Abstract
Significant phenotypic diversity was observed when we examined the abilities of a number of Cronobacter sakazakii natural isolates to cope with various sublethal stress conditions (acid, alkaline, osmotic, oxidative, or heat stress). Levels of catalase activity and use of acetate as a carbon source, phenotypes commonly used as indirect assays to predict RpoS function, revealed a high correlation between predicted RpoS activity and tolerance to acid, alkaline, osmotic, and oxidative treatments. The rpoS genes were sequenced and analyzed for polymorphisms. Loss-of-function mutations were found in two strains; C. sakazakii DPC 6523 and the genome-sequenced strain C. sakazakii ATCC BAA-894. The complementation of these strains with a functional rpoS gene resulted in an increase in bacterial tolerance to acid, osmotic, and oxidative stresses. The pigmentation status of strains was also assessed, and a high variability in carotenoid content was observed, with a functional rpoS gene being essential for the production of the characteristic yellow pigment. In conclusion, the evidence presented in this study demonstrates that rpoS is a highly polymorphic gene in C. sakazakii, and it supports the importance of RpoS for the tolerance under stress conditions that C. sakazakii may encounter in the food chain and in the host during infection.
INTRODUCTION
Cronobacter sakazakii is a Gram negative, peritrichous, motile, non-spore-forming, facultative anaerobic microorganism that is a member of the Enterobacteriaceae family. It is an opportunistic pathogen and the etiological agent of rare but life-threatening cases of meningitis, necrotizing enterocolitis, and sepsis in infants. Although it has been isolated from a wide variety of food sources (milk, cheese, kefir, tofu, meats, vegetables, rice, fermented bread, dried foods, tea, herbs, and spices), powdered infant formula (PIF) and powdered milk (PM) are the most common vehicles implicated in C. sakazakii infections (7, 22, 28, 49), and the microorganism has been shown to survive well in those environments (45). Available information on the taxonomy, biochemical characteristics, epidemiology, pathogenicity, and clinical features of C. sakazakii has been compiled in several review articles (13, 30, 32, 35, 39, 46).
Similar to other pathogens, C. sakazakii is likely to encounter numerous suboptimal conditions during its transition from the environment to the host gastrointestinal tract. For example, the pathogen may have to survive in dried foods and tolerate the variations in pH and osmolarity encountered in the intestine. Elucidation of the mechanisms used by C. sakazakii to survive stressful conditions will therefore be important for the development of control and treatment strategies.
The alternative sigma factor σs, encoded by the rpoS gene, modulates a large regulon that controls expression of ∼10% of the genome in Gram-negative bacteria, including genes that govern the general stress response of stationary-phase cells (33, 57). Several authors have shown rpoS to be located in a highly polymorphic segment of the chromosome (24, 25). In addition, rpoS has been shown to be a highly mutable gene in Escherichia coli and Salmonella spp. (9, 20, 38, 51, 52, 56), and its expression level has been reported to contribute to the overall intraspecies variability in stress resistance for E. coli (14, 52, 56). However, to our knowledge, the relationship between rpoS and stress tolerance has not been investigated in C. sakazakii.
The current study was undertaken to investigate the growth or survival of a number of natural isolates of C. sakazakii under various sublethal stress conditions. Furthermore, analyses of rpoS genes were performed in order to examine if phenotypic diversity could be correlated with RpoS status among C. sakazakii strains.
MATERIALS AND METHODS
Bacterial strains, media, chemicals, and culture conditions.
The bacterial isolates, plasmids, and primers used throughout this study are shown in Table 1. Master stocks of all strains were maintained in cryovials in the presence of 40% glycerol as cryoprotectant and stored at −80°C. Bacteria were resuscitated in tubes containing 10 ml of Luria-Bertani (LB) broth (Merck) by incubation at 37°C for 24 h followed by streaking on LB agar plates, which were incubated under the same conditions and stored at 4°C. Stationary-phase cell suspensions were obtained by inoculating 10 ml of fresh LB with an isolated colony from the stock LB agar plates and incubating it overnight at 37°C. Chloramphenicol (Sigma) was made up as a concentrated stock and added to the medium at the required levels. For solid medium, agar (Merck) was added to 1.5%.
Table 1.
Bacterial strains, plasmids, and primers
| Strain, plasmid, or primer | Origin, characteristic, or sequence | Sourcea |
|---|---|---|
| C. sakazakiistrains (no.) | ||
| NCTC08155 (1) | DPC | |
| ATCC 12868 (2) | ATCC | DPC |
| ATCC 29004 (3) | ATCC | DPC |
| DSM4485 (4) | DPC | |
| NCTC11467 (5) | DPC | |
| DPC 6522 (6) | Blood | DPC |
| DPC 6523 (7) | Cerebrospinal fluid | DPC |
| DPC 6524 (8) | Stool | DPC |
| DPC 6525 (9) | Urine | DPC |
| DPC 6526 (10) | Blood | DPC |
| DPC 6527 (11) | Blood | DPC |
| DPC 6528 (12) | Cerebrospinal fluid | DPC |
| DPC 6529 (13) | Tracheal aspirate | DPC |
| DPC 6530 (14) | Bronchial alveolar lavage fluid | DPC |
| ATCC BAA-894 (15) | ATCC | DPC |
| ATCC BAA-894 + rpoSb | This study | |
| DPC 6523 + rpoSb | This study | |
| Plasmid | ||
| pNZ44 | Cmrc | 43 |
| Primers | ||
| RPOS-1 | TGATTACCTGAGTGCCTACG | |
| RPOS-2 | TGAACTTCATGAGGGAGAGC | |
| RPOS-3 | GATCGAAAGTAACCTGCGTC | |
| RPOS-4 | TCCGGAACCAGTTCAACACG | |
| RPOS-5 | CGCAGATAGACGTTAAGCTC | |
| RPOS-6 | TGTCTGGCGGCATGTAATTC | |
| RPOS-7 | CTAGCCATGGATCAGAATACGCTGAAAG | |
| RPOS-8 | GTCATCTAGAGTGATTATTCGCGGAACAG |
All strains were obtained from the Dairy Products Research Centre (DPC), Fermoy, County Cork, Ireland. Strains 6 to 14 were originally obtained from M. Ardino, Centers for Disease Control and Prevention, Atlanta, GA.
rpoS was amplified from C. sakazakii DPC 6529.
Cmr, chloramphenicol resistance.
Indirect assays for the assessment of RpoS status. (i) Catalase activity.
The catalase activity of C. sakazakii strains was examined by dropping 20 μl of 6% (wt/vol) hydrogen peroxide (Sigma) onto fresh colonies that were grown on LB agar for 24 h at 37°C. Immediate vigorous bubbling indicated positive catalase activity. Strains were considered to have moderate reactivity to hydrogen peroxide when a delay in bubbling was observed. When both a delay and a reduction in bubbling were observed, the strains were considered strains with low reactivity. Moreover, no bubbling indicated negative catalase activity. Catalase activity was also measured using a spectrophotometric approach, following an adapted version of the protocol described by den Besten and coworkers (17). Briefly, cells in the mid-exponential growth phase were pelleted by centrifugation, washed with phosphate-buffered saline (PBS; Gibco), and finally resuspended in PBS containing 40 mM H2O2. The decrease in absorbance at 240 nm was measured over time at 30°C with a spectrophotometer (Beckman Coulter). The optical density of the exponential-phase cultures was measured at 600 nm and normalized, so for all strains the amount of cells used was similar. Plate count data confirmed this (data not shown).
