Abstract
The spread of antibiotic-resistant microorganisms is widely recognized, but data about their sources, presence, and significance in marine environments are still limited. We examined 109 Escherichia coli strains from coastal marine sediments carrying virulence genes for antibiotic susceptibility, specific resistance genes, prevalence of class 1 and 2 integrons, and sequence type. Antibiotic resistance was found in 35% of strains, and multiple resistances were found in 14%; the resistances detected most frequently were against tetracycline (28%), ampicillin (16.5%), trimethoprim-sulfamethoxazole (13%), and streptomycin (7%). The highest prevalence of resistant strains was in phylogenetic group A, whereas phylogroup B2 exhibited a significantly lower frequency than all the other groups. Sixty percent of multiresistant strains harbored class 1 or 2 integrase genes, and about 50% carried resistance genes (particularly dfrA and aadA) linked to a class 1 integron. Multilocus sequence typing of 14 selected strains identified eight different types characteristic of extraintestinal pathogens and three new allelic combinations. Our data suggest that coastal marine sediment may be a suitable environment for the survival of pathogenic and antimicrobial-resistant E. coli strains capable of contributing to resistance spread via integrons among benthic bacteria, and they highlight a role for these strains in the emergence of new virulent genotypes.
INTRODUCTION
Escherichia coli is naturally part of the intestinal flora of warm-blooded animals, including humans, and is considered a reliable indicator of fecal pollution of aquatic environments and of the presence of pathogens of intestinal origin (14).
Some strains of E. coli have evolved as pathogenic strains and can cause a wide range of extraintestinal and intestinal diseases (17). The extraintestinal pathogenic strains belong predominantly to the B2 and, to a lesser extent, the D phylogenetic group, while commensal strains generally belong to phylogroups A and B1 (3, 21, 28). Strains of these four groups bear different phenotypic and genotypic traits and appear to occupy different ecological niches (9, 21). B2 and D group strains, which are recovered less frequently from aquatic environments than group A and B1 strains, are believed to originate from a variety of sources, including humans, wild and farm animals, and wastewater. They are stored in “reservoirs” (e.g., sediment particles and the surface of algae) that facilitate survival and growth outside the host (15). In this secondary habitat, E. coli is generally considered a transient member of the natural microbiota. However, it has recently been suggested that some strains that persist outside the host can become naturalized members of native bacterial communities. Although specific genetic targets can probably be selected for by the environment to enhance adaptation and persistence outside the host, a role for secondary habitats in generating and maintaining genomic diversity in E. coli populations may also be hypothesized (1, 2). The presence and persistence of enteric bacteria in aquatic environments can be a sanitary risk; this risk can be significantly raised if such bacteria are antibiotic resistant (AR).
Although drug resistance has been recognized since the early 1940s, the problem continues to grow and evolve, and the extensive use and misuse of antibiotics in human and animal treatments and in agriculture has contributed to the spread of AR bacteria (19, 22). AR fecal bacteria have been described in seawater, freshwater, and wastewater (30). According to recent studies, several marine bacteria that are normal components of the microbial assemblages in coastal marine waters, like those belonging to the genera Pseudomonas, Pseudoalteromonas, Vibrio, and Roseobacter, can also harbor resistance genes, especially those conferring resistance against tetracycline (8). The presence of AR bacteria in coastal environments is a serious health risk and can contribute to the spread and evolution of antibiotic resistance, especially when resistance genes are carried on mobile genetic elements (MGE). However, little is known about the distribution of these genes among fecal bacteria from coastal marine environments, since most works report phenotypic studies in areas subjected to strong selective pressure (5, 18, 35).
In this study, 109 E. coli strains isolated from coastal marine sediments in the Adriatic Sea and previously characterized for the presence of virulence factors (23; C. Vignaroli, G. M. Luna, C. Rinaldi, A. Di Cesare, R. Danovaro, and F. Biavasco, unpublished data) were examined to assess (i) the phenotypic resistance to 10 common antimicrobials used in human treatments, (ii) the presence of specific resistance determinants and their possible linkage to MGE, and (iii) the epidemiological significance of virulent and/or multidrug-resistant (MDR) strains, to gain information about the presence of harmful clones capable of surviving or even emerging in this environment.
MATERIALS AND METHODS
Bacterial strains.
A total of 109 E. coli strains previously isolated from marine coastal sediments in the Adriatic Sea and assigned to phylogenetic groups A (n = 38), B1 (n = 27), B2 (n = 21), and D (n = 23) were used in this study. All details regarding sampling sites and procedure, microbiological tests (including strain isolation from the sediment matrix) and the presence of virulence factors in B2 and D strains were reported by Luna et al. (23). Data regarding some virulence genes frequently found in commensal and enteropathogenic strains, i.e., intimin (eaeA), toxins (east1, cnf1), and iron acquisition systems (aer, fyuA, iutA, iroN), were available for A and B1 group strains (Vignaroli et al., unpublished).
