Abstract
In this study, we undertook a functional characterization and transcriptome analysis that enabled a comprehensive study of the mating type loci of the mushroom Schizophyllum commune. Induced expression of both the bar2 receptor and the bap2(2) pheromone gene within 6 to 12 h after mates' contact was demonstrated by quantitative real-time PCR. Similar temporal expression patterns were confirmed for the allelic bbr1 receptor and bbp1 pheromone-encoding genes by Northern hybridization. Interestingly, the fusion of clamp connections to the subterminal cell was delayed in mating interactions in which one of the compatible partners expressed the bar2 receptor with a truncated C terminus. This developmental delay allowed the visualization of a green fluorescent protein (Gfp)-labeled truncated receptor at the cell periphery, consistent with a localization in the plasma membrane of unfused pseudoclamps. This finding does not support hypotheses envisioning a receptor localization to the nuclear membrane facilitating recognition between the two different nuclei present in each dikaryotic cell. Rather, Gfp fluorescence observed in such pseudoclamps indicated a role of receptor-pheromone interaction in clamp fusion. Transcriptome changes associated with mating interactions were analyzed in order to identify a role for pheromone-receptor interactions. We detected a total of 89 genes that were transcriptionally regulated in a mating type locus A-dependent manner, employing a cutoff of 5-fold changes in transcript abundance. Upregulation in cell cycle-related genes and downregulation of genes involved in metabolism were seen with this set of experiments. In contrast, mating type locus B-dependent transcriptome changes were observed in 208 genes, with a specific impact on genes related to cell wall and membrane metabolism, stress response, and the redox status of the cell.
INTRODUCTION
The filamentous fungus Schizophyllum commune is a widely distributed mushroom that lives saprophytically and can cause white rot on wood. It has been extensively used to study mating interactions for almost 100 years (47). In addition to studies involving the mushroom Coprinopsis cinerea and the corn smut Ustilago maydis, the mating type genes have been extensively analyzed for this tetrapolar basidiomycete (4, 29). The recent publication of the S. commune genome sequence enables global expression analyses (41). During the life cycle of this fungus, compatible haploid monokaryons can mate to produce a fertile dikaryon with two nuclei per cell, one derived from each mating partner. Under appropriate environmental conditions, the dikaryon is able to form the sexual reproductive fruiting bodies (40). While the fusion of two monokaryotic hyphae (plasmogamy) is independent of mating types, specific steps of sexual development are regulated by the mating type genes encoded in the loci A and B (25, 47). For each of these loci, two linked, multiallelic subloci (termed α and β) have been determined by recombination analyses (27, 49, 51, 65). The A loci encode homeodomain transcription factors, while the B loci code for multiallelic pheromone receptors and pheromones (55, 63, 66). Both the A and B pathways can be activated by the combination of different allelic specificities in at least one sublocus derived from each of the two mates (46, 52, 53). Thus, for a compatible mating interaction to occur, different A and B mating types (A≠B≠) in the partners are essential.
The migration of nuclei is triggered by the presence of different B mating types after the fusion of two compatible monokaryons and occurs reciprocally from one mating partner throughout the mycelium of the other (54). Subsequent nuclear pairing, as well as the initiation of clamp cell development, is regulated by the A mating type. The formation of clamp cells at the septa of the developing dikaryon results in a regular distribution of the two different nuclei during mitosis within the dikaryon (4, 39). The last step of clamp fusion is dependent on different B factors and involves Ras signaling (31, 59). This sequence of events can be observed in semicompatible mating interactions where a heterokaryon with an activated B locus (Bon or A=B≠) undergoes constant nuclear migration associated with irregular distribution of nuclei (26, 38). This phenotype, termed flat, results in reduced aerial mycelia, malformed hyphae, and no formation of clamps (43, 48). The second type of semicompatible mating interaction occurs with an activated A locus (Aon or A≠B=) and is evident in the formation of pseudoclamps and a die-off in the contact zone (barrage phenotype) (38, 43, 44).
For the pheromone-receptor system encoded in the B locus of S. commune, the pheromone signaling is triggered by the interaction of nonself-pheromones with G-protein-coupled receptors (GPCRs) of class D (1, 20, 70). All pheromone receptors of basidiomycetes belong to the Saccharomyces cerevisiae Ste3-related pheromone receptor family and bind a-factor-related lipopeptide pheromones (12, 32, 67, 64). Multiple different cognate pheromones capable of activating a single receptor in a multistate model of interaction have evolved in S. commune, most likely by recombination between copies of the mating type locus (15, 20). The function of pheromone signaling is thus linked to the processes of nuclear migration, nuclear identity, and clamp fusion.
A model that links pheromone-receptor interactions to the distance between nuclei in dikaryons had been proposed. Under this model, productive interaction between non-self-pheromones and their cognate receptor occurs only when the nuclei are in close proximity to each other (60). Another hypothesis (5) proposed that receptor localization to the nuclear envelope was necessary for detection of intracellular pheromone(s). This would necessitate an inverse orientation of the receptor within the membrane. In order to investigate the role of the pheromone receptor and to distinguish between the different models described above, receptor localization has been of great interest for a number of years. A plasma membrane localization was found for pheromone receptors in single cells of the corn pathogen U. maydis (17). In mushroom-forming homobasidiomycetes like S. commune or C. cinerea, however, cellular localization of the pheromone receptor has not yet been determined.
The role of pheromone perception in S. cerevisiae includes both mate attraction and the formation of shmoo cells that show growth directed toward the mating partner (33). In contrast, attraction and fusion of S. commune hyphae can be seen independent of mating type. Directional growth toward a mate has been observed at very short distances (54). In an earlier investigation on the role of Ras in sexual development of S. commune, we demonstrated that a deletion of a Ras-specific GTPase-activating protein, Δgap1, results in a lack of clamp fusion in Δgap1 homoallelic dikaryons (59). This uninucleate state is rescued by development of a branch, which is then able to fuse with the clamp cell after prolonged growth. We hypothesized that this situation in the mutant represents two monokaryotic cells which show clear attraction toward each other. Thus, clamp fusion in dikaryons can be viewed as a system in which mate attraction can be visualized at very short distances.
The intracellular signaling via GPCRs has been shown to be controlled by regulators of G-protein signaling (RGS) in other studies of S. commune (6, 13). The RGS protein Thn1 negatively regulates the activity of the G-protein α subunit by acting as a GTPase-activating protein (GAP) (42). A loss-of-function mutation due to transposon integration into thn1 results in hyphae with a characteristic corkscrew morphology which are unable to accept nuclei of a compatible mating partner (13, 61). For S. cerevisiae, RGS gene SST2 was functionally linked to mating interactions (7, 8).
In the study presented here, we show the localization of the G-protein-coupled pheromone receptor protein in hyphal filaments of S. commune. Furthermore, we report on the role of the C-terminal region of the pheromone receptor Bar2 in mating interactions. In combination with transcriptome analyses, a comprehensive study of the pheromone-receptor system for a mushroom-forming basidiomycete is presented for the first time.
