Abstract
The purpose of this study was to investigate the aerosolization of particles (micro- and macroconidia and fragments) from Botrytis cinerea cultures in relation to potential human inhalation in indoor environments. The influence of the following factors on the aerosolization of B. cinerea particles was studied: exposure to airflow, relative humidity (rh), changing rh, and plant or building materials. The aerodynamic diameter (da) and the respirable fraction of the aerosolized particles were determined. Conidia and fragments of B. cinerea were not aerosolized as a response to a decrease in the rh. In contrast, both micro- and macroconidia and fungal fragments were aerosolized when exposed to an airflow of 1.5 m s−1 or 0.5 m s−1. Significantly more particles of microconidial size and fragment size were aerosolized at a low rh (18 to 40% rh) than at a higher rh (60 to 80% rh) when cultures were exposed to airflow. The size of the respirable fraction of the aerosolized particles was dependent on the rh but not on the growth material. At high rh, about 30% of the aerosolized particles were of respirable size, while at low rh, about 70% were of respirable size. During low rh, more fungal (1→3)-β-d-glucan and chitinase were aerosolized than during high rh. In conclusion, exposure to external physical forces such as airflow is necessary for the aerosolization of particles from B. cinerea. The amount and size distribution are highly affected by the rh, and more particles of respirable sizes were aerosolized at low rh than at high rh.
INTRODUCTION
Airborne fungi are found in elevated concentrations in buildings with water damage and are related to health problems (2, 46, 55). Botrytis cinerea has been found in indoor air (47), including in damp buildings (15), and it needs a minimum water activity of 0.9 for growth (11, 13). Relatively many people are allergic to the fungus B. cinerea in spite of its low airborne prevalence (26). For example, 24% of 180 suspected mold-allergic patients (49) and 4% of 104 greenhouse workers (20) reacted positively to Botrytis in a skin prick test. Fungal growth, aerosolization of fungal material, and inhalation are steps in the process causing human exposure of the airways. For some fungi, it is known that their growth on agar-based media is not dependent on the relative humidity (rh) of the air but on free water on or in the agar medium (41). Laboratory studies have investigated the aerosolization of fungal particles from some fungal species on agar media (16, 42), gypsum boards (29, 35), and ceiling tiles (16) and from naturally occurring fungi on straw and wood chips (35) when the cultures were subjected to mechanical handling or to airflow. Studies investigating aerosolization from gypsum boards have shown that the aerosolization of spores from different species is affected by air velocity differently (29). Furthermore, repeated exposure of fungal cultures to the activity causing the aerosolization resulted in more particles becoming aerosolized during the first exposure period than during the following periods (4, 5, 35). The use of agar media in aerosolization studies has been criticized, as aerosolization may be dependent on the growth medium (45). In addition to spores, particles smaller than fungal spores are shown to be aerosolized from fungal cultures (16, 28, 37). These particles are referred to as fungal microparticles (measured sizes between 0.3 and 1.3 μm in da) (37), fungal fragments (measured sizes between 0.3 and 1.5 μm in optical diameter) (16), or fungal hyphal or conidial fragments (sizes not measured) (18) and can constitute a large part of bioaerosols in terms of numbers (16, 18, 36, 52).
The aerodynamic diameter (da) of an airborne particle influences the site of deposition in the respiratory system and the possible health effects it can cause (10, 22, 23), as well as its ability to stay airborne and to penetrate small cracks in, e.g., building constructions. Fungal spore sizes are typically between 2 and 10 μm (10, 36). B. cinerea can produce globose microconidia with a physical size of 2.5 to 3.0 μm, smooth-walled, obovoid macroconidia of 8 to 14 by 6 to 9 μm, and larger sclerotia which are irregular in size and shape (7). The aerodynamic diameters of particles aerosolized from B. cinerea cultures are not known.
B. cinerea is able to grow parasitically or saprophytically on many plant materials (11, 33), and indoor plantings are described as sources of indoor exposure of fungi in general (3, 51). It is not known whether B. cinerea grows on common building materials, such as gypsum boards or floor paper. However, airborne, culturable B. cinerea units have been found in water-damaged buildings (43), and the exposure source may have been building materials or household waste.
In this study, we investigate whether the aerosolization of particles from B. cinerea cultures in terms of size distribution and number are affected by rh, changing rh, airflow, and growth media. As growth media, we have chosen gypsum boards and floor paper as representatives of indoor building materials and a vegetable to represent household waste. All may be considered common potential growth media for fungi in indoor environments such as homes.
MATERIALS AND METHODS
Cultivation on building materials and aubergines.
An isolate of Botrytis cinerea Pers.:Fr. (DSM5144; Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany) was used. The building materials gypsum boards and floor paper were used, as they are commonly present in indoor environments. As a vegetable, aubergine (Solanum melongena var. Esculentum, Valstar Westland, Holland), also called eggplant, was chosen, as it is used globally for fresh consumption and as B. cinerea is a common postharvest pathogen on aubergines (9). It is the most widely grown aubergine variety in Europe and North America; it has an elongated ovoid shape, 12 to 23 cm long and 6 to 9 cm wide with dark purple skin.
B. cinerea was cultivated on pieces (area = 800 cm2) of sterilized, wet gypsum boards without wallpaper (Knauf, Danogips, Denmark), pieces of sterilized, water-saturated floor paper (Icopal, Herlev, Denmark) (area = 132 cm2), and round slices of surface-sterilized aubergine (area = 32 to 36 cm2, thickness = 1 cm) and incubated at a relative humidity (rh) of approximately 95% as described below. Distilled Milli-Q water was used for wetting the boards and floor paper. Inoculation was performed by placing four sclerotia per 100 cm2 or spraying 50 macroconidia per 100 cm2. The gypsum boards, floor paper, and aubergine slices were placed in sterilized stainless steel boxes with tightly fitting glass covers. A saturated solution of K2SO4 controlled the rh (29, 57). The B. cinerea-inoculated materials were incubated at 20°C in darkness. The cultures were, as a standard, incubated for 8 to 10 weeks on gypsum boards, for 10 to 12 weeks on floor paper, and for 2 to 6 weeks on aubergines. In one experiment, cultures on floor paper were incubated for 200 days.