Antimicrobial susceptibility.
Antimicrobial susceptibility to ampicillin (AMP) (10 μg), amoxicillin-clavulanic acid (AMC) (20/10 μg), cefotaxime (CTX) (30 μg), gentamicin (GEN) (10 μg), streptomycin (STR) (10 μg), tetracycline (TET) (30 μg), ciprofloxacin (CIP) (5 μg), nalidixic acid (NAL) (30 μg), trimethoprim-sulfamethoxazole (SXT) (1.25/23.75 μg), and chloramphenicol (CHL) (30 μg) was established by the disk diffusion method according to Clinical and Laboratory Standards Institute (CLSI) interpretive criteria (6). Briefly, a bacterial suspension in Mueller-Hinton broth (Oxoid, Basingstoke, United Kingdom) adjusted to a turbidity of 0.5 McFarland standard was spread on Mueller-Hinton agar (Oxoid) with a sterile cotton swab. The antimicrobial disks were placed onto the surface of inoculated agar, and plates were incubated for 24 h at 37°C. After incubation, isolates were scored as susceptible, intermediate, or resistant to a given antimicrobial based on the inhibition zone diameter around the disk and according to CLSI breakpoints (6). Strains resistant to β-lactams were also evaluated for extended-spectrum β-lactamase (ESBL) production using CLSI screening and confirmatory tests (6). The antibiotic disks were purchased from Oxoid except for cefotaxime-clavulanic acid (30/10 μg) and ceftazidime-clavulanic acid (30/10 μg) (BBL Sensi-disc, Becton Dickinson & Co., Sparks, MD) used in ESBL testing. Susceptibility to ampicillin, streptomycin, tetracycline, ciprofloxacin, nalidixic acid, sulfamethoxazole, and chloramphenicol (Sigma-Aldrich, Milano, Italy) was tested by the broth microdilution method. Serial 2-fold dilutions of the antibiotics were prepared in Mueller-Hinton broth cation adjusted (MHBCA) (BBL, Becton Dickinson & Co) in a 96-well standard tray. Bacterial suspensions in MHBCA were adjusted to a turbidity of 0.5 McFarland standard and further diluted 100-fold prior to addition of the final inoculum in each well (0.05 ml containing 1 × 106 CFU/ml). The MIC for each isolate was recorded after incubation for 24 h at 37°C as the lowest concentration of the drug that inhibited bacterial growth. MIC results were interpreted according to CLSI breakpoints (6). E. coli ATCC 25922 was used as the control strain in all assays.
Detection of resistance genes and integrons.
The presence of TET resistance genes (tetA, tetB, tetC, tetD, tetE, tetG) was determined by multiplex PCR using primers and conditions described by Jun et al. (16). Genes associated with resistance to AMP (blaTEM and blaSHV), SXT (dfrA1), sulfonamides (sul1, sul2, sul3), and STR (strA, strB, aadA1) and the intI1 and intI2 genes—encoding the integrase of class 1 and class 2 integrons, respectively—were detected by single PCR assays (20). The variable region of class 1 integrons was characterized by PCR, and sequencing of the amplicons was obtained. For primer pairs, see Table S1 in the supplemental material. Each PCR assay was performed in a 50-μl final reaction volume containing 1× buffer (10 mM Tris-HCl [pH 8.8], 50 mM KCl, and 0.1% Triton X-100), 1.5 mM MgCl2, 0.5 μM each primer, 200 μM each deoxynucleoside triphosphate (dNTP), 1 U of DyNAzyme DNA polymerase (Finnzymes, Espoo, Finland), and 5 μl of DNA template obtained from crude lysates of bacterial cultures grown overnight in brain heart infusion broth (Oxoid). The amplification program was as follows: 1 cycle of 4 min at 94°C, followed by 30 cycles of 45 s at 94°C, 45 s at an annealing temperature specific for each primer pair (58 to 60°C), and 45 s at 72°C, and a final extension step of 10 min at 72°C. E. coli strains from our laboratory collection, testing positive by PCR, were used as positive controls after sequencing of their PCR products; antimicrobial-susceptible strains of E. coli were the negative controls.
Multilocus sequence typing (MLST).
Multilocus sequence typing of 14 isolates (3 group A, 3 group B1, 3 group B2, and 5 group D strains) was performed by sequence analysis of internal fragments of the seven housekeeping genes adk, fumC, gyrB, icd, mdh, purA, and recA according to the protocol reported in the E. coli MLST website (http://mlst.ucc.ie/mlst/mlst/dbs/Ecoli/documents). The amplicons obtained for each locus were purified using the GenElute PCR CleanUp kit (Sigma-Aldrich) and sequenced. The allelic profiles of the seven gene sequences, ST, and sequence complexes were obtained from the E. coli MLST website database. New allele numbers and ST designations were given by the curator of the E. coli MLST database.
eBURST diagram.