MATERIALS AND METHODS
Strains and culture conditions.
The S. commune strains 4-40, 23, 684, 1792-114-10, W21, W22, W22-thin, V153-21 (Bnull), 12-43, and 4-39 were obtained from the Jena Microbial Resource Center (JMRC) or the strain collection of University of Turku. S. commune receptor transformants generated in this study (Vbar2f, Vbar2t, Vbar2tG1, Vbar2tG11) and strains relevant for microarray analysis are listed in Table 1. S. commune was routinely grown at 28°C on minimal medium (MM) (50) or complex yeast medium (CYM) (62) with 2% (wt/vol) glucose, with or without 1.8% (wt/vol) agar. Liquid cultures were shaken at 150 rpm. For RNA extraction, cultures were grown on a cellophane membrane placed on solid medium. The S. commune strains investigated by Northern blotting or quantitative real-time PCR (RT-PCR) were cultured using a modified sandwich method as described previously (67). Interacting mycelia were grown for 3 to 72 h at 28°C before harvest. For transcriptome analysis, mycelia from a monokaryon or a mating interaction were transferred onto a cellophane membrane placed on fresh CYM plates and grown for 3 days.
Table 1.
Schizophyllum commune strains and mating interactions
| Strains and crosses | Mating type, relevant genotype/description |
|---|---|
| H4-8 | A4,6;B3,2 |
| W21 | A1,1;B1,1 |
| W22 | A4,6;B3,2 |
| W22-thin | A4,6;B3,2; thin (thina) |
| V153-21 | A3,5;Bnull; trp1− |
| Vbar2f | A3,5;Bnull::bar2 trp1 |
| Vbar2t | A3,5; Bnull::bar2ΔPstI trp1 |
| Vbar2tG1 | A3,5;Bnull::bar2ΔPstI-HA-Gfp trp1 |
| Vbar2tG11 | A3,5;Bnull::bar2ΔPstI-HA-Gfp trp1 |
| 12-43 | A3,5;B2,3; ura1−; monokaryon (Mon1a) |
| 4-39 | A1,1;B3,2; monokaryon (Mon2a) |
| W22 × 12-43 | Dikaryon (Dika) |
| W22 × 4-39 | Semicompatible interaction: A≠B= (Aona) |
| W21 × 4-39 | Semicompatible interaction: A=B≠ (Bona) |
| Vbar2f × 4-39 | Dikaryon of receptor transformant with full-length receptor (Dik-Vbar2fa) |
| Vbar2t × 4-39 | Dikaryon of receptor transformant with truncated receptor (Dik-Vbar2ta) |
Term for microarray analysis.
Functional analysis and receptor localization.
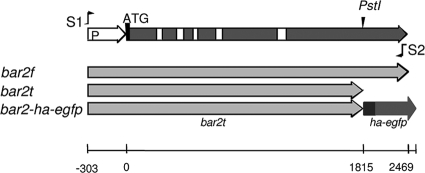
We generated the transformants that are expressing Vbar2f using a Bnull strain, which untransformed does not expresses mating pheromones or receptors (14). For amplification of the pheromone receptor gene bar2f (NCBI accession number X91168.4), we used primers S1 (5′-GGATCCGCCCATTGTCC-3′) and S2 (5′-TCACACCGACGCGCGGT-3′) to produce an amplicon starting from approximately 300 bp upstream of ATG and extending to the last 17 bases of bar2 (Fig. 1). Comparison of the last 17 bases of bar2 with those in bar1 and bar3 shows that they are identical, except for a different stop codon in bar3. The pheromone receptor gene bar2f encodes a full-length protein of 636 amino acids (aa). In addition, we generated the strain Vbar2t, which was transformed with a mutated gene encoding a truncated receptor of Bα2 specificity with 518 aa in length; the sequence of bar2t is truncated at a 3′ PstI site (20) (NCBI accession number X91168.2, GI23954358). The truncated bar2t receptors of strains Vbar2tG1 and Vbar2tG11 have been tagged with extended green fluorescent protein (eGfp) for in vivo studies and with hemagglutinin (3× HA) for receptor localization by indirect immunostaining. The HA-eGfp sequence (845 bp) was amplified with the primers 5′-GGC TGC AGG GAT ACC CGT ATG ATG TTC CGG ATT ACG CTG GCT ACC CAT ACG ACG TCC CAG ACT ACG CTG GCT ACC CAT ACG ACG TCC CAG ACT ACG CTG GCG CAC CTG GAG CCA TGG TGA GCA AGG GCG AGG AGC-3′and 5′-GTT GGA ATT CTG CAG TCG CGG CCG CTT TAC TTG-3′ using the vector pegfp (Clontech, The Netherlands) as template and ligated into the PstI site at the C terminus of bar2t. All transformed pheromone receptor genes were expressed under their native promoter.
Fig 1.
Gene bar2 of Schizophyllum commune. The primers S1 and S2 were used for amplification of full-length gene bar2f (NCBI accession number X91168.4) with promoter region (P). The gene bar2t was truncated at the 3′ PstI restriction site (see also NCBI accession number X91168.2). The 3× HA-eGfp tag (ha-egfp) is indicated at the C terminus of the bar2t gene. The white squares indicate introns.
Fluorescence microscopy and immunostaining.
For microscopic analysis, sterile glass coverslips were placed on an agar plate such that hyphae could attach to and grow onto the slides after 2 to 4 days of cultivation. Mycelia of S. commune were fixed for 90 min at room temperature with methanol and PME {50 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)], pH 6.7, 25 mM EGTA, pH 8.0, 5 mM MgSO4} plus 3.7% formaldehyde. Coverslips were then washed with PME and the cells were treated with 3% lysis enzyme (Trichoderma harzianum; L1412; Sigma, Germany) and 50% egg white. The cells were permeabilized with 0.3% Triton X-100 in phosphate-buffered saline (PBS; 137 mM NaCl, 2.68 mM KCl, 8.09 mM Na2HPO4, 1.76 mM KH2PO4, pH 7.4). Nonspecific binding sites were blocked with 3% bovine serum albumin (BSA). The 1st antibody (mouse anti-HA 2367; Cell Signaling) was diluted 1:200 in PBS with 3% BSA, and coverslips were incubated overnight at 4°C. The fluorescein isothiocyanate (FITC)-labeled 2nd antibody (FITC-antimouse; F4018; Sigma) was diluted 1:100 in PBS and incubated for 60 min at 37°C. Following every incubation step, the coverslips were washed with PBS at least once. Nuclei were stained with DAPI (4′,6-diamidino-2′-phenylindole dihydrochloride; 0.1 to 1 μg/ml) added to the mounting medium (0.1 M Tris HCl, pH 8.0, 50% glycerol, and 1 mg/ml phenylenediamine). The mycelia were examined with a fluorescence microscope (Axioplan2) and confocal laser scanning microscope (LSM 5 Live) (Carl Zeiss MicroImaging, Jena, Germany). Images were processed with SPOT imaging software (Diagnostic Instruments, Sterling Heights, MI) and ZEN software (Carl Zeiss, Jena, Germany).
