Abstract
The entomopathogenic bacteria Xenorhabdus nematophila and Photorhabdus temperata subsp. temperata suppress insect immune responses by inhibiting the catalytic activity of phospholipase A2 (PLA2), which results in preventing biosynthesis of immune-mediating eicosanoids. This study identified PLA2 inhibitors derived from culture broths of these two bacteria. Both X. nematophila and P. temperata subsp. temperata culture broths possessed significant PLA2-inhibitory activities. Fractionation of these bacterial metabolites in the culture broths using organic solvent and subsequent chromatography purified seven potent PLA2 inhibitors, three of which (benzylideneacetone [BZA], proline-tyrosine [PY], and acetylated phenylalanine-glycine-valine [FGV]) were reported in a previous study. Four other compounds (indole, oxindole, cis-cyclo-PY, and p-hydroxyphenyl propionic acid) were identified and shown to significantly inhibit PLA2. X. nematophila culture broth contained these seven compounds, while P. temperata subsp. temperata culture broth contained three compounds (BZA, acetylated FGV, and cis-cyclo-PY). BZA was detected in the largest amount among these PLA2 compounds in both bacterial culture broths. All seven bacterial metabolites also showed significant inhibitory activities against immune responses, such as phenoloxidase activity and hemocytic nodulation; BZA was the most potent. Finally, this study characterized these seven compounds for their insecticidal activities against the diamondback moth, Plutella xylostella. Even though these compounds showed relatively low toxicities to larvae, they significantly enhanced the pathogenicity of Bacillus thuringiensis. This study reports bacterial-origin PLA2 inhibitors, which would be applicable for developing novel insecticides.
INTRODUCTION
Insect immune responses consist of innate cellular and humoral components (2). Upon pathogen infection, pattern recognition receptors recognize nonself and the recognition signal is transferred by immune mediators to effector tissues, such as hemocytes and fat body (7). Eicosanoids form a chemical group of oxygenated polyunsaturated C20 fatty acids, which are derived from arachidonic acid, a catalytic product of phospholipase A2 (PLA2) on phospholipid (PL) substrate (3). Two types of eicosanoids, prostaglandins and leukotrienes, are known to mediate the nonself recognition signals against various insect pathogens, including bacteria, fungi, viruses, and parasitoid eggs in insects (38). Inhibition of PLA2 prevents eicosanoid biosynthesis and suppresses immune responses in response to microbial pathogens (23, 37).
Xenorhabdus nematophila and Photorhabdus temperata subsp. temperata are insect pathogens that are symbiotic to the entomopathogenic nematodes Steinernema carpocapsae and Heterorhabditis megidis, respectively (1, 13, 25). Infective juvenile-stage nematodes enter target insect hemocoel through natural openings (such as mouth, anus, and spiracles) and then release their symbiotic bacteria from the nematode intestine. The released bacteria suppress insect immune responses by inducing hemolysis, degrading antimicrobial peptides, suppressing prophenoloxidase activation, and inhibiting eicosanoid biosynthesis (17, 36). Under immunosuppressive conditions, bacteria multiply and kill host insects. In the insect cadaver, infective juveniles can grow and reproduce to form new generations. Subsequently, developed infective juveniles reassociate with bacteria by the bacteria's specific process of colonizing the nematode intestine and the juveniles emerge from the insect cadaver to look for other target insects (1). In terms of host immunosuppression, three bacterial metabolites were previously identified from X. nematophila bacterial culture broth: benzylideneacetone (BZA), proline-tyrosine (PY) dipeptide, and acetylated phenylalanine-glycine-valine (FGV) tripeptide (Ac-FGV) (11, 35, 37). P. temperata subsp. temperata culture broth also possesses immunosuppressive activity that inhibits hemocyte-spreading behavior and nodule formation (33). Moreover, the bacterial culture broth showed a synergistic effect on the pathogenicity of Bacillus thuringiensis (12). An organic extract of P. temperata subsp. temperata culture broth contains PLA2-inhibitory factor(s) (34). These studies suggest a possibility of additional identification of PLA2-inhibitory compounds originating from these bacterial culture broths.
This study was conducted to identify a new bacterial metabolite(s) that is responsible for PLA2 inhibition. To this end, both X. nematophila and P. temperata subsp. temperata culture broths were sequentially fractionated and analyzed for PLA2 inhibition. Purified compounds possessing PLA2-inhibitory activity were chemically identified using gas chromatography and mass spectrometry (GC-MS) and nuclear magnet resonance (NMR) analyses. The identified PLA2 inhibitors were then analyzed for their inhibitory activities against cellular immune responses and their insecticidal effects in order to develop novel pesticides.
MATERIALS AND METHODS
Insect and bacterial culture.
Larvae of Plutella xylostella originated from a cabbage field and were reared on cabbage in the laboratory under conditions of 25 ± 1°C and 16 h of light/8 h of darkness. The fourth-instar larvae were collected from cohorts at 8 days after hatching. Larvae of Spodoptera exigua were reared on an artificial diet (23). P. temperata subsp. temperata was isolated from an entomopathogenic nematode, H. megidis (13). X. nematophila was isolated from S. carpocapsae (25). Bacteria were cultured in Luria-Bertani (LB; Bacto tryptone, 10 g/liter; yeast extract, 5 g/liter; sodium chloride, 10 g/liter) medium for 48 h at 28°C on a shaking (200-rpm) incubator (JS-SKI-N900; Johnsam, Seoul, Republic of Korea).
Chemicals.
A PLA2 surrogate substrate, 1-hexadecanoyl-2-(1-pyrenedecanoyl)-sn-glycerol-3-phosphatidylcholine, was purchased from Molecular Probes, Inc. (Eugene, OR). Bovine serum albumin (BSA), dimethyl sulfoxide (DMSO), l-3,4-dihydroxyphenylalanine (l-DOPA), (E)-4-phenylbut-3-en-2-one acetic acid (BZA), 1H-benzo[b]pyrrole (indole), 2-indolinone (oxindole), 3-(p-hydroxyphenyl)-propionic acid (PHPP), chloroform, ethanol, ether, ethyl acetate, hexane, and methanol were purchased from Sigma-Aldrich Korea (Seoul, Republic of Korea).
Fractionation of bacterial culture broth.