P-FLEC measurements.
The P-FLEC (particle-field and laboratory emission cell) (Chematec, Denmark) was used for measuring the aerosolization of airborne particles from surfaces as described by Kildesø et al. (29). The lid from the steel box was removed, and the P-FLEC was placed on the B. cinerea cultures. Airflow was directed toward the surface at an angle of 45°. The jets were scanned over the surface, covering a surface area of 130 cm2 gypsum board or floor paper or 32 to 36 cm2 aubergine slice. A bar with 10 0.8-mm nozzles was rotated 1.0 cm over the surface; one complete rotation was 60 s, and each measurement lasted 300 s in this study. A flow of 5.0 liters min−1 through the 10 nozzles gave a mean velocity over the surface of 1.5 m s−1, while a flow of 1.7 liters min−1 gave a mean velocity over the surface of 0.5 m s−1. The particles were transported by the airflow to the outlet at the top. Particles were either measured every second with an aerodynamic particle sizer (APS 3320; TSI Inc.) in 51 size ranges between 0.54 and 19.8 μm and in one fraction of particles smaller than 0.52 μm or collected on a polycarbonate filter (pore size, 0.8 μm) using a GSP sampler (Gesamtstaubprobenahme; conical inhalable sampler [CIS] by BGI, Inc., Waltham, MA) or a Triplex cyclone (BGI, MA). The sampling was performed for 300 s. The Triplex cyclone sampled airborne particulate matter with a Da50 of 1 μm (PM1) particles (flow rate, 3.5 l min−1) and PM2.5 (flow rate, 1.5 l min−1). The Triplex cyclone has a well-defined, sharp penetration curve, and at a flow rate of 3.5 liters min−1, only about 1% of particles with a da between 1.7 and 2.0 μm penetrates the cyclone (21). The GSP samples inhalable particles (flow, 3.5 liters min−1). The filters were analyzed for N-acetyl-β-d-glucosaminidase (NAGase) activity and (1→3)-β-d-glucan (β-glucan) content and by microscopy. The respirable fractions of particles were calculated from the APS data according to EN481 (6, 23). The fraction of particles smaller than 0.52 μm is not included in the calculation of the size of the respirable fraction because the measurement of this fraction is less precise than the measurements of the other fractions. The measured particles are also categorized in fractions of particles smaller than 0.52 μm, 0.54 to 1.6 μm (particles smaller than microconidial size), 1.8 to 3.3 μm (particles covering the sizes of microconidia), and 3.5 to 10.4 μm (particles covering the sizes of macroconidia).
Effects of changing rh on aerosolization.
Aerosolization of particles from gypsum boards, floor paper, and aubergines was studied when affected by a decreasing rh but with no exposure to airflow. This was studied for 5, 13, and 8 h for, respectively, gypsum boards, floor paper, and aubergines. The rh was lowered using silica gel. The silica gel absorbs approximately 14 g water per 100 g silica gel (P. Kruse, personal communication). The rh of the air in the P-FLEC was measured using a humidity and temperature probe (Vaisala HM141; Vaisala, Finland), and the rh of the gypsum boards and aubergines were measured using a moisture/temperature meter (Testo 606-2; Testo, Germany).
The release was also studied on floor paper and aubergine slices during an increasing rh.
Effects of rh and airflow on aerosolization.
Four days before the exposure to an airflow of 1.5 m s−1, the rh of the air in the incubation boxes with gypsum boards, floor paper, or aubergines was adjusted using different amounts of silica gel. In an experiment with only aubergines, cultures on aubergines were exposed to an airflow of 0.5 m s−1 also at different rh's.
Effects of repeated exposure on aerosolization.
The particle aerosolization was studied for B. cinerea on gypsum boards to see how it was affected by repeated agitation of the same area by an airflow of 1.5 m s−1 14 days after the first exposure.
Extraction of particles.
The B. cinerea particles on polycarbonate filters were extracted in 6.0 ml sterile 0.05% Tween 80 and 0.85% NaCl aqueous solution by shaking it for 15 min (500 rpm) at room temperature. The suspensions were then stored at −80°C until analysis of β-glucan, NAGase, and microscopy was performed.
Microscopy.
The particle suspension was incubated with wheat germ agglutinin (WGA) (L-4895; Sigma) in phosphate-buffered saline, pH 7.2, for 20 min in darkness, with subsequent filtration through a polycarbonate filter (25 mm, 0.4 μm; Nuclepore, Cambridge, MA) to label chitin. The presence of microconidia and macroconidia was confirmed by microscopy at a magnification of 1,250 times using epifluorescence microscopy (Orthoplan; Leitz, Wetzlar, Germany).
The (1→3)-β-d-glucan and NAGase assay.
Concentrations of β-glucan were extracted using 0.3 M NaOH for 60 min (34). After extraction with NaOH, β-glucan was quantified in duplicate using the kinetic Fungitic G test (Seikagaku Co., Tokyo, Japan). A standard curve with β-glucan (Pachyman derived from the sclerotia of Poria cocos) ranging from 4.0 to 100 pg ml−1 was used.