Quantitative real-time PCR and Northern hybridization.
Mycelia were harvested, frozen in liquid nitrogen, and ground with mortar and pestle to a powder. Total RNA was isolated from not more than 100 mg of tissue powder using a commercial kit (RNeasy plant minikit; Qiagen, Hilden, Germany). Reverse transcription was performed using 1 μg of total RNA in a 20-μl reaction mix (iScript cDNA synthesis kit; Bio-Rad, Munich, Germany). For each real-time PCR, we used 2 μl of cDNA as template in a 25-μl reaction mix that additionally contained 12.5 μl 2× reaction mix [1.25 U Hot Taq DNA polymerase, 0.4 mM deoxynucleoside triphosphates, 40 mM Tris HCl, pH 8.55, 32 mM (NH4)2SO4, 0.02% Tween 20, and 4 mM MgCl2; peqGold hot start mix Y; Peqlab], 7.5 μl water (nuclease free), 1 μl of each primer (10 pmol/μl), and 1 μl SYBR green I (using a 1:2,000 dilution of the stock reagent; Molecular Probes, Invitrogen) using a SmartCycler II thermocycler (Cepheid). The PCR program consisted of 5 steps (initial denaturation for 120 s at 94°C; 45 cycles of denaturation for 20 s at 94°C, annealing for 20 s at 50 or 55°C, and extension for 20 s at 72°C; and a final melting curve analysis at 60 to 95°C with 0.2°C/s). The SYBR green fluorescence was detected (excitation, 450 to 495 nm; emission, 510 to 527 nm) during both the extension phase of each cycle and the subsequent melting curve analysis. For amplification of the target genes bar2 and bap2(2), as well as the reference genes act1 and tef1, the following oligomers were used: 5′-ATTACTCTTGGCGCCTCTGTA-3′ and 5′-AATGAGAGCGTCGACCATGACT-3′ for bar2 (yielding a product of 138 bp), 5′-TTACTGATAGTCACAGATA-3′ and 5′-ATGGCGAACCGGAC-3′ for bap2(2) (87 bp), 5′-GTCCGCCCTCGAGAAGAGTTA-3′ and 5′-TTGTACGTCGTCTCGTGGATA-3′ for act1 (141 bp), and 5′-AGCTTGGCAAGGGTTCCTTCA-3′ and 5′-AACTTCCAGAGGGCGATATCA-3′ for tef1 (97 bp). The primers for bar2, act1, and tef1 span an intron to identify potential genomic DNA contamination. The gene bap2(2) has a total length of only 87 bp and has no intron. Annealing was performed at 55°C for all genes except bap2(2), which was amplified at an annealing temperature of 50°C. The calculation of the gene-specific efficiencies was based on the slope of a standard curve generated from a series of different cDNA concentrations (0.04, 0.2, 1, 5, 25, 50, 100, and 200 ng cDNA/25-μl PCR mixture). cDNA was derived from a mating interaction between two compatible wild-type strains (12-43 × 4-39). The PCR efficiency for bar2 was 1.83 (corresponding to 83% of exponential expression), that for bap2(2) was 1.53 (53%), that for act1 was 2.1 (110%), and that for tef1 was 2.0 (100%).
Since the receptor and pheromone genes between different strains are not alike at the DNA sequence level but belong to the same family of gene products, the primer combinations were tested with templates of different Bα specificities. The receptor and pheromone primers gave no amplification with specificities other than Bα2 (data not shown). The genes act1, coding for actin, and tef1, coding for translation elongation factor EF1α, were suitable reference genes with stable expression levels under the tested conditions.
The expression of the target genes was determined relative to the expression of reference genes, which were used for the normalization of expression between samples. We measured expression levels in mycelia isolated at a progression of different times during the mating interaction (after 3, 6, 9, 12, 24, 30, 48, 54, and 72 h; samples t3 through t72). These times correspond to mycelia of different nuclear distributions. Sample t0 corresponds to a mix of two monokaryons which have been brought together directly before sampling (t0 = monokaryon, 0 h) and reflects the basal level of expression (control). For each time and individual mating interaction, the total RNAs of three independently grown mycelia have been isolated and transcribed into cDNA in duplicate, and each one was used as a template in real-time PCR, also measured in duplicate. The expression of the target genes was normalized and quantified relative to the expression of the reference genes and was also corrected for the calculated efficiency (45, 68). Northern hybridization experiments and the labeling of the receptor and pheromone cDNA fragments were performed as described previously (21).
Microarray-based transcriptome analysis.
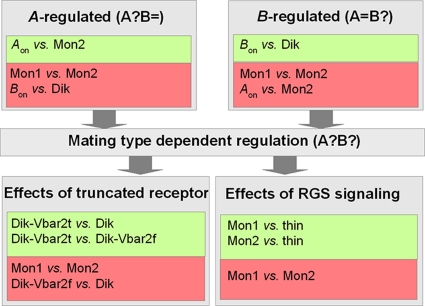
Quantification of transcriptome changes associated with mating interactions was performed in order to identify signaling components and targets of pheromone response. In addition, an S. commune strain showing the thin phenotype was investigated to resolve the role of Thn1 in mating. The microarrays (febit biomed GmbH, Heidelberg, Germany) used in the present study contain probes for all 13,181 predicted genes of S. commune. It is based primarily upon the November 2008 version of the Schizophyllum commune (version 1.0) genome using strain H4-8 (A4,6;B3,2). The probes (oligonucleotides of ∼50 bases in length) have been spotted on Geniom Biochips. The strains and mating interactions were investigated in microarray analysis in two biological or technical replicates (Table 1; see also Table S1 in the supplemental material). Genes which show regulation (fold change, ≥2) between the two monokaryotic strains 12-43 and 4-39 were eliminated from all other comparisons as strain-specific differences (Fig. 2).
Fig 2.
Diagram of interactions screened to identify genes differentially regulated. The comparison of assumed different (green) and similar (red) interactions was used to identify genes regulated in transcriptome profiling.
A comparison of the Aon condition versus 4-39 (Mon2) yielded what were defined to be A-regulated genes. In contrast, B-regulated genes were defined by comparing Bon versus Dik: this assumes that hyphae activated for B-dependent regulation (Bon) are more related to dikaryotic hyphae containing nuclei of both mating partners after nuclear migration. At the same time, no gene was included from the former set (A-regulated genes) so as not to involve cross-pathway signaling between A and B (Fig. 2).
