Abstract
Cry1Fa insecticidal protein was successfully radiolabeled with 125I-Na. Specific binding to brush border membrane vesicles was shown for the lepidopteran species Ostrinia nubilalis, Spodoptera frugiperda, Spodoptera exigua, Helicoverpa armigera, Heliothis virescens, and Plutella xylostella. Homologous competition assays were performed to obtain equilibrium binding parameters (Kd [dissociation constant] and Rt [concentration of binding sites]) for these six insect species.
TEXT
Since the commercial introduction of genetically modified crops for the control of insect pests in 1996, numerous plant species of agronomic interest have been transformed to express cry genes from the bacterium Bacillus thuringiensis. Bacillus thuringiensis Cry1A proteins confer high protection against most of the economically important lepidopteran pests, as shown by the unprecedented fast adoption of corn and cotton expressing Cry1A proteins (Bt corn and Bt cotton, respectively) by growers worldwide (www.isaaa.org). Because some lepidopteran pests are rather tolerant to Cry1A insecticidal proteins, and because of the threat of development of resistance to these proteins, other B. thuringiensis genes for insecticidal proteins targeting lepidopterans have been introduced in plants. Corn expressing Cry1Fa was registered in 2001 (TC1507 maize; Dow AgroSciences), and cotton expressing Cry1Fa in combination with Cry1Ac has been commercialized since 2005 (Widestrike; Dow AgroSciences). Cry1Fa has high activity against many lepidopteran species, such as Plutella xylostella, Heliothis virescens, Cacyreus marshalli, Ostrinia nubilalis, Conopomorpha cramarella, Sesamia nonagrioides, Cydia pomonella, Spodoptera exigua, Spodoptera frugiperda, and Prays oleae (www.glfc.forestry.ca/bacillus).
Bacillus thuringiensis Cry proteins exert their toxicity through binding to specific sites in the midgut of insect larvae. The affinity for these sites and their abundance in the midgut contribute to the specificity and the toxicity of Cry proteins to the target. Furthermore, binding competition experiments among Cry proteins are useful to confirm the physiological basis of cross-resistance and to predict its emergence (1, 2). For example, a common binding site for Cry1Fa, Cry1Ja, and Cry1A proteins has been proposed for several lepidopteran species based on competition binding assays among these proteins, which is in agreement with their cross-resistance patterns (3).
Among the different alternatives to label Cry proteins for binding assays, radiolabeling is the only one that allows an accurate calculation of binding parameters. Furthermore, it provides the highest sensitivity in competition experiments. Unfortunately, attempts at radiolabeling Cry1Fa with 125I-Na were discouraged after the study performed by Luo et al. (5), and labeling of Cry1Fa for binding studies has been since restricted to biotinylation. Luo et al. showed an almost complete reduction in the activity against S. frugiperda of Cry1Fa and other Cry proteins after labeling with 127I (the common and nonradioactive isotope of iodine) according to a protocol for radiolabeling Cry proteins with the 125I isotope with some modifications. The loss of in vivo activity after labeling with 127I-Na was accompanied by a lack of specific binding of the 125I-labeled Cry1Fa, which led the authors to conclude that labeling of Cry1Fa with iodine destroyed its biological functionality.
The aim of the present study was to obtain specific binding of 125I-labeled Cry1Fa to brush border membrane vesicles (BBMV) from S. frugiperda larvae and extend it to other lepidopteran species. We also followed the loss of toxicity of Cry1Fa throughout the steps of purification and labeling with the nonradioactive isotope under different labeling conditions.
