Abstract
Bioturbated sediments are thought of as areas of increased denitrification or fixed-nitrogen (N) loss; however, recent studies have suggested that not all N may be lost from these environments, with some N returning to the system via microbial dinitrogen (N2) fixation. We investigated denitrification and N2 fixation in an intertidal lagoon (Catalina Harbor, CA), an environment characterized by bioturbation by thalassinidean shrimp (Neotrypaea californiensis). Field studies were combined with detailed measurements of denitrification and N2 fixation surrounding a single ghost shrimp burrow system in a narrow aquarium (15 cm by 20 cm by 5 cm). Simultaneous measurements of both activities were performed on samples taken within a 1.5-cm grid for a two-dimensional illustration of their intensity and distribution. These findings were then compared with rate measurements performed on bulk environmental sediment samples collected from the lagoon. Results for the aquarium indicated that both denitrification and N2 fixation have a patchy distribution surrounding the burrow, with no clear correlation to each other, sediment depth, or distance from the burrow. Field denitrification rates were, on average, lower in a bioturbated region than in a seemingly nonbioturbated region; however, replicates showed very high variability. A comparison of denitrification field results with previously reported N2 fixation rates from the same lagoon showed that in the nonbioturbated region, depth-integrated (10 cm) denitrification rates were higher than integrated N2 fixation rates (∼9 to 50 times). In contrast, in the bioturbated sediments, depending on the year and bioturbation intensity, some (∼6.2%) to all of the N lost via denitrification might be accounted for via N2 fixation.
INTRODUCTION
Nitrogen (N) is an essential element contributing to the biological processes of all organisms (16), yet biologically available (fixed) N is often limiting for marine productivity (17, 78). Sediments play a key role in the global N cycle, yet our understanding of the benthic microbial processes involved in the N cycle, especially in regard to the sinks and sources of fixed N (16, 17), is incomplete and often debated. In coastal marine sediments, N is lost through the production of dinitrogen gas (N2), typically through the microbial processes of denitrification or through the conversion of ammonium and nitrite to N2 (anaerobic ammonium oxidation [anammox]) (26, 36). In sediments impacted by bioturbation (the biological reworking and ventilation of sediments by infauna [see reference 49 for a comprehensive overview of the current definition of bioturbation]), the rates of coupled nitrification-denitrification are believed to be increased (28), resulting in an even greater loss of N from sediments. It has been known that N2 fixation, the input of N into the system, plays a role in specific benthic environments, primarily within the photic zone (photosynthetic microbial mats, coral reef sediments, and sediments vegetated by sea grasses and marsh plants) (13, 14, 17, 34, 35). Only recently was it shown that subsurface sediments in bioturbated areas can represent habitats of significant N2 fixation by sulfate-reducing bacteria (SRB) (5, 6), further demonstrating that our current understanding of N cycling in benthic environments is limited.
Animal-sediment-microbe interactions and the environmental factors controlling these interactions are of great interest to benthic ecologists. In particular, the study of bioturbation has gained momentum in many areas of science (e.g., geology, sedimentology, and paleontology) because burrowing organisms may affect the majority of all surfaces on Earth (54), potentially >20,700 km3 of sediments worldwide (76), and over geological time scales (10, 65, 77). Bioturbating organisms can be regarded as “ecosystem engineers,” organisms that significantly modify the environment in such a way as to greatly change the availability of resources for other organisms (39, 54). Macrofaunal burrows are characterized by a species-specific architecture (32, 48) and vary substantially in size and permanence (4, 24, 32, 58, 84), making it challenging to evaluate the impact of bioturbation (the movement of particles) or bioirrigation (the movement of fluids) on the geochemistry and microbial ecology of inhabited sediments. The construction and maintenance of burrows increase the oxic-anoxic interface, which may enhance microbial activity and result in increased cell abundances (1, 58, 59). Processes within the N cycle are particularly linked to the oxic-anoxic interface. Ammonium, which is released through the remineralization of organic matter, is reoxidized to nitrate in the oxic zone (nitrification). The denitrification of nitrate, in the absence of oxygen, to nitrite and further to N2 is considered to be the major loss of N from the benthic system. Bioturbation, by enhancing coupled nitrification-denitrification activities, is thought to further increase the loss of N2. In a previous study (5), we showed that subsurface N2 fixation by SRB can be an important source of N in bioturbated sediments. The question is whether the input of fixed N balances the loss of N via denitrification in bioturbated systems.
In this study, we used field and laboratory measurements to examine the influence of bioturbation on denitrification and N2 fixation in coastal sediments bioturbated by the bay ghost shrimp Neotrypaea californiensis Dana 1854 (Crustacea: Decapoda: Thalassinidea). N. californiensis inhabits intertidal areas along the west coast of North America from Alaska to Baja California (51) and belongs to a group of decapods, Thalassinidea, of which there are more than 550 known taxa worldwide (25). Thalassinidean burrow morphology is species specific (23, 24, 31, 73), with some thalassinids being capable of creating 3-m-deep burrows (60). The burrow structure relates to the feeding mode (i.e., deposit feeders, drift catchers, or filter or suspension feeders) of the thalassinid (32, 73), but environmental and biological conditions, such as grain size or population density, can also influence burrow morphology (31). N. californiensis is known to construct a highly branching and deep-reaching (∼80-cm) burrow system (51, 75) and spends the majority of its life subsurface, constantly reworking and maintaining its burrow while deposit feeding (12, 51). We investigated the denitrification and N2 fixation activity associated with burrow systems created by N. californiensis in an intertidal lagoon on Santa Catalina Island off the coast of Los Angeles, CA. The overall goal of these detailed and high-resolution measurements was to quantify N inputs and losses in a bioturbated system to evaluate the role of coastal sediments as sinks or sources of fixed N.
MATERIALS AND METHODS
Study site.
Investigations were carried out in an intertidal lagoon located in Catalina Harbor, Catalina Island, CA (33°25.23′N, 118°19.42′W), in July and August 2009. The lagoon is a shallow (<2-m) area consisting of muddy sand (with the majority of grains being <500 μm). Tides at this location are mixed, with the higher high water preceding the lower low water and a range of ∼1.7 m (19). During sampling, the water temperature varied little and was typically in the range of 18°C to 20°C, and salinity was 34.5‰.
Two intertidal sampling areas, one with N. californiensis bioturbation (∼500 burrow openings m−2 seafloor) and one without (0 burrow openings m−2 seafloor), were chosen for detailed investigations. The same areas (within a few meters) had previously been investigated to determine N2 fixation rates in June 2007 and May 2008 (5). The burrow density at each sampling location was determined by counting the number of burrow openings within a 25-cm by 25-cm frame, with 10 replicates counted. Note that the nonbioturbated area received higher levels of subsurface organic carbon input from the root systems of a surrounding marsh area and was characterized by slightly coarser sediment. At the bioturbated location, N. californiensis burrows reached ∼20 cm deep into the sediment (4). Typically, each burrow system has multiple branches and 3 to 4 openings to the sediment surface. The burrows consist of shafts (∼1-cm diameter) and chambers (∼2-cm diameter) that the shrimp maintains and frequently flushes with oxygen-rich water.