Differentially expressed genes of the thin phenotype were up- or downregulated in both of the comparisons made: 12-43 (Mon1) versus W22-thin and 4-39 (Mon2) versus W22-thin (Fig. 2). To identify regulated genes influenced by the C-terminal region of Bar2, we defined two criteria: genes had to be up- or downregulated in both comparisons: Dik-Vbar2t versus Dik and Dik-Vbar2t versus Dik-Vbar2f. In addition, these genes were unregulated in Dik-Vbar2f versus Dik (Fig. 2).
For each array, 1 μg of total RNA was labeled using the MessageAmp-biotin enhanced RNA kit from Ambion. Hybridization was performed automatically for 16 h at 45°C using RT-Analyzer (febit, Heidelberg, Germany). The biotin-labeled nucleic acid was detected using streptavidin-phycoerythrin (SAPE) in combination with consecutive signal enhancement (CSE). Feature recognition using the Cy3 filter set and signal calculation were automatically analyzed within milliseconds using the Geniom RT-Analyzer (febit, Heidelberg, Germany). For preprocessing, all data analyses of the febit microarrays were performed using the LIMMA (linear models for microarray data) packages of the Bioconductor software (18). Background correction was performed using the intensities of blank probes that consisted of only a single T nucleotide. The median background intensity was subtracted from the spot intensity. After converting any negative values to a low-positive value (8), signal intensities were log2 transformed, and duplicate spots were averaged. The data obtained were processed using quantile normalization. To identify the genes with the greatest evidence of differential expression, a linear model fit was applied for each gene using LIMMA. Candidate genes were selected for further analysis on the basis of their fold change (≥5) in expression and their P value (≤0.05).
Microarray data accession number.
The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus (9) and are accessible through GEO Series accession number GSE26401 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE26401).
RESULTS
Expression of pheromone receptor and pheromone genes during mating interactions.
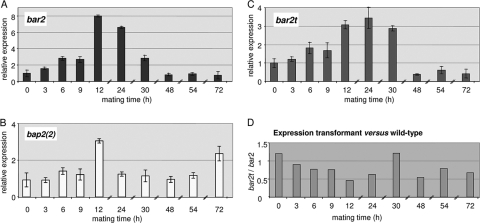
In order to assess the expression of both pheromone receptor and pheromone genes during the life cycle of S. commune, a specific pheromone receptor gene, bar2, and a corresponding self-pheromone gene, bap2(2), were analyzed by quantitative real-time PCR. As expected, expression levels for both mating type-specific genes were low in monokaryons (t0), and expression was specifically induced during mating interactions. In a time series from 0 to 72 h (t0 to t72) during a compatible mating interaction, the expression of the receptor gene bar2 increased gradually to a 3-fold higher level in the first 9 h and reached a maximal change of 8-fold after 12 h (Fig. 3). Subsequently, the expression of bar2 gradually decreased to a low level comparable to monokaryotic expression levels. The expression of the pheromone gene bap2(2) increased to a maximum of a 3-fold increase over baseline after 12 h, decreasing shortly thereafter (Fig. 3). In contrast to the receptor, however, pheromone expression increased steadily and then again up to 2.5-fold after 72 h. Overall, the expression levels of receptor gene bar2 and pheromone gene bap2(2) in wild type were extremely low, reaching the threshold level only after 26 and 29 to 32 cycles, respectively, while the reference genes act1 and tef1 amplified with mean threshold cycles of 15 and 21, respectively.
Fig 3.
(A and B) Expression of the pheromone receptor gene bar2 (A) and the pheromone gene bap2(2) (B) in a wild-type mating (12-43 × 4-39); (C) expression of a truncated version of pheromone receptor gene bar2t in the transformant Vbar2t in a mating with the wild-type strain 4-39. The gene expression levels were determined by quantitative real-time PCR of compatible mating interactions over a time period of 72 h. The expression levels in monokaryons (t0) were normalized to 1, and all other mating times (t3 to t72) are shown relative to t0. (D) Ratio of bar2t/bar2 expression, illustrating the effect of a C-terminal truncation of the pheromone receptor.
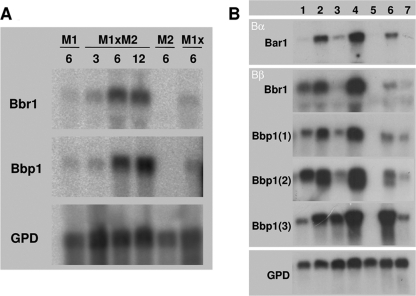
The quantitative RT-PCR measurements of bar2 and bap2(2) were in accordance with those from Northern blot experiments determining both bbr1 receptor and bbp1(1) pheromone gene expression in a time series from 3 to 12 h (Fig. 4). Induction of genes encoding receptors bar1 and bbr1 as well as pheromones bbp1(1), bbp1(2), and bbp1(3) was obtained in Aon and Bon semicompatible matings, although the signals in these interactions were lower than those in a compatible mating leading to dikaryon formation (Fig. 4).
Fig 4.
Northern hybridization of receptor and pheromone gene expression during compatible and semicompatible matings. (A) Expression of the Bbr1 receptor and the Bbp1 pheromone-encoding genes in a compatible mating, 4-40 (M1; A4,6;B1,1) × 4-39 (M2; A1,1;B3,2), and the two respective monokaryons, as well as an incompatible cross between identical monokaryons (M1x). RNA extraction was performed after the indicated times (3, 6, 12 h). (B) Expression of the receptor genes encoding Bar1 and BbrI and also those encoding the pheromones Bbp1(1), Bbp1(2), and Bbp1(3). Lane 1, expression in Aon semicompatible mating between strains 23 (A4,6;B3,1) × 684 (A2,6;B3,1); no signal was expected for bar1 since neither of the B loci encodes the bar1 receptor; lanes 2 and 3, Bon semicompatible matings between strains 1792-114-10 (A4,6;B3,6) × 4-40 (A4,6;B1,1) and 43/26 (A4,6;B3,1) × 4-40 (A4,6;B1,1), respectively; lane 4, Aon;Bon fully compatible mating between strains 4-40 (A4,6;B1,1) × 4-39 (A1,1;B3,2); lane 5, strain 4-39 (A1,1;B3,2); no signal was expected in this control since no sequences encoding either the Bar1 and Bbr1 receptor or any of the three Bbp1 pheromones are present in strain 4-39; lane 6, strain 4-40 (A4,6;B1,1); lane 7, strain 23 (A4,6;B3,1). All strains were grown for 8 h after mating. Each well contains 20 μg of total RNA. Expression of glyceraldehyde-3-phosphate dehydrogenase (GPD) was monitored as a loading control.