(ii) Utilization of acetate as a carbon substrate.
C. sakazakii strains were screened for their growth in M9 (minimal medium) broth (Fluka) and M9 agar plates supplemented with various concentrations (ranging from 0.04 to 0.4%) of acetate (Sigma) as a carbon source. The growth ability in the presence of acetate was used as an indirect assay for the assessment of RpoS status, since it has been shown for E. coli strains that high rpoS expression/activity is associated with a reduced ability to compete for poor growth substrates, like acetate, or even good substrates, like glucose, at suboptimal concentrations (26). In fact, it has been shown that rpoS mutants show increased growth abilities in the presence of acetate as the sole carbon source (38).
Evaluation of bacterial behavior under heat, acid, alkaline, osmotic, and oxidative sublethal stress conditions.
All stress tolerance experiments were performed in LB broth. When required, the challenge medium was supplemented by adding 3 N HCl (Sigma), 3 N NaOH (Merck), or 30% H2O2. Heat treatments were carried out at 60°C. Once the temperature of the treatment medium (1 ml of LB) was stabilized, an inoculum of 0.01 ml of each bacterial suspension was added. During heating, samples were removed and plated on LB agar. For acid, alkaline, and oxidative treatments, aliquots of stationary-phase cultures were inoculated (1% [vol/vol] inoculation concentration) into LB broth supplemented with HCl (pH 2.5), NaOH (pH 11.0), or H2O2 (30 mM). After incubation at room temperature, the survival was monitored periodically. For osmotic treatments, 100-μl aliquots of stationary-phase cultures were transferred to 96-well culture plates (Genetix). The plates were kept without lids in a 25°C incubator for air drying. Under these conditions the time to dry of the sample was approximately 3 h. Subsequently, plates were incubated at room temperature for up to 8 days, and bacterial survival was determined after the rehydration of samples by addition of 100 μl PBS.
Under all treatment conditions, 10-fold serial dilutions were produced in sterile PBS solution, and suitable dilutions were plated in duplicate on LB agar plates. Viable cells at each point in time were enumerated following incubation of the plates at 37°C for 48 h (longer incubation times did not have any influence on the count).
In order to test the growth of the C. sakazakii strains under several sublethal stress conditions, overnight cultures were inoculated into LB broth containing various concentrations of HCl, NaOH, H2O2, and NaCl (Sigma) in 96-well culture plates (inoculation level of 1%). Cell growth at 37°C was measured spectrophotometrically by determining the optical density at 600 nm (OD600) using a temperature-controlled automatic plate reader (Multiscan FC; Thermo Scientific), and the time to detection (TTD), chosen as the time (in hours) at which the culture reached an OD600 of 0.2, was determined for each strain under the different conditions tested.
DNA manipulation and cloning procedures.
Genomic DNA was isolated using the PureLink genomic DNA kit (Invitrogen). Plasmid DNA was isolated with the Qiagen QIAprep spin miniprep kit (Qiagen). DNA was extracted from agarose gels using the Qiaex II gel extraction kit (Qiagen). PCR reagents (KOD DNA polymerase [Novagen], deoxynucleoside triphosphates [dNTPs; Novagen]) and T4 DNA ligase (Roche) were used according to the manufacturers' instructions.
C. sakazakii strains 7 and 15 were complemented with a functional rpoS gene. The rpoS gene was amplified from C. sakazakii 13 by using primers RPOS-7 and RPOS-8. The PCR product was purified, digested with NcoI and XbaI, and ligated to similarly digested plasmid pNZ44. Electrocompetent C. sakazakii cells were transformed by electroporation, and transformants were selected on LB agar plates supplemented with 10 μg/ml chloramphenicol at 37°C.
Sequencing of the rpoS gene from C. sakazakii strains.
In order to sequence the rpoS gene from the 15 C. sakazakii strains, a 1.3-kb PCR fragment specific to rpoS was amplified from chromosomal DNA preparations by using primers RPOS-1 and RPOS-2 (Table 1). For each strain, several PCR products were purified and sequenced by using different combinations of primers RPOS-1 to RPOS-5, also shown in Table 1. A PCR product was not obtained for strain 7, and a new primer (RPOS-6 [Table 1]), designed in the upstream region of the nlpD gene, which precedes the rpoS gene, was used for amplification and sequencing of the rpoS gene for that strain. Sequencing was performed by MWG Biotechnologies (Germany). The sequences were analyzed using the Clone Manager professional suite (Sci-Ed Central), and multiple local alignments were carried out with ClustalW software (54).
Assessment of pigmentation status.
The production of carotenogenic pigment by C. sakazakii strains was determined both visually and spectrophotometrically. The color of colonies grown on LB agar was examined after 72 h of incubation at 25°C. Yellow colonies indicated the production of pigment. The carotenoid content was quantitatively measured using the spectrophotometric approach described by Morikawa and coworkers (44). Briefly, the cellular mass collected from bacterial colonies was resuspended in 1.5 ml of PBS until a suspension with an OD600 of 1.0 was obtained. Bacterial suspensions were centrifuged (6,000 × g, 5 min) and resuspended in 0.5 ml of methanol (Sigma). Following incubation for 6 min at 55°C and centrifugation for 3 min at 10,000 × g, the supernatant was collected and the carotenoid content of that extract was determined by measuring the absorbance at 465 nm.
Statistical analysis.
Experimental results were compared by performing Student's t test for independent samples with the Statistica for Windows version 7.0 program (Statsoft, Inc., Tulsa, OK).
RESULTS
Assessment of RpoS activity.
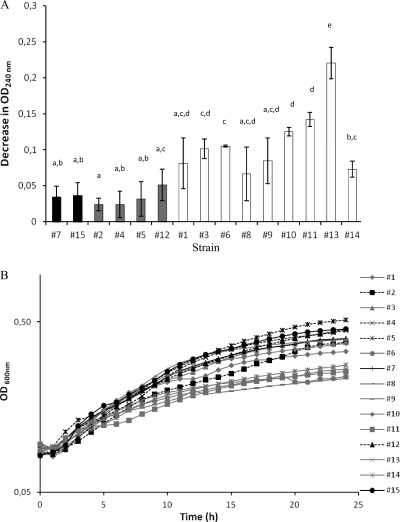
The catalase activities of colonies were examined by adding 6% (wt/vol) hydrogen peroxide. For the majority of strains (strains 1, 3, 6, 8, 9, 10, 11, 13, and 14), immediate vigorous bubbling was observed. The remaining strains showed moderate (strains 4 and 5), low (strains 2 and 12), or no (strains 7 and 15) reactivity to hydrogen peroxide. Therefore, the strains were grouped into one of three categories, i.e., strains with no rpoS activity (highlighted in black in all figures), strains with low/moderate rpoS activity (highlighted in gray in all figures), and strains with high rpoS activity (highlighted in white in all figures). Spectrophotometric studies corroborated these results (Fig. 1A). The strains that showed the lowest decrease in absorbance at 240 nm in the presence of 40 mM hydrogen peroxide and consequently the lowest catalase activity are likely to have low RpoS activity. On the other hand, strains 10, 11, and especially 13 (13 was significantly different from the rest of the strains [P < 0.05]) showed the highest catalase activities and may be considered the strains with the greatest rpoS activity.