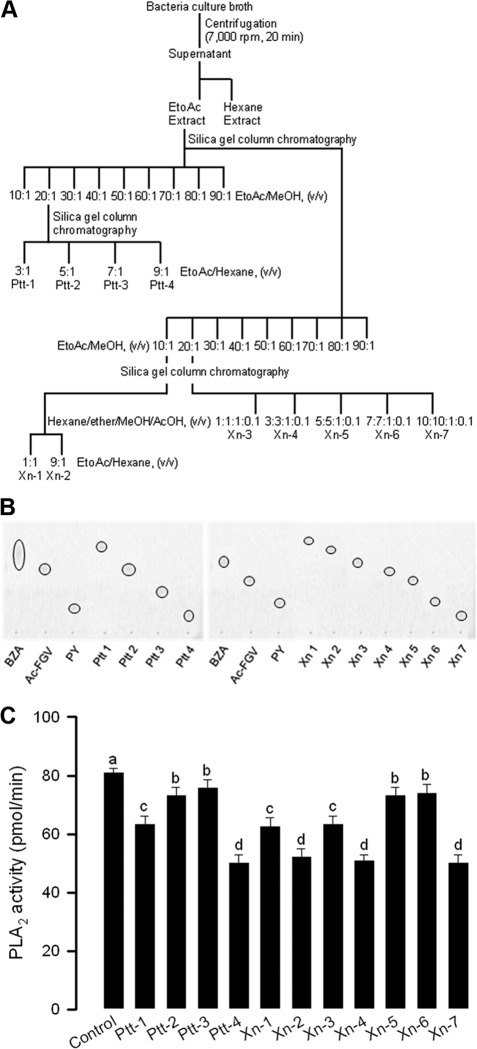
Culture broths (48 h) of X. nematophila and P. temperata subsp. temperata were centrifuged at 12,500 × g for 30 min, and the supernatants were used for subsequent fractionation (see Fig. 2A). At the first step, the same volume of hexane was mixed with the supernatant and separated into organic and aqueous fractions. The aqueous phase was combined with the same volume of ethyl acetate. The resulting organic fraction was combined and dried with a rotary evaporator (Sunil Eyela, Seongnam, Republic of Korea) at 40°C for 5 min. The ethyl acetate extract was subjected to chromatography in a chromatograph filled with silica gel (70 to 230 mesh; Merck, Germany) using an ethyl acetate/methanol (99:1, vol/vol) ratio with increasing amounts of methanol. Each resulting subfraction was analyzed by an in vitro PLA2 activity assay (see below). The active subfraction was separated by silica gel chromatography with hexane-ether-methanol-acetic acid (10:10:1:0.1, vol/vol/vol/vol) for X. nematophila and ethyl acetate-methanol (20:1, vol/vol) for P. temperata subsp. temperata as respective eluents. The active fractions were confirmed with respect to significant inhibition of PLA2 activity.
Fig 2.
Fractionation of bacterial metabolites of Xenorhabdus nematophila (Xn) or Photorhabdus temperata subsp. temperata (Ptt) and their PLA2-inhibitory activities. (A) Diagram showing purification steps of PLA2 inhibitors. Ethyl acetate (EtOAc) and methanol (MeOH) were used in the fraction. (B) The final 11 fractions were analyzed by thin-layer chromatography (TLC) to confirm a single compound using a TLC eluent composed of hexane and methanol in a 40:10 (vol/vol) ratio. (C) PLA2-inhibitory activities of each purified sample (1 mg/ml). The PLA2 assay used a pyrene-labeled phospholipid as a substrate (28). PLA2 was extracted from hemocytes of fifth-instar Spodoptera exigua as described in Materials and Methods. The hemocyte PLA2 sample (10 μg) was incubated with 10 μl of purified metabolite at 25°C for 10 min. Then, the substrate solution was added and the residual PLA2 activity was monitored at 348 nm for excitation and 390 nm for emission using a spectrofluorometer. Each measurement was replicated three times with three independent samplings. Different letters above standard deviation bars indicate significant differences among means at a type I error of 0.05 (LSD test).
TLC.
Thin-layer chromatography (TLC) was performed to analyze organic extracts of bacterial culture broth. Each organic extract was spotted at the bottom of a silica gel plate (20 by 20 cm; Merck, Darmstadt, Germany) and then placed in a shallow pool of the mixture of isopropyl alcohol (Hayashi, Tokyo, Japan) and sterilized water (7:3, vol/vol) as an eluent in a development chamber, which was then allowed to run by capillary action until the solvent reached the top end of the plate. The silica gel plate was removed and dried, and the separated components were stained with a mixture (19:1, wt/wt) of sea sand (Merck, Darmstadt, Germany) and iodine (Duksan, Ansan, Republic of Korea).
Reverse-phase HPLC analysis.
Purified samples were analyzed by high-performance liquid chromatography (HPLC; Waters, Milford, MA). Samples were cleaned with a C18 cartridge (Advantec, Inc., Anyang, Republic of Korea). The clean sample (10 μl) was injected into an HPLC equipped with a C18 column (Deltapak; 15 mm, 300 A, 300 by 7.8 mm). The samples were then separated with a mobile phase of methanol-water (50:50, vol/vol) at a flow rate of 0.5 ml/min for 30 min with a UV detector at 254 nm.
GC-MS analysis.
Purified samples were analyzed by GC-MS (7890A-5975C GC/MSD; Agilent, Santa Clara, CA) equipped with a Supelco Wax 10-fused silica capillary (30-m length by 0.25-mm inside diameter [i.d.]; Supelco, Bellefonte, PA). The carrier gas used helium at a constant flow rate of 1.0 ml/min. Two microliters of purified fraction was injected into the column using a 10:1 split injection mode. The oven temperature was initially held at 100°C for 3 min, then raised to 300°C for 5 min, and finally held at 300°C for 48 min. Temperatures of the injector and detector were 200°C and 240°C, respectively. The mass detector was operated in an electron impact mode with an ionization energy of 70 eV, a scanning range of 33 to 550 atomic mass units (amu), and a scan rate of 1.4 scans/s. Purified samples were respectively identified by comparing mass spectra and retention indices in the Wiley mass spectral database (Hewlett-Packard, Palo Alto, CA).
NMR spectroscopy.
The NMR spectrum of each (5-mg) purified compound (Xn-1, Xn-2, Xn-4, Xn-7, and Ptt-4) was obtained on an NMR spectrometer (Advance Digital, Bruker, Massachusetts) operating at 400 MHz (1H, 13C).
Chemical synthesis of cyclo-(proline-tyrosine) (cPY).
All details of chemical synthesis were described in the other study (19). Briefly, N-9-fluorenylmethoxy carbonyl (Fmoc)–l-proline was mixed with l-tyrosine methyl ester hydrochloride to synthesize PY dipeptide. Cyclization of this peptide was performed by heating it at 150°C, and it was purified by column chromatography using a silica gel.
PLA2 enzyme assay.
PLA2 activity was measured by spectrofluorometry using a pyrene-labeled phospholipid (PL) substrate in the presence of BSA (28). The PL was dissolved to 10 mM in 100% ethanol while a 10% solution of BSA was prepared in 50 mM sodium phosphate buffer containing 0.7% NaCl (phosphate-buffered saline [PBS], pH 7.4). To prepare the enzyme sample, hemolymph was collected from fifth-instar larvae of S. exigua and separated into plasma and hemocyte by centrifugation at 500 × g for 10 min. The hemocytes were then sonicated in PBS and centrifuged at 12,500 × g for 10 min at 4°C. The supernatant was used for PLA2 measurement. A reaction mixture (2 ml) was prepared in a cuvette by sequentially adding 50 mM PBS, 10% BSA, 1 M CaCl2, and an enzyme preparation. The reaction was initiated by addition of 10 mM PL substrate and subsequently monitored for fluorescence intensity in an Aminco Bowmen Series 2 luminescence spectrometer (FA257; Spectronic Instruments, Madison, WI) using excitation and emission wavelengths of 345 and 398 nm, respectively.
