirming the existence of a third Glt decarboxylase (possibly GadD3), apart from GadD1 and GadD2. We have repeatedly tried to mutate gadD3, without success, but in new evidence showing that this is possible (2), we continue our attempts. However, the deletions in ΔgadD1D2 are nonpolar (7, 9), and therefore, GadD3 should be able to function synergistically with the antiporters which are intact. This suggests that GadD3 is a part of GADi but not of GADe, deviating from the model of the Glt decarboxylases cooperating with the antiporters, as described for GadB, the homologue of GadD2 in E. coli (4). GadD3 could also be responsible for the initiation of GADi at milder pH values than initiation of GADe acting on readily available pools of Glti (Fig. 1). Although the contribution of the gadD1T1 operon to acid survival was minor, similar to GadD3, GadD1 was upregulated in EGD-e and 10403S (Fig. 5; see Fig. S1 in the supplemental material), possibly in an attempt to compensate for the low initial transcriptional levels of all Glt decarboxylases. In all strains, the transcription of gadT1 was significantly higher than that of gadD1, as it has been observed previously (26).
The work described above establishes for the first time the concept of GADi in a revised model for the function of GAD and demonstrates its important role in the acid resistance of L. monocytogenes (Fig. 1). Further study of this revised model could have important implications in the understanding of the acid resistance of various bacteria but also other microorganisms that possess the GAD system and encompass all kingdoms of microbial life (fungi, yeasts, and archaea).
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to our colleagues and especially to Conor Feehily in Microbiology at NUI Galway for helpful discussions and comments on the manuscript. We also thank David Cullen and David Reddington for technical support, Paul Cotter (Teagasc, Cork, Ireland) and Colin Hill (University College Cork, Cork, Ireland) for providing the gad mutants, Martin Cormican (University College Hospital, National University of Ireland, Galway, Ireland) for providing the clinical isolates used in this study, and Cormac Gahan (University College Cork, Cork, Ireland) for useful discussions.
This project was supported by a Science Foundation Ireland Starting Investigator Research Grant (SIRG) awarded to K.-A. G. Karatzas (09/SIRG/B1570). L. Suur was supported by an SFI-UREKA program grant (08/UR/B1350).
Footnotes
Published ahead of print 9 March 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Amezaga M-R, et al. 1995. The role of peptide metabolism in the growth of Listeria monocytogenes ATCC 23074 at high osmolarity. Microbiology 141:41–49 [DOI] [PubMed] [Google Scholar]
- 2. Begley M, Cotter PD, Hill C, Ross RP. 2010. Glutamate decarboxylase-mediated nisin resistance in Listeria monocytogenes. Appl. Environ. Microbiol. 76:6541–6546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bruno JC, Jr, Freitag NE. 2010. Constitutive activation of PrfA tilts the balance of Listeria monocytogenes fitness towards life within the host versus environmental survival. PLoS One 5:e15138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Capitani G, et al. 2003. Crystal structure and functional analysis of Escherichia coli glutamate decarboxylase. EMBO J. 22:4027–4037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castanie-Cornet M-P, Penfound TA, Smith D, Elliott JF, Foster JW. 1999. Control of acid resistance in Escherichia coli. J. Bacteriol. 181:3525–3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cotter PD, O'Reilly K, Hill C. 2001. Role of the glutamate decarboxylase acid resistance system in the survival of Listeria monocytogenes LO28 in low pH foods. J. Food Prot. 64:1362–1368 [DOI] [PubMed] [Google Scholar]
- 7. Cotter PD, Gahan CGM, Hill C. 2001. A glutamate decarboxylase system protects Listeria monocytogenes in gastric fluid. Mol. Microbiol. 40:465–475 [DOI] [PubMed] [Google Scholar]
- 8. Cotter PD, Hill C. 2003. Surviving the acid test: responses of Gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 67:429–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cotter PD, Ryan S, Gahan CGM, Hill C. 2005. Presence of GadD1 glutamate decarboxylase in selected Listeria monocytogenes strains is associated with an ability to grow at low pH. Appl. Environ. Microbiol. 71:2832–2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Foster JW. 2004. Escherichia coli acid resistance: tales of an amateur acidophile. Nat. Rev. Microbiol. 2:898–907 [DOI] [PubMed] [Google Scholar]