Phylogenetic analysis of MLST data was performed with the eBURST algorithm using the eBURSTv3 software, available at http://eburst.mlst.net.
Statistical analysis.
Analysis of similarity (ANOSIM) was performed using the PRIMER 6+ software (Plymouth Marine Laboratory, United Kingdom) using a permutation/randomization method on a Bray-Curtis similarity matrix. To create the similarity matrix the susceptibility/resistance patterns of each isolate were transformed into presence/absence data. Differences were considered significant at P values of <0.05.
RESULTS
Antimicrobial resistance of E. coli strains.
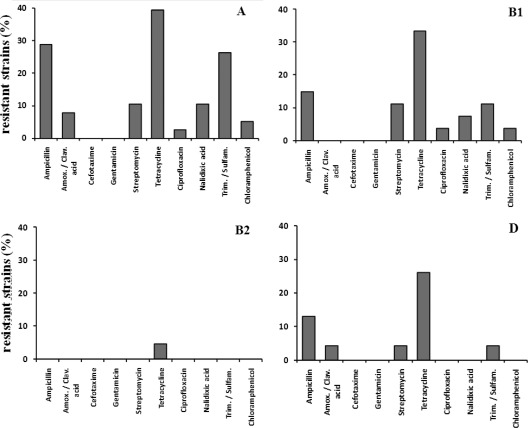
The 109 isolates from marine sediment were tested for their susceptibility to 10 antimicrobials by the disk diffusion method (Fig. 1). Overall, 67% were susceptible to all 10 antimicrobials, 15% were resistant to a single agent, and 14% were MDR, defined here as those showing simultaneous resistance to more than two antibiotics. Resistance to tetracycline was the most frequent (28.4%), followed by resistance to ampicillin (16.5%) and to trimethoprim-sulfamethoxazole (12.8%) regardless of the phylogenetic group. Resistance to gentamicin or cefotaxime was not detected. Strains of groups A and B1 were most often resistant to tetracycline (39.4% and 33.3%, respectively), followed by ampicillin (28.9% and 14.8%), trimethoprim-sulfamethoxazole (26.3% and 11.1%), streptomycin (10.5% and 11.1%), and nalidixic acid (10.5% and 7.4%) (Fig. 1). The prevalence of AR strains was highest in group A isolates. Only tetracycline resistance was detected in B2 strains (Fig. 1).
Fig 1.
Antimicrobial resistances detected in 109 E. coli strains from four phylogenetic groups (A, B1, B2, and D). Group A, 38 strains; group B1, 27 strains; group B2, 21 strains; group D, 23 strains.
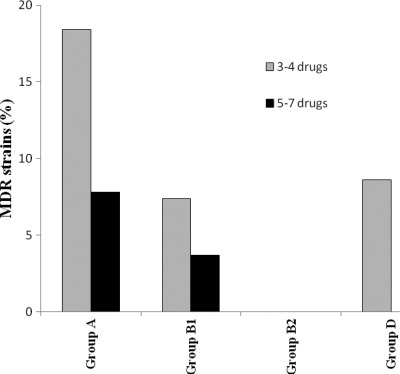
There were 15 MDR isolates, of which 11 were resistant to three or four drugs and 4 were resistant to up to seven drugs. The MIC results of the 7 antimicrobials against which the MDR strains showed resistance on the disk diffusion test are listed in Table 1.
Table 1.