Sampling and sediment characteristics.
A set of three parallel sediment push cores (diameter of 5.4 cm; 30 cm long) were collected during high tide from each sampling location. Each core was collected randomly, with no specific orientation toward N. californiensis burrow openings. Two cores from each set were sliced in 2-cm intervals down to a depth of 20 cm under an N2 atmosphere, and each section was subsampled for further geochemical processing, as follows. Pore water was collected from each interval of one core by centrifugation (10 min at 3,500 rpm) using 50-ml Macrosep centrifugal cell concentrators (Pall Corporation, Life Sciences). Pore water samples (∼3 ml) were immediately frozen at −20°C for later determinations of fixed N concentrations: ammonium by flow injection analysis modified for small sample volumes (33) and nitrate by reduction to nitrite with spongy cadmium followed by spectrophotometry (40). On the second core, the total organic carbon (TOC), calculated as the loss on ignition (LOI), was determined for each 2-cm section by drying a known volume of sediment at 65°C for 24 h and then combusting the sample at 450°C for 24 h.
Catalina Harbor denitrification.
The third sediment core from each location was sliced in 2-cm intervals and analyzed for denitrification rates by use of an acetylene (C2H2) inhibition method in which C2H2 blocks the transformation of N2O to N2 in the denitrification pathway (70), causing an accumulation of N2O, which can be measured by gas chromatography (20) or by using N2O microsensors (7, 63). Two known potential drawbacks of the inhibition method are that C2H2 inhibition may be incomplete (66), especially when hydrogen sulfide is present (47), and that C2H2 may inhibit nitrification, causing a decrease in levels of NO3− over time (20, 37). To help alleviate this second problem, it was suggested previously that NO3− be added to incubation mixtures (44). Both potential drawbacks would lead to an underestimation rather than an overestimation.
In this study, triplicate 5-cm3 samples from each location and each depth were placed into 9-ml serum vials that were flushed with N2 and contained 400 μl of 110 μM potassium nitrate. This addition of potassium nitrate led to a final concentration of ∼20 μM NO3− in the samples, which is half the highest NO3− concentration seen previously at this study site (4). Prior to the start of incubation, initial N2O concentrations in each vial were determined by using a N2O microsensor that was inserted into the sediment (Unisense, Aarhus, Denmark). Microsensor signals were amplified and transformed into millivolts by a 2-channel picoammeter (PA 2000; Unisense) and directly recorded on a computer by using Profix software (Unisense). Each vial was then closed with a butyl stopper, crimp sealed, and injected with 1 ml of C2H2. Vials were kept in the dark and at the in situ temperature (20°C). After approximately 6 h, an analysis of N2O with microsensors was performed again as an endpoint measurement. Additionally, over the course of the experiment, another set of vials was prepared similarly, using sediment from the 3-cm-depth horizon of the bioturbated sampling location. The sediment was thoroughly mixed, and 36 5-cm3 samples were placed into 9-ml serum vials that were flushed with N2 and contained 400 μl of 110 μM potassium nitrate. Each vial was closed with a butyl stopper, crimp sealed, injected with 1 ml of C2H2, and kept in the dark and at the in situ temperature. Every hour, over the course of 36 h, the N2O in one vial was measured by using the N2O microsensor to ensure that N2O production remained linear over this time frame.
Laboratory mesocosm studies.
A detailed examination of an N. californiensis burrow was performed by using a narrow aquarium (Fig. 1) that was kept in a wet laboratory at the Wrigley Institute for Environmental Studies, located on Santa Catalina Island. The aquarium was 15 cm tall by 20 cm wide by 5 cm deep, providing enough space for the ghost shrimp to establish a burrow system. The aquarium was filled with sediment collected from the study site that was sieved through a 500-μm sieve to remove large sediment particles and sediment-dwelling macrofauna. One shrimp was added to the aquarium after 24 h, when the sediment had sufficiently settled. The narrow aquarium was placed into a larger container so that the sediment surface was continually flushed with seawater pumped to the laboratory from an offshore location. Before it reached the laboratory, the seawater passed through a rough sieve to remove larger particles. The shrimp was not fed in order to keep the laboratory setup as similar to natural conditions as possible (i.e., to not alter the amount of organic carbon available). Because the shrimp feeds on suspended matter that is supplied in the ventilation current, as well as on sediment microbiota, the shrimp had access to its natural nutrition sources. The entire setup was left for 1 month, allowing enough time to establish a burrow system. Once a burrow was established, transparencies were placed against the aquarium so that the visible burrow structures could be traced and exact sampling locations could be selected and recorded. Photographs of the aquarium were also taken with a Nikon model D40 digital camera (Fig. 1). The front wall of the aquarium was perforated with holes in a 1-cm grid that were filled with aquarium silicone. The silicone-filled holes served as ports for pore water sampling, especially for targeting specific burrow structures. This front wall was also easily removable so that samples could be collected from the exposed sediment in selected areas.
Fig 1.
Photograph of the narrow aquarium and burrow system, with the bioturbating ghost shrimp visible.
Pore water (3 ml) was drawn from 6 different burrow compartments for the determination of ammonium (33) and nitrate (40) concentrations within the burrow system. Vertical microprofiles of the redox potential (Unisense, Aarhus, Denmark) were performed at 1-mm increments from the overlying water into the sediment and in 2-cm horizontal intervals across the width of the narrow aquarium. The sensor was attached to a motorized micromanipulator (Märzhäuser, Wetzlar, Germany) controlled by a computer and connected to a high-impedance mV meter (WTW-pH 340; WTW GmbH, Weilheim, Germany).
Mesocosm microbial rate measurements.
To map variations in denitrification and N2 fixation (measured as nitrogenase activity [NA]) around an individual shrimp burrow, sediment samples were taken from the aquarium in a grid with a 1.5-cm spatial resolution. Because denitrification and NA measurements both use acetylene (C2H2), we were able to perform both analyses on the same individual samples, as was done in previous studies (e.g., see reference 44). For sediment sampling, the aquarium was laid on a slight incline so that all sediment and pore water would remain undisturbed while the front wall of the aquarium was removed. A center portion of the aquarium containing visible burrow structures was selected for sampling (13.5 cm wide and 9.5 cm high), avoiding edge effects. The sediment was cored by using 5-ml plastic syringes with the tip cut off following a 1.5-cm resolution grid. Single 5-cm−3 samples were placed into 9-ml serum vials that were flushed with N2 and contained 200 μl of 220 μM potassium nitrate, again resulting in a final concentration of ∼20 μM NO3− in the samples. A higher concentration of potassium nitrate was used to ensure that denitrification measurements could run longer. Prior to the start of incubation, initial measurements of the N2O level in each vial were made by using the N2O microsensor (Unisense, Aarhus, Denmark). Each vial was then crimp sealed and injected with 1 ml of C2H2. Vials were kept in the dark, at an in situ temperature, and were incubated over a 36-h time period.