Fig 1.
(A) Catalase activities of the 15 C. sakazakii strains. Cells in the mid-exponential phase of growth were pelleted by centrifugation, washed once in PBS, and resuspended in PBS containing 40 mM H2O2. The decrease in absorbance at 240 nm was positively correlated to the catalase activity of the strain. The average of two independent experiments ± the standard deviations are shown. The strains with a presumptive lack of RpoS activity are shown in black. The strains with a presumptive low/moderate RpoS activity are shown in gray. The strains with a presumptive high RpoS activity are shown in white. Strains sharing at least one lowercase letter did not have significantly different results (P ≥ 0.05). (B) Growth curves of the 15 C. sakazakii strains in M9 broth supplemented with 0.4% acetate (results from one of three trials). Black symbols and continuous lines are used for strains with a presumptive lack of RpoS activity. Black symbols and discontinuous lines are used for strains with a presumptive low/moderate RpoS activity. Gray symbols and continuous gray lines are used for strains with a presumptive high RpoS activity.
When the abilities of the strains to use acetate as a sole carbon source were tested, it was observed that, in general, those strains with low catalase activity and presumptive low RpoS activity grew faster than strains with high catalase and presumptive high RpoS activities (Fig. 1B).
Heterogeneity in stress tolerance.
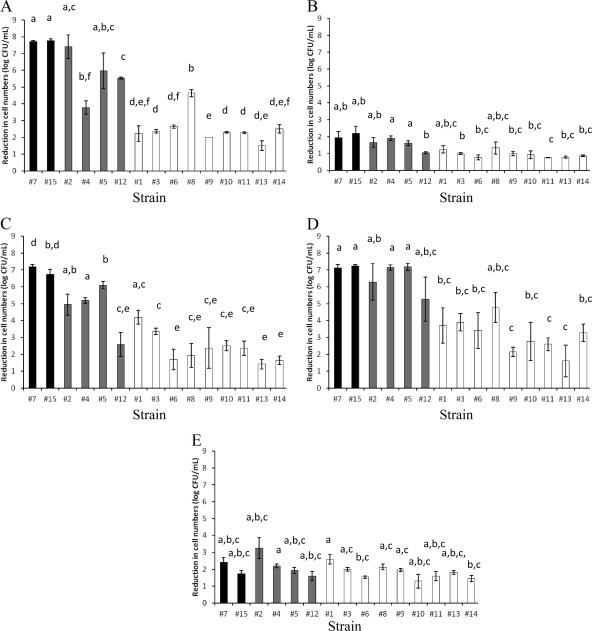
In order to study the variability in acid tolerance among the 15 C. sakazakii strains, cells grown for 24 h at 37°C in LB broth were treated in LB medium adjusted to pH 2.5 using hydrochloric acid. Figure 2A shows the log cycles of inactivation achieved after a 60-min treatment. Significant heterogeneity in acid tolerance was observed. For example, a reduction of less than 2 log-cycles was observed for strain 13, whereas approximately 7 log-cycles of inactivation were observed in the cases of strains 2, 7, and 15. A lower variability was observed for cells treated under alkaline stress conditions (60-min treatment in LB broth adjusted to pH 11.0 using sodium hydroxide [Fig. 2B]). This treatment caused low lethality, with approximately 1 log-cycle reduction for most strains. Strains 4, 7, and 15, with approximately 2 log-cycles of inactivation, were the most alkaline-sensitive strains. C. sakazakii strains were osmotically challenged by means of dehydration by air drying and subsequent incubation at room temperature for up to 8 days (Fig. 2C). The most osmotic stress-resistant strains, 6, 8, 13, and 14, only showed a reduction of less than 2 log-cycles. On the other hand, more than 6 log-cycles of inactivation were achieved for the most sensitive strains, 5, 7, and 15. Significant variability among strains was also observed for cells exposed to lethal oxidative stress conditions (60-min treatment in LB broth containing 30 mM hydrogen peroxide [Fig. 2D]). Whereas strain 13, with approximately 1.5 log-cycles of inactivation, was the most resistant strain, an approximately 7 log-cycles reduction was achieved for the most oxidative stress-sensitive strains, 4, 5, 7, and 15. In the case of lethal heat treatments (exposure to 60°C for 5 min [Fig. 2E]), a lower heterogeneity in stress tolerance was found. Strains 2, 1, and 7, with 3.3, 2.6, and 2.4 log-cycles of inactivation, respectively, were the most heat-sensitive strains. On the other hand, the most heat-resistant strains, 6, 10, and 14, only showed a reduction of around 1.5 log-cycles.
Fig 2.
Reduction in cell numbers (in log CFU/ml) for the 15 C. sakazakii strains after different lethal treatments. The averages of two independent experiments ± standard deviations are shown. (A) Acid stress (60-min treatment in LB broth adjusted to pH 2.5 using HCl). (B) Alkaline stress (60-min treatment in LB broth adjusted to pH 11.0 using NaOH). (C) Osmotic stress (dehydration for 8 days at room temperature). (D) Oxidative stress (60-min treatment in LB broth supplemented with 30 mM H2O2). (E) Heat stress (5-min treatment in LB broth at 60°C). The strains with a presumptive lack of RpoS activity are shown in black. The strains with a presumptive low/moderate RpoS activity are shown in gray. The strains with a presumptive high RpoS activity are shown in white. Strains sharing at least one lowercase letter (a to f) did not have significantly different results (P ≥ 0.05).
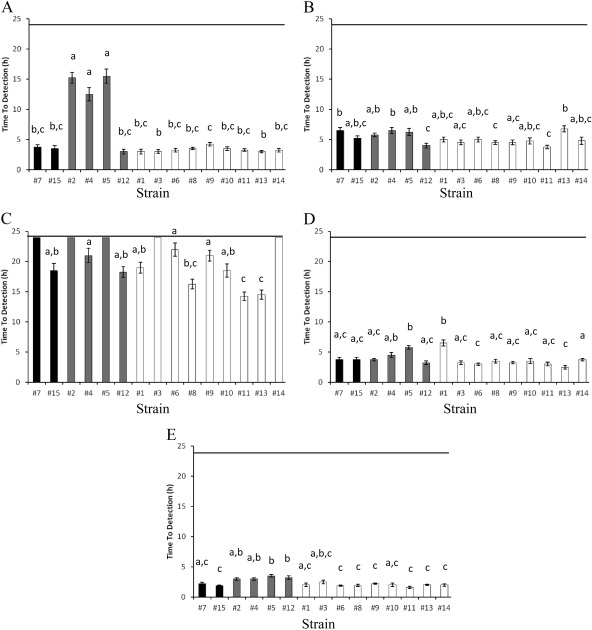
The growth of the 15 C. sakazakii strains under a set of sublethal stress conditions was compared by using the TTD at 600 nm. The TTD is defined as the time until the threshold OD600 value of 0.2 is reached (10). This threshold value was chosen because it is well above the detection limit of the optical reader, and such a threshold helps avoid false-positive growth samples due to fluctuations in optical density close to the detection limit. High TTD values reflect a delay in bacterial growth rather than differences in growth rates. Strains 2, 4, and 5 showed a significant delay in growth (P < 0.05) under sublethal acid environments (LB broth at pH 4.6) (Fig. 3A), with TTDs of 15.25, 12.5, and 15.5 h, respectively, whereas TTDs between 3.0 and 4.3 h were observed for the remainder of the strains. Under alkaline stress conditions (LB broth at pH 9.5 [Fig. 3B]), strains 11 and 12 showed the greatest growth abilities (TTDs of 3.8 and 4.0 h, respectively). On the other hand, the highest TTDs were observed for strains 4, 5, 7, and 13 (TTDs ranging from 6.3 to 6.8 h). With regard to osmotic stress environments (LB broth supplemented with 8% NaCl [Fig. 3C]), no significant growth was detected for five strains after 24 h of incubation (strains 2, 3, 5, 7, and 14). On the contrary, strains 8, 11, and 13, with TTDs ranging from 14.3 to 16.3 h, showed the best growth capacities. Under sublethal oxidative stress conditions (LB broth supplemented with 2 mM hydrogen peroxide [Fig. 3D]) strains 1, 4, and 5 were the most sensitive strains (TTDs ranging from 4.5 to 6.5 h), while TTDs of 3.0 to 3.8 h were observed for the remaining strains. Finally, faster growth was observed under sublethal heat stress (LB broth at 45°C [Fig. 3E]), with TTDs ranging from 1.6 to 3.5 h. In this case, strains 2, 4, 5, and 12 showed the lowest TTDs.