Resistance phenotype and genotype of the 15 MDR strains of Escherichia coli
| MDR strain | Phylogroup | MIC (μg/ml)a |
Resistance genes | Integrase gene (class 1 or 2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AMP | STR | TET | CIP | NAL | SUL | CHL | ||||
| PE site | ||||||||||
| PE9i6 | A | >128 | 8 | 128 | ≤0.125 | 2 | >512 | 8 | blaTEMtetAsul2 | |
| PE9i8 | A | >128 | >128 | 128 | 8 | >128 | >512 | 8 | blaTEMstrAaadA1tetAsul1sul2dfrA1 | intI1 |
| PE9i42 | A | >128 | 16 | 128 | ≤0.125 | 0.5 | >512 | 4 | blaTEMtetAsul2 | |
| PE9i19 | A | 32 | 32 | >128 | ≤0.125 | >128 | >512 | 128 | aadA1tetAsul1sul2 | intI1 |
| PE9i26 | A | >128 | 16 | 128 | ≤0.125 | 2 | >512 | 128 | tetAsul2 | |
| PE9i39 | A | >128 | 16 | >128 | ≤0.125 | 2 | >512 | 8 | blaTEMtetA sul2 | |
| PE9i45 | A | >128 | 8 | >128 | ≤0.125 | 2 | >512 | 8 | blaTEMtetAsul2 | |
| PE11i1 | B1 | 2 | >128 | >128 | ≤0.125 | 4 | >512 | 8 | strAstrBaadA1tetAsul2dfrA1 | intI2 |
| PE11i7 | D | >128 | >128 | >128 | ≤0.125 | 2 | >512 | 4 | blaTEMstrAstrBtetAsul2 | intI1 |
| PE9i27 | D | >128 | 4 | 128 | ≤0.125 | 2 | >512 | 8 | blaTEMtetA | |
| FE site | ||||||||||
| FE5E4 | A | >128 | >128 | >128 | ≤0.125 | 2 | >512 | >128 | blaTEMstrAaadA1tetAsul1sul2dfrA1 | intI1 |
| FE5E11 | A | >128 | 16 | >128 | ≤0.125 | 2 | >512 | 128 | blaTEMtetA sul1dfrA1 | intI1 |
| FE6E4 | A | >128 | 4 | >128 | ≤0.125 | >128 | >512 | 4 | blaTEMsul2dfrA1 | intI1 |
| FE7E1 | B1 | >128 | 128 | >128 | 32 | >128 | >512 | 128 | blaTEMstrAstrBaadA1tetAsul3 | intI1 |
| CF site | ||||||||||
| CF12i5 | B1 | >128 | 8 | 128 | ≤0.125 | 128 | >512 | 8 | blaTEMtetA | intI1 |
AMP, ampicillin; STR, streptomycin; TET, tetracycline; CIP, ciprofloxacin; NAL, nalidixic acid; SUL, sulfamethoxazole; CHL, chloramphenicol. MIC interpretive standard (S, susceptible; I, intermediate; R, resistant) (6): AMP, S ≤ 8, I = 16, R ≥ 32; STR, no MIC interpretive standard available; TET, S ≤ 4, I = 8, R ≥ 16; CIP, S ≤ 1, I = 2, R ≥ 4; NAL, S ≤ 16, R ≥ 32; SUL, S ≤ 256, R ≥ 512; CHL, S ≤ 8, I = 16, R ≥ 32.
Most MDR strains (25%) were phylogenetic group A, and none were group B2 (Fig. 2, Table 1). Interestingly, 14 of the 15 MDR strains were from just two of the seven coastal sites sampled in the Adriatic region (23): 10 strains had been collected at a depth of 2 m (2 stations of site PE) and 4 at a depth of 5 m (3 stations of site FE). The last MDR isolate was recovered from a third site (CF) at a depth of 2 m. None of the 15 MDR strains were positive for the ESBL screening test.
Fig 2.
Prevalence of MDR strains among the 109 E. coli isolates analyzed in this study.
However, the proportion of AR strains was statistically higher in phylogroups A, B1, and D than in group B2 (ANOSIM test, P < 0.05 for each comparison), whereas an association between AR strains and sites or sampling depths was not detected (ANOSIM test, P > 0.05).
Detection of resistance genes and integrons in MDR strains.
The 15 MDR strains were tested for the most common determinants related to each resistance phenotype and for the genes encoding class 1 and 2 integrases. The results are shown in Table 1.
All strains were uniformly resistant to tetracycline, and all but one carried the tetA gene. PCR products specific for ampicillin (blaTEM) and trimethoprim-sulfamethoxazole (dfrA1) resistance genes were detected in 12 and 5 strains, respectively. Resistance to streptomycin, which was seen in 6/15 strains, was associated to the presence of genes strA, strB, and/or aadA1. The sulfonamide resistance genes sul1 and sul2 were found, alone or together, in 12/15 isolates, sul2 being found in 11 strains; sul3 was detected in a single strain carrying neither sul1 nor sul2. Class 1 and class 2 integrase genes were detected in eight strains and in one strain, respectively. Only four (Fe5E4, PE9i8, CF12i5, PE9i19) of these eight isolates could be amplified with primers (see Table S1 in the supplemental material) specific for the variable regions (1.5 to 2.0 kbp). Sequencing of the amplicons showed three different gene cassette arrangements: dfrA1-aadA1 in two strains (Fe5E4, PE9i8) and dfrA12-aadA2 and dfrA17-aadA5 in one strain each (CF12i5 and PE9i19, respectively). With the exception of blaTEM, tetA, and sul2, all resistance genes were detected in strains that also carried intI1 or intI2 (Table 1).
Multilocus sequence typing (MLST).