NA measurements of the aquarium samples were obtained by using a C2H2 reduction assay (15). C2H2 is a substrate analog of N2 gas and is preferentially reduced by the conventional (molybdenum-based) nitrogenase enzyme to ethylene (C2H4), which is easily quantified by gas chromatography using a flame ionization detector (FID). It is important to note that C2H2 has been found to inhibit a number of physiological groups, including some nitrifiers, methylotrophs, and methanogens, and caution is advised for interpreting data for environments where those groups are important (57).
In this experiment, C2H4 was assayed over a 36-h incubation period (while the NA was linear) with a Shimadzu Mini-2 gas chromatograph (GC) fitted with an FID and a 6-ft Alltech column packed with Hayesep A. Data were taken from a total of 5 time points, including sampling at time zero. At each time point, 100 μl of headspace gas from each vial was sampled and directly injected into the GC. N2 fixation rates were calculated from the NA results by using a conversion factor of 3 moles of C2H4 to 1 mole of N2. After the C2H4 measurements were finished, analysis of N2O with microsensors was performed again as an endpoint for denitrification. Both results of the determination of rates were then plotted on a two-dimensional (2-D) contour graph. The location of the burrow (as traced on transparencies) was overlaid onto these graphs so that direct comparisons between the measured rates in relation to where the sample was taken could be made.
Testing of the effect of the addition of seawater and potassium nitrate on C2H2 reduction.
Perturbation experiments are generally performed by the addition of the test compound diluted in seawater (e.g., in this experiment, NO3− was added). To test the effect of the addition of the seawater itself on the reduction of the C2H2 concentration, a set of vials was prepared by using sediment from the 0- to 2-cm-depth horizon of the selected bioturbated location from our field studies. The sediment was thoroughly mixed, and 4 sets of triplicate 4-cm3 samples were placed into 9-ml serum vials that were flushed with N2. Each set was then subjected to a different volume of filtered anoxic seawater: 0, 0.5, 2, and 4 ml. Each vial was crimp sealed and injected with 1 ml of C2H2, and the increase in the level of C2H4 was assayed over a 16-h period with a GC. Over this time period, data from a total of 4 time points were taken, including sampling at time zero. Incubation mixtures were kept in the dark and at the in situ temperature (20°C). Afterwards, the percent decrease due to the addition of water was calculated by using the 0-ml addition as the starting point. These calculations accounted for the differences in volume in each vial as well as the partitioning of C2H4 between the water and gas phases. Similarly, to test the effect of the addition of potassium nitrate on the measurements of the reduction of C2H2 levels, another set of vials was prepared by using sediment from the 0- to 5-, 5- to 10-, and 10- to 15-cm-depth horizons from the same bioturbated sampling location. Samples were collected down to 15 cm so as to match the depth of our aquarium setup. The sediment from each depth was thoroughly mixed, and 4-cm3 samples were placed into 9-ml serum vials that were flushed with N2. For each depth, 2 sets of 5 replicates were prepared. Four hundred microliters of 110 μM potassium nitrate in filtered anoxic seawater was added to one set, and 400 μl of filtered anoxic seawater was added to the other set. Each vial was then crimp sealed and injected with 1 ml of C2H2, and the increase in the C2H4 level was assayed over a 24-h period by gas chromatography. Over this time period, samples were taken at a total of 3 time points, including the sampling at time zero. Incubation mixtures were kept in the dark and at the in situ temperature (20°C). Statistical comparisons of the NA between samples after the addition of potassium nitrate and samples after the addition of seawater from the same sediment depth horizon were performed by using a one-tailed t test. The results of these methodological tests showed that the addition of anoxic filtered seawater to the sediment slurry caused the NA to decrease by ∼63% on average, regardless of the amount of seawater added (Table 1). In contrast, the addition of nitrate to slurry incubation mixtures did not significantly increase or decrease NA measurements compared to samples with only seawater added (Table 2).
Table 1.
Comparison of NAs between slurry and nonslurry incubation mixtures
| Amt of water added (ml) | Mean NA (nmol C2H4 cm−3 h−1) ± SE | % decrease in NA |
|---|---|---|
| 0 | 0.48 ± 0.15 | |
| 0.5 | 0.17 ± 0.07 | 64 |
| 2 | 0.16 ± 0.03 | 66 |
| 4 | 0.20 ± 0.07 | 58 |
Table 2.
Effect of the addition of nitrate on NA
| Sampling location depth (cm) | % decrease in NA | P valuea |
|---|---|---|
| 0–5 | 5.7 | 0.53 |
| 5–10 | 8.4 | 0.31 |
| 10–15 | −9.2 | 0.07 |
Determined by a two-tailed t test.
RESULTS
Environmental parameters of Catalina Harbor cores.
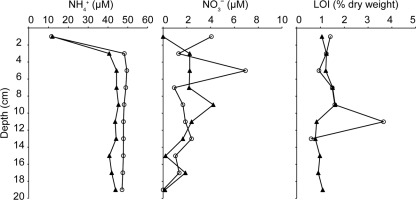
Ammonium (NH4+) concentrations in the top 2 cm from both locations (the bioturbated area, with ∼500 burrow openings m−2, and the nonbioturbated area, with 0 burrow openings m−2) were characterized by very similar values of ∼10 μM (Fig. 2). Below this depth, both locations showed increased NH4+ concentrations down to a 3-cm depth, and below this depth, the concentrations remained stable at ∼40 μM for the bioturbated location and ∼50 μM for the nonbioturbated location. Nitrate (NO3−) concentrations, although varying with depth, showed no clear pattern and were below 8 μM for both locations (Fig. 2). The loss on ignition (LOI) at both sites typically ranged from 1 to 2% sediment dry weight (Fig. 2). The nonbioturbated location showed a large peak in the LOI (3.6% dry weight) at an 11-cm depth, where an oily tar-like substance and an abundance of plant roots were noted during core slicing.
Fig 2.
Depth profiles of ammonium (NH4+), nitrate (NO3−), and total organic carbon calculated by the loss on ignition (LOI) in sediment cores collected from a bioturbated area (○) and a nonbioturbated area (▲).
N2O production in Catalina Harbor sediments over time.