NAGase activity was quantified according to the assay described by Møller et al. (39), with minor modifications. Briefly, 100 μl of 200 μM 4-methylumbelliferyl (MUF) N-acetyl-B-d-glucosaminide (Sigma-Aldrich) was added to 1.0 ml 50 mM Tris-maleate buffer (pH 5). An amount of 50 μl of test sample was then added to the solution and incubated at 25°C for 30 min. The enzymatic reaction was stopped by adding ice-cold 96% ethanol. Tubes were then centrifuged for 5 min (4,000 rpm; 2°C), and Tris buffer (2.5 M; pH 10) was added to the supernatant to reach pH 10. An amount of 200 μl of this solution was added to a black microtiter plate in replicas of three. Fluorescence at 446 nm derived from the release of 4-methylumbelliferone (4-MU) was quantified by a spectrometer using an excitation wavelength of 377 nm. Activity of NAGase was calculated by comparing sample fluorescence with that of a standard curve containing 4-MU (0 to 35.5 nmol ml−1).
Treatment of data.
In total, B. cinerea was cultured on 15 pieces of gypsum boards, 18 pieces of floor paper, and 36 aubergine slices. The number of aerosolized particles, NAGase activity (30 samples), and amount of β-glucan (30 samples) were standardized to the exposed area and are expressed in unit cm−2 exposed area. All experiments with the P-FLEC were performed in triplicate. The number of aerosolized particles, NAGase activity, and amount of β-glucan as affected by different rh's were log transformed and compared using the analysis of variance procedure (PROC ANOVA) in SAS (SAS 9.1), and the standard deviation (s*) was calculated (32). The amount of β-glucan in PM1 per amount in the inhalable fraction was normally distributed, and the standard deviation was calculated.
RESULTS
B. cinerea was able to grow on sterilized gypsum boards, floor paper, and slices of aubergines. On all three materials it produced hyphae, micro- and macroconidia, and, over time, also sclerotia. It colonized aubergine slices faster than gypsum boards, and it colonized gypsum boards faster than floor paper; therefore, different incubation times were used for the three materials.
Influence of changing rh on aerosolization of particles.
The particle aerosolization from a B. cinerea culture on a gypsum board not exposed to airflow was studied for 5 h; no particles were aerosolized during this period. During the measurement period, the air rh decreased continuously from 83 to 62% while the temperature increased from 25 to 28°C.
The aerosolization of particles from B. cinerea on floor paper was studied for a 1-h period, during which the rh increased from 71 to 78%, and subsequently for a 13-h period, during which it decreased from 78 to 20%. The temperatures during the periods were between 25 and 28°C. During the 14 h, almost no particles were aerosolized. During a decrease in rh from 33 to 30%, only 0.015, 0.023, and 0.015 particles of fungal fragment size, microconidial size, and macroconidial size, respectively, were released per cm2. This corresponds to 0.022% of the particles of fungal fragment size, 0.11% of microconidial size, and 0.11% of macroconidial size aerosolized per minute relative to the number aerosolized during exposure to airflow (1.5 m s−1, rh = 34%). The fastest decrease in rh was a decrease from 76 to 56% within 38 min. In that period, no particles smaller than microconidial size were aerosolized; a fraction of 0.034% of both microconidial and macroconidial sizes were aerosolized per minute relative to the number aerosolized during exposure to airflow (1.5 m s−1, rh = 62%).
The aerosolization of B. cinerea particles from aubergines without exposure to airflow was studied for 8 h during an increase in rh from 30 to 55% and during a subsequent decrease from 55 to 29%; the temperature was between 26 and 28°C. During the whole period, almost no particles were aerosolized, and the very few particles that were aerosolized were of macroconidial size.
Influence of rh on the aerosolization of particles when affected by airflow.
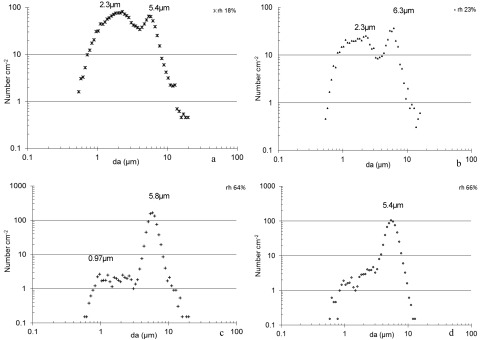
The rh had a significant influence on the number and size distribution of particles aerosolized when B. cinerea cultures on gypsum boards, floor paper, and aubergines were affected by an airflow of 1.5 m s−1. At low humidities (air rh = 18 to 34%), many particles smaller than 1.6 μm were aerosolized, while at higher humidities, fewer particles were aerosolized (Fig. 1, 2, and 3). Microscopy revealed that both micro- and macroconidia were present in the inhalable fraction of the aerosols. The fraction of the particles being of respirable size was highest for particles aerosolized at the low rh. During low-rh conditions, many particles smaller than 0.52 μm were aerosolized (Table 1).
Fig 1.
(a to d) The effects of an airflow of 1.5 m s−1 and air humidity on the aerosolization of particles from Botrytis cinerea cultures grown on gypsum boards. Numbers over the curves are the aerodynamic diameters (da) of particles constituting the dominating size fraction in terms of numbers.
Fig 2.
(a and b) The effects of an airflow of 1.5 m s−1 and air humidity on the aerosolization of particles from Botrytis cinerea cultures grown on floor paper. Numbers over the curves are the aerodynamic diameters (da) of particles constituting the dominating size fraction in terms of numbers.
Fig 3.
(a to d) The effects an airflow of 1.5 m s−1 and air humidity on the aerosolization of particles from Botrytis cinerea cultures grown on aubergines. Numbers over the curves are the aerodynamic diameters (da) of particles constituting the dominating size fraction in terms of numbers.
Table 1.