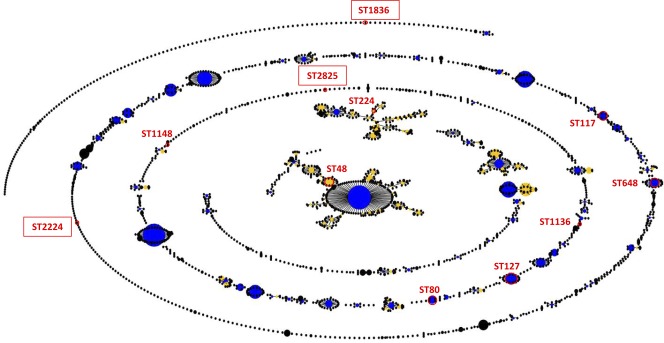
Fourteen strains representative of all phylogroups were analyzed for sequence type (ST) determination. They were selected for their resistance (this study) or virulence profile (23; Vignaroli et al., unpublished) among those recovered from sites PE, FE, and CF. Isolates showing no drug resistance were selected for MLST typing among those carrying the highest number of virulence genes, as shown in Table 2. Ten strains (PE9i19, PE9i39, FE5E8, PE9i12, PE9i17, PE11i7, CF13E1, PE9i35, PE11i1, FE5E4) were assigned to ST reported in the MLST database as typical of E. coli from extraintestinal diseases of humans and animals (Table 2). One strain (FE7E1) was assigned to ST224, which includes nonpathogenic strains. The remaining three strains (CF12i1, CF12i5, and CF12i7), isolated from the same site, exhibited new allelic combinations and were assigned to three new ST, designated ST1836, ST2825, and ST2224 (http://mlst.ucc.ie/). The eBURST diagram shown in Fig. 3 represents the population snapshot obtained from all ST (n = 2,090) reported in the E. coli MLST database at the time of the study and displays the correlations of the ST obtained in this study with the whole MLST database.
Table 2.
Sequence type and other data for selected MDR and virulent Escherichia coli strains
| Strain | Phylogroup | Virulence/resistance genesb | Sequence type | Host typea | Pathogen type(s)a,c | Diseasea,d |
|---|---|---|---|---|---|---|
| PE9i19 | A | traT/aadA1tetA sul1 sul2 | ST48 | Human/animal | ETEC, EPEC, EAEC, UPEC | Diarrhea, UTI, sepsis |
| PE9i39 | A | ibeA fyuA/blaTEMtetAsul2 | ||||
| FE5E4 | A | −/blaTEMstrA aadA1 tetA sul1 sul2 dfrA1 | ||||
| FE7E1 | B1 | −/blaTEMstrA strB aadA1 tetA sul3 | ST224 | Human | Nonpathogen | None |
| PE11i1 | B1 | aer/strAstrB aadA1tetAsul2 dfrA1 | ST1136 | Human | UPEC | UTI |
| CF12i5 | B1 | −/blaTEMtetA | ST 2825 | Unknown | Unknown | None |
| FE5E8 | B2 | hylA cnf1 sfa ibeA fyuA aer iroN traT/− | ST80 | Human/animal | NMEC, ExPEC, UPEC | UTI, meningitis |
| PE9i12 | B2 | hylA cnf1 sfa pap fyuA iroN traT/− | ST127 | Human/animal | ExPEC, UPEC | Sepsis, UTI |
| PE9i17 | B2 | cnf1 sfa pap eaeA ibeA fyuA iroN traT/− | ||||
| PE11i7 | D | iutA aer iroN traT/blaTEMstrA strB tetA sul2 | ST648 | Human/animal | Commensal, UPEC | Sepsis, UTI |
| CF12i1 | D | east1 iutAiroNtraT/− | ST1836 | Unknown | Unknown | None |
| CF12i7 | D | eaeA ibeA fyuA traT/− | ST2224 | Unknown | Unknown | None |
| CF13E1 | D | eaeA ibeA fyuA traT/− | ST1148 | Animal | ExPEC | Mastitis |
| PE9i35 | D | ibeAfyuAaertraT/blaTEMtetA | ST117 | Human/animal | APEC, UPEC | Sepsis, UTI |
Data regarding host, pathogen type, and commonly associated diseases are from the MLST database.
Reference 23 and Vignaroli et al., unpublished data. −, no gene detected .
ETEC, enterotoxigenic E. coli; EPEC, enteropathogenic E. coli; EAEC, enteroaggregative E. coli; UPEC, uropathogenic E. coli; NMEC, neonatal meningitis E. coli; ExPEC, extraintestinal pathogenic E. coli; APEC, avian pathogenic E. coli.
UTI, urinary tract infection.
Fig 3.
Population snapshot showing the clusters of related ST and the individual unrelated ST within the MLST E. coli database (3,725 isolates). The ST indicated are those found in this study. The three new ST are boxed.