PO activity measurement.
Hemolymph phenoloxidase (PO) activity was determined with a DOPA substrate. Hemolymph was collected by cutting the abdominal proleg. Hemocytes and plasma were separated by centrifugation at 200 × g for 10 min. The hemocytes were then resuspended in PBS. Ten microliters of each sample was added to 990 μl of PO substrate solution containing 1 μg/μl of DOPA in PBS. Initial absorbance change was monitored at 495 nm.
Nodulation assay.
The nodulation assay was performed by injecting 3.2 × 104 cells of Escherichia coli into hemocoel of P. xylostella larvae using a microinjector (Nanojet II; WPI, Inc., Sarasota, FL). After 4 h of incubation at room temperature, melanized nodules were counted using a stereomicroscope (SZX9; Olympus, Tokyo, Japan) at a ×50 magnification. Injection solution was prepared in 5 × 10−3, 5 × 10−2, 5 × 10−1, 5, and 50 μM inhibitor concentrations. From this solution, 25 nl was injected into each larva along with the bacteria, and nodulation was checked as described above.
Insecticidal bioassay.
All pathogenic bioassays used a dipping method, in which Chinese cabbage was cut into small pieces (1 cm2) and soaked in a predetermined concentration of test solutions for 5 min. A commercial product of Bacillus thuringiensis subsp. kurstaki (Bt; Thuricide; Misung Agrochemical Inc., Daejon, Republic of Korea) was purchased and used for B. thuringiensis treatment. After brief drying under dark conditions, the treated diet was supplied to the test larvae. Each dose of bacterial concentration was replicated three times with 10 larvae per replicate. Mortality was determined at 48 h after treatment. Larvae were considered dead if they were unable to move in a coordinated manner when prodded with a blunt probe.
Data analysis.
Survival data were transformed by the square root and arcsine method for normalization. Treatment means and variances of the transformed data were analyzed by PROC GLM of the SAS program (32). Comparison among means used a least significant difference (LSD) test at a type I error of 0.05.
RESULTS
PLA2-inhibitory activities of both bacterial culture broths.
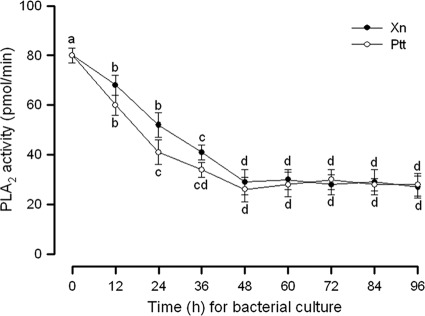
Inhibitory activities of the culture broths on insect PLA2 were measured during X. nematophila and P. temperata subsp. temperata culture (Fig. 1). As the culture time increased, both bacterial culture broths showed increased inhibitory activities against PLA2 activity. However, there was no further significant increase of PLA2-inhibitory activities after 48 h in both cultures. Subsequent chemical purification of PLA2 inhibitor(s) used 48-h cultures in both bacterial species.
Fig 1.
PLA2-inhibitory activities of bacterial culture broth of Xenorhabdus nematophila (Xn) or Photorhabdus temperata subsp. temperata (Ptt). For X. nematophila, 2.6 × 104 cells were cultured in 1 liter of LB culture medium at 28°C. For P. temperata subsp. temperata, 6.3 × 105 cells were cultured in 1 liter of LB culture medium at 28°C. The PLA2 assay used a pyrene-labeled phospholipid as a substrate (28). PLA2 was extracted from hemocytes of fifth-instar Spodoptera exigua as described in Materials and Methods. The hemocyte PLA2 sample (10 μg) was incubated with 10 μl of bacterial culture broth at 25°C for 10 min. Then, the substrate solution was added and the residual PLA2 activity was monitored at 348 nm for excitation and 390 nm for emission using a spectrofluorometer. Each measurement was replicated with three independent samplings. Different letters above standard deviation bars indicate significant differences among means at a type I error of 0.05 (LSD test).
Fractionation of bacterial metabolites in culture broths and PLA2-inhibitory activities.
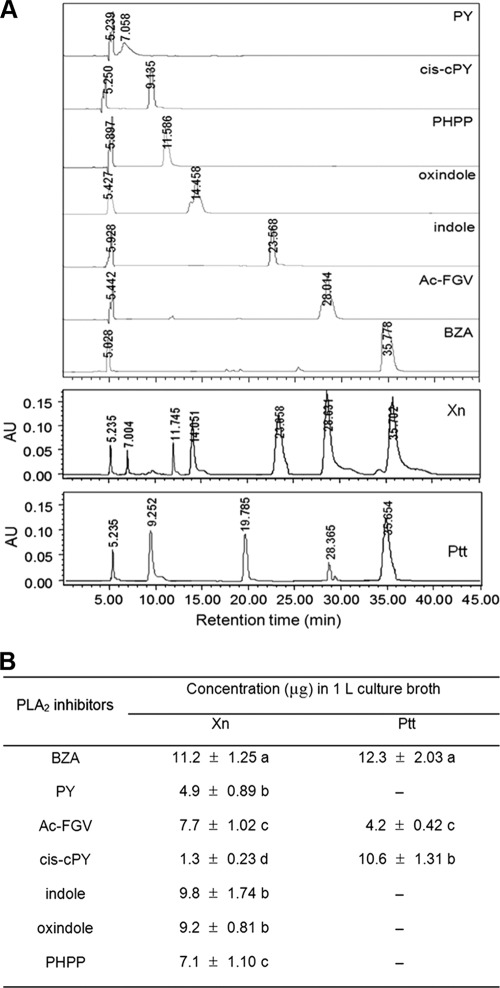
The 48-h culture broths of both X. nematophila and P. temperata subsp. temperata were separated using ethyl acetate into organic and aqueous fractions (Fig. 2A). Only the organic fraction showed PLA2-inhibitory activity. The active organic fraction molecules were further fractionated on a silica column with a gradient of hydrophobic eluents using different mixtures of ethyl acetate and methanol. Two subfractions of X. nematophila broth and one subfraction of P. temperata subsp. temperata broth showed significant inhibitory activities against PLA2. These molecules in the active fractions were further fractionated on a silica column with different mixtures of hexane and ethyl acetate. Four P. temperata subsp. temperata and seven X. nematophila subfractions were purified into single compounds and showed significant PLA2-inhibitory activities (Fig. 2B and C).