Influence of relative humidity on the number of particles aerosolized per cm2 gypsum board, floor paper, or aubergine when exposed to an airflow of 1.5 m s−1 for 5 min (n = 3)
| Material | rh (%) | No. of particlesa with an aerodynamic diam of: |
Respirable fraction (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fragment size <0.52 μm (s*) | Fragment sizeb 0.54–1.6 μm (s*) | Microconidial size 1.8–3.3 μm (s*) | Macroconidial size 3.5–10.4 μm (s*) | Sum 0.54–19.8 μm (s*) | ||||||||
| Gypsum board | 19 | 176 A | (1.32) | 460 A | (1.12) | 675 A | (1.37) | 534 B | (1.22) | 1,669 A | (1.39) | 74 |
| 23 | 39 B | (1.24) | 321 A | (1.23) | 362 A | (1.37) | 891 AB | (1.29) | 1,574 A | (1.40) | 70 | |
| 65 | 54 B | (1.31) | 19 B | (1.34) | 27 B | (1.41) | 736 AB | (1.34) | 784 B | (1.19) | 31 | |
| 72 | 26 C | (1.21) | 38 B | (1.40) | 49 B | (1.32) | 1,202 A | (1.36) | 1,378 AB | (1.86) | 22 | |
| Floor paper | 34 | 99 A | (1.33) | 283 A | (1.34) | 9,801 A | (1.31) | 6,325 A | (1.43) | 16,420 A | (1.37) | 69 |
| 43 | 31 B | (1.30) | 41 B | (1.41) | 76 B | (1.30) | 4,688 A | (1.39) | 4,809 B | (1.41) | 37 | |
| 79 | 1.2 C | (2.11) | 1.3 C | (2.09) | 5.3 C | (2.00) | 327 B | (1.31) | 337 C | (2.03) | 28 | |
| Aubergine | 37 | 670 A | (1.26) | 211 A | (1.34) | 6,312 A | (1.29) | 952 B | (1.66) | 8,348 A | (1.34) | 79 |
| 50 | 329 B | (1.23) | 16 B | (1.39) | 1,182 B | (1.31) | 237 C | (1.50) | 1,441 B | (1.49) | 79 | |
| 55 | 155 C | (1.35) | 30 B | (1.34) | 926 B | (1.55) | 4,020 A | (1.30) | 4,987 A | (1.57) | 39 | |
| 60 | 4.0 D | (1.93) | 15 B | (1.75) | 6.3 C | (1.88) | 226 C | (1.83) | 318 C | (1.42) | 33 | |
| 65 | 1.2 D | (1.87) | 12 B | (1.72) | 9.0 C | (1.76) | 432 BC | (1.71) | 456 BC | (1.73) | 29 | |
Comparison (within a material, within a certain size fraction) of numbers of aerosolized particles at different rh's. Numbers followed by the same letter are not significantly different (P > 0.05). s*, standard deviation.
The sizes are the aerodynamic diameters measured by an APS; the respirable fraction is calculated for particles between 0.54 and 19.8 μm. The cultures on the different materials have different ages.
The effect of exposure to airflow at different rh's was also studied for 200-day-old B. cinerea cultures on floor paper. For these old cultures, the same patterns for particle release were seen as those shown in Fig. 2 (data not shown).
The amount of β-glucan measured in the inhalable fraction aerosolized from B. cinerea-colonized gypsum boards was not affected significantly by the rh of the air (Fig. 4a), while the NAGase activity and amount of β-glucan in PM1 and PM2.5 decreased with increasing rh (Fig. 4a and b). The amounts of β-glucan in PM1 per amount in the inhalable fraction were 0.59 ± 0.07, 0.58 ± 0.10, and 0.25 ± 0.04 at rh's of 22, 59, and 66%, respectively. The activity of NAGase and amount of β-glucan measured in the inhalable fraction as well as in PM1 aerosolized from floor paper (Fig. 4c and d) and aubergines (Fig. 4e and f) decreased with increasing rh. From both floor paper and aubergines, the amounts of β-glucan in PM1 per amount in the inhalable fraction were between 0.36 and 0.41 independently of the rh's.
Fig 4.
(a to f) Content of β-glucan or NAGase in aerosols from Botrytis cinerea-colonized gypsum boards, floor paper, or slices of aubergines. The total column is the inhalable fraction sampled with the GSP sampler, the middle fraction of the column is the fraction called PM2.5 (only measured for gypsum board), and the bottom fraction is called PM1. The numbers of each size fraction followed by the same letter on each figure are not significantly different (P > 0.05; n = 3). s*, standard deviation. The cultures on the three materials are different ages. Percentages on the x axis indicate relative air humidity.
Low airflow.
When exposing a culture of B. cinerea on aubergine slices to an airflow of 0.5 m s−1, only very few particles were aerosolized. At rh's of 34 to 37%, only 0.04% of the total numbers of particles aerosolized when exposed to an airflow of 1.5 m s−1 were aerosolized. The aerosolization was dependent on the rh. At an rh of 34%, a fraction of 51% of the total number of particles aerosolized at an rh of 27% were aerosolized. The dominating size fraction in terms of numbers had das between 2.3 and 3.1 μm.
Repeated exposure.
Throughout the 5 min of exposure to airflow, particles were aerosolized from B. cinerea cultures on all three materials, but the numbers of aerosolized particles decreased during periods of air exposure. This was seen at both low (18 to 25%) and high (60 to 72%) humidities. There was a tendency to a higher relative decrease at lower humidities. In Fig. 5a and b, the following example is shown for B. cinerea on gypsum boards: the average number of particles of each size fraction aerosolized during the fourth and fifth minute at an rh of 18 and 64% constituted 41 and 59% of the average number of particles aerosolized during the first 2 min of air exposure.
Fig 5.