The protocol for 125I radiolabeling of Cry proteins indicates a molar ratio of 1:4 of Cry protein to 125I-Na, with a final concentration of both Cry protein and NaI on the order of 10−6 M (7). However, the conditions used by Luo et al. (5) for the labeling of Cry1Fa with 127I were significantly different, with a ratio of Cry protein to 127I-Na of 1:1,000 (10−6 M protein and 10−3 M NaI). The reason for increasing the ratio was probably to minimize the presence of unlabeled molecules that could contribute to the toxicity. In our study, the toxicity of trypsin-activated Cry1Fa against S. frugiperda was tested after chromatography purification and after labeling with 127I under two regimens: low NaI and high NaI. Trypsin-activated and chromatographically purified Cry1Fa (see Fig. S1 in the supplemental material) was labeled with 127I-Na by the chloramine T method (7) under high or low NaI concentration. Overlay bioassays with artificial diet were performed in duplicate using seven serial dilutions of Cry1Fa (50 μl of each dilution per well). A single neonate larva was placed in each well, with a total of 16 larvae per dilution. Assay plates were incubated at 25 ± 2°C, with relative humidity of 65% ± 5% and a photoperiod of 16:8 (light:dark). Larval mortality was scored after 5 days and analyzed using the POLO-PC probit analysis program (LeOra Software, Berkeley, CA). Toxicity assays showed that the toxicity of Cry1Fa after chromatography purification was reduced approximately nine times with respect to the activity of the nonpurified trypsin-activated Cry1Fa (Table 1). The lower toxic activity obtained with the chromatography-purified sample indicates that this purification step involves the inactivation of part of Cry1Fa molecules. This inactivation may be due to a conformational change during the processes of binding to and/or release from the column. In addition, important different toxicities of 127I-Cry1Fa were found, depending on the final concentration of 127I-Na used in the labeling reaction. When a low concentration of NaI was used (10−6 M; conditions used for 125I labeling), 127I-Cry1Fa showed no loss of toxicity compared with that of the chromatographically purified Cry1Fa (Table 1). In contrast, if the incubation of the toxin was done with a high concentration of NaI (10−3 M; conditions used by Luo et al. [5]), the 127I-Cry1Fa protein showed a substantial loss of toxicity (Table 1). Since our results indicated that, under common radiolabeling conditions (10−6 M NaI), Cry1Fa did not lose toxicity against S. frugiperda, Cry1Fa was labeled with 125I-Na and tested for specific binding with BBMV from several lepidopteran species.
Table 1.
Toxicity of trypsin-activated Cry1Fa protein following various treatments administered to neonate larvae of S. frugiperda (measured after 5 days)a
| Protein | LC50 (ng/cm2) | 95% FL | Slope ± SEM |
|---|---|---|---|
| Nonpurified Cry1Fab | 51 | 25–131 | 0.9 ± 0.1 |
| Chromatography-purified Cry1Fab | 460c | 264–1,040 | 0.9 ± 0.1 |
| 127I-Cry1Fa (incubated with 10−6 M 127I-Na) | 255c | 155–509 | 1.6 ± 0.1 |
| 127I-Cry1Fa (incubated with 10−3 M 127I-Na) | >1,250 |
LC50, 50% lethal concentration; FL, fiducial limit at the 95% level.
Protein concentration was estimated by densitometry of Cry1Fa band in SDS-PAGE.
Differences between LC50 values were not statistically significant. The relative potency of purified Cry1Fa/127I-Cry1Fa was 1.4 (FL of 0.4 to 4.9).
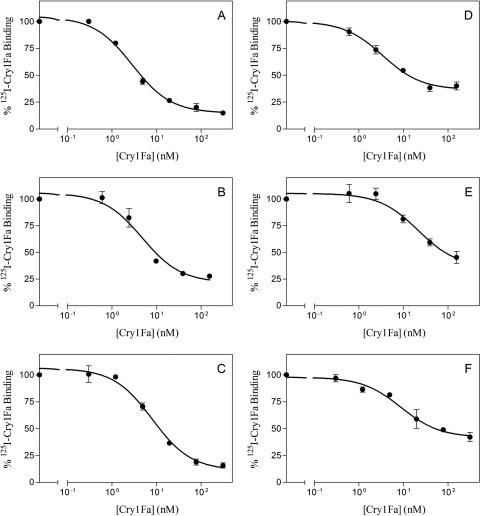
For binding assays, BBMV were prepared by the differential magnesium precipitation method (8) from last-instar larva guts of O. nubilalis, S. frugiperda, S. exigua, Helicoverpa armigera, and H. virescens and whole last-instar larvae of P. xylostella. After labeling of Cry1Fa protein with 125I, the estimated specific activity of 125I-Cry1Fa was 0.5 μCi/μg and was calculated as previously described (4). Binding parameters, Kd (dissociation constant) and Rt (concentration of binding sites), were estimated with the LIGAND computer program (6). To test for specific binding, increasing amounts of BBMV were incubated with 4.5 nM 125I-Cry1Fa in a final volume of 0.1 ml of binding buffer (8 mM Na2HPO4, 2 mM KH2PO4, 150 mM NaCl, pH 7.4, 0.1% bovine serum albumin [BSA]) for 1 h at 25°C. An excess (80-fold) of unlabeled toxin was used to calculate the nonspecific binding. An increase in specific binding of Cry1Fa was obtained with increasing concentrations of BBMV from all the species tested (data not shown). Homologous competition assays performed with a fixed amount of 125I-Cry1Fa (4.5 nM) and BBMV (see Fig. 1 legend) and increasing concentrations of unlabeled Cry1Fa confirmed the specific binding of radiolabeled 125I-Cry1Fa (Fig. 1). The competition curves fitted a single-site model equation and allowed the estimation of Kd and Rt values (Table 2). Kd values corresponded to high-affinity binding sites for Cry1Fa in all the species tested.