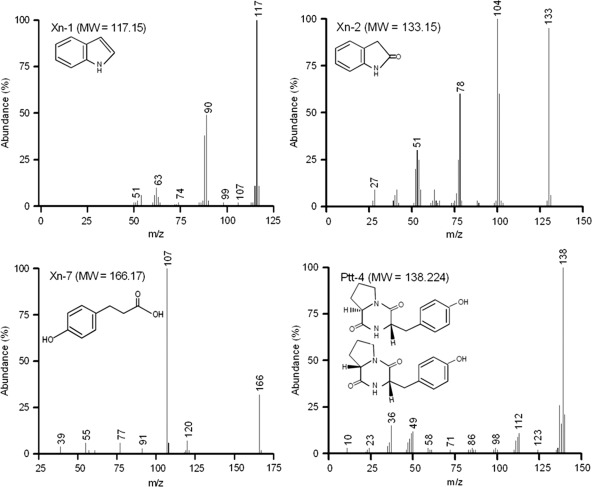
Four subfractions (Ptt-1, Xn-3, Xn-5, and Xn-6) corresponded to three known PLA2 inhibitors on the basis of TLC analysis (Fig. 2B). Five subfractions (Xn-1, Xn-2, Xn-4, Xn-7, and Ptt-4) showed relatively high inhibitory activities against PLA2, of which Ptt-4 and Xn-7 showed identical migrations in TLC analysis. Subsequent chemical identifications were focused on four compounds (Xn-1, Xn-2, Xn-4, and Ptt-4). GC-MS analyses of these four purified compounds suggested that Xn-1 was indole, Xn-2 was oxindole, Xn-4 was 3-(4-hydroxyphenyl)propionic acid (PHPP), and Ptt-4 was cPY (Fig. 3). These chemical identifications were further supported by NMR data: indole, 1H NMR (CDCl3, 400 MHz), δ – 8.01 (s, 1H), 7.58 (d, J – 7.6 Hz, 1H), 7.31 (d, J – 7.6 Hz, 1H), 7.16 to 7.10 (m, 2H), 7.05 (t, J – 7.6 Hz, 1H), 6.49 to 6.47 (m, 1H); indole, 13C NMR (CDCl3, 100 MHz), δ – 135.9, 127.9, 124.3, 122.1, 120.9, 119.9, 111.2, 102.7; oxindole, 1H NMR (CDCl3, 400 MHz), δ – 9.13 (s, 1H), 7.17 to 7.13 (m, 2H), 6.94 (t, J – 7.0 Hz, 1H), 6.84 (t, J – 7.0 Hz, 1H), 3.48 (s, 2H); oxindole, 13C NMR (CDCl3, 100 MHz), δ – 178.7, 143.0, 128.4, 125.7, 125.0, 122.8, 110.3, 36.7; PHPP, 1H NMR (DMSO-D6, 400 MHz), δ – 6.99 (d, J – 6.4 Hz, 2H), 6.65 (d, J – 6.4 Hz, 2H), 2.68 (t, J – 6.2 Hz, 2H), 2.39 (t, J – 6.2 Hz, 2H); PHPP, 13C NMR (DMSO-D6, 100 MHz), δ – 174.3, 155.4, 131.3, 129.0, 115.2, 36.5, 29.9; cPY, 1H NMR (DMSO-D6, 400 MHz), δ – 9.19 (s, 1H), 7.87 (s, 1H), 7.06 (d, J – 8.4 Hz, 2H), 6.65 (d, J – 8.4 Hz, 2H), 4.26 to 4.24 (m, 1H), 4.05 (t, J – 6.8 Hz, 1H), 3.44 to 0.336 (m, 2H), 3.29 to 3.23 (m, 1H), 2.98 to 2.29 (m, 1H), 2.05 to 1.98 (m, 1H), 1.20 to 1.71 (m, 2H), 1.46 to 1.31 (m, 1H); cPY, 13C NMR (DMSO-D6, 100 MHz), δ – 168.89, 165.09, 155.88, 130.80, 127.05, 114.76, 58.38, 56.00, 44.54, 34.69, 27.83, 21.85.
Fig 3.
Chemical identification of four purified bacterial metabolites of Xenorhabdus nematophila (Xn) or Photorhabdus temperata subsp. temperata (Ptt). Each GC-MS chromatogram was obtained with 100 μg of a purified sample, which was injected into the column using a 10:1 split injection mode. The oven temperature was initially held at 100°C for 3 min, then raised to 300°C for 5 min, and finally held at 300°C for 48 min. The temperatures of the injector and the detector were 200°C and 240°C, respectively, using GC-MS (7890A-5975C GC/MSD; Agilent, Santa Clara, CA). m in m/z is representative of molecular or atomic mass, and z is representative of the number of elementary charges carried by the ion. MW, molecular weight.
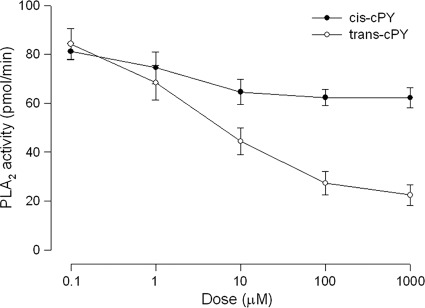
There might be two kinds of stereoisomers in cPY into cis-cPY (S,S isomer) and trans-cPY (R,S isomer) on the basis of two stereogenic sites (Fig. 3, bottom). TLC and 1H/13C NMR spectra clearly indicated that purified cPY was in the cis form. Further analysis using X-ray crystallography showed that two stereogenic centers were S,S, while synthetic cPY also produced an R,S isomer due to the change of l-proline to d-proline at a high synthetic reaction temperature (150°C) (data not shown). Furthermore, when these isomers were analyzed for their PLA2-inhibitory activity, only the cis isomer significantly inhibited the enzyme activity (Fig. 4).
Fig 4.
Comparison of two stereoisomers of cPY in inhibition of PLA2 activity. PLA2 was extracted from hemocytes of fifth-instar Spodoptera exigua as described in Materials and Methods. The hemocyte PLA2 sample (10 μg) was incubated with 10 μl of bacterial culture broth at 25°C for 10 min. Then, the substrate solution was added and the residual PLA2 activity was monitored at 348 nm for excitation and 390 nm for emission using a spectrofluorometer. Each measurement was replicated with three independent samplings. Error bars represent standard deviations.
Stoichiometry of PLA2 inhibitors in bacterial culture broth.
Ethyl acetate extracts of X. nematophila and P. temperata subsp. temperata bacterial culture broths were analyzed using reverse-phase HPLC (Fig. 5). Seven bacterial metabolites were clearly separated with different retention times: BZA at 35 min, Ac-FGV at 28 min, indole at 23 min, oxindole at 14 min, PHPP at 11 min, cis-cPY at 9 min, and PY at 7 min (Fig. 5A). Among seven PLA2 inhibitors identified as originating from bacterial culture broths, all seven compounds were detected in X. nematophila culture broth, while only three compounds were detected in P. temperata subsp. temperata culture broth. However, there were unknown minor peaks in both bacterial culture broths. When these identified bacterial metabolites were quantified in the bacterial culture broths, BZA turned out to be the most abundant metabolite (Fig. 5B).