Aerosolization of particles from Botrytis cinerea cultures on gypsum boards when exposed to airflow (1.5 m s−1). The average numbers of particles of each size fraction aerosolized during the first and second minute of air exposure and during the fourth and fifth minute at an rh of 18% (a) and of 64% (b), from a culture exposed twice with a 10-day interval between each 5-min exposure period (c), and during the first and second minute of exposure to air jets (d) are shown. The sizes in the figures are the aerodynamic diameters (da) of the dominating fractions in terms of numbers during the first or second exposure minutes (a, b) or minute (d) or period (c).
When a culture on gypsum board (rh 71 to 72%) was exposed twice to airflow for 5 min (10 days between the two exposures), a portion between 25 and 50% of the number of particles aerosolized during the first 5-min period was aerosolized during the second 5-min period (example in Fig. 5c).
Under all conditions, particles with das around 5 μm were aerosolized. In 89% of 39 studies, the das of these aerosolized particles were larger during the first minute of air exposure than in the following minutes (example in Fig. 5d).
DISCUSSION
The release of conidia from some fungal species is described to be regulated by a hygroscopic mechanism after a drop in humidity (7, 25). In this study, we saw that a decrease or increase in rh did not in itself cause aerosolization of conidia. In contrast, exposure to an airflow of 1.5 m s−1 caused an immediate aerosolization of both macro- and microconidia and fungal fragments. A survey of the mycological flora of wine cellars has shown a larger amount of airborne B. cinerea after grape-pressing activity than before (48). Furthermore, an investigation of airborne fungi in water-damaged buildings showed culturable B. cinerea spores during demolition activity but neither before nor after demolition (43). These studies together with the present study show that exposure to airflow or mechanical handling such as human activity is important in the aerosolization of B. cinerea conidia. This study further shows that an air jet exposure is also necessary for aerosolization of small fragments of B. cinerea.
Particles of microconidial size were aerosolized in large amounts at low rh, and microscopy revealed that microconidia were present. Microconidia are uninucleate and function primarily as spermatia (12, 58) and are not able to germinate and grow on agar media (14, 19). In studies of aerobiology, fungi are traditionally quantified as culturable units on agar media and hence microconidia are not quantified. The lack of germinability of microconidia and the importance of exposure to airflow or mechanical handling to cause particle aerosolization may be reasons why, as concluded in a review paper, B. cinerea is not measured to be among the dominating fungi in indoor air (26). The facts that B. cinerea is able to grow on building materials and on many common fruits and vegetables, that it produces microconidia and particles smaller than conidial size, and that relatively many people are allergic to B. cinerea (24, 27, 30, 31) may indicate that exposure to B. cinerea is more common than what has been measured in exposure studies. The allergenic properties of B. cinerea spores are higher if they are germinated (17), but how often they are present in the air as germinated micro- or macroconidia is not known.
In this study, we measured the influence of rh of the air between 19 and 83%. The rh of the air in buildings varies according to activities in the building, season, number of persons in the building, climate zone, etc. In a study in Baltimore, MD, the average rh in 85 homes measured during four seasons was 36% (38), and in a British study performed in 1,095 dwellings during the winter, the median rh in living rooms was 43% and in bedrooms was 49% (40). According to the present study, many of the aerosolized B. cinerea particles will be aerosolized as respirable particles at these average rh's found in indoor air. During drying of water-damaged building materials colonized by B. cinerea, conidia of this fungus will be aerosolized only if it is concurrently exposed to external forces such as airflow. Thus, the drying out may increase release of B. cinerea microconidia from conidiophores or hyphae and the airflow may cause aerosolization.
We have shown that not only plant material but also water-damaged gypsum boards and floor paper are probable growth materials for B. cinerea and thus sources of exposure to B. cinerea in indoor air. Microscopy showed that micro- and macroconidia and sclerotia were produced on all three materials. It was also possible to cultivate B. cinerea on slices of aubergines without contamination by other microorganisms; furthermore, it was possible to get slices of aubergines of almost the same size and to fit the colonized slices of aubergines into the P-FLEC–APS-GSP or Triplex cyclone system, and thus aubergines seem to be a good model plant or model household waste material for studying aerosolization of B. cinerea. This system may also be used with other fungi, e.g., Rhizopus stolonifer, and it may be used in relation to aerosolization and exposure in, e.g., kitchens, vegetable storage, work with household rubbish, and vegetable fields. B. cinerea is the most common postharvest plant pathogen (58), and thus, exposure can also potentially occur from other commonly sold plant materials such as strawberries, grapes, tomatoes, and onions.
Throughout the 5 min of exposure to airflow, particles were aerosolized from B. cinerea cultures on all three materials. However, most particles were aerosolized during the first 1 or 2 min. For another fungus, Penicillium chrysogenum, it has been shown that the aerosolization of spores growing in an air handling system duct (4) and on wallpapered gypsum boards (35) or placed on flooring materials (5) was also higher in the beginning of the exposure period than later. For B. cinerea, this paper shows that at low rh's, the number of aerosolized particles decreased faster than during higher rh's. This is probably because the particle source is drained faster at low rh's. Thus, the exposure period may be shorter and the immediate exposure higher at low rh's than at high rh's.
Fragments of B. cinerea and particles of microconidial size were aerosolized in the largest amounts at low rh when concurrently exposed to an airflow of 1.5 m s−1. The number of particles aerosolized of macroconidial size was different at different rh's. In another study, B. cinerea on grapes was exposed to an airflow of 0.6 m s−1 and conidia were only aerosolized during incubation at an rh of 94%, not during incubation at rh's of 90% and 69% (54). The aerosolization of Aspergillus fumigatus, Penicillium sp., and Cladosporium sp. from an agar medium and measured as culturable units has also been shown to be affected by both airflow and rh but not in a simple way. For example, when exposed to an airflow of 1.5 m s−1, A. fumigatus aerosolized more culturable units at 37 to 42% rh than at 12 to 18% and 71 to 73%, while Penicillium sp. aerosolized more at an rh of 12 to 18% than at the two other rh's, and Cladosporium sp. aerosolized most at an rh of 71 to 73% (42). In the present study, we also saw an effect of rh when B. cinerea was exposed to airflow with a low flow (0.5 m s−1).