The pheromone receptors of S. commune contain long intracellular C termini and are considerably longer than those of the S. cerevisiae Ste3 pheromone receptor. Thus, specific intracellular binding sites for regulatory proteins could be present and might also perform a function in the regulation of expression. This prompted us to measure expression levels with quantitative RT-PCR in the transformant Vbar2t, which carries only the one truncated receptor gene (bar2t) but lacks all other B mating genes. The expression level of bar2t was determined to be slightly higher than the expression level of bar2 in a wild-type strain at t0 (Fig. 3D). The induction of expression upon mating was lower, however. The truncated gene also showed a slower increase in expression, which prompted us to carefully reexamine its phenotype.
C-terminal truncation of the pheromone receptor affects clamp fusion.
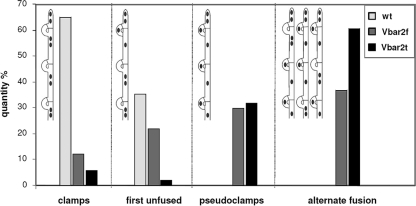
We examined the Bnull transformants containing either the entire, full-length pheromone receptor gene bar2f or the truncated version, bar2t, in order to investigate the potential functions of the long intracellular C terminus of the receptor. Integration of either bar2f or bar2t reconstituted the wild-type phenotype of mating competence in monokaryotic, vegetative mycelium. Pheromone receptor transformants developed mycelia (substrate and aerial) that grew in a normal fashion, but with a slightly smaller colony diameter than wild-type strains (data not shown). Pheromone receptor transformants mated with fully compatible partners (A≠B≠) and showed the expected unilateral nuclear migration and formation of apparently clamped mycelium on the side of the receptor transformant. Both versions of the receptor recognized pheromones of all other Bα specificities but the self-specificity Bα2, and clamp structures were formed at every septum. In transformants carrying a truncated receptor gene, a delay in development was seen, with these matings taking 1 to 2 days longer before clamp cells became visible, correlating well with the delayed upregulation of receptor and pheromone gene expression measured by RT-PCR. Closer examination of all fully compatible crosses (A≠B≠) involving transformants carrying either bar2f or bar2t alleles revealed the formation of pseudoclamps with a trapped nucleus in the unfused hook cells (Fig. 5). The tip cells of mated receptor transformants contained two nuclei and hence were dikaryotic. Subterminal cells often contained only one nucleus, while the other remained trapped in the unfused pseudoclamp (Fig. 5). For analysis, we classified four states of clamp fusion: (i) three successive clamps behind the tip cell are fused clamps, (ii) only the first clamp (closest to the tip cell) is still unfused, (iii) all three clamp connections are pseudoclamps, and (iv) an alternate distribution of pseudoclamps and fused clamps occurs (Fig. 6). Using this classification, it was observed that in wild-type mating interactions, clamp fusion in tip cells was not (yet) completed in 35% of the cases, while all subterminal cells had only fused clamps. In transformants carrying either bar2f or bar2t, however, all four patterns occurred, albeit with a higher rate of clamps in the transformant with the full-length gene bar2f (Fig. 6). Thus, the 118 aa missing from the C terminus in Vbar2t influence the correct and timely development of dikaryotic hyphae.
Fig 5.
Pseudoclamp formation in transformants carrying the truncated pheromone receptor gene bar2t after a compatible mating. (a) Pseudoclamp with a DAPI-stained nucleus trapped in the unfused clamp. Bar, 5 μm. (b) Bright-field micrograph of hyphae and DAPI staining of nuclei showing a nuclear pair in the tip cell and the two nuclei separated in the unfused clamp and subterminal cell (asterisk). The tip of the hypha is at the extreme right of the figure, and nuclei are indicated by an arrow. Bar, 20 μm.
Fig 6.
States of clamp fusion in a wild-type (wt) dikaryon (12-43 × 4-39) and in two pheromone receptor transformants carrying either the full-length gene bar2f (Vbar2f) or the truncated version, bar2t (Vbar2t), after interacting with a compatible mating partner (strain 4-39); n = 100.
Truncation of Bar2 affects fruit body development and spore production.
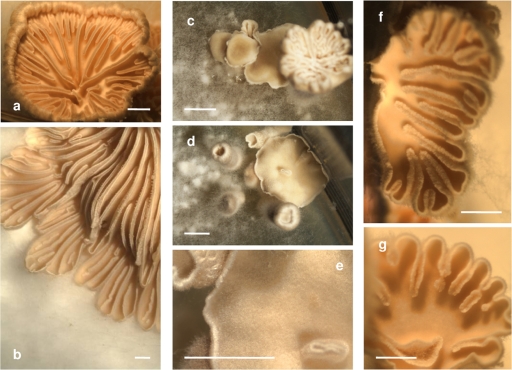
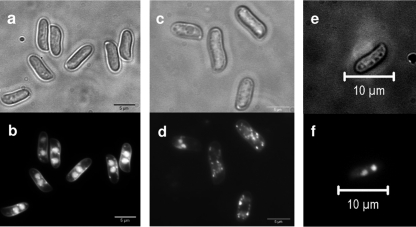
Despite the formation of pseudoclamps, the receptor transformants Vbar2f and Vbar2t were able to develop fruit bodies. However, fruit body development often stopped before maturity. Lamellae were either absent or malformed, but production of spores was recorded nevertheless (Fig. 7). The spores produced from transformants carrying bar2t lacked nuclei in high proportions (>70%). The majority of the spores of receptor transformant Vbar2t showed only mitochondrial DNA staining, appearing as small spots distributed all over the cell (Fig. 8). However, a small proportion of nucleated spores was formed in transformant Vbar2t, and these were able to germinate and to develop into monokaryotic mycelia. This is in contrast to the wild type, where spores generally contain two nuclei per spore (Fig. 8) and germinated at almost 100%. Transformants carrying bar2f showed an intermediate phenotype, with anucleated spores and wild-type-like spores being observed (Fig. 8).
Fig 7.
Fruiting bodies of Schizophyllum commune in the wild type (a and b), pheromone receptor transformant Vbar2t with the truncated receptor (c to e), and the pheromone receptor transformant Vbar2f with the full-length receptor gene (f and g). All strains have been mated with the compatible partner strain 4-39. While the wild type forms fruit bodies with ordinary pseudolamellae, the transformants show defects in fruit body development, resulting in fewer or absent pseudolamellae. Bars, 0.5 cm.
Fig 8.
Spores of Schizophyllum commune in the wild type (a and b) or pheromone receptor transformants encoding either the truncated receptor (Vbar2t) (c and d) or the full-length receptor (Vbar2f) (e and f). Spores were obtained from fruiting bodies generated from compatible crosses with strain 4-39. While almost all wild-type spores contained two nuclei (b), more than 70% of the spores from the truncated receptor transformants did not contain nuclei and only mitochondrial DNA was stained (d). The spores derived from outcrosses of the full-length receptor transformant Vbar2f showed a higher incidence of two nuclei than spores derived from outcrosses with the truncated version (f).
Cellular localization of pheromone receptor.