Fig 5.
Comparison of identified bacterial metabolites in 48-h culture broths of Xenorhabdus nematophila (Xn) and Photorhabdus temperata subsp. temperata (Ptt). Abbreviations: BZA, benzylideneacetone; Ac-FGV, acetylated FGV tripeptide; PY, PY dipeptide; cis-cPY, cis-cyclo-PY; indole, 1H-benzo[b]pyrrole; oxindole, 2-indolinone; PHPP, p-hydroxyphenyl propionic acid. (A) Reverse-phase HPLC chromatograms of ethyl acetate extracts of the bacterial culture broths. (B) Measurements of seven bacterial metabolites in the bacterial culture broth. Each measurement was independently estimated three times. Means followed by different letters indicate significant differences among means at a type I error of 0.05 (LSD test).
Inhibitory activities of PLA2 inhibitors on PO and nodule formation.
Eicosanoids are required for inducing hemocyte melanization in S. exigua by activating PO precursor (36). Hemocyte nodule formation is a form of cellular immunity of S. exigua and accompanied by melanization reactions (23). Seven PLA2 inhibitors were compared in their inhibitory activities for immune-associated characteristics (Fig. 6). All seven compounds significantly inhibited both PLA2 and PO catalytic activities in a dose-dependent manner (Fig. 6A). These seven compounds also significantly inhibited hemocyte nodulation in response to bacterial challenge. When these inhibitory activities were compared by estimating median inhibitory activity, BZA was the most potent in three assays (Fig. 6B).
Fig 6.
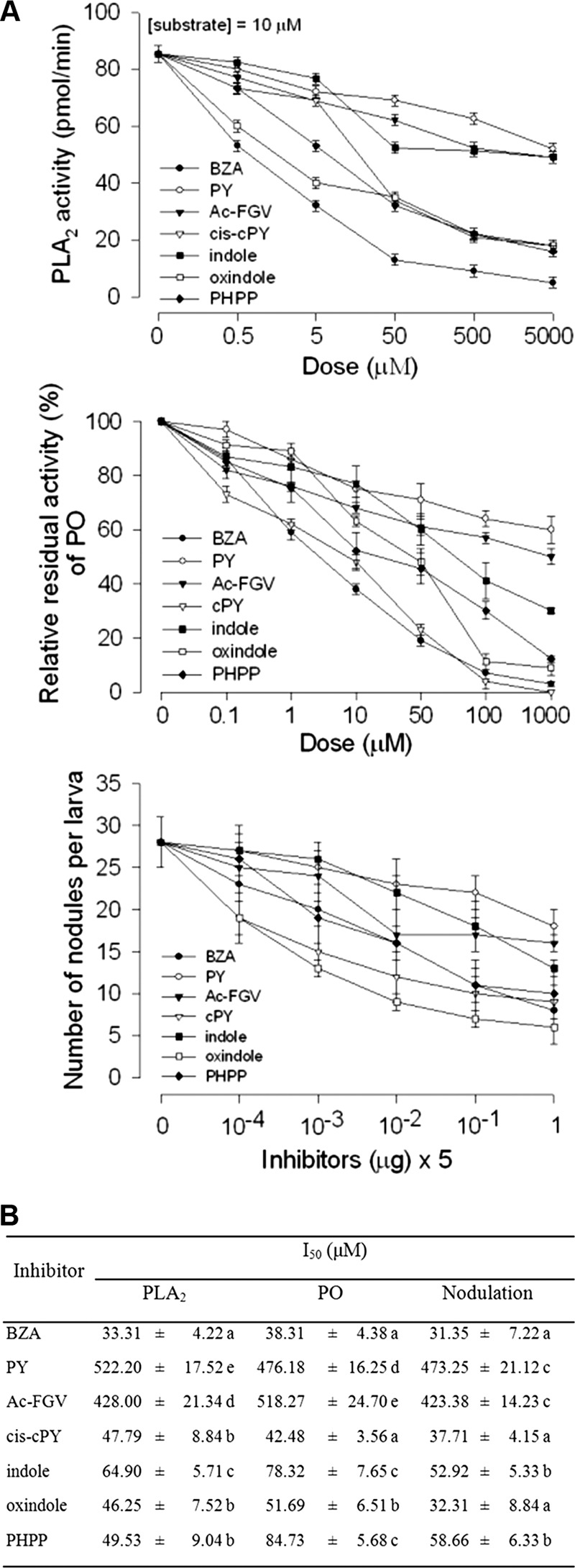
Biological characteristics of seven bacterial metabolites of Xenorhabdus nematophila (Xn) and Photorhabdus temperata subsp. temperata (Ptt). Abbreviations: BZA, benzylideneacetone; Ac-FGV, acetylated FGV tripeptide; PY, PY dipeptide; cis-cPY, cis-cyclo-PY; indole, 1H-benzo[b]pyrrole; oxindole, 2-indolinone; PHPP, p-hydroxyphenyl propionic acid. (A) Inhibitory activities of seven compounds against immune-associated responses of Spodoptera exigua. The PLA2 assay used a pyrene-labeled phospholipid as a substrate (28). PLA2 was extracted from hemocytes of fifth-instar Spodoptera exigua as described in Materials and Methods. The hemocyte PLA2 sample (10 μg) was incubated with 10 μl of bacterial culture broth at 25°C for 10 min. Then, the substrate solution was added and the residual PLA2 activity was monitored at 348-nm excitation and 390-nm emission using a spectrofluorometer. Each measurement was replicated three times with three independent samplings. For phenoloxidase (PO) analysis, 10 μl of hemolymph was collected from the fifth-instar S. exigua larvae and incubated with 10 μl of the bacterial metabolite. Then, substrate solution (980 μl) containing l-DOPA in PBS was added and the residual activity was monitored at 495 nm. For the nodule assay, Escherichia coli (3.2 × 104 cells) was mixed with the bacterial compound in a 10-μl volume and injected into the fifth-instar S. exigua. After 8 h of incubation at 25°C, the formed nodules were counted under a stereomicroscope at a magnification of ×50. (B) Median inhibitory concentrations (I50) of seven bacterial compounds against three endpoints. Each treatment was replicated three times. Means followed by different letters indicate significant differences at a type I error of 0.05 (LSD test).
Insecticidal activities of seven PLA2 inhibitors.