Some fungi are described to be hygroscopic (42) or slightly hygroscopic (44). For B. cinerea, two or three size fractions were dominating and the da of the aerosolized particles in the numerically dominating fraction was dependent on the rh. However, this seems not to be due mainly to hygroscopic extensions because a dominating fraction of particles with a da of 5.4 μm could be found at rh's of both 18 and 66% (Fig. 1). It is more likely that particles agglomerate at a higher rh or that macroconidia are adapted to be aerosolized at a higher rh than microconidia because of their different roles in the life cycles of B. cinerea. Microscopy showed that many macroconidia were released at high rh. Nonfungal particles of nanosizes have also been shown to agglomerate at high rh (56). In this study, the aerosolization of the smallest measured fraction of particles (da, <0.52 μm) was also highly affected by the rh.
We found surprisingly high concentrations of β-glucan in the PM1 fraction compared to the inhalable fraction and compared to what was expected from the APS data. This high β-glucan concentration in PM1 may partly come from fragments of B. cinerea conidia, conidiophores, or hyphae. For the fungi Trichoderma harzianum and Chaetomium globosum, the release of fungal fragments is seen to increase during autolysis of hyphae (37). It has been shown that B. cinerea during growth produces extracellular β-glucan, which forms an adhering capsule around the hyphae (50). The β-glucan found in the PM1 fraction may also come from this extracellular capsule of β-glucan, and when the capsule dries out, it may become airborne if exposed to airflow. This hypothesis is further supported by the measurement showing a higher concentration of β-glucan in PM1 from aubergines than from floor paper and gypsum boards because, according to Stahmann et al. (50), a degradation of the extracellular β-glucan occurs during low nutrient conditions. The amount of β-glucan in PM1 per amount in the inhalable fraction was between 0.3 and 0.6. This is higher than between the amount of β-glucan in PM1 per amount in PM2.5 in aerosols of Stachybotrys chartarum and Apergillus versicolor growing on agar media and gypsum boards (45). The presence of β-glucan in the PM1 fraction causing a potential exposure to small particles containing β-glucan is of importance because exposure to β-glucan has been related to airway symptoms and inflammation (8).
Higher activity of NAGase as a marker of fungal exposure has been found in homes of patients with sarcoidosis (53), and NAGase can stimulate exposed cells to interleukin-8 secretion (1). In this study, activity of NAGase was found in all particle size fractions and the aerosolization of NAGase increased with decreasing rh.
In conclusion, B. cinerea grows well on both gypsum boards and floor paper as representatives of indoor building materials and on a vegetable representing household waste. Exposure to external physical forces such as airflow was necessary for the release of particles from B. cinerea. The amount and size distribution of the particles were highly affected by the rh, and more particles of respirable size were released at low rh than at high rh. This is of relevance in relation to human inhalation of indoor air in general and specifically during remediation of water-damaged buildings. Drying out a material does not in itself cause aerosolization of B. cinerea particles but may cause the release of the particles from the hyphae and conidiophores, thus making the particles easier to aerosolize. Part of the β-glucan found in the PM1 fraction may come from the extracellular β-glucan produced by B. cinerea.
ACKNOWLEDGMENTS
I thank Margit W. Frederiksen for technical assistance, Claudia W. Jürgensen and Peder Wolkoff for valuable discussions, and Kira Tendal for linguistic assistance.
This study was part of the Centre for Indoor Air and Health in Dwellings (CISBO), which was supported by the REALDANIA foundation.
Footnotes
Published ahead of print 23 March 2012
REFERENCES
- 1. Allermann L, Wilkins CK, Madsen AM. 2006. Inflammatory potency of dust from the indoor environment and correlation to content of NAGase and fungi. Toxicol. In Vitro 20:1522–1531 [DOI] [PubMed] [Google Scholar]
- 2. Bloom E, Nyman E, Must A, Pehrson C, Larsson L. 2009. Molds and mycotoxins in indoor environments—a survey in water-damaged buildings. J. Occup. Environ. Hyg. 6:671–678 [DOI] [PubMed] [Google Scholar]
- 3. Burge HA, Solomon WR, Muilenberg ML. 1982. Evaluation of indoor plantings as allergen exposure sources. J. Allergy Clin. Immunol. 70:101–108 [DOI] [PubMed] [Google Scholar]
- 4. Buttner MP, Cruz-Perez P, Garrett PJ, Stetzenbach LD. 1999. Dispersal of fungal spores from three types of air handling system duct material. Aerobiologia 15:1–8 [Google Scholar]
- 5. Buttner MP, Cruz-Perez P, Stetzenbach LD, Garrett PJ, Luedtke AE. 2002. Measurement of airborne fungal spore dispersal from three types of flooring materials. Aerobiologia 18:1–11 [Google Scholar]
- 6. CEN Standard EN 481 1993. Workplace atmospheres. Size fraction definitions for measurement of airborne particles, Brussels, Belgium. British Standards Institution (BSI) Publishers, London, United Kingdom [Google Scholar]