DISCUSSION
E. coli, a widespread commensal and pathogenic bacterium of vertebrates, is considered a sensitive indicator of fecal pollution in marine and freshwater environments (23, 26, 31). Its decay rates in aquatic environments are believed to be affected by a complex array of interactions both with the environment, due to broad fluctuations in its physicochemical characteristics, and with the aquatic biota (11). Recent work has shown that many E. coli strains can persist outside the host, probably as a result of selective pressure and of favorable environmental conditions. Environmental selection has been suggested to enhance the genetic diversity of E. coli populations, favoring strains with characteristics enabling persistence in the environment and enhancing their fitness in the primary host (1, 2). The survival and persistence of E. coli in marine and freshwater environments are greater in sediments than in the overlying water column, probably in relation to the more favorable conditions provided by this environment, such as the higher availability of organic carbon and the protection from protozoan predation and from viral infections (4, 7, 24, 32).
The E. coli strains found in coastal marine sediment originate from terrestrial inputs, including urban waste; virulent and/or AR strains may exchange genetic information with resident microbiota and contribute to the emergence of new virulent and resistant genotypes of clinical interest.
However, data about benthic E. coli are much more limited compared with those on the water column. In this study, 109 E. coli strains isolated from marine sediments (23) were tested for susceptibility to 10 antimicrobials, for the presence of specific resistance determinants linked to MGE, and for their relationship with known pathogenic E. coli strains. AR and MDR strains were 35% and 14%, respectively. The few available studies of antimicrobial resistance of E. coli strains from aquatic environments (10, 30, 34) describe similar or higher prevalence values. The most prevalent resistances detected in our strains (to TET, AMP, SXT, and STR) are those commonly found in E. coli strains isolated from humans or from food-producing animals, such as poultry and swine (13, 20, 27, 33). These findings are in accordance with the extensive use of these antimicrobials in many settings (human and veterinary medicine, agriculture, and aquaculture) and with the reported spread of the corresponding resistances in the environment (8, 19). The highest prevalence of AR strains was found in group A isolates, as described in other studies (10, 27).
B2 strains showed a significantly lower frequency of antimicrobial resistances compared to all the other phylogroups; in contrast, no significant differences were found between the proportion of AR strains and the sampling site or sampling depth, suggesting a widespread spatial distribution of the most frequent resistances in the coastal area investigated and the need for a greater understanding of the drivers of the spatial distribution of antibiotic resistance in marine sediments. However, it should be noted that one of the sites (PE), where 10 of the 15 MDR strains were recovered, was the most polluted from urban wastes, as previously reported (23), whereas the FE site, where four MDR strains were recovered, exhibited no significant differences compared with the other sites (23).
For the vast majority of genes a clear association was seen between AR determinants and the presence of MGE, particularly integrons. As many as 60% of MDR strains harbored class 1 or 2 integrase genes, and 80% carried sul genes, which are usually found on integrons, transmissible plasmids, or other MGE (33). Moreover, the genes most commonly involved in resistance to STR and STX were found only in integrase-positive MDR strains. Sequencing of the variable region of the class 1 integron demonstrated that these resistance genes (particularly aadA and dfrA) were inside the integron in about half of the integron-positive MDR strains. This is the first study reporting a linkage between AR genes and MGE in E. coli strains from marine sediment.
Our data support the hypothesis that integrons may contribute to the dissemination of antimicrobial resistance among Gram-negative bacteria—including autochthonous species of marine coastal sediments—via horizontal transfer, and they suggest a role for the sediment reservoir of AR E. coli in the spread of antibiotic resistances in the coastal marine environment. MLST analysis of 14 selected virulent and MDR strains showed that there was no similarity among isolates, either from the same site or from different sites. Only the three group A strains (PE9i19, PE9i39, and FE5E4) from sites PE and FE were found in the same ST (ST48), but they had different virulence and resistance patterns. Most strains showed ST which comprise several pathogenic extraintestinal human or animal strains of E. coli (http://mlst.ucc.ie/). In particular, ST48 is part of clonal complex CC10, the largest group of closely related ST in the database, which includes both extraintestinal pathogens and intestinal or nonpathogenic strains, while ST127 (strains PE9i12 and PE9i17) has recently been reported as a virulent clone widespread in hospital and community urinary tract infections (12).
Two group D strains (CF12i1 and CF12i7) and a group B1 strain (CF12i5), remarkably isolated from the same site (CF), exhibited new allelic combinations. The first two were susceptible to all 10 antimicrobials and carried four different virulence genes (east1, iutA, iroN, traT in CF12i1; eaeA, ibe10, fyuA, traT in CF12i7) (23; Vignaroli et al., unpublished), whereas CF12i5, though carrying no virulence genes, harbored resistance genes (bla and tet genes) very common also in natural environments (8, 19). It has been suggested that virulence and some resistance determinants may be genetic traits enhancing bacterial survival and fitness outside the host (25, 29). Persistence in a secondary habitat, as reported by Anderson et al. (1), is not related to a specific phylogenetic group but seems to be strain-dependent.