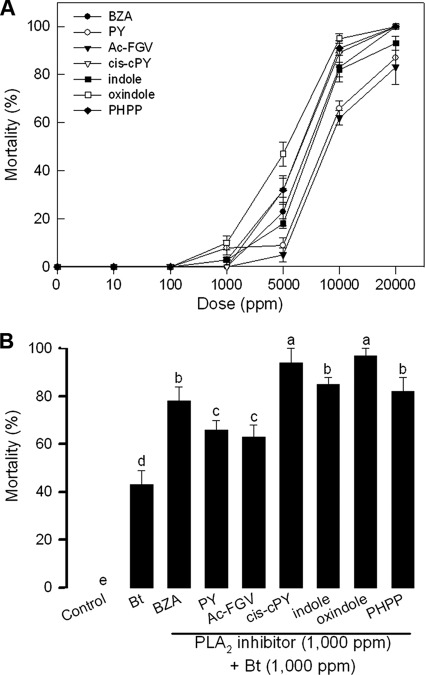
To test any insecticidal activity of these seven identified PLA2 inhibitors, different doses of these inhibitors were fed to larvae of P. xylostella (Fig. 7A). All PLA2 inhibitors showed significant insecticidal activities at relatively high doses. The 50% lethal concentration (LC50) of BZA was 5,735.6 ppm (95% confidence interval [CI], 5,531.5 to 5,831.2). The LC50 of PY was 7,694.8 ppm (95% CI, 7,341.1 to 7,948.6). The LC50 of Ac-FGV was 8,347.6 ppm (95% CI, 7,847.8 to 8,546.2). The LC50 of cis-cPY was 5,948.7 ppm (95% CI, 5,756.2 to 6,218.7). The LC50 of indole was 6,274.3 ppm (95% CI, 5,995.4 to 6,435.3). The LC50 of oxindole was 4,832.5 ppm (95% CI, 4,714 to 5,002.1). The LC50 of PHPP was 6,643.9 ppm (95% CI, 6,223.5 to 6,985.9). However, when these compounds at 1,000 ppm were added to B. thuringiensis insecticide, they significantly enhanced the B. thuringiensis pathogenicity (Fig. 7B).
Fig 7.
Insecticidal activities of seven bacterial compounds identified from bacterial culture broths of Xenorhabdus nematophila (Xn) and Photorhabdus temperata subsp. temperata (Ptt). Abbreviations: BZA, benzylideneacetone; Ac-FGV, acetylated FGV tripeptide; PY, PY dipeptide; cis-cPY, cis-cyclo-PY; indole, 1H-benzo[b]pyrrole; oxindole, 2-indolinone; PHPP, p-hydroxyphenyl propionic acid. The bioassay used a leaf-dipping method in test solution. Fourth-instar larvae were fed with cabbage leaves that had been soaked in different inhibitor solutions. Mortality was estimated at 48 h after treatment. Each treatment used 10 larvae and was replicated three times. (A) Dose-mortality curve of each bacterial compound. (B) Synergistic effect of seven compounds on pathogenicity of Bacillus thuringiensis (Bt). Different letters above standard deviation bars represent significant differences among means at a type I error of 0.05 (LSD test).
DISCUSSION
Two entomopathogenic bacteria, X. nematophila and P. temperata subsp. temperata, analyzed in this study are known to be potent biological agents for killing lepidopteran insects (1, 13). A previous study (17) showed that these two bacteria suppress insect immune responses by commonly inhibiting PLA2 enzyme activity. Catalytic activity of PLA2 is required for biosynthesis of eicosanoids (5). Eicosanoids play a crucial role in insect immune responses by activating PO (36) or by stimulating migration of hemocytes to infection foci (20). Thus, inhibiting PLA2 can prevent de novo supply of eicosanoids in response to pathogen infection, which leads to substantial enhancement to susceptibility of target insects to microbial pesticides. This study showed that X. nematophila and P. temperata subsp. temperata synthesized and released these PLA2 inhibitors to culture medium. By fractionation of these culture broths, this study identified seven PLA2 inhibitors, in which three are already known in chemical structures in the X. nematophila culture medium. Four unknown PLA2 inhibitors were chemically identified and analyzed for their biological activities in inhibition of insect immune responses, such as inducible PLA2 activity, PO activation, and hemocyte nodule formation. Finally, this study showed that these PLA2 inhibitors could potentially be applied to develop novel insect pesticides by showing their synergistic effects on B. thuringiensis pathogenicity.
PLA2 inhibitory factor(s) was synthesized and secreted to culture broth. With increase of culture period, the PLA2-inhibitory activity significantly increased and reached a maximal level at 48 h in both X. nematophila and P. temperata subsp. temperata cultures. Seo and Kim (34) showed that both bacteria grow and reach stationary phase at 48 h when they are cultured at 28°C with an initial bacterial inoculation of 5.0 × 106 CFU. This suggests that both bacteria synthesized and released PLA2 inhibitor(s) during the bacterial growth phase.
Ethyl acetate extracts of both bacterial culture broths contained significantly inhibitory effects against PLA2 activities of S. exigua hemocytes. Further fractionation of metabolites using CHCl3-methanol showed that PLA2 inhibitors were eluted in hydrophobic fractions. These suggested that both bacterial cultures might have similar metabolites possessing PLA2-inhibitory effects. A previous study predicted that a novel PLA2-inhibitory compound(s) originated from the entomopathogenic bacteria because an organic extract of X. nematophila culture broth inhibited various PLA2s derived from a wide range of organisms, including prokaryotes, insects, reptiles, and mammals (24). Later, chemical fractionation of the X. nematophila culture broth led to identification of three PLA2-inhibitory compounds, including BZA, PY, and Ac-FGV (11, 35). This current study finally purified seven compounds from X. nematophila and three compounds from P. temperata subsp. temperata culture broth, in which the three previously reported compounds were also detected. Further chemical analysis of highly PLA2-inhibitory fractions identified four novel PLA2 inhibitors of cis-PY, indole, oxindole, and p-hydroxyphenyl propionic acid.
cPY is known to be synthesized from another nematode-associated bacterium, Pseudomonas fluorescens, isolated from the pine wood nematode, Bursaphelenchus xylophilus, and shows toxicities to both suspension cells and seedlings of Pinus thunbergii (8). In a phytopathogen, Alternaria alternata, cPY is known as a phytotoxin called maculosin (22). It appears to bind to and inhibit ribulose-1,5-diphosphate carboxylase, an enzyme catalyzing carbon dioxide-fixing reaction in photosynthesis (21, 27). To our best knowledge, no report has been published on PLA2 inhibition of cPY. Interestingly, a relatively large amount of cPY was detected in P. temperata subsp. temperata culture broth, but little in X. nematophila culture broth. However, X. nematophila culture broth contained a significant amount of PY instead of cPY. We speculate that cPY may be synthesized from PY by an unknown cyclase activity that is highly active in P. temperata subsp. temperata compared to X. nematophila. This speculation was supported by significantly higher inhibitory activities of cPY compared to PY against PLA2, PO, and hemocyte nodulation. This current study also showed that cPY can form two stereoisomers probably during circularization of dipeptide PY, in which only the cis form was active in inhibiting PLA2 activity, suggesting that the cyclase may be stereospecific.