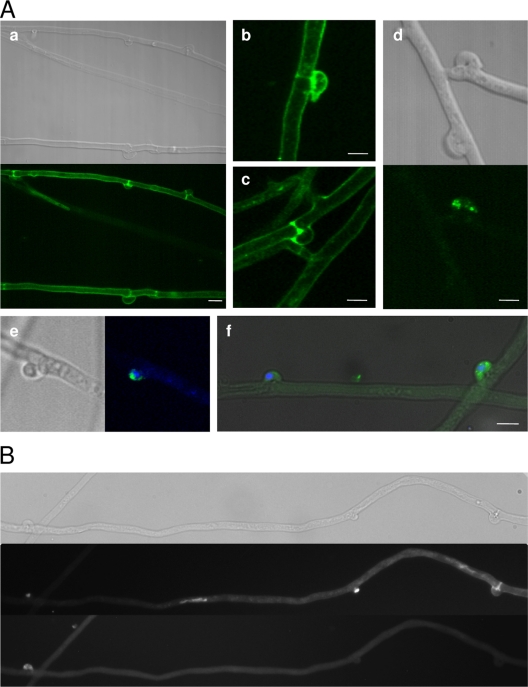
We decided to utilize the enhanced level of pseudoclamps for in vivo and in situ localization of receptor molecules. The prolonged presence of unfused clamp cells allowed us to examine the short-distance attraction between interacting cells. For this purpose, the receptor gene bar2t C-terminally fused to both Gfp and HA allowed us to perform both in vivo observations and immunofluorescence staining of fixed cells, respectively. When this fusion construct was transformed into the Bnull recipient strain, complementation was observed for mating functions with all non-self-Bα specificities, as was the case for the untagged pheromone receptors. The hyphae produced from the matings showed the expected occurrence of pseudoclamps, with no apparent effect of the Gfp-HA tag on receptor function.
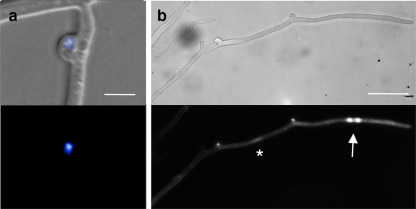
Expression of the Gfp-tagged receptor was induced by a mating interaction with a compatible wild-type strain, and fluorescence could be detected in pseudoclamps, at the cell periphery, and in vesicles (Fig. 9). Thus, in vivo visualization confirmed the presence of receptor molecules within pseudoclamps, while staining of the nuclear envelope was never observed. To independently confirm the results, the same transformants were used for immunofluorescence detection of the receptor. At the low expression levels observed for the receptor gene, we reasoned that the signal enhancement inherent in antibody detection would be of benefit to our analyses. Localization of the receptor with predominant occurrence at the cell periphery was consistent with our observations of membrane localization in pseudoclamps and clamp connections, utilizing an FITC-labeled antibody specific for the HA tag (Fig. 9). The fluorescence signal was especially intense at the septa, which we interpret to be a result of cell membranes on both sides of a single septum. For some pseudoclamps, detection of Gfp-tagged receptor failed, presumably for those trapping a wild-type nucleus not carrying Gfp.
Fig 9.
(A) Micrographs of HA-Gfp-tagged pheromone receptor localization in dikaryotic S. commune transformants by immunostaining (Aa to Ad) and in vivo (Ae and Af) in dikaryotic mycelium (Vbar2tG1 or Vbar2tG11 mated to 4-39); (B) differentiation of pseudoclamps showing strong receptor staining while others are nonfluorescent (top, bright field; middle, DAPI and calcofluor; bottom, Gfp). Bars, 5 μm.
Since the expression of pheromone receptor protein was shown to be induced in compatible mating interactions, fluorescence analyses were performed predominantly on mycelia derived from mated cultures. When monokaryotic, vegetative mycelium of the tagged Gfp-receptor transformants was investigated, no clear fluorescence signal was detectable for either Gfp or HA, because of the extremely low expression levels under these conditions (data not shown). Mycelia of untagged wild-type strains were used as negative controls in fluorescence microscopy.
Downstream signaling and target genes of pheromone response.
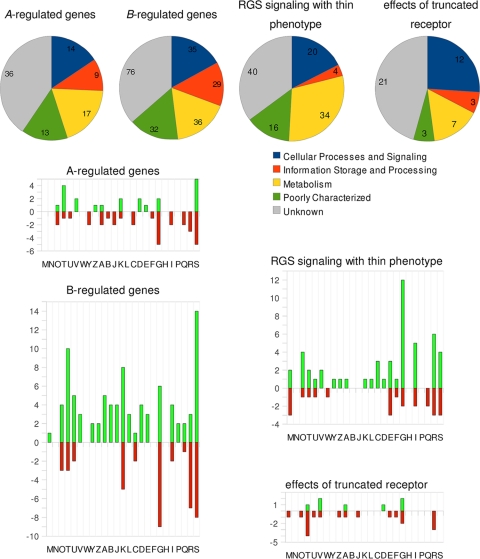
In order to identify genes responding to pheromone signaling at the transcriptional level, we used whole-genome microarrays hybridized with cDNA derived from different mating interactions. These interactions included the heterokaryotic Aon, heterokaryotic Bon, and dikaryotic (Aon and Bon) conditions. Overall, 26% of the entire genome was determined to be transcriptionally regulated (fold change, ≥2; P ≤ 0.05) due to mating interactions. This can be broken down into 974 genes (7% of the genome) regulated by activation of the A pathway, 1,480 genes (11% of the genome) regulated by the B pathway, and 1,016 genes (8% of the genome) regulated by both A and B. The last type of regulation was not analyzed further, since mushroom formation would likely override or obscure any direct effects of combined A and B regulation. A higher threshold for transcriptional regulation (change, ≥5-fold) yielded 89 A-regulated genes (41 up, 48 down; see Tables S2 to S4 in the supplemental material) and 208 B-regulated genes (138 up, 70 down; see Tables S2, S5, and S6 in the supplemental material), corresponding to 0.7% and 1.6% of the genome, respectively. According to KOG (euKaryotic Orthologous Groups) classification, putative protein domains and general functions for the obtained regulated genes were classified (Fig. 10).
Fig 10.
Transcriptional regulation of genes associated with various mating interactions in S. commune. Functional groups of regulated genes (change, ≥5-fold; P ≤ 0.05; green, upregulation; red, downregulation). KOG classification: cellular processes and signaling (M, cell wall/membrane/envelope biogenesis; N, cell motility; O, posttranslational modification, protein turnover, chaperones; T, signal transduction; U, intracellular trafficking, secretion, and vesicular transport; V, defense mechanisms; W, extracellular structures; Y, nuclear structure; Z, cytoskeleton), information storage and processing (A, RNA processing and modification; B, chromatin structure and dynamics; J, translation, ribosomal structure and biogenesis; K, transcription; L, replication, recombination, and repair), metabolism (C, energy production and conversion; D, cell cycle control, cell division, chromosome partitioning; E, amino acid transport and metabolism; F, nucleotide transport and metabolism; G, carbohydrate transport and metabolism; H, coenzyme transport and metabolism; I, lipid transport and metabolism; P, inorganic ion transport and metabolism; Q, secondary metabolites biosynthesis, transport and catabolism), and poorly characterized (R, general function prediction only; S, function unknown).