It may thus be hypothesized that isolates CF12i1, CF12i5, and CF12i7 are not clinical strains reaching the marine environment through hospital or other waste: this is also supported by their antibiotic susceptibility profile and by their belonging to ST never reported among pathogenic strains. The virulence or resistance determinants carried by the three strains may have been acquired from the environment and may contribute to their evolution as human pathogens.
In conclusion, the large proportion of resistant E. coli found in coastal sediments may constitute a health risk, as a reservoir for the dissemination of antibiotic resistance genes among native microbial communities. The involvement of the marine environment in the evolution of new virulent E. coli genotypes clearly warrants further investigation.
Supplementary Material
ACKNOWLEDGMENTS
MLST analysis was carried out using the data freely available at http://mlst.ucc.ie, which is currently supported by a grant from the Science Foundation of Ireland (05/FE1/B882).
This work was supported by the Italian Ministry of Research and Education (contract PRIN 2008–I31J10000050001FYXAXL_003) and by ISPRA (Ministero Ambiente).
Footnotes
Published ahead of print 23 March 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Anderson KL, Whitlock JE, Harwood VJ. 2005. Persistence and differential survival of fecal indicator bacteria in subtropical waters and sediments. Appl. Environ. Microbiol. 71:3041–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bergholz PW, Noar JD, Buckley DH. 2011. Environmental patterns are imposed on the population structure of Escherichia coli after fecal deposition. Appl. Environ. Microbiol. 77:211–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bingen E, et al. 1998. Phylogenetic analysis of Escherichia coli strains causing neonatal meningitis suggests horizontal gene transfer from a predominant pool of highly virulent B2 group strains. J. Infect. Dis. 177:642–650 [DOI] [PubMed] [Google Scholar]
- 4. Boehm AB, et al. 2009. Faecal indicator bacteria enumeration in beach sand: a comparison study of extraction methods in medium to coarse sands. J. Appl. Microbiol. 107:1740–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chelossi E, et al. 2003. Antibiotic resistance of benthic bacteria in fish-farm and control sediments of the Western Mediterranean. Aquaculture 219:83–97 [Google Scholar]
- 6. Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing. Twentieth informational supplement. Document M100-S20, vol 30, no 1. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 7. Craig DL, Fallowfield J, Cromar NJ. 2002. Enumeration of fecal coliforms from recreational coastal sites: evaluation of techniques for the separation of bacteria from sediments. J. Appl. Microbiol. 93:557–565 [DOI] [PubMed] [Google Scholar]
- 8. Dang H, Ren J, Song L, Sun S, An L. 2008. Dominant chloramphenicol-resistant bacteria and resistance genes in coastal marine waters of Jiaozhou Bay, China. World J. Microbiol. Biotechnol. 24:209–217 [Google Scholar]
- 9. Escobar-Páramo P, et al. 2006. Identification of forces shaping the commensal Escherichia coli genetic structure by comparing animal and human isolates. Environ. Microbiol. 8:1975–1984 [DOI] [PubMed] [Google Scholar]
- 10. Garcia-Aljaro C, Moreno E, Andreu A, Prats G, Blanch AR. 2009. Phylogroups, virulence determinants and antimicrobial resistance in stx2 gene-carrying Escherichia coli isolated from aquatic environments. Res. Microbiol. 160:585–591 [DOI] [PubMed] [Google Scholar]
- 11. Garzio-Hadzick A, et al. 2010. Survival of manure-borne E. coli in streambed sediment: effects of temperature and sediment properties. Water Res. 44:2753–2762 [DOI] [PubMed] [Google Scholar]
- 12. Gibreel TK, et al. 2011. Population structure, virulence potential and antibiotic susceptibility of uropathogenic Escherichia coli from Northwest England. J. Antimicrob. Chemother. doi:10.1093/jac/dkr451. [DOI] [PubMed]
- 13. Ho PL, Wong RC, Chow KH, Que TL. 2009. Distribution of integron-associated trimethoprim-sulfamethoxazole resistance determinants among Escherichia coli from humans and food-producing animals. Lett. Appl. Microbiol. 49:627–634 [DOI] [PubMed] [Google Scholar]