Indole and derivatives have been identified in culture broths of several Xenorhabdus spp. (26). These indole compounds showed antibacterial activity by inhibiting bacterial RNA synthesis through accumulation of a stress signal, guanosine-5′,5′-bis-pyrophosphate (39). Indole compound also showed a nematicidal effect against Aphelenchoides rhytium, Bursaphelenchus spp., and Caenorhabditis elegans (9, 10). However, no information was available for the PLA2-inhibitory effect of indole compound before this current study. In comparison, oxindole is an indole derivative containing carbonyl at position 2 of the five-membered ring. Its bacterial origin was reported as Penicillium sp., and it showed a significant inhibition of human synovial PLA2 (41).
p-Hydroxyphenyl propionic acid is known to be produced as a bacterial metabolite by degradation of proanthocyanidins, which are polyphenols especially profuse in grape seed (40). It possesses a medicinal effect on the prevention of chronic diseases probably by inhibition of platelet aggregation and inhibition of cyclo-oxygenase 2 in HT-29 colon cells, reduction in the synthesis of prostanoids in colon cells, and antiproliferative activity in prostate and cancer cells (6, 15, 30, 31). However, no direct evidence of PLA2 inhibition of PHPP was reported before this current study.
All seven PLA2 inhibitors identified from X. nematophila and P. temperata subsp. temperata culture broths also showed significant inhibition against PO activity and hemocyte nodulation of S. exigua. Hemocyte nodule formation is a biphasic process consisting of hemocyte aggregation and melanization (29). Hemocyte migration to pathogen can be modulated by eicosanoids (20). PO activity is required for melanization (4, 14). Thus, inhibitory activities of these seven bacterial metabolites against PLA2 and PO would result in suppression of hemocyte nodule formation in response to bacterial challenge. Among seven identified PLA2 inhibitors, BZA was the most active inhibitor against PLA2, PO, and hemocyte nodulation. Both bacteria synthesized and released BZA as the major metabolite. Injection or oral administration of BZA induced significant immunosuppression and developmental retardation in S. exigua (16, 18). BZA was also known to have potent antibiotic activity (11). Furthermore, any structural changes of BZA, such as hydroxylation on the phenyl ring or modification of the carboxyl group, reduced its inhibitory activity against PLA2 (35). These suggest that BZA plays a crucial role in pathogenesis of these bacteria in target insects.
Finally, this current study provides an application of these PLA2 inhibitors as additions to B. thuringiensis biopesticide. This is evident from immunosuppression induced by PLA2 inhibitors, which would be favored by the bacterial pathogen Bacillus thuringiensis. BZA was known to enhance B. thuringiensis pathogenicity in S. exigua (18). In addition, X. nematophila or P. temperata subsp. temperata culture broth showed significant enhancement of B. thuringiensis pathogenicity in S. exigua (12). These facts support the applicability of the seven PLA2 inhibitors for development as additives to other microbial biopesticides.
In summary, seven PLA2 inhibitors are identified in both X. nematophila and P. temperata subsp. temperata culture broths. These also showed significant inhibition against PO activity and hemocyte nodule formation in response to bacterial challenge. These immunosuppressive activities of PLA2 inhibitors can be applied to develop novel insecticides.
ACKNOWLEDGMENTS
This study was funded by an AGENDA grant from the Rural Development Administration, Suwon, Republic of Korea, to Y.K. S.S. was supported by the second-stage BK21 program of the Ministry of Education, Science and Technology, Republic of Korea.
Footnotes
Published ahead of print 23 March 2012
REFERENCES
- 1. Akhurst RJ. 1980. Morphological and functional dimorphism in Xenorhabdus ssp., bacteria symbiotically associated with the insect pathogenic nematodes Neoaplectana and Heterorhabditis. J. Gen. Microbiol. 121:303–309 [DOI] [PubMed] [Google Scholar]
- 2. Beckage NE. 2008. Insect immunology. Academic Press, New York, NY [Google Scholar]
- 3. Burke JE, Dennis EA. 2009. Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 50:5237–5242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cerenius L, Söderhäll K. 2004. The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 198:116–126 [DOI] [PubMed] [Google Scholar]
- 5. Dennis EA. 1997. The growing phospholipase A2 super family of signal transduction enzymes. Trends Biochem. Sci. 22:1–2 [DOI] [PubMed] [Google Scholar]
- 6. Gao K, et al. 2006. Of the major phenolic acid formed during human microbial fermentation of tea, citrus, and soy flavonoid supplements, only 3,4-dihydroxyphenylacetic acid has antiproliferative activity. J. Nutr. 136:52–61 [DOI] [PubMed] [Google Scholar]
- 7. Gillespie JP, Kanost MR, Trenzek T. 1997. Biological mediators of insect immunity. Annu. Rev. Entomol. 42:611–643 [DOI] [PubMed] [Google Scholar]
- 8. Guo Q, Guo D, Zhao B, Xu J, Li R. 2007. Two cyclo dipeptides from Pseudomonas fluorescens GcM5-1A carried by the pine wood nematode and their toxicities to Japanese black pine suspension cells and seedlings in vitro. J. Nematol. 39:243–247 [PMC free article] [PubMed] [Google Scholar]
- 9. Hu K, Li J, Webster JM. 1996. 3,5-Dihydroxy-4-isopropylstilbene: a selective nematicidal compound from the culture filtrate of Photorhabdus luminescence. Can. J. Plant Pathol. 18:104–116 [Google Scholar]
- 10. Hu K, Li J, Webster JM. 1999. Nematicidal metabolites produced by Photorhabdus luminescens (Enterobacteriaceae), bacterial symbionts of entomopathogenic nematodes. Nematology 1:457–469 [Google Scholar]
- 11. Ji D, et al. 2004. Identification of an antibacterial compound, benzylideneacetone, from Xenorhabdus nematophila against major plant-pathogenic bacteria. FEMS Microbiol. Lett. 239:241–248 [DOI] [PubMed] [Google Scholar]
- 12. Jung C, Kim Y. 2006. Synergistic effect of entomopathogenic bacteria (Xenorhabdus sp. and Photorhabdus temperata subsp. temperata) on the pathogenicity of Bacillus thuringiensis ssp. aizawai against Spodoptera exigua (Lepidoptera: Noctuidae). Environ. Entomol. 35:1584–1589 [Google Scholar]