Fig 3.
Growth capacity (time to detection) of the 15 C. sakazakii strains under different sublethal stress conditions. The averages of two independent experiments ± standard deviations are shown. (A) Acid stress (LB broth adjusted to pH 4.6 using HCl). (B) Alkaline stress (LB broth adjusted to pH 9.5 using NaOH). (C) Osmotic stress (LB broth supplemented with 8% NaCl). (D) Oxidative stress (LB broth supplemented with 2 mM H2O2). (E) Heat stress (LB broth at 45°C). The strains with a presumptive lack of RpoS activity are shown in black. The strains with a presumptive low/moderate RpoS activity are shown in gray. The strains with a presumptive high RpoS activity are shown in white. Strains sharing at least one lowercase letter (a to c) were not significantly different (P ≥ 0.05).
Relationship between rpoS status and stress tolerance.
Several strains highly resistant to multiple stresses were identified. These included strains 9, 10, 11, 13, and 14, which constantly showed the greatest tolerance to acid, alkaline, osmotic, and oxidative lethal stress environments. Among them, strain 13 was particularly noteworthy, as it was the most acid-, osmotic stress-, and oxidative-tolerant strain. On the other hand, strains 2, 4, 5, 7, and 15 could be considered strains sensitive to multiple stresses, and strains 7 and 15 consistently showed the lowest capacities to withstand the stress conditions. In the growth experiments, lower variability was observed among the 15 C. sakazakii strains. However, it is important to note that strains 2, 4, and 5, which are sensitive to multiple stresses, showed the lowest growth abilities under most of the sublethal stress conditions tested, and this effect was especially marked for cells exposed to acid environments.
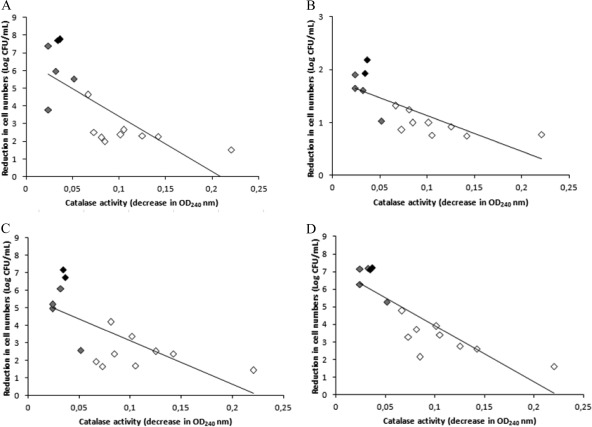
Correlation analyses (Fig. 4) revealed a positive correlation between the catalase activity (functioning as an indirect indicator of RpoS activity) and bacterial tolerance to acid, alkaline, osmotic, and oxidative lethal stress exposures, with r values of 0.55, 0.57, 0.47, and 0.73, respectively. Thus, those strains previously identified as resistant to multiple stresses (strains 9, 10, 11, 13, and 14) belonged to the group of strains with higher catalase/RpoS activities, and strain 13, with the highest catalase activity, also had the greatest tolerance to the imposed acid, osmotic, and oxidative stress conditions.
Fig 4.
Relationship between catalase activity, an indirect indicator of RpoS activity, and bacterial tolerance (log-cycles of inactivation) to lethal stress exposures. (A) Acid stress (60-min treatment in LB broth adjusted to pH 2.5 using HCl). (B) Alkaline stress (60-min treatment in LB broth adjusted to pH 11.0 using NaOH. (C) Osmotic stress (dehydration for 8 days at room temperature). (D) Oxidative stress (60-min treatment in LB broth supplemented with 30 mM H2O2). The strains with a presumptive lack of RpoS activity are shown in black. The strains with a presumptive low/moderate RpoS activity are shown in gray. The strains with a presumptive high RpoS activity are shown in white.
Analysis of rpoS alleles.
The rpoS genes were amplified and sequenced. Polymorphisms were observed in the rpoS sequences of the different natural isolates (Table 2), and strains 7 and 15, both multiply stress-sensitive strains with no catalase activity, showed significant disruptions in the rpoS nucleotide sequence. An 843-bp deletion encompassing 609 bp of the upstream nlpD gene, 60 bp of the intergenic region, and the first 174 bp of the rpoS open reading frame was present in strain 7. Strain 15 has undergone a single-base substitution at nucleotide 601 of the rpoS open reading frame, which resulted in the introduction of a stop codon (TAG). Regarding the other strains, whereas some of the polymorphisms observed did not change the amino acid composition of the RpoS protein, amino acid substitutions were observed at positions 562 to 564 (glutamine/lysine) and 802 to 804 (proline/alanine) of the open reading frame.
Table 2.
Variation in rpoS sequences of the C. sakazakii strains tested
| Strain(s) | Bases (amino acid)a at positions: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 370–372 | 556–558 | 562–564 | 601–603 | 613–615 | 619–621 | 682–684 | 712–714 | 802–804 | 823–825 | 874–876 | |
| 1, 9, 11, 13 | AAT (N) | TCC (S) | AAA (K) | CAG (Q) | CCA (P) | GAC (D) | GGC (G) | CTG (L) | GCC (A) | GCC (A) | GGC (G) |
| 3, 10, 12, 14 | AAT (N) | TCC (S) | CAA (Q) | CAG (Q) | CCA (P) | GAC (D) | GGC (G) | CTG (L) | GCC (A) | GCC (A) | GGC (G) |
| 4, 5 | AAC (N) | TCG (S) | AAA (K) | CAG (Q) | CCG (P) | GAT (D) | GGT (G) | CTG (L) | GCC (A) | GCC (A) | GGT (G) |
| 6 | AAT (N) | TCC (S) | CAA (Q) | CAG (Q) | CCA (P) | GAC (D) | GGC (G) | CTG (L) | CCC (P) | GCC (A) | GGC (G) |
| 7b | AAT (N) | TCC (S) | AAA (K) | CAG (Q) | CCA (P) | GAC (D) | GGC (G) | CTG (L) | GCC (A) | GCC (A) | GGC (G) |
| 8 | AAC (N) | TCG (S) | AAA (K) | CAG (Q) | CCA (P) | GAC (D) | GGT (G) | TTG (L) | GCC (A) | GCT (A) | GGC (G) |
| 15 | AAT (N) | TCC (S) | AAA (K) | TAG(Stop codon) | CCA (P) | GAC (D) | GGC (G) | CTG (L) | GCC (A) | GCC (A) | GGC (G) |
A, alanine; D, aspartic acid; G, glycine; K, lysine; L, leucine; N, asparagines; P, proline; Q, glutamine; S, serine. Results shown in boldface indicate relevant changes in the amino acid sequence of the RpoS protein.