- 14. Ibekwe AM, Murinda SE, Graves AK. 2011. Genetic diversity and antimicrobial resistance of Escherichia coli from human and animal sources uncovers multiple resistances from human sources. PLoS One 6:e20819 doi:10.1371/journal.pone.0020819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ishii S, Sadowsky MJ. 2008. Escherichia coli in the environment: implications for water quality and human health. Microb. Environ. 23:101–108 [DOI] [PubMed] [Google Scholar]
- 16. Jun LJ, et al. 2004. Detection of tetracycline-resistance determinants by multiplex polymerase chain reaction in Edwardsiella tarda isolated from fish farms in Korea. Aquaculture 240:89–100 [Google Scholar]
- 17. Kaper JB, Nataro JP, Mobley HLT. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123–140 [DOI] [PubMed] [Google Scholar]
- 18. Kimiran-Erdem A, et al. 2007. Isolation and identification of enterococci from seawater samples: assessment of their resistance to antibiotics and heavy metals. Environ. Monit. Assess. 125:219–228 [DOI] [PubMed] [Google Scholar]
- 19. Kümmerer K. 2004. Resistance in the environment. J. Antimicrob. Chemother. 54:311–320 [DOI] [PubMed] [Google Scholar]
- 20. Lapierre L, Cornejo J, Borie C, Toro C, San Martin B. 2008. Genetic characterization of antibiotic resistance genes linked to class 1 and class 2 integrons in commensal strains of Escherichia coli isolated from poultry and swine. Microb. Drug Res. 14:265–272 [DOI] [PubMed] [Google Scholar]
- 21. Le Gall T, et al. 2007. Extraintestinal virulence is a coincidental by-product of commensalism in B2 phylogenetic group Escherichia coli strains. Mol. Biol. Evol. 24:2373–2384 [DOI] [PubMed] [Google Scholar]
- 22. Levy SB, Marshall B. 2004. Antibacterial resistance worldwide: causes, challenges and responses. Nat. Med. 10:S122–S129 [DOI] [PubMed] [Google Scholar]
- 23. Luna GM, et al. 2010. Extraintestinal Escherichia coli carrying virulence genes in coastal marine sediments. Appl. Environ. Microbiol. 76:5659–5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luna GM, Dell'Anno A, Pietrangeli B, Danovaro R. 2012. A new molecular approach based on qPCR for the quantification of fecal bacteria in contaminated marine sediments. J. Biotechnol. 157:446–453 [DOI] [PubMed] [Google Scholar]
- 25. Muir R, Tan MW. 2006. Evolution of pathogens in soil, p 131–146. In Seifert HS, Dirita VJ. (ed), Evolution of microbial pathogens. ASM Press, Washington, DC [Google Scholar]
- 26. Noble RT, Blackwood AD, Griffith JF, McGee CD, Weisberg SB. 2010. Comparison of rapid quantitative PCR-based and conventional culture-based methods for enumeration of Enterococcus spp. and Escherichia coli in recreational waters. Appl. Environ. Microbiol. 76:7437–7443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Obeng AS, Rickard H, Ndi O, Sexton M, Barton M. 2011. Antibiotic resistance, phylogenetic grouping and virulence potential of Escherichia coli isolated from the faeces of intensively farmed and free range poultry. Vet. Microbiol. doi:10.1016/j.vetmic.2011.07.010. [DOI] [PubMed]
- 28. Picard B, et al. 1999. The link between phylogeny and virulence in Escherichia coli extraintestinal infection. Infect. Immun. 67:546–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pruzzo C, Vezzulli L, Colwell RR. 2008. Global impact of Vibrio cholerae interactions with chitin. Environ. Microbiol. 10:1400–1410 [DOI] [PubMed] [Google Scholar]
- 30. Servais P, Passerat J. 2009. Antimicrobial resistance of fecal bacteria in waters of the Seine river watershed (France). Sci. Total Environ. 408:365–372 [DOI] [PubMed] [Google Scholar]
- 31. Shanks OC, et al. 2008. Quantitative PCR for detection and enumeration of genetic markers of bovine fecal pollution. Appl. Environ. Microbiol. 74:745–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smith J, Edwards J, Hilger H, Steck TR. 2008. Sediment can be a reservoir for coliform bacteria released into streams. J. Gen. Appl. Microbiol. 54:173–179 [DOI] [PubMed] [Google Scholar]
- 33. Soufi L, et al. 2011. Escherichia coli of poultry food origin as reservoir of sulphonamide resistance genes and integrons. Int. J. Food Microbiol. 144:497–502 [DOI] [PubMed] [Google Scholar]
- 34. Vieira RH, et al. 2010. Antimicrobial susceptibility of Escherichia coli isolated from shrimp (Litopenaeus vannamei) and pond environment in northeastern Brazil. J. Environ. Sci. Health B 45:198–203 [DOI] [PubMed] [Google Scholar]
- 35. Zhao J, Dang H. 2011. Identification of a globally distributed clinical streptomycin-resistance plasmid and other resistance determinants in a coastal bay of China. Lett. Appl. Microbiol. 52:1–8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.