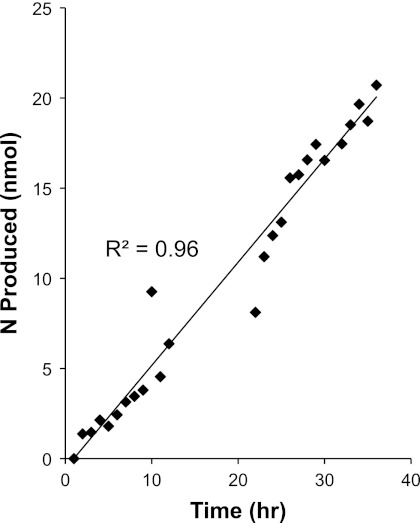
Over the course of 36 h, N2O production (Fig. 3) remained linear (R2 = 0.96), indicating that our Catalina Harbor and mesocosm denitrification rates were not compromised by the incubation time. These results support data reported previously by Jenkins and Kemp (38), who found that 15N2 production in intact estuarine sediment cores remained linear over a 50-h time span.
Fig 3.
Nitrogen production in serum vials measured by an N2O microsensor. These measurements were performed to test the linearity of denitrification over a 36-h time period.
Catalina Harbor denitrification activity.
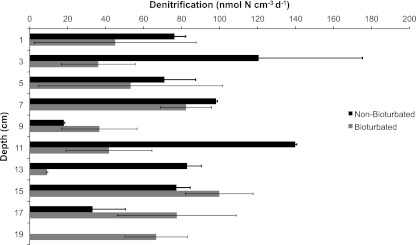
Denitrification rates for both locations were highly variable with depth, ranging from 18.0 ± 0.5 to 139.9 ± 0.8 nmol N cm−3 day−1 for the nonbioturbated area and from 9.1 ± 0.5 to 99.9 ± 17.9 nmol N cm−3 day−1 for the bioturbated area (Fig. 4). On average, the bioturbated location showed more variability in denitrification rates at each depth, with the exception of the 3- and 13-cm depths. The two depths that showed the greatest difference were the 11- and 13-cm depths, where the nonbioturbated rates were much higher than the bioturbated rates (Fig. 4). These depths coincided with the large observed peak in the organic carbon concentration (or LOI).
Fig 4.
Denitrification measurements in sediment cores collected from a bioturbated area (light) and a nonbioturbated area (dark). Error bars represent standard errors (n = 3).
Mesocosm ammonium and nitrate concentrations.
The NH4+ and NO3− concentrations within the aquarium did not vary greatly between locations in the burrow and in the overlying water (Table 3). The deepest sampling location (a burrow compartment at a 9-cm depth) had no detectable NO3− and the highest NH4+ concentrations (8.27 ± 0.24 μM). However, this NH4+ concentration was lower than what was measured in the field at the same depth (∼45 μM), suggesting that the shrimp periodically ventilates its burrow, keeping the burrow at far lower NH4+ concentrations than those in the surrounding sediment. Vertical profiles of redox potential (mV) measurements depicted as a contour plot (Fig. 5) showed an oxidized zone (positive redox potential) at the sediment surface. With depth, the redox potential decreased and eventually became negative (indicating a reduced zone) near part of the burrow at around a 5-cm depth.
Table 3.
Ammonium and nitrate concentrations of selected burrow compartments
| Location | Mean NH4+ concn (μM) ± SE | NO3− concn (μM) |
|---|---|---|
| Overlying water | 4.15 ± 0.13 | 1.35 |
| Burrow opening | 3.95 ± 0.17 | 1.41 |
| Burrow opening | 4.41 ± 0.00 | 1.82 |
| 4-cm-deep burrow compartment | 4.15 ± 0.17 | 1.20 |
| 8-cm-deep burrow compartment | 5.92 ± 0.35 | 2.19 |
| 9-cm-deep burrow compartment | 8.27 ± 0.24 | 0.00 |
Fig 5.
Contour plots of redox potential (A), denitrification (B), N2 fixation (C), and ratio of denitrification to N2 fixation (denit:NF) (D) measured within an aquarium inhabited with a single ghost shrimp. The figures on the right show those portions of the burrow (in white) that were visible at the aquarium wall at the time of sampling, and dots indicate where sampling was performed.
Mesocosm microbial rates.
Denitrification rates surrounding the burrow (Fig. 5) showed a patchwork distribution, with no clear trend in relation to distance from the burrow or with sediment depth. Integrated denitrification rates at each sampling row ranged from 71 to 118 nmol N cm−2 day−1. N2 fixation rates also showed a patchwork distribution, with no statistically significant relation to distance from the burrow or with sediment depth, but many of the areas displaying the highest N2 fixation rates appeared to occur along the underside of the burrow. Integrated N2 fixation rates at each sampling row ranged from 3.6 to 4.6 nmol C2H4 cm−2 day−1 (or 2.4 to 3.1 nmol N cm−2 day−1). Looking at the individual sampling locations, the ratio of denitrification to N2 fixation (Fig. 5) ranged from 0.73 to 240.9, with the highest value seen for the samples from the area located beneath the burrow at a 6-cm sediment depth. Similar to the rate values themselves, the ratio showed no statistically significant distribution in relation to distance from the burrow or with sediment depth. Additionally, a comparison of denitrification versus N2 fixation at each location showed no correlation (Fig. 6). For a comparison of the 70 locations where both rates were measured, the denitrificaiton-to-N2 fixation ratios were <10 for 5 locations and <50 for 38 niches, and a ratio of >100 was measured for only 9 locations. However, both denitrification and N2 fixation were measured in the same vials, which contained 200 μl of 220 μM potassium nitrate. While the addition of nitrate did not show an effect on measurements of the NA, the addition of water showed an adverse effect on the NA, averaging a 63% reduction in measured rates. When the N2 fixation results were corrected according to this percentage, the ratios of denitrification to N2 fixation shifted to 0.46 to 151.7, with 9 locations showing a ratio of <10, 56 locations showing a ratio of <50, and only 1 location exhibiting a ratio of >100.
Fig 6.
Denitrification versus N2 fixation from aquarium sediment samples that were incubated in the same serum vials.
DISCUSSION
N in benthic environments.
Marine sediments play an extremely important, if complicated and not-well-understood, role in oceanic N cycling. It has been estimated that the remineralization of organic matter in benthic environments can supply up to 80% of the N required by phytoplankton in coastal waters (9, 11, 21). When bioturbation or bioirrigation is present, the flux of dissolved N from the sediment into the water column can greatly increase. A recent study showed that 76% of the benthic efflux of NH4+ within a shallow bay was due to bioirrigation (22). In some areas of the world ocean, according to isotope mass balance, up to 75% of the N lost from the marine system, via the conversion of fixed N into N2 by denitrification, occurs in benthic environments (18, 68). It was suggested previously that this loss of N may indirectly regulate primary production in the coastal ecosystem (46, 56). Conversely, climate-induced decreases in primary production may cause heterotrophic marine sediments to switch from being a net sink, via denitrification, to being a net source of N, via N2 fixation, which is explained by a decreased export of organic matter to the seafloor under these conditions and, possibly, N limitation (27). However, most estimates of benthic N processes are based on overall fluxes of N species across the sediment-water interface, with few studies directly exploring the N cycling occurring subsurface within different microniches that are important in bioturbated sediments (4).