As is evident from this analysis, more genes were regulated by activation via action of the B mating type genes than through the A-dependent pathways. We found that the A mating type genes, which code for homeodomain transcription factors, primarily activate genes involved in signal transduction, defense mechanisms, transcription, and cell cycle control, while genes involved in carbohydrate metabolism are downregulated. Specifically, the increased expression of genes coding for a splicing coactivator subunit (protein identifier [ID] 234140) and the large subunit of an RNA polymerase II (ID 112761) hints to an enhancement of transcriptional activity (Table 2).
Table 2.
Identification of genes differentially expressed under A-regulated development ordered by KOG group
| Regulation, KOG groupa: (gene name) protein function/biological process/cellular component | Protein ID | Fold change in expression |
|---|---|---|
| Upregulated | ||
| O: E3 ubiquitin ligase interacting with arginine methyltransferase, Zn finger, CCHC type | 107850 | 8.3 |
| T: (aay4) large RNA-binding protein (RRM superfamily), A-α-Y mating type-dependent binding region, A-α-Y4 protein; HD2 | 231556b | 11.9 |
| T: (clp3) Clp1-like protein, mitochondrial carrier | 233611 | 5.9 |
| T: G-protein beta WD-40 repeat | 107647 | 5.8 |
| T: cAMP-dependent protein kinase catalytic subunit (PKA) | 233600 | 5.5 |
| T: (bpl1) pheromone precursor | 112470 | 5.5 |
| V: heme peroxidase, plant/fungal/bacterial, response to oxidative stress | 106700 | 11.1 |
| V: (von Willebrand factor and related) coagulation proteins | 256712 | 5.1 |
| Z: dystonin, GAS (growth-arrest-specific protein), and related proteins | 232734 | 7.7 |
| A: splicing coactivator SRm160/300, subunit SRm300 | 234140 | 5.5 |
| K: RNA polymerase II, large subunit | 112761 | 5.2 |
| D: warts/lats-like serine threonine kinases, serine/threonine protein kinase | 60342 | 20.3 |
| D: checkpoint kinase and related serine/threonine protein kinases, serine/threonine protein kinase | 111399 | 9.9 |
| E: peptidase M, neutral zinc metallopeptidases, zinc-binding site | 235599 | 7.5 |
| G: chitinase | 110277 | 18.2 |
| G: glycoside hydrolase, chitinase active site | 234329b | 5.1 |
| S: cyclin-like F box | 108012 | 21.0 |
| S: Zn finger domain, MYND type | 237371 | 11.1 |
| S: cyclin-like F box | 234092 | 9.3 |
| S: Zn finger, C2H2 type, nucleic acid binding | 110202 | 6.5 |
| S: flank to A genes, hypothetical proteins in Laccaria bicolor (NCBI accession no. XP_001886997.1) and C. cinerea (NCBI accession no. XP_001840121.2) by BLASTp analysis | 241485 | 6.1 |
| O: transport protein Sec61, alpha subunit, protein secretion, P—P bond hydrolysis-driven protein transmembrane transporter activity | 234899 | −76.0 |
| O: molecular chaperone (DnaJ superfamily), heat shock protein binding | 49803 | −5.7 |
| T: EPS15 homology, synaptic vesicle protein EHS-1 and related EH domain proteins | 113426 | −14.7 |
| U: transport protein particle (TRAPP) complex subunit, Bet3 | 14103 | −16.0 |
| Y: nucleolar GTPase/ATPase p130 | 234188 | −83.9 |
| Y: nucleolar GTPase/ATPase p130 | 233086 | −5.5 |
| A: helix-turn-helix, Fis type, splicing factor 1/branch point binding protein (RRM superfamily) | 54550 | −8.7 |
| A: RNase, RNase III | 30567 | −5.1 |
| B: predicted histone tail methylase containing SET domain | 107557 | −5.2 |
| J: translation initiation factor 5B (eIF-5B), phosphotransferases with an alcohol group as acceptor | 14724 | −17.3 |
| J: mitochondrial ribosomal protein L16 | 33729 | −7.3 |
| K: thyroid hormone receptor-associated protein complex, subunit TRAP230 | 47014 | −9.6 |
| C: NADH-cytochrome b5 reductase, flavoprotein pyridine nucleotide cytochrome reductase | 231100 | −25.0 |
| C: (R,R)-butanediol dehydrogenase, FAD-linked oxidase, N terminal | 63691 | −8.8 |
| F: dihydroorotase and related enzymes, urease | 16734 | −26.7 |
| G: cellulose-binding region, fungal, esterase, poly-β-hydroxybutyrate depolymerase, esterase/lipase/thioesterase | 236921 | −24.0 |
| G: glycosyltransferase, family 8 glycogenin, glycogenin glucosyltransferase | 73723 | −14.6 |
| G: dimeric dihydrodiol dehydrogenase, trans-1,2-dihydrobenzene-1,2-diol dehydrogenase | 54187 | −9.8 |
| G: glycoside hydrolase, family 61 | 58521 | −6.2 |
| G: predicted transporter (major facilitator superfamily), l-arabinose isomerase | 76389 | −5.8 |
| I: cytochrome P450 CYP4/CYP19/CYP26 subfamilies, unspecific monooxygenase | 67567 | −6.7 |
| I: peroxisomal 3-ketoacyl coenzyme A-thiolase P-44/SCP2, thiolase | 67156 | −6.1 |
| Q: zinc-containing alcohol dehydrogenase superfamily, sorbitol dehydrogenase, l-iditol 2-dehydrogenase | 64180 | −13.5 |
| Q: dehydrogenases with different specificities (related to short-chain alcohol dehydrogenases), glucose/ribitol dehydrogenase | 234748 | −9.0 |
| R: predicted transporter (major facilitator superfamily), similar to drug-resistant transporter EmrB/QacA | 44593 | −13.5 |
| R: monodehydroascorbate/ferredoxin reductase, FAD-dependent pyridine nucleotide-disulfide oxidoreductase | 82230 | −10.4 |
| R: predicted yippee-type zinc-binding protein | 58559 | −8.0 |
| S: mitochondrial carrier domain | 258521c | −126.6 |
| S: [ribulose-bisphosphate-carboxylase]-lysine N-methyltransferase, nuclear protein SET | 59023 | −14.0 |
| S: winged helix-turn-helix transcription repressor DNA binding | 113352 | −7.3 |
| S: dimethylmenaquinone methyltransferase | 57620 | −5.9 |
| S: Zn finger domain, MYND type | 109469 | −5.9 |
KOG groups are defined in the legend to Fig. 10.