Fig 1.
Homologous competition binding assays between 125I-Cry1Fa and increasing concentrations of unlabeled Cry1Fa to BBMV from different species. (A) O. nubilalis (0.9 mg/ml); (B) S. frugiperda (0.8 mg/ml); (C) S. exigua (0.6 mg/ml); (D) H. armigera (0.3 mg/ml); (E) H. virescens (0.8 mg/ml); (F) P. xylostella (0.7 mg/ml). Each data point is the mean of two (A, B, and F) or three (C, D, and E) independent replicates. The concentration of BBMV proteins in the reaction mixture is given in parentheses.
Table 2.
Kd (dissociation constant) and Rt (concentration of binding sites) binding parameters estimated from Cry1Fa homologous competition assays with BBMV from O. nubilalis, S. frugiperda, S. exigua, H. armigera, H. virescens, and P. xylostella
| Insect species | Mean ± SEMa |
|
|---|---|---|
| Kd (nM) | Rt (pmol/mg)b | |
| O. nubilalis | 0.8 ± 0.2 | 0.44 ± 0.04 |
| S. frugiperda | 1.5 ± 0.6 | 0.67 ± 0.11 |
| S. exigua | 5.3 ± 0.7 | 1.38 ± 0.14 |
| H. armigera | 2.0 ± 0.7 | 0.90 ± 1.16 |
| H. virescens | 8.3 ± 3.9 | 1.38 ± 0.30 |
| P. xylostella | 2.7 ± 1.4 | 0.27 ± 0.07 |
Values were obtained from two replicates of the assays with O. nubilalis, S. frugiperda, and P. xylostella and from three replicates of the assays with S. exigua, H. armigera, and H. virescens.
Values are expressed in picomoles per milligram of BBMV protein.
The successful radiolabeling of Cry1Fa with 125I-Na has directly shown the specific binding of this protein to high-affinity binding sites in the midgut of O. nubilalis, S. frugiperda, S. exigua, H. armigera, H. virescens, and P. xylostella. This has allowed us, for the first time, to estimate the binding affinity and the concentration of binding sites for this protein. Labeling of Cry1Fa with 125I-Na will allow the performance of reciprocal heterologous competition studies (in which 125I-Cry1Fa is competed by other unlabeled Cry proteins) to verify receptor models proposed for Cry1Fa, such as the occurrence of shared binding sites among Cry1Fa and Cry1A toxins. This information will be very useful for the sustainable use of B. thuringiensis products combining several Cry toxins as well as for the design of effective transgenic crops that would help delay the evolution of insect resistance.
Supplementary Material
Footnotes
Published ahead of print 23 March 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Ferré J, Van Rie J. 2002. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 47:501–533 [DOI] [PubMed] [Google Scholar]
- 2. Ferré J, Van Rie J, MacIntosh SC. 2008. Insecticidal genetically modified crops and insect resistance management (IRM), p 41–85 In Romeis J, Shelton AM, Kennedy GG. (ed), Integration of insect resistant genetically modified crops within IPM programs. Springer, New York, NY [Google Scholar]
- 3. Hernández CS, Ferré J. 2005. Common receptor for Bacillus thuringiensis toxins Cry1Ac, Cry1Fa, and Cry1Ja in Helicoverpa armigera, Helicoverpa zea, and Spodoptera exigua. Appl. Environ. Microbiol. 71:5627–5629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hernández-Rodríguez CS, Van Vliet A, Bautsoens N, Van Rie J, Ferré J. 2008. Specific binding of Bacillus thuringiensis Cry2A insecticidal proteins to a common site in the midgut of Helicoverpa species. Appl. Environ. Microbiol. 74:7654–7659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luo K, Banks D, Adang MJ. 1999. Toxicity, binding, and permeability analyses of four Bacillus thuringiensis Cry1 delta-endotoxins using brush border membrane vesicles of Spodoptera exigua and Spodoptera frugiperda. Appl. Environ. Microbiol. 65:457–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Munson P, Rodbard D. 1980. LIGAND: a versatile computerized approach for characterization of ligand-binding systems. Anal. Biochem. 107:220–239 [DOI] [PubMed] [Google Scholar]
- 7. Van Rie J, Jansens S, Höfte H, Degheele D, Van Mellaert H. 1989. Specificity of Bacillus thuringiensis δ-endotoxins. Importance of specific receptors on the brush border membrane of the midgut of target insects. Eur. J. Biochem. 186:239–247 [DOI] [PubMed] [Google Scholar]
- 8. Wolfersberger MG, et al. 1987. Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae). Comp. Biochem. Physiol. 86A:301–308 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.