Strain with an 843-bp deletion, including the first 174 bp of the rpoS open reading frame.
Numerous attempts were made to sequence the rpoS gene of strain 2. Several combinations of primers were used (5 primer sets in total), and PCR products of the correct size were amplified in all cases. These products were purified from agarose gels and sequenced. Although sequence analysis confirmed that it was the rpoS gene that had been amplified, the sequence data were not of sufficient quality to make definite conclusions with regard to polymorphisms. The reason why consistently poor sequence data were obtained for this strain is unknown.
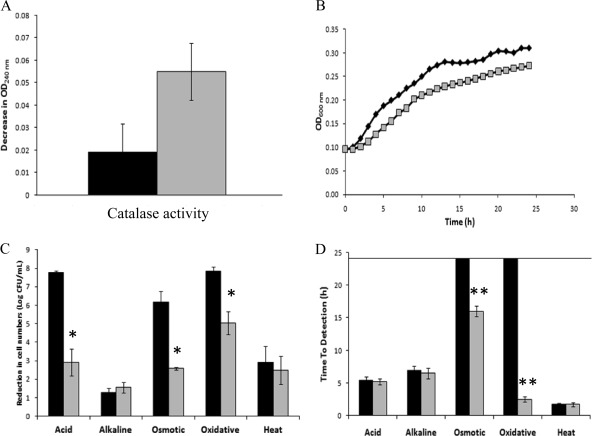
The contribution of RpoS to C. sakazakii stress tolerance was confirmed by complementing strains 7 and 15 with a functional rpoS gene amplified from strain 13, the strain with the highest rpoS activity, inserted into the pNZ44 vector. Transformants were subsequently characterized regarding their RpoS activities and stress tolerances (Fig. 5; see also Fig. S1 in the supplemental material). The complemented strains showed significantly higher catalase activities and lower abilities to grow in minimal medium in the presence of acetate than their wild-type counterparts, which is indicative of increased RpoS activity. In addition, the complementation of both strains with a functional rpoS gene resulted in a significant increase in bacterial tolerance to acid, oxidative, and osmotic lethal stress conditions. Finally, the complemented strains were able to grow under some stress conditions close to the growth boundaries that inhibited the growth of their wild-type counterparts (e.g., it was possible to detect growth for the complemented strain 15 in LB broth supplemented with 8% sodium chloride and 4 mM hydrogen peroxide).
Fig 5.
Characterization of strain 15 C. sakazakii ATCC BAA-894 (black) and its counterpart complemented with a functional rpoS gene (gray). (A) Catalase activity (decrease in OD240 after incubation with 40 mM H2O2). (B) Growth curves in M9 broth supplemented with 0.4% acetate. (C) Inactivation rate (log CFU/ml) after lethal acid (60 min at pH 2.5), alkaline (60 min at pH 11.0), osmotic (dehydration for 8 days at room temperature), oxidative (60 min at 30 mM H2O2), or heat treatment (5 min at 60°C). (D) Growth capacity (time to detection) under sublethal acid (pH 4.0), alkaline (pH 9.75), osmotic (8% NaCl), oxidative (4 mM H2O2), or heat (45°C) stress conditions. Asterisks indicate the complemented strain was significantly different from its counterpart: *, P < 0.05; **, P < 0.01.
Assessment of pigmentation status.
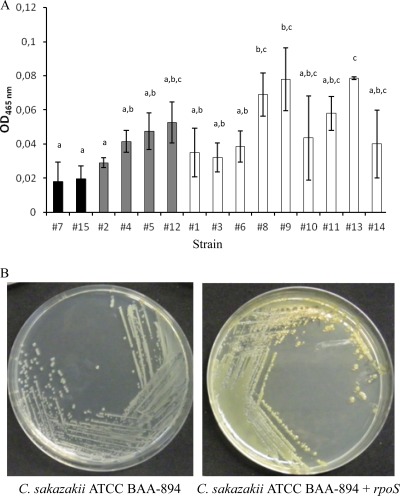
In order to test whether there is a relationship between yellow pigmentation, RpoS status, and stress tolerance, the pigmentation statuses of the different C. sakazakii isolates were determined after growth in LB agar (72 h at 25°C), using both a visual and a spectrophotometric approach. Colonies with the characteristic yellow pigmentation were obtained for the different isolates, with the exception of strains 7 and 15, both of which are sensitive to multiple stresses and with no rpoS activity and produced colorless colonies. The carotenogenic pigment was extracted, and its relative concentration was determined by measuring the absorbance of the extract at 465 nm (Fig. 6A). As expected, the lowest absorbances were obtained for the two strains with colorless colonies. On the other hand, the highest absorbances were observed for strains 8, 9, and 13, all multiply stress-resistant strains with high rpoS activity that showed a carotenoid content significantly higher (P < 0.05) than that of the two colorless strains. In addition to the previous evidence, complementation of the colorless strains, 7 and 15, with a functional rpoS gene restored the yellow pigmentation (Fig. 6B [for data for strain 15]).
Fig 6.
(A) Carotenoid content of the 15 C. sakazakii strains. The carotenogenic pigment was extracted, and its relative concentration was determined by measuring the absorbance of the extract at 465 nm. The averages of two independent experiments ± standard deviations are shown. The strains with a presumptive lack of RpoS activity are shown in black. The strains with a presumptive low/moderate RpoS activity are shown in gray. The strains with a presumptive high RpoS activity are shown in white. Strains sharing at least one lowercase letter (a to c) were not significantly different (P ≥ 0.05). (B) Visual assessment of the production of yellow pigment by C. sakazakii ATCC BAA-894 and C. sakazakii ATCC BAA-894 plus rpoS.