- 7. Domsch KH, Gams W, Anderson T-H. 1993. Compendium of soil fungi. IHW-Verlag, Regensburg, Germany [Google Scholar]
- 8. Douwes J. 2005. (1–>3)-β-d-glucans and respiratory health: a review of the scientific evidence. Indoor Air 15:160–169 [DOI] [PubMed] [Google Scholar]
- 9. Droby S, Lichter A. 2007. Post-harvest botrytis infection: etiology, development and management, p 349–367 Botrytis: biology, pathology and control. Springer, Dordrecht, the Netherlands [Google Scholar]
- 10. Eduard W. 2009. Fungal spores: a critical review of the toxicological and epidemiological evidence as a basis for occupational exposure limit setting. Crit. Rev. Toxicol. 39:799–864 [DOI] [PubMed] [Google Scholar]
- 11. Elad Y, Shtienberg D. 1995. Botrytis cinerea in greenhouse vegetables: chemical, cultural, physiological and biological controls and their integration. Integ. Pest Manag. Rev. 1:15–29 [Google Scholar]
- 12. Faretra F, Antonacci E, Pollastro S. 1988. Sexual behaviour and mating system of Botryotinia fuckeriana, teleomorph of Botrytis cinerea. J. Gen. Microbiol. 134:2543–2550 [Google Scholar]
- 13. Flannigan B, Miller JD. 2001. Microbial growth in indoor environments, p 35–67 In Flannigan B, Samson RA, Miller JD. (ed), Microorganisms in home and indoor work environments. Taylor & Francis, London, United Kingdom [Google Scholar]
- 14. Fukumori Y, Nakajima M, Akutsu K. 2004. Microconidia act the role as spermatia in the sexual reproduction of Botrytis cinerea. J. Gen. Plant Pathol. 70:256–260 [Google Scholar]
- 15. Garrett MH, Rayment PR, Hooper MA, Abramson MJ, Hooper BM. 1998. Indoor airborne fungal spores, house dampness and associations with environmental factors and respiratory health in children. Clin. Exp. Allergy 28:459–467 [DOI] [PubMed] [Google Scholar]
- 16. Górny RL, et al. 2002. Fungal fragments as indoor air biocontaminants. Appl. Environ. Microbiol. 68:3522–3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Green BJ, Mitakakis TZ, Tovey E. 2003. Allergen detection from 11 fungal species before and after germination. J. Allergy Clin. Immunol. 11:285–289 [DOI] [PubMed] [Google Scholar]
- 18. Green BJ, Sercombe JK, Tovey ER. 2005. Fungal fragments and undocumented conidia function as new aeroallergen source. J. Allergy Clin. Immunol. 115:1043–1048 [DOI] [PubMed] [Google Scholar]
- 19. Grindle M. 1979. Phenotypic Differences Between Natural and Induced Variants of Botrytis cinerea. J. Gen. Microbiol. 111:109–120 [Google Scholar]
- 20. Groenewoud GCM, de Jong NW, van Oorschot-van Nes AJ, Vermeulen AM. 2002. Prevalence of occupational allergy to bell pepper pollen in greenhouses in the Netherlands. Clin. Exp. Allergy 33:434–440 [DOI] [PubMed] [Google Scholar]
- 21. Gussman RA, Kenny LC. 2000. Design and calibration of a cyclone for PM-1 ambient air sampling. J. Aerosol Sci. 31:194–195 [Google Scholar]
- 22. Harada RN, Repine JE. 1985. Pulmonary host defense mechanisms. Chest 87:247–252 [DOI] [PubMed] [Google Scholar]
- 23. Hinds WC. 1999. Respiratory deposition, p 233–259 Aerosol technology properties, behavior, and measurement of airborne particles. Wiley-Interscience, New York, NY [Google Scholar]
- 24. Immonen J, Meklin T, Taskinen T, Nevalainen A, Korppi M. 2001. Skin-prick test findings in students from moisture- and mould-damaged schools: a 3-year follow-up study. Pediatr. Allergy Immunol. 12:87–94 [DOI] [PubMed] [Google Scholar]
- 25. Jarvis WR. 1980. Epidemiology, p 219–250 In Coley-Smith JR, Verhoeff K, Jarvis WR. (ed), The biology of Botrytis. Academic Press, London, United Kingdom [Google Scholar]
- 26. Jürgensen CW, Madsen AM. 2009. Exposure to the airborne mould Botrytis and its health effects. Ann. Agric. Environ. Med. 16:183–196 [PubMed] [Google Scholar]
- 27. Karlsson-Borgå Å, Jonsson P, Rolfsen W. 1989. Specific IgE antibodies to 16 widespread mold genera in patients with suspected mold allergy. Ann. Allergy 63:521–526 [PubMed] [Google Scholar]
- 28. Kildesø J, Würtz H, Nielsen KF, DAMBIB group 1999. Quantification of the release of fungal spores from water damaged plasterboards. Danish-Finnish Workshop on Moulds in Buildings, Helsingør, Denmark, 7 to 8 October 1999 [Google Scholar]
- 29. Kildesø J, et al. 2003. Determination of fungal spore release from wet building materials. Indoor Air 13:148–155 [DOI] [PubMed] [Google Scholar]
- 30. Koivikko A, Viander M, Lanner A. 1991. Use of the extended Phadebas RAST panel in the diagnosis of mould allergy in asthmatic children. Allergy 46:85–91 [DOI] [PubMed] [Google Scholar]
- 31. Korhonen K, et al. 2006. Skin test reactivity to molds in pre-school children with newly diagnosed asthma. Pediatr. Int. 48:577–581 [DOI] [PubMed] [Google Scholar]
- 32. Limpert E, Stahel WA, Abbt M. 2001. Log-normal distributions across the sciences: keys and clues. Bioscience 51:341–352 [Google Scholar]
- 33. Lopez-Herrera CJ. 1988. Levels of airborne Botrytis cinerea conidia trapped among pepper (Capsicum annum L.) and eggplant (Solanum melongena L.) crops cultivated in polyethylene greenhouses on the Málaga Coastel Plain (Southern Spain). J. Phytopathol. 122:274–280 [Google Scholar]