Marine sediments are generally characterized by geochemical gradients created by the consumption of electron acceptors coupled to the oxidation of organic carbon. Oxygen, the most energetically favorable electron acceptor, is rapidly consumed at the sediment-water interface, followed by other electron acceptors in the order of decreasing energy gain (3, 42). As macrofauna move through the sediment, particles are rapidly transported between oxic and anoxic conditions, causing redox oscillations that disrupt the local electron acceptor succession with depth (2, 45). Those redox oscillations produced by bioturbation may result in a faster and more complete decomposition of organic matter (74), as well as producing unique microbial communities surrounding the bioturbated area (52, 53, 62). The formation of this unique microbial community structure can have major impacts on the local benthic N cycle through enhanced microbial remineralization processes (see reference 50 and references therein). Previous studies have shown that bioturbation can greatly increase coupled nitrification-denitrification rates through the extension of the sediment-water interface, an increase in solute transport, and the oxygenation of sediments (28, 29, 34). Other studies have shown that bioturbation can significantly increase subsurface benthic N2 fixation rates, linked to sulfate reduction (5, 6), showing that N cycling in bioturbated sediments may be more complex than previously thought.
Bioturbation and organic carbon availability.
In Catalina Harbor sediments, denitrification appears to be more closely related to organic carbon availability than to bioturbation activity alone. The highest average denitrification rate occurred in the nonbioturbated location at an 11-cm sediment depth (Fig. 4), where a spike in the organic carbon concentration (LOI) was observed (Fig. 4). This result is not surprising, as organic matter is known to enhance denitrification. Additionally, van Luijn et al. (79) reported previously that denitrification rates in muddy versus sandy sediments were greatly influenced by the level of fresh organic matter. Bioturbation itself can increase the organic matter content of sediments, and this has been shown to increase sulfate reduction activity in the vicinity of burrows (5, 6, 30). The product of sulfate reduction (hydrogen sulfide) can, in turn, have inhibitory effects on nitrification and thus potentially limit the loss of N through coupled nitrification-denitrification (43). However, in iron-rich sediments, such as those in Catalina Harbor, hydrogen sulfide is rapidly precipitated as iron oxides, and although sulfate reduction rates are high, hydrogen sulfide concentrations in the pore water remain very low (at or below the detection limit of hydrogen sulfide microsensors). More importantly, SRB have the genetic capability to fix N2 (82), which was supported by data from previously reported culture and field studies (e.g., see references 55, 69, and 71), and have been shown to be responsible for the majority of N2 fixation in the bioturbated sediments of our study site (5). We speculate that the localized increase in the amount of organic matter enhanced N2 fixation by SRB, while the resulting sulfide inhibited nitrification and a loss of N through subsequent denitrification. In contrast, in the nonbioturbated location, where sulfate reduction rates have been shown to be lower than those found in a bioturbated area, supporting a bioturbation intensity similar to that seen in this study (5), the majority of the organic matter may be supporting increased denitrification rates.
Impacts of microniche formation on benthic N cycling.
The bioturbated location of Catalina Harbor typically showed extremely variable denitrification rates, indicating high levels of sediment heterogeneity and the possible presence of denitrifying microniches. This high spatial variability was also seen in the measurements of denitrification and N2 fixation rates in the narrow aquarium (Fig. 5). Interestingly, a correlation between denitrification, N2 fixation, sediment depth, and distance from the burrow could not be seen (Fig. 6). Because denitrification is an anaerobic process, one would expect that denitrification rates would increase with distance from the burrow as the sediment becomes more reduced. However, denitrification has been shown to occur under aerobic conditions within intact fecal pellets that form an anaerobic organic-rich microniche within the sediment (64). In theory, the diameter of these fecal pellets would need to be >2 mm to maintain a reduced microniche within an oxygen-saturated environment (41). While thalassinids often deposit their fecal pellets on the sediment surface, pellets can sometimes be pushed into the burrow wall as a substrate (8). Thalassinids are known to produce rod-shaped fecal pellets that can range from 0.75 to 4 mm in diameter and 2 to 10 mm in length (61, 67), meaning that thalassinid fecal pellets could be large enough to produce an anaerobic microniche where denitrification could occur. These reduced microniches in N. californiensis-bioturbated sediments can also support increased sulfate reduction rates (6) and, through N2 fixation by SRB, may cause an increase in N2 fixation instead of denitrification, which may be inhibited by the increased sulfide concentrations (43) within the microniche.
Beyond fecal pellets, in particular, thalassinidean shrimp are known to secrete a mucus (polysaccharides), which, when mixed with sediment particles, is used to stabilize burrow walls (32), enriching burrow lining in organic carbon (Corg) and possibly creating organic-rich microniches. Furthermore, microniches can also form around decaying organisms (83), algal aggregates (81), other forms of organic matter (such as marine snow) that are deposited on the sediment surface and transported deeper into the sediment via bioturbation (72), and organic particles in seawater that are carried into the burrow via bioirrigation. As the sediment is bioturbated, these microniche-forming organic aggregates can be shifted, causing a complex and patchy distribution of microniches throughout the sediment column that can be seen by using whole-core resin embedding (80). This patchiness in organic aggregates may be the reason why there appeared to be no correlation between denitrification, N2 fixation, sediment depth, and distance from the burrow (Fig. 6) within the experimental setup. These findings support the notion that N cycling within bioturbated sediments is extremely complex and most likely driven by the formation of microniches surrounding the burrow system.
Denitrification versus N2 fixation in Catalina Harbor sediments.
The above-mentioned N2 fixation study of these same Catalina Harbor sediments (5) showed that summer (May through June) benthic N2 fixation rates, integrated down to 10 cm, can vary from 0.25 to 8.05 mmol N m−2 day−1, depending on the bioturbation intensity and the presence of a microbial mat at the surface. In contrast, it was shown that an area without bioturbation supported integrated N2 fixation rates of only 0.15 to 0.80 mmol N m−2 day−1. The integration of the denitrification rates found in this study down to a sediment depth of 10 cm revealed areal denitrification rates (denitrification rates per surface area) of 7.68 mmol N m−2 day−1 for the nonbioturbated location and 5.07 mmol N m−2 day−1 for the bioturbated location (Fig. 4). Comparing these rates with the rates previously reported for Catalina Harbor N2 fixation (5), the denitrification rate at the nonbioturbated location was ∼9 to 50 times higher than N2 fixation rates, suggesting a possible loss of fixed N. For the bioturbated location, the rate of denitrification was ∼20 times higher than the lowest rate reported previously (5) for N2 fixation (0.25 mmol N m−2 day−1), while the highest reported (5) N2 fixation rates (8.05 mmol N m−2 day−1) were higher than the denitrification rates by ∼2.98 mmol N m−2 day−1. Because these denitrification and N2 fixation measurements were made in separate years, these results should be taken as an estimate of possible fixed N loss or gain and not as an absolute budget.