DISCUSSION
Since the recognition of C. sakazakii as an important emerging food-borne pathogen, several research papers have focused on the characterization of the tolerance of C. sakazakii to relevant environmental stress conditions (1, 2, 3, 4, 11, 16, 21, 50). However, most of these works focused on a single strain or a very limited number of C. sakazakii strains, and there is a lack of comprehensive studies addressing the heterogeneity in stress tolerance among natural isolates of C. sakazakii. The current study was undertaken to examine the tolerance of a number of C. sakazakii isolates to several food- and host-related stresses (acid, alkaline, osmotic, oxidative, and heat stresses) in order to evaluate the intraspecies variability. In addition, after taking into account the recognized role of the alternative sigma factor RpoS in the general stress response of Gram-negative bacteria (18, 33), the rpoS alleles were analyzed in order to examine how much of any phenotypic diversity could be attributed to variation in rpoS status among C. sakazakii strains. Our results demonstrated that C. sakazakii isolates had significant heterogeneity in their abilities to tolerate stress, which was predominantly dependent on the stress condition tested. We observed wide variations in tolerance (differences of 5.5 to 6.5 log cycles of inactivation between the most and the less stress-resistant strain) for acid-, osmotic stress-, and oxidative stress-treated cells, but less heterogeneity was observed for the alkaline and heat treatments (differences of 1.5 to 2.0 log cycles of inactivation between the most tolerant strain and the less stress-tolerant strain). On several occasions it has been proposed that strains more resistant to a given stress tend to be more resistant to various other types of inactivation agents (5, 34). However, there are some reported exceptions to this general behavior (31, 55). In our case, it was possible to find several strains tolerant to multiple stresses: strains 9, 10, 11, 13, and 14. On the other hand, strains 2, 4, 5, 7, and 15 were relatively sensitive to all the inactivation agents tested. Overall, our findings suggest it is important to examine large numbers of isolates, or cocktails of strains, when performing studies aimed to predict stress resistance abilities, since studies focused on a few isolates or a single strain may seriously underestimate the variabilities that underlie important phenotypic variations in pathogen populations.
Several authors previously reported variations in rpoS activity in natural populations of E. coli, ranging from null to full expression (8, 9, 38, 40, 52, 56). Furthermore, rpoS has been shown to be located in a highly polymorphic segment of the chromosome (15, 24). Different indirect assays have been recently used to assess the variation at the rpoS locus, such as analysis of temperature tolerance, the catalase assay, detection of synthesis of glycogen, evaluation of β-galactosidase activity, or assessment of the bacterial nutritional profiles (9, 38, 40). In our study, we indirectly examined the RpoS activity of C. sakazakii strains by determining their catalase activities and by testing their nutritional profiles. We observed a wide variation in rpoS activity among C. sakazakii strains, and this was closely linked to their tolerance to environmental stress conditions. Thus, those C. sakazakii strains with low, moderate, or no RpoS activity belonged to the group of strains sensitive to multiple stresses, and C. sakazakii strain 13, with the highest catalase and RpoS activities, consistently showed the greatest tolerance to the range of lethal treatments tested.
Some authors have previously shown rpoS to be a highly mutable gene in E. coli populations (14, 20, 52, 56). In the most comprehensive single study, Waterman and Small (56) found that over 20% of 58 E. coli strains had mutations in the rpoS gene that were linked to a reduced stress tolerance. The majority of rpoS mutations are loss-of-function mutations with little or no residual RpoS protein and include stop codons, deletions, and insertions as well as point mutations, although partial or attenuated rpoS mutations have also been described (25, 48). In our study, the rpoS gene was sequenced, and several polymorphisms were detected for the different isolates, including two loss-of-function mutations in C. sakazakii strains 7 and 15, with an 843-bp deletion and a single-base nonsense mutation, respectively. These strains were sensitive to multiple stresses and showed an especially high sensitivity to acid, osmotic, and oxidative treatments. Some other single-base substitutions resulting in modifications in the amino acid sequence of the RpoS protein were observed. However, none of these polymorphisms could be associated with a stress-sensitive phenotype. Therefore, for the remainder of strains sensitive to multiple stress conditions and with low/moderate rpoS activity, the phenotype observed could not be attributed to mutations in the rpoS gene open reading frame. The low RpoS activities found for these strains could be due to mutations in the rpoS promoter region or alterations in the regulation of rpoS expression. Therefore, our results suggest that despite its recognized importance for the cell, rpoS is a highly polymorphic gene in C. sakazakii. Maintenance of a defective or less active RpoS may appear paradoxical, as it is likely to affect cell survival under stressful environments. However, rpoS mutations may confer a selective advantage under certain conditions. rpoS polymorphisms in E. coli have been shown to influence the trade-off between self-preservation and nutritional competence (SPANC). For example, King and coworkers (38) demonstrated that E. coli rpoS mutant strains were better able to use novel and alternative carbon sources for their growth, which might allow the mutant population to dominate the culture under nutrient-limited environments. It is also worth mentioning that evolution by natural selection has been shown to occur in cultures of E. coli maintained under carbon starvation. Mutants of increased fitness express a growth advantage in stationary phase (GASP) phenotype, enabling them to grow and displace the parent as the majority population (27). The first GASP mutation was identified as a loss-of-function allele of rpoS (58). This rpoS-associated GASP phenotype has been shown to be dependent on pH and the nutrient environment (23).
The role of the alternative sigma factor RpoS in the general stress response of Gram-negative microorganisms is well recognized (18, 33). However, this is the first study to highlight the importance of this global regulator for coping with harsh environments in C. sakazakii. The current study describes a significant positive correlation between RpoS activity (albeit indirectly estimated) and the bacterial tolerance to lethal acid, alkaline, osmotic, and oxidative stress conditions. In addition, the increased resistance to acid, osmotic, and oxidative treatments shown by the rpoS mutant strains 7 and 15 when complemented with a functional rpoS gene suggests that this alternative sigma factor may play an important role in C. sakazakii survival throughout the food chain and subsequently in the host during infection. However, it should be noted that an intact rpoS gene is obviously not essential for the virulence of some C. sakazakii strains, as the rpoS gene of the sequenced strain ATCC BAA-894 (strain 15), originally associated with an outbreak in a neonatal intensive care unit, possesses a premature stop codon, and the rpoS gene of C. sakazakii strain 7, isolated from cerebrospinal fluid, has a 174-bp deletion in the rpoS open reading frame. Variations in the role of RpoS in the virulence of different strains of a given species have been observed for other bacterial pathogens (19).
C. sakazakii strains generally produce a carotenogenic yellow pigment on solid medium. However, several studies have recently identified colorless or cream-white C. sakazakii strains at prevalence rates ranging from 8 to 21% (6, 12, 36). Two recent elegant studies have identified the genes involved in pigment expression in C. sakazakii (37, 41). Lehner and coworkers (41) identified a gene cluster comprised of seven genes in the carotenoid operon (crtE-idi-crtXYIBZ) as responsible for carotenoid biosynthesis. Johler and colleagues (37) created a knockout library using random transposon mutagenesis and identified 30 colorless mutants. The mapping of the transposon insertion sites revealed insertions in not only the carotenoid operon (crtE-idi-crtXYIBZ) but also in various other genes involved in signal transduction and energy metabolism, including the rpoS gene. Those authors also evaluated the impact of pigmentation on persistence and growth under conditions of environmental stress by comparing white mutants (ΔctrE, ΔctrX, and ΔctrY) to the yellow wild type in a variety of growth and inactivation experiments, a macrophage assay, and a phenotype array, and they found that colorless mutants showed an increased susceptibility to desiccation and UV irradiation, which suggests the importance of the carotenoid pigment for cellular protection against harsh conditions. This protective effect could be attributed to the role of carotenoids as membrane stabilizers, which influence membrane fluidity, or as antioxidants, reducing the oxidative damage (29, 53, 59). To our knowledge, the timing of carotenoid biosynthesis in C. sakazakii has not yet been investigated. However, studies of other microorganisms have reported that the induction of carotenoid biosynthesis occurs at the beginning of the stationary phase (42), suggesting that carotenoid production is associated with the age and lower growth rate of the culture. Interestingly, this timing of biosynthesis matches with the habitual time of expression of rpoS in Gram-negative bacteria (47). Our study showed the existence of high variability in the content of carotenoids among C. sakazakii isolates and demonstrated that a functional rpoS gene is essential for the production of the characteristic yellow pigment. Thus, the pigmentation status of strains or the determination of the carotenoid content could be considered novel indirect assays for the prediction of RpoS activity in natural populations of C. sakazakii.