- 34. Madsen AM, Frederiksen MW, Allermann L, Pejtersen JH. 2011. (1 → 3)-beta-d-glucan in different background environments and seasons. Aerobiologia 27:173–179 [Google Scholar]
- 35. Madsen AM, Kruse P, Schneider T. 2006. Characterization of microbial particle release from biomass and building material surfaces for inhalation exposure risk assessment. Ann. Occup. Hyg. 50:175–187 [DOI] [PubMed] [Google Scholar]
- 36. Madsen AM, Schlünssen V, Olsen TT, Sigsgaard T, Avci H. 2009. Airborne fungal and bacterial components in PM1 dust from biofuel plants. Ann. Occup. Hyg. 53:749–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Madsen AM, Wilkins CK, Poulsen OM. 2005. Micro-particles from fungi, p 276–291 In Johanning E. (ed), Bioaerosols, fungi, bacteria, mycotoxins and human health: patho-physiology, clinical effects, exposure assessment, prevention and control in indoor environments and work. Fungal Research Group Foundation, Inc., Albany, NY [Google Scholar]
- 38. Mazique D, et al. 2011. Predictors of airborne endotoxin concentrations in inner city homes. Environ. Res. 111:614–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Møller J, Miller M, Kjøller A. 1999. Fungal-bacterial interaction on beech leaves: infuence on decomposition and dissolved organic carbon quality. Soil Biol. Biochem. 31:367–374 [Google Scholar]
- 40. Oreszczyn T, Ridley I, Hong SH, Wilkinson P. 2011. Mould and winter relative humidity in low income households in England. Indoor Built Environ. 15:125–135 [Google Scholar]
- 41. Pasanen A-L, Kalliokoski P, Pasanen P. 1991. Laboratory studies on the relationship between fungal growth and atmospheric temperature and humidity. Environ. Int. 17:225–228 [Google Scholar]
- 42. Pasanen A-L, Pasanen P, Jantunen MJ, Kalliokoski P. 1991. Significance of air humidity and air velocity for fungal spore release into the air. Atmos. Environ. 25:459–462 [Google Scholar]
- 43. Rautiala S, et al. 1996. Exposure to airborne microbes during the repair of moldy buildings. Am. Ind. Hyg. Assoc. J. 57:279–284 [DOI] [PubMed] [Google Scholar]
- 44. Reponen T, Willeke K, Ulevicius V, Reponen A, Grinshpun SA. 1996. Effect of relative humidity on the aerodymamic diameter and respiratory deposition of fungal spores. Atmos. Environ. 30:3967–3974 [Google Scholar]
- 45. Seo SC, Reponen T, Levin L, Borchelt T, Grinshpun SA. 2008. Aerosolization of particulate (1→3)-beta-d-glucan from moldy materials. Appl. Environ. Microbiol. 74:585–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Seuri M, et al. 2000. An outbreak of respiratory diseases among workers at a water-damaged building—a case report. Indoor Air 10:138–145 [DOI] [PubMed] [Google Scholar]
- 47. Shelton BG, Kirkland KH, Flanders WD, Morris GK. 2002. Profiles of airborne fungi in buildings and outdoor environments in the United States. Appl. Environ. Microbiol. 68:1743–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Simeray J, Mandin D, Mercierr M, Chaumont J-P. 2001. Survey of viable airborne fungal propagules in French wine cellars. Aerobiologia 17:19–24 [Google Scholar]
- 49. Spieksma FT, Nolard N, Beaumont F, Vooren PH. 1987. Concentrations of airborne Botrytis conidia, and frequency of allergic sensitization to Botrytis extract. Experientia Suppl. 51:165–167 [DOI] [PubMed] [Google Scholar]
- 50. Stahmann KP, Pielken P, Schimz KL, Sahm H. 1992. Degradation of extracellular beta-(1,3)(1,6)-d-glucan by Botrytis cinerea. Appl. Environ. Microbiol. 58:3347–3354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Summerbell RC, Krajden S, Kane J. 1989. Potted plants in hospitals as reservoirs of pathogenic fungi. Mycopathologia 106:13–22 [DOI] [PubMed] [Google Scholar]
- 52. Tendal K, Madsen AM. 2011. Exposure to airborne microorganisms, hyphal fragments, and pollen in a field of organically grown strawberries. Aerobiologia 27:13–23 [Google Scholar]
- 53. Tercelj M, Salobir B, Harlander M, Rylander R. 2011. Fungal exposure in homes of patients with sarcoidosis—an environmental exposure study. Environ. Health. doi:10.1186/1476-069X-10-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Thomas CS, Marois JJ, English JT. 1988. The effects of wind speed, temperature, and relative humidity on development of aerial mycelium and conidia of Botrytis cinerea on grape. Phytopathology 78:260–265 [Google Scholar]
- 55. Trout D, Bernstein J, Martinez K, Biagini R, Wallingford K. 2001. Bioaerosol lung damage in a worker with repeated exposure to fungi in a water-damaged building. Environ. Health Perspect. 109:641–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tsai SJ, Huang RF, Ellenbecker MJ. 2010. Airborne nanoparticle exposures while using constant-flow, constant-velocity, and air-curtain-isolated fume hoods. Ann. Occup. Hyg. 54:78–87 [DOI] [PubMed] [Google Scholar]
- 57. Weast RC, Melvin AJ. 1981. CRC handbook of chemistry and physics. CRC Press, Inc., Boca Raton, FL [Google Scholar]
- 58. Williamson B, Tudzynski B, Tudzynski P, Kan JALV. 2007. Botrytis cinerea: the cause of grey mould disease. Mol. Plant Pathol. 8:561–580 [DOI] [PubMed] [Google Scholar]