- 13. Kang S, Han S, Kim Y. 2004. Identification of an entomopathogenic bacterium, Photorhabdus temperata subsp. temperata, in Korea. J. Asia Pac. Entomol. 7:331–337 [Google Scholar]
- 14. Kanost M, Jiang H, Yu X. 2004. Innate immune responses of a lepidopteran insects, Manduca sexta. Immunol. Rev. 198:97–105 [DOI] [PubMed] [Google Scholar]
- 15. Karlsson P, et al. 2005. Human fecal water inhibits COX-2 in colonic HT-29 cells: role of phenolic compounds. J. Nutr. 135:2343–2349 [DOI] [PubMed] [Google Scholar]
- 16. Kim J, Kim Y. 2011. Three metabolites from an entomopathogenic bacterium, Xenorhabdus nematophila, inhibit larval development of Spodoptera exigua (Lepidoptera: Noctuidae) by inhibiting a digestive enzyme, phospholipase A2. Insect Sci. 18:282–288 [Google Scholar]
- 17. Kim Y, Ji D, Cho S, Park Y. 2005. Two groups of entomopathogenic bacteria, Photorhabdus and Xenorhabdus, share an inhibitory action against phospholipase A2 to induce host immunodepression. J. Invertebr. Pathol. 89:258–264 [DOI] [PubMed] [Google Scholar]
- 18. Kwon B, Kim Y. 2008. Benzylideneacetone, an immunosuppressant, enhances virulence of Bacillus thuringiensis against beet armyworm (Lepidoptera: Noctuidae). J. Econ. Entomol. 101:36–41 [DOI] [PubMed] [Google Scholar]
- 19. Lee S. 2012. Identification, synthesis, and biological activities of cyclic PY. Andong National University, Andong, Republic of Korea [Google Scholar]
- 20. Merchant D, Ertl RL, Rennard SI, Stanley DW, Miller JS. 2008. Eicosanoids mediate insect hemocyte migration. J. Insect Physiol. 54:215–221 [DOI] [PubMed] [Google Scholar]
- 21. Miziorko HM, Lorimer GH. 1983. Ribulose-1,5-bisphosphate carboxylase-oxygenase. Annu. Rev. Biochem. 52:507–535 [DOI] [PubMed] [Google Scholar]
- 22. Park SH, Strobel GA. 1994. Cellular protein receptors of maculosin, a host specific phytotoxin of spotted knapweed (Centaurea maculosa L.). Biochim. Biophys. Acta 1199:13–19 [DOI] [PubMed] [Google Scholar]
- 23. Park Y, Kim Y. 2000. Eicosanoids rescue Spodoptera exigua infected with Xenorhabdus nematophila, the symbiotic bacteria to the entomopathogenic nematode Steinernema carpocapsae. J. Insect Physiol. 46:1469–1476 [DOI] [PubMed] [Google Scholar]
- 24. Park Y, Kim Y, Stanley D. 2004. The bacterium Xenorhabdus nematophila inhibits phospholipase A2 from insect, prokaryote, and vertebrate sources. Naturwissenschaften 91:371–373 [DOI] [PubMed] [Google Scholar]
- 25. Park Y, Kim Y, Yi Y. 1999. Identification and characterization of a symbiotic bacterium associated with Steinernema carpocapsae in Korea. J. Asia Pac. Entomol. 2:105–111 [Google Scholar]
- 26. Paul VJ, Frautschy S, Fenical W, Nealson KH. 1981. Antibiotics in microbial ecology: isolation and structure assignment of several new antibacterial compounds from the insect-symbiotic bacteria, Xenorhabdus spp. J. Chem. Ecol. 7:589–597 [DOI] [PubMed] [Google Scholar]
- 27. Prasad C. 1995. Bioactive cyclic dipeptides. Peptides 16:151–164 [DOI] [PubMed] [Google Scholar]
- 28. Radvanyi F, Jordan L, Russo-Marie F, Bon C. 1989. A sensitive and continuous fluorometric assay for phospholipase A2 using pyrene-labeled phospholipids in the presence of serum albumin. Anal. Biochem. 177:103–109 [DOI] [PubMed] [Google Scholar]
- 29. Ratcliffe NA, Rowley AF. 1979. A comparative synopsis of the structure and function of the blood cells of insects and other invertebrates. Dev. Comp. Immunol. 3:189–221 [DOI] [PubMed] [Google Scholar]
- 30. Rechner AR, Kroner C. 2005. Anthocyanins and colonic metabolites of dietary polyphenols inhibit platelet function. Thromb. Res. 116:327–334 [DOI] [PubMed] [Google Scholar]
- 31. Russell WR, Drew JE, Scobbie L, Duthie GG. 2006. Inhibition of cytokine-induced prostanoid biogenesis by phytochemicals in human colonic fibroblasts. Biochim. Biophys. Acta 1762:124–130. [DOI] [PubMed] [Google Scholar]
- 32. SAS Institute 1989. SAS/STAT user's guide, release 6.03. SAS Institute, Cary, NC [Google Scholar]
- 33. Seo S, Kim Y. 2009. Two entomopathogenic bacteria, Xenorhabdus nematophila K1 and Photorhabdus temperata subsp. temperata ANU101 secrete factors enhancing Bt pathogenicity against the diamondback moth, Plutella xylostella. Kor. J. Appl. Entomol. 38:385–392 [Google Scholar]
- 34. Seo S, Kim Y. 2010. Study on development of novel biopesticides using entomopathogenic bacterial culture broth of Xenorhabdus and Photorhabdus. Kor. J. Appl. Entomol. 49:241–249 [Google Scholar]
- 35. Shrestha S, Hong YP, Kim Y. 2010. Two chemical derivatives of bacterial metabolites suppress cellular immune responses and enhance pathogenicity of Bacillus thuringiensis against the diamondback moth, Plutella xylostella. J. Asia Pac. Entomol. 13:55–60 [Google Scholar]
- 36. Shrestha S, Kim Y. 2008. Eicosanoids mediate prophenoloxidase release from oenocytoids in the beet armyworm, Spodoptera exigua. Insect Biochem. Mol. Biol. 38:99–112 [DOI] [PubMed] [Google Scholar]
- 37. Shrestha S, Kim Y. 2009. Biochemical characteristics of immune-associated phospholipase A2 and its inhibition by an entomopathogenic bacterium, Xenorhabdus nematophila. J. Microbiol. 47:774–782 [DOI] [PubMed] [Google Scholar]
- 38. Stanley DW. 2000. Eicosanoids invertebrate signal transduction systems. Princeton University Press, Princeton, NJ [Google Scholar]
- 39. Sundar L, Chang FN. 1992. The role of guanosine-3′,5′-bispyrophosphate in mediating antimicrobial activity of the antibiotic 3,5-dihydroxy-4-ethyl-trans-stilbene. Antimicrob. Agents Chemother. 38:2645–2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ward NC, Croft KD, Puddey IB, Hodgson JM. 2004. Supplementation with grape seed polyphenols results in increased urinary excretion of 3-hydroxyphenylpropionic acid, an important metabolite of proanthocyanidins in humans. J. Agric. Food Chem. 52:5545–5549 [DOI] [PubMed] [Google Scholar]
- 41. Witter L, Anke T, Sterner O. 1998. A new inhibitor of synovial phospholipase A2 from fermentations of Penicillium sp. 62-92. Z. Naturforsch. C 53:60–64 [DOI] [PubMed] [Google Scholar]