In summary, we have detected significant heterogeneity in RpoS activity and stress tolerance among natural isolates of C. sakazakii. It was possible to identify some strains resistant to multiple stresses and some sensitive strains. This variability in C. sakazakii stress resistance could be explained, at least in part, by heterogeneity in rpoS expression, since good correlations were found between RpoS activity and the tolerance of C. sakazakii strains to acid, alkaline, osmotic, and oxidative stresses. Sequence analysis of rpoS genes allowed us to detect the presence of several single-base substitutions in the open reading frames. Loss-of-function mutations were found for two C. sakazakii strains, namely, 7 and 15. The complementation of these strains with a functional rpoS gene gave rise to an increase in bacterial tolerance to acid, osmotic, and oxidative stresses linked to an increase in rpoS activity. Therefore, the results obtained indicate that RpoS plays an important role in the C. sakazakii response to a wide range of stress environments and contributes to the phenotypic diversity of natural populations.
Supplementary Material
ACKNOWLEDGMENTS
A. Álvarez-Ordóñez gratefully acknowledges financial support from the “Alfonso Martín Escudero” Foundation. We acknowledge the funding received by the Alimentary Pharmabiotic Centre under the Science Foundation of Ireland Centres for Science Engineering and Technology (CSET) program. The Food for Health Ireland research center is funded by Enterprise Ireland under grant number CC20080001.
Footnotes
Published ahead of print 23 March 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Adekunte A, et al. 2010. Resistance of Cronobacter sakazakii in reconstituted powdered infant formula during ultrasound at controlled temperatures: a quantitative approach on microbial responses. Int. J. Food Microbiol. 142:53–59 [DOI] [PubMed] [Google Scholar]
- 2. Al-Holy MA, Lin M, Abu-Ghoush MM, Al-Qadiri HM, Rasco BA. 2009. Thermal resistance, survival and inactivation of Enterobacter sakazakii (Cronobacter spp.) in powdered and reconstituted infant formula. J. Food Saf. 29:287–301 [Google Scholar]
- 3. Arroyo C, Condón S, Pagán R. 2009. Thermobacteriological characterization of Enterobacter sakazakii. Int. J. Food Microbiol. 136:110–118 [DOI] [PubMed] [Google Scholar]
- 4. Arroyo C, Cebrián G, Pagán R, Condón S. 2010. Resistance of Enterobacter sakazakii to pulsed electric fields. Innov. Food Sci. Emerg. 11:314–321 [Google Scholar]
- 5. Benito A, Ventoura G, Casadei M, Robinson T, Mackey B. 1999. Variation in resistance of natural isolates of Escherichia coli O157 to high hydrostatic pressure, mild heat and other stresses. Appl. Environ. Microbiol. 65:1564–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Besse NG, Leclercq A, Maladen V, Tyburski C, Lombard B. 2006. Evaluation of the International Organization for Standardization-International Dairy Federation (ISO-IDF) draft standard method for detection of Enterobacter sakazakii in powered infant formulas. J. AOAC Int. 89:1309–1316 [PubMed] [Google Scholar]
- 7. Beuchat LR, et al. 2009. Cronobacter sakazakii in foods and factors affecting its survival, growth and inactivation. Int. J. Food Microbiol. 136:204–213 [DOI] [PubMed] [Google Scholar]
- 8. Bhagwat AA, et al. 2005. Characterization of enterohemorrhagic Escherichia coli strains based on acid resistance phenotypes. Infect. Immun. 73:4993–5003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bhagwat AA, et al. 2006. Functional heterogeneity of RpoS in stress tolerance of enterohemorrhagic Escherichia coli strains. Appl. Environ. Microbiol. 72:4978–4986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Biesta-Peters EG, Reij MW, Joosten H, Gorris LGM, Zwietering MH. 2010. Comparison of two optical-density-based methods and a plate count method for estimation of growth parameters of Bacillus cereus. Appl. Environ. Microbiol. 76:1399–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Breeuwer P, Lardeau A, Peterz M, Joosten HM. 2003. Desiccation and heat tolerance of Enterobacter sakazakii. J. Appl. Microbiol. 95:967–973 [DOI] [PubMed] [Google Scholar]
- 12. Cawthorn DM, Botha S, Witthuhn RC. 2008. Evaluation of different methods for the detection and identification of Enterobacter sakazakii isolated from South African infant formula milks and the processing environment. Int. J. Food Microbiol. 127:129–138 [DOI] [PubMed] [Google Scholar]
- 13. Chenu JW, Cox JM. 2009. Cronobacter (“Enterobacter sakazakii”): current status and future prospects. Lett. Appl. Microbiol. 49:153–159 [DOI] [PubMed] [Google Scholar]
- 14. Chiang SM, Dong T, Edge TA, Schellhorn HE. 2011. Phenotypic diversity caused by differential RpoS activity among environmental Escherichia coli isolates. Appl. Environ. Microbiol. 77:7915–7923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Culham DE, Wood JM. 2000. An Escherichia coli reference collection group B2- and uropathogen-associated polymorphism in the rpoS-mutS region of the E. coli chromosome. J. Bacteriol. 182:6272–6276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dancer GI, Mah JH, Rhee MS, Hwang IG, Kang DH. 2009. Resistance of Enterobacter sakazakii (Cronobacter spp.) to environmental stresses. J. Appl. Microbiol. 107:1606–1614 [DOI] [PubMed] [Google Scholar]
- 17. den Besten HMW, et al. 2010. Short- and long-term biomarkers for bacterial robustness: a framework for quantifying correlations between cellular indicators and adaptive behavior. PLoS One 5:e13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dodd CER, Aldsworth TG. 2002. The importance of RpoS in the survival of bacteria through food processing. Int. J. Food Microbiol. 74:189–194 [DOI] [PubMed] [Google Scholar]
- 19. Dong T, Schellhorn HE. 2010. Role of RpoS in virulence of pathogens. Infect. Immun. 78:887–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dong T, Chiang SM, Joyce C, Yu R, Schellhorn HE. 2009. Polymorphism and selection of rpoS in pathogenic Escherichia coli. BMC Microbiol. 9:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Edelson-Mammel S, Porteous MK, Buchanan RL. 2006. Acid resistance of twelve strains of Enterobacter sakazakii, and the impact of habituating the cells to an acidic environment. J. Food Sci. 71:M201–M207 [Google Scholar]
- 22. Farber JM. 2004. Enterobacter sakazakii: new foods for thought? Lancet 363:5–6 [DOI] [PubMed] [Google Scholar]
- 23. Farrell MJ, Finkel SE. 2003. The growth advantage in stationary-phase phenotype conferred by rpoS mutations is dependent on the pH and nutrient environment. J. Bacteriol. 185:7044–7052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferenci T. 2003. What is driving the acquisition of mutS and rpoS polymorphisms in Escherichia coli? Trends Microbiol. 11:457–461 [DOI] [PubMed] [Google Scholar]
- 25. Ferenci T. 2008. The spread of a beneficial mutation in experimental bacterial populations: the influence of the environment and genotype of the fixation of rpoS mutations. Heredity 100:446–452 [DOI] [PubMed] [Google Scholar]