Conclusions.
An understanding of N cycling in coastal environments is critical for an understanding of global element cycling because nitrification and denitrification rates are significantly higher in these shallow-water environments than in the deep sea (43). However, the question of whether these systems represent a sink or source of N is difficult to answer, because bioturbation activity impacts not only nitrification-denitrification activity but also subsurface N2 fixation. Although this heterogeneity of redox processes induced by macrofaunal activity represents a challenge in determining a net loss or gain, we can conclude that in bioturbated sediments, it is possible for the loss of N to be balanced by a gain of N via subsurface N2 fixation. Thus, shallow-water sediment environments can represent a source of bioavailable N. The processes within the benthic N cycle are impacted greatly by a change in environmental factors (temperature, salinity, oxygen availability, and nutrient and organic carbon loading); therefore, it is critical that more detailed studies are carried out to improve our understanding of the role of coastal environments in global N cycling.
ACKNOWLEDGMENTS
We acknowledge the entire staff of the USC Wrigley Institute for Environmental Studies (WIES) on Catalina Island, especially Lauren Czarnecki, for continuing support for our laboratory and field studies. We thank Douglas Capone for helpful discussions on methodology and use of equipment on Catalina Island. Additionally, we thank Michael Morando and Amanda Liss for their assistance with field and aquarium sampling.
This work was supported by a USC WIES graduate research fellowship awarded to V.J.B., USC start-up funds provided to W.Z., and USC Provost's undergraduate research fellowship and summer undergraduate research fund awarded to C.M.
Footnotes
Published ahead of print 23 March 2012
REFERENCES
- 1. Aller JY, Aller RC. 1986. Evidence for localized enhancement of biological activity associated with tube and burrow structures in deep-sea sediments at the HEBBLE site, western North Atlantic. Deep Sea Res. 33:755–790 [Google Scholar]
- 2. Aller RC. 1994. Bioturbation and remineralization of sedimentary organic matter: effects of redox oscillation. Chem. Geol. 114:331–345 [Google Scholar]
- 3. Berner RA. 1980. Early diagenesis—a theoretical approach. Princeton University Press, Princeton, NJ [Google Scholar]
- 4. Bertics VJ, Ziebis W. 2009. Biodiversity of benthic microbial communities in bioturbated coastal sediments is controlled by geochemical microniches. ISME J. 3:1269–1285 [DOI] [PubMed] [Google Scholar]
- 5. Bertics VJ, et al. 2010. Burrowing deeper into benthic nitrogen cycling: the impact of bioturbation on nitrogen fixation coupled to sulfate reduction. Mar. Ecol. Prog. Ser. 409:1–15 [Google Scholar]
- 6. Bertics VJ, Ziebis W. 2010. Bioturbation and the role of microniches for sulfate reduction in coastal marine sediments. Environ. Microbiol. 12:3022–3034 [DOI] [PubMed] [Google Scholar]
- 7. Binnerup SJ, Jensen K, Revsbech NP, Jensen MH, Sørensen J. 1992. Denitrification, dissimilatory reduction of nitrate to ammonium, and nitrification in a bioturbated estuarine sediment as measured with 15N and microsensor techniques. Appl. Environ. Microbiol. 58:303–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bishop GA, Williams AB. 2005. Taphonomy and preservation of burrowing thalassinidean shrimps. P. Biol. Soc. Wash. 118:218–236 [Google Scholar]
- 9. Blackburn TH, Henriksen K. 1983. Nitrogen cycling in different types of sediments from Danish waters. Limnol. Oceanogr. 28:447–493 [Google Scholar]
- 10. Bottjer DJ, Hagadorn JW, Dornbos SQ. 2000. The Cambrian substrate revolution. GSA Today 10:1–7 [Google Scholar]
- 11. Boynton WR, Kemp WM. 1985. Nutrient regeneration and oxygen consumption by sediments along an estuarine salinity gradient. Mar. Ecol. Prog. Ser. 23:44–55 [Google Scholar]
- 12. Brenchley GA. 1981. Disturbance and community structure, an experimental study of bioturbation in marine soft-bottom environments. J. Mar. Res. 39:767–790 [Google Scholar]
- 13. Capone DG. 1983. Benthic nitrogen fixation, p 105–137 In Carpenter EJ, Capone DG. (ed), Nitrogen in the marine environment. Springer, New York, NY: [DOI] [PubMed] [Google Scholar]
- 14. Capone DG. 1988. Benthic nitrogen fixation, p 85–123 In Blackburn TH, Sørensen J. (ed), Nitrogen cycling in coastal marine environments. Springer, New York, NY [Google Scholar]
- 15. Capone DG. 1993. Determination of nitrogenase activity in aquatic samples using the acetylene reduction procedure, p 621–631 Kemp PF, Sherr BF, Sherr EB, Coles JJ. (ed), Handbook of methods in aquatic microbial ecology. CRC Press, Boca Raton, FL [Google Scholar]
- 16. Capone DG, Knapp AN. 2007. A marine nitrogen cycle fix? Nature 445:159–160 [DOI] [PubMed] [Google Scholar]
- 17. Carpenter EJ, Capone DG. 2008. Nitrogen fixation in the marine environment, p 141–198 In Capone DG, Bronk DA, Mulholland MR, Carpenter EJ. (ed), Nitrogen in the marine environment. Academic Press, Elsevier, San Diego, CA [Google Scholar]
- 18. Codispoti LA. 2007. An oceanic fixed nitrogen sink exceeding 400 Tg N a−1 vs the concept of homeostasis in the fixed-nitrogen inventory. Biogeosciences 4:233–253 [Google Scholar]
- 19. Colbert SL, Berelson WM, Hammond DE. 2008. Radon-222 budget in Catalina Harbor, California. 2. Flow dynamics and residence time in a tidal beach. Limnol. Oceanogr. 53:659–665 [Google Scholar]
- 20. Cornwell JC, Kemp WM, Kana TM. 1999. Denitrification in coastal ecosystems: methods, environmental controls, and ecosystem level controls, a review. Aquat. Ecol. 33:41–54 [Google Scholar]
- 21. Dale AW, Prego R. 2002. Physico-biogeochemical controls on benthic-pelagic coupling of nutrient fluxes and recycling in a coastal upwelling system. Mar. Ecol. Prog. Ser. 235:15–28 [Google Scholar]