- 11. Joseph B, et al. 2006. Identification of Listeria monocytogenes genes contributing to intracellular replication by expression profiling and mutant screening. J. Bacteriol. 188:556–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karatzas K-AG, Brennan O, Heavin S, Morrissey J, O'Byrne CP. 2010. Intracellular accumulation of high levels of γ-aminobutyrate by Listeria monocytogenes 10403S in response to low pH: uncoupling of γ-aminobutyrate synthesis from efflux in a chemically defined medium. Appl. Environ. Microbiol. 76:3529–3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karatzas K-AG, Bennik MHJ. 2002. Characterization of a Listeria monocytogenes Scott A isolate with high tolerance towards high hydrostatic pressure. Appl. Environ. Microbiol. 68:3183–3189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karatzas K-AG, et al. 2008. Effects of repeated cycles of acid challenge and growth on the phenotype and virulence of Salmonella enterica. J. Appl. Microbiol. 105:1640–1648 [DOI] [PubMed] [Google Scholar]
- 15. Karatzas K-AG, Valdramidis VP, Wells-Bennik MHJ. 2005. Contingency locus in ctsR of Listeria monocytogenes Scott A: a strategy for occurrence of abundant piezotolerant isolates within clonal populations. Appl. Environ. Microbiol. 71:8390–8396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karatzas K-AG, et al. 2003. The CtsR regulator of Listeria monocytogenes contains a variant glycine repeat region that affects piezotolerance, stress resistance, motility and virulence. Mol. Microbiol. 49:1227–1238 [DOI] [PubMed] [Google Scholar]
- 17. Karatzas K-AG, Zervos A, Tassou CC, Mallidis CG, Humphrey TJ. 2007. Piezotolerant small-colony variants with increased thermotolerance, antibiotic susceptibility, and low invasiveness in a clonal Staphylococcus aureus population. Appl. Environ. Microbiol. 73:1873–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kazmierczak MJ, Mithoe SC, Boor KJ, Wiedmann M. 2003. Listeria monocytogenes σB regulates stress response and virulence functions. J. Bacteriol. 185:5722–5734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim H-J, Mittal M, Sonenshein AL. 2006. CcpC-dependent regulation of citB and lmo0847 in Listeria monocytogenes. J. Bacteriol. 188:179–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murray EGD, Webb RA, Swann MBR. 1926. A disease of rabbit characterised by a large mononuclear leucocytosis, caused by a hitherto undescribed bacillus: Bacterium monocytogenes. J. Pathol. Bacteriol. 29:407–439 [Google Scholar]
- 21. O'Byrne CP, Feehily C, Ham R, Karatzas K-AG. 2011. A modified rapid enzymatic microtiter plate assay for the quantification of intracellular γ-aminobutyric acid and succinate semialdehyde in bacterial cells. J. Microbiol. Methods 84:137–139 [DOI] [PubMed] [Google Scholar]
- 22. O'Byrne CP, Karatzas K-A. 2008. The role of sigma B (sigma B) in the stress adaptations of Listeria monocytogenes: overlaps between stress adaptation and virulence. Adv. Appl. Microbiol. 65:115–140 [DOI] [PubMed] [Google Scholar]
- 23. Ofer A, et al. 2011. Implications of the inability of Listeria monocytogenes EGD-e to grow anaerobically due to a deletion in the class III NrdD ribonucleotide reductase for its use as a model laboratory strain. J. Bacteriol. 193:2931–2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsukatani T, Higuchi T, Matsumoto K. 2005. Enzyme-based microtiter plate assay for γ-aminobutyric acid: application to the screening of γ-aminobutyric acid-producing lactic acid bacteria. Anal. Chim. Acta 540:293–297 [Google Scholar]
- 25. Waterman SR, Small PLC. 2003. The glutamate-dependent acid resistance system of Escherichia coli and Shigella flexneri is inhibited in vitro by l-trans-pyrrolidine-2,4-dicarboxylic acid. FEMS Microbiol. Lett. 224:119–125 [DOI] [PubMed] [Google Scholar]
- 26. Wemekamp-Kamphuis HH, et al. 2004. Identification of sigma factor σB-controlled genes and their impact on acid stress, high hydrostatic pressure, and freeze survival in Listeria monocytogenes EGD-e. Appl. Environ. Microbiol. 70:3457–3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.