- 26. Ferenci T, Spira B. 2007. Variation in stress responses within a bacterial species and the indirect costs of stress resistance. Ann. N. Y. Acad. Sci. 1113:105–113 [DOI] [PubMed] [Google Scholar]
- 27. Finkel SE, Kolter R. 1999. Evolution of microbial diversity during prolonged starvation. Proc. Natl. Acad. Sci. U. S. A. 96:4023–4027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Friedemann M. 2007. Enterobacter sakazakii in food and beverages (other than infant formula and milk powder). Int. J. Food Microbiol. 116:1–10 [DOI] [PubMed] [Google Scholar]
- 29. Gruszecki WI, Strzalka K. 2005. Carotenoids as modulators of lipid membrane physical properties. Biochim. Biophys. Acta 1740:108–115 [DOI] [PubMed] [Google Scholar]
- 30. Gurtler JB, Kornacki JL, Beuchat LR. 2005. Enterobacter sakazakii: a coliform of increased concern to infant health. Int. J. Food Microbiol. 104:1–34 [DOI] [PubMed] [Google Scholar]
- 31. Hauben KJA, et al. 1997. Escherichia coli mutants resistant to inactivation by high hydrostatic pressure. Appl. Environ. Microbiol. 63:945–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Healy B, et al. 2010. Cronobacter (Enterobacter sakazakii): an opportunistic foodborne pathogen. Foodborne Pathog. Dis. 7:339–350 [DOI] [PubMed] [Google Scholar]
- 33. Hengge-Aronis R. 2000. The general stress response in Escherichia coli, p 161–178 In Storz G, Hengge-Aronis R. (ed), Bacterial stress responses. ASM Press, Washington, DC [Google Scholar]
- 34. Humphrey TJ, Slater E, McAlpine K, Rowbury RJ, Gilbert R. 1995. Salmonella enteritidis phage type 4 isolates more tolerant of heat, acid, or hydrogen peroxide also survive longer on surfaces. Appl. Environ. Microbiol. 61:3161–3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Iversen C, Forsythe S. 2003. Risk profile for Enterobacter sakazakii, an emergent pathogen associated with infant milk formula. Trends Food Sci. Technol. 14:443–454 [Google Scholar]
- 36. Iversen C, et al. 2007. Identification of “Cronobacter” spp. (Enterobacter sakazakii). J. Clin. Microbiol. 45:3814–3816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johler S, Stephan R, Hartmann I, Kuehner KA, Lehner A. 2010. Genes involved in yellow pigmentation of Cronobacter sakazakii ES5 and influence of pigmentation on persistence and growth under environmental stress. Appl. Environ. Microbiol. 76:1053–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. King T, Ishihama A, Kori A, Ferenci T. 2004. A regulatory trade-off as a source of strain variation in the species Escherichia coli. J. Bacteriol. 186:5614–5620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lai KK. 2001. Enterobacter sakazakii infections among neonates, infants, children, and adults. Case reports and a review of the literature. Medicine (Baltimore) 80:113–122 [DOI] [PubMed] [Google Scholar]
- 40. Large TM, Walk ST, Whittam TS. 2005. Variation in acid resistance among Shiga toxin-producing clones of pathogenic Escherichia coli. Appl. Environ. Microbiol. 71:2493–2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lehner A, et al. 2006. Cloning and characterization of Enterobacter sakazakii pigment genes and in situ spectroscopic analysis of the pigment. FEMS Microbiol. Lett. 265:244–248 [DOI] [PubMed] [Google Scholar]
- 42. Lodato P, et al. 2007. Expression of the carotenoid biosynthesis genes in Xanthophyllomyces dendrorhous. Biol. Res. 40:73–84 [DOI] [PubMed] [Google Scholar]
- 43. McGrath S, Fitzgerald GF, van Sinderen D. 2001. Improvement and optimization of two engineered phage resistance mechanisms in Lactococcus lactis. Appl. Environ. Microbiol. 67:608–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Morikawa K, et al. 2001. Overexpression of sigma factor, σB, urges Staphylococcus aureus to thicken the cell wall and to resist β-lactams. Biochem. Biophys. Res. Commun. 288:385–389 [DOI] [PubMed] [Google Scholar]
- 45. Morrissey R, et al. 2011. Real-time monitoring of luciferase-tagged Cronobacter sakazakii in reconstituted infant milk formula. J. Food Prot. 74:573–579 [DOI] [PubMed] [Google Scholar]
- 46. Mullane NR, et al. 2007. Enterobacter sakazakii an emerging bacterial pathogen with implications for infant health. Minerva Pediatr. 59:137–148 [PubMed] [Google Scholar]
- 47. Navarro Llorens JM, Tormo A, Martínez-García E. 2010. Stationary phase in gram-negative bacteria. FEMS Microbiol. Rev. 34:476–495 [DOI] [PubMed] [Google Scholar]
- 48. Notley-McRobb L, King T, Ferenci T. 2002. rpoS mutations and loss of general stress resistance in Escherichia coli populations as a consequence of conflict between competing stress responses. J. Bacteriol. 184:806–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Osaili T, Forsythe S. 2009. Desiccation resistance and persistence of Cronobacter species in infant formula. Int. J. Food Microbiol. 136:214–220 [DOI] [PubMed] [Google Scholar]
- 50. Pérez MCP, Aliaga DR, Bernat CF, Enguidanos MR, López AM. 2007. Inactivation of Enterobacter sakazakii by pulsed electric field in buffered peptone water and infant formula milk. Int. Dairy J. 17:1441–1449 [Google Scholar]
- 51. Robbe-Saule V, Algorta G, Rouilhac I, Norel F. 2003. Characterization of the RpoS status of clinical isolates of Salmonella enterica. Appl. Environ. Microbiol. 69:4352–4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Robey M, et al. 2001. Variation in resistance to high hydrostatic pressure and rpoS heterogeneity in natural isolates of Escherichia coli O157:H7. Appl. Environ. Microbiol. 67:4901–4907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sedkova N, Tao L, Rouviere PE, Cheng Q. 2005. Diversity of carotenoid synthesis clusters from environmental Enterobacteriaceae strains. Appl. Environ. Microbiol. 71:8141–8146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Uhlich GA, Sinclair JR, Warren NG, Chmielecki WA, Fratamico P. 2008. Characterization of Shiga toxin-producing Escherichia coli isolates associated with two multistate food-borne outbreaks that occurred in 2006. Appl. Environ. Microbiol. 74:1268–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Waterman SR, Small PLC. 1996. Characterization of the acid resistance phenotype and rpoS alleles of Shiga-like toxin-producing Escherichia coli. Infect. Immun. 64:2808–2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Weber H, Polen T, Heuveling J, Wendisch VF, Hengge R. 2005. Genome-wide analysis of the general stress response network in Escherichia coli: σS-dependent genes, promoters, and σ factor selectivity. J. Bacteriol. 187:1591–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zambrano MM, Siegele DA, Almiron M, Tormo A, Kolter R. 1993. Microbial competition: Escherichia coli mutants that take over stationary phase cultures. Science 259:1757–1760 [DOI] [PubMed] [Google Scholar]
- 59. Ziegelhoffer EC, Donohue TJ. 2009. Bacterial responses to photooxidative stress. Nat. Rev. Microbiol. 7:856–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.