- 22. Dale AW, et al. 2011. Rates and regulation of nitrogen cycling in seasonally-hypoxic sediments during winter (Boknis Eck, SW Baltic Sea): sensitivity to environmental variables. Estuar. Coast Shelf Sci. 95:14–28 [Google Scholar]
- 23. Dworschak PC. 1983. The biology of Upogebia pusilla Petagna (Decapoda, Thalassinidea). 1. The burrows. Mar. Ecol. 4:19–43 [Google Scholar]
- 24. Dworschak PC. 2001. The burrows of Callianassa tyrrhena (PETAGNA, 1792) (Decapoda: Thalassinidea). Mar. Ecol. 22:155–166 [Google Scholar]
- 25. Dworschak PC. 2005. Global diversity in the Thallassinidea (Decapoda): an update (1998–2004). Nauplius 13:57–63 [Google Scholar]
- 26. Fennel K, et al. 2008. Modeling denitrification in aquatic sediments. Biogeochemistry 93:159–178 [Google Scholar]
- 27. Fulweiler RW, Nixon SW, Buckley BA, Granger SL. 2007. Reversal of the net dinitrogen gas flux in coastal marine sediments. Nature 448:180–182 [DOI] [PubMed] [Google Scholar]
- 28. Gilbert F, Bonin P, Stora G. 1995. Effect of bioturbation on denitrification in a marine sediment from the West Mediterranean littoral. Hydrobiologia 304:49–58 [Google Scholar]
- 29. Gilbert F, Stora G, Bonin P. 1998. Influence of bioturbation on denitrification activity in Mediterranean coastal sediments: an in situ experimental approach. Mar. Ecol. Prog. Ser. 163:99–107 [Google Scholar]
- 30. Goldhaber MB, et al. 1977. Sulfate reduction, diffusion, and bioturbation in Long Island Sound sediments: report of the FOAM group. Am. J. Sci. 277:193–237 [Google Scholar]
- 31. Griffis RB, Chavez FL. 1988. Effects of sediment type on burrows of Callianassa californiensis Dana and C. gigas Dana. J. Exp. Mar. Biol. Ecol. 117:239–253 [Google Scholar]
- 32. Griffis RB, Suchanek TH. 1991. A model of burrow architecture and trophic modes in thalassinidean shrimps (Decapoda: Thalassinidea). Mar. Ecol. Prog. Ser. 79:171–183 [Google Scholar]
- 33. Hall PO, Aller RC. 1992. Rapid small-volume flow injection analysis for ΣCO2 and NH+4 in marine and fresh waters. Limnol. Oceanogr. 37:1113–1119 [Google Scholar]
- 34. Herbert RA. 1999. Nitrogen cycling in coastal marine ecosystems. FEMS Microbiol. Rev. 23:563–590 [DOI] [PubMed] [Google Scholar]
- 35. Howarth RW, Marino R, Lane J, Cole JJ. 1988. Nitrogen fixation in freshwater, estuarine, and marine ecosystems. 1. Rates and importance. Limnol. Oceanogr. 33:669–687 [Google Scholar]
- 36. Hulth S, et al. 2005. Nitrogen removal in marine environments: recent findings and future research challenges. Mar. Chem. 94:125–145 [Google Scholar]
- 37. Hynes RK, Knowles R. 1978. Inhibition by acetylene of ammonia oxidation in Nitrosomonas europea. FEMS Microbiol. Lett. 4:319–321 [DOI] [PubMed] [Google Scholar]
- 38. Jenkins MC, Kemp WM. 1984. The coupling of nitrification and denitrification in two estuarine sediments. Limnol. Oceanogr. 29:609–619 [Google Scholar]
- 39. Jones CG, Lawton JH, Shachak M. 1994. Organisms as ecosystem engineers. Oikos 69:373–386 [Google Scholar]
- 40. Jones MN. 1984. Nitrate reduction by shaking with cadmium—alternative to cadmium columns. Water Res. 18:643–646 [Google Scholar]
- 41. Jørgensen BB. 1977. Bacterial sulfate reduction within reduced microniches of oxidized marine sediments. Mar. Biol. 41:7–17 [Google Scholar]
- 42. Jørgensen BB. 2000. Bacteria and marine biogeochemistry, p 173–208 In Schulz HD, Zabel M. (ed), Marine geochemistry. Springer-Verlag, Berlin, Germany [Google Scholar]
- 43. Joye SB, Hollibaugh JT. 1995. Influence of sulfide inhibition of nitrification on nitrogen regeneration in sediments. Science 270:623–625 [Google Scholar]
- 44. Joye SB, Smith SV, Hollibaugh JT, Paerl HW. 1996. Estimating denitrification rates in estuarine sediments: a comparison of stoichiometric and acetylene based methods. Biogeochemistry 33:197–215 [Google Scholar]
- 45. Jumars PA, Mayer LM, Deming JW, Baross JA, Wheatcroft RA. 1990. Deep-sea deposit-feeding strategies suggested by environmental and feeding constraints. Philos. Trans. R. Soc. A 331:85–102 [Google Scholar]
- 46. Kaspar HF, Asher RA, Boyer IC. 1985. Microbial nitrogen transformations in sediments and inorganic nitrogen fluxes across the sediment/water interface on the South Island West Coast, New Zealand. Estuar. Coast Shelf Sci. 21:245–255 [Google Scholar]
- 47. Koike I, Sørensen J. 1988. Nitrate reduction and denitrification in marine sediments, p 251–274 In Blackburn TH, Sørensen J. (ed), Nitrogen cycling in coastal marine environments. John Wiley & Sons, New York, NY [Google Scholar]
- 48. Kristensen E, Kostka JE. 2005. Macrofaunal burrows and irrigation in marine sediment: microbiological and biogeochemical interactions, p 125–157 In Kristensen E, Haese RR, Kostka JE. (ed), Interactions between macro- and microorganisms in marine sediment. Coastal and estuarine studies, vol 60 American Geophysical Union, Washington, DC [Google Scholar]
- 49. Kristensen E, et al. 2012. What is bioturbation? The need for a precise definition for fauna in aquatic sciences. Mar. Ecol. Prog. Ser. 446:285–302 [Google Scholar]
- 50. Laverock B, Gilbert JA, Tait K, Osborn AM, Widdicombe S. 2011. Bioturbation: impact on the marine nitrogen cycle. Biochem. Soc. Trans. 39:315–320 [DOI] [PubMed] [Google Scholar]
- 51. MacGinitie GE. 1934. The natural history of Callianassa californiensis Dana. Am. Midl. Nat. 15:166–177 [Google Scholar]
- 52. Mermillod-Blondin F, Rosenberg R, François-Carcaillet F, Norling K, Mauclaire L. 2004. Influence of bioturbation by three benthic infaunal species on microbial communities and biogeochemical processes in marine sediment. Aquat. Microb. Ecol. 36:271–284 [Google Scholar]
- 53. Meyers MB, Fossing H, Powell EN. 1987. Micro-distribution of interstitial meiofauna, oxygen and sulfide gradients, and the tubes of macro-infauna. Mar. Ecol. Prog. Ser. 35:223–241 [Google Scholar]
- 54. Meysman FJR, Middelburg JJ, Heip CHR. 2006. Bioturbation: a fresh look at Darwin's last idea. Trends Ecol. Evol. 21:688–695 [DOI] [PubMed] [Google Scholar]
- 55. Nielsen LB, et al. 2001. Sulfate reduction and nitrogen fixation rates associated with roots, rhizomes and sediments from Zostera noltii and Spartina maritima meadows. Environ. Microbiol. 3:63–71 [DOI] [PubMed] [Google Scholar]
- 56. Nixon SW. 1981. Remineralisation and nutrient cycling in coastal marine systems, p 111–138 In Neilson BJ, Cronin LE. (ed), Estuaries and nutrients. Humana Press, Clifton, NJ [Google Scholar]
- 57. Oremland RS, Capone DG. 1988. Use of specific inhibitors in biogeochemistry and microbial ecology, p 285–383 In Marshall KC. (ed), Advances in microbial ecology, vol 10 Plenum Press, New York, NY [Google Scholar]
- 58. Papaspyrou S, Gregersen T, Cox RP, Thessalou-Legaki M, Kristensen E. 2005. Sediment properties and bacterial community in the burrows of the mud shrimp Pestarella tyrrhena (Decapoda: Thalassinidea). Aquat. Microb. Ecol. 38:181–190 [Google Scholar]
- 59. Papaspyrou S, Gregersen T, Kristensen E, Christensen B, Cox RP. 2006. Microbial reaction rates and bacterial communities in sediment surrounding burrows of two nereidid polychaetes (Nereis diversicolor and N. virens). Mar. Biol. 148:541–550 [Google Scholar]
- 60. Pemberton SG, Risk MJ, Buckley DE. 1976. Supershrimp: deep bioturbation in the Strait of Canso, Nova Scotia. Science 192:790–791 [DOI] [PubMed] [Google Scholar]
- 61. Pryor WA. 1975. Biogenic sedimentation and alteration of argillaceous sediments in shallow marine environments. Geol. Soc. Am. Bull. 86:1244–1254 [Google Scholar]
- 62. Reichardt W. 1989. Microbiological aspects of bioturbation. Sci. Mar. 53:301–306 [Google Scholar]
- 63. Revsbech NP, Christensen PB, Nielsen LP, Sørensen J. 1989. Denitrification in a trickling filter biofilm studied by a microsensor for oxygen and nitrous oxide. Water Res. 23:867–871 [Google Scholar]
- 64. Sayama M, Kurihara Y. 1983. Relationship between burrowing activity of the polychaetous annelid, Neanthes Japonica (Izuka) and nitrification-denitrification processes in the sediments. J. Exp. Mar. Biol. Ecol. 72:233–241 [Google Scholar]
- 65. Seilacher A, Pflüger F. 1994. From biomats to benthic agriculture: a biohistortic revolution, p 97–105 In Krumbein WE, Paterson DM, Stal LJ. (ed), Biostabilization of sediments. Universität Oldenburg, Oldenburg, Germany [Google Scholar]
- 66. Seitzinger SP, Nielsen LP, Caffrey J, Christensen PB. 1993. Denitrification measurements in aquatic sediments: a comparison of three methods. Biogeochemistry 23:147–167 [Google Scholar]
- 67. Shinn EA. 1968. Burrowing in recent lime sediments of Florida and the Bahamas. J. Paleontol. 42:879–894 [Google Scholar]
- 68. Sigman DM, et al. 2003. Distinguishing between water column and sedimentary denitrification in the Santa Barbara Basin using the stable isotopes of nitrate. Geochem. Geophys. Geosyst. 4:1040 doi: 10.1029/2002GC000384 [Google Scholar]
- 69. Sisler FD, ZoBell CE. 1951. Nitrogen fixation by sulfate-reducing bacteria indicated by nitrogen/argon ratios. Science 113:511–512 [DOI] [PubMed] [Google Scholar]
- 70. Sørensen J. 1978. Denitrification rates in a marine sediment as measured by the acetylene inhibition technique. Appl. Environ. Microbiol. 36:139–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Steppe TF, Paerl HW. 2002. Potential N2 fixation by sulfate-reducing bacteria in a marine intertidal microbial mat. Aquat. Microb. Ecol. 28:1–12 [Google Scholar]
- 72. Stockdale A, Davison W, Zhang H. 2010. Formation of iron sulfide at faecal pellets and other microniches within suboxic surface sediments. Geochim. Cosmochim. Acta 74:2665–2676 [Google Scholar]
- 73. Suchanek TH. 1985. Thalassinid shrimp burrows: ecological significance of species-specific architecture, p 205–210 In In Proceedings of the 5th International Coral Reef Congress Tahiti EPHE, Moorea, French Polynesia [Google Scholar]
- 74. Sun MY, Aller RC, Lee C, Wakeham SG. 1999. Enhanced degradation of algal lipids by benthic macrofauna activity: effect of Yoldia limatula. J. Mar. Res. 57:775–804 [Google Scholar]
- 75. Swinbanks DD, Murray JW. 1981. Biosedimentological zonation of Boundary Bay tidal flats, Fraser River Delta, British Columbia. Sedimentology 28:201–237 [Google Scholar]
- 76. Teal LR, Bulling M, Parker ER, Solan M. 2008. Global patterns of bioturbation intensity and mixed depth of marine soft sediments. Aquat. Biol. 2:207–218 [Google Scholar]
- 77. Thayer CW. 1979. Biological bulldozers and the evolution of marine benthic communities. Science 203:458–461 [DOI] [PubMed] [Google Scholar]
- 78. Thomas WH. 1966. Surface nitrogenous nutrients and phytoplankton in the northeastern tropical Pacific ocean. Limnol. Oceanogr. 15:393–400 [Google Scholar]
- 79. van Luijn F, Boers PCM, Lijklema L, Sweerts J-PRA. 1999. Nitrogen fluxes and processes in sandy and muddy sediments from a shallow eutrophic lake. Water Res. 33:33–42 [Google Scholar]
- 80. Watling L. 1988. Small-scale features of marine sediments and their importance to the study of deposit-feeding. Mar. Ecol. Prog. Ser. 47:135–144 [Google Scholar]
- 81. Widerlund A, Davison W. 2007. Size and density distribution of sulfide-producing microniches in lake sediments. Environ. Sci. Technol. 41:8044–8049 [DOI] [PubMed] [Google Scholar]
- 82. Zehr JP, et al. 1995. Diversity of heterotrophic nitrogen fixation genes in a marine cyanobacterial mat. Appl. Environ. Microbiol. 61:2527–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhu QZ, Aller RC, Fan YZ. 2006. Two-dimensional pH distributions and dynamics in bioturbated marine sediments. Geochim. Cosmochim. Acta 70:4933–4949 [Google Scholar]
- 84. Ziebis W, Forster S, Huettel M, Jørgensen BB. 1996. Complex burrows of the mud shrimp Callianassa truncata and their geochemical impact in the sea bed. Nature 382:619–622 [Google Scholar]