Additionally upregulated in thin phenotype.
Additionally downregulated in thin phenotype.
In contrast to the A pathway, the response to B activation was associated with more than 2-fold the number of transcriptionally regulated genes. Most of the differentially expressed genes are upregulated and involved in information storage, metabolism, and signal transduction via 3′-5′-cyclic AMP (cAMP) phosphodiesterase and small G proteins (for example, S. cerevisiae Pde1 [ID 105393]; GAP [ID 108327], and a signaling protein [ID 62504]). Fewer downregulated genes were detected, and these were mostly classified to either the metabolism of carbohydrates (IDs 31488, 55688, 13089, 70398, 108884, 13397, 110470, 109961, 236244), transcription (IDs 61956, 35685, 65707, 114395, 256713), or posttranslational modification, protein turnover, and chaperones (IDs 56996, 17256, 83759) (Table 3).
Table 3.
Identification of genes differentially expressed under B-regulated development ordered by KOG group
| Regulation, KOG groupa: (gene name) protein function/biological process/cellular component | Protein ID | Fold change in expression |
|---|---|---|
| Upregulated | ||
| M: chitinase | 231665 | 14.7 |
| O: molecular chaperone (DnaJ superfamily), heat shock protein DnaJ | 231463 | 18.1 |
| O: AAA+-type ATPase | 109412 | 8.6 |
| O: nuclear AAA ATPase, peptidase S16, Lon protease | 58751 | 8.2 |
| O: HSP90 cochaperone CPR7/cyclophilin, peptidyl-prolyl cis-trans isomerase, cyclophilin type | 55359 | 6.1 |
| T: indoleamine 2,3-dioxygenase | 33516 | 198.5 |
| T: signaling protein RIC-8/synembryn (regulates neurotransmitter secretion) | 62504 | 20.1 |
| T: G protein beta WD-40 repeat | 104106 | 18.6 |
| T: GTPase activator protein, Rab GTPase activator activity | 108327 | 13.8 |
| T: serine/threonine protein kinase | 111639 | 13.5 |
| T: casein kinase (serine/threonine/tyrosine protein kinase), protein kinase | 106153 | 13.5 |
| T: 3′,5′-cyclic nucleotide phosphodiesterase, 3′,5′-cyclic AMP phosphodiesterase activity, similar to S. cerevisiae Pde1 | 105393 | 10.6 |
| T: serine/threonine protein kinase | 103735 | 10.5 |
| T: armadillo/beta-catenin/plakoglobin, uridine kinase, phosphoribulokinase | 71870 | 10.2 |
| T: signal transduction serine/threonine kinase with PAS/PAC sensor domain, protein kinase | 16935 | 6.2 |
| U: translocase of outer mitochondrial membrane complex, subunit TOM37/metaxin 1 | 83604 | 13.9 |
| U: G-protein beta WD-40 repeat, prolactin regulatory element-binding protein/protein transport protein SEC12p | 49920 | 9.3 |
| U: endoplasmic reticulum-Golgi vesicle-tethering protein p115 | 110288 | 7.9 |
| U: peptide exporter, ABC superfamily | 51416 | 7.4 |
| U: vacuolar sorting protein VPS24, Snf7, protein transport | 47850 | 5.8 |
| V: predicted transporter (major facilitator superfamily), tetracycline resistance protein TetB, hydrogen antiporter activity | 41682 | 12.2 |
| V: Von Willebrand factor and related coagulation proteins, various SH3 domains (protein destination) | 255861 | 11.4 |
| V: predicted transporter (major facilitator superfamily), tetracycline resistance protein TetB, similar to Mfs1.1 | 233429b | 5.2 |
| Y: nucleolar GTPase/ATPase p130 | 51001 | 12.2 |
| Y: nucleolar GTPase/ATPase p130 | 256072 | 6.3 |
| Z: spindle pole body protein Sad1p, peptidase C19, ubiquitin carboxyl-terminal hydrolase 2 | 80616 | 25.6 |
| Z: myosin class V heavy chain, myosin head, motor region | 70377 | 11.0 |
| A: splicing coactivator SRm160/300, subunit SRm300 | 232813 | 33.0 |
| A: splicing coactivator SRm160/300, subunit SRm300 | 113731 | 25.4 |
| A: polyadenylation factor I complex, subunit, Yth1 (cleavage and polyadenylation specific factor [CPSF] subunit), Zn finger | 13540 | 15.6 |
| A: splicing coactivator SRm160/300, subunit SRm300 | 256249 | 7.9 |
| A: tuftelin-interacting protein TIP39, contains G-patch domain, D111/G patch (mouse protein) | 36337 | 5.7 |
| B: SWI-SNF chromatin remodeling complex, Snf5 subunit, SNF5/SMARCB1/INI1, chromatin remodeling | 53495 | 18.2 |
| B: chromatin remodeling protein, contains PHD (plant homeodomain) Zn finger | 233026 | 14.9 |
| B: histone acetyltransferase SAGA, TRRAP/TRA1 component, phosphatidylinositol 3 and 4 kinases | 12680 | 14.7 |
| B: SWI/SNF chromatin-remodeling complex protein, prion protein, aminoacyl-tRNA synthetase | 106231 | 6.9 |
| J: 60s acidic ribosomal protein P1, structural constituent of ribosome | 233634 | 11.5 |
| J: tRNA methyltransferase, N2,N2-dimethylguanosine tRNA methyltransferase, tRNA (guanine-N2-)-methyltransferase | 81492 | 10.0 |
| J: pseudouridylate synthase, tRNA pseudouridine synthase | 42694 | 8.9 |
| J: translation initiation factor 2C (eIF-2C) and related proteins, argonaute and dicer protein, PAZ domain | 257069 | 6.1 |
| K: RNA polymerase II, large subunit, DNA-directed RNA polymerase | 105543 | 16.8 |
| K: RNA polymerase I, large subunit, DNA-directed RNA polymerase | 255115 | 14.8 |
| K: transcription regulator XNP/ATRX, DEAD box superfamily, shugoshin, N terminal | 256320 | 13.8 |
| K: transcription coactivator, double-stranded RNA binding | 104274 | 11.5 |
| K: DNA-directed RNA polymerase subunit E′, RNA polymerase Rpb7, N terminal | 234509 | 7.6 |
| K: transcription initiation factor IIF, small subunit (RAP30); transcription initiation factor IIF, beta subunit | 78184 | 6.5 |
| K: transcription factor XBP-1, basic leucine zipper (bZIP) transcription factor | 236086 | 5.7 |
| K: GCN5-related N-acetyltransferase, transferring groups other than amino acyl groups | 52412 | 5.5 |
| L: DNA repair protein, SNF2 family, helicase, C terminal, SNF2 related | 81511 | 306.0 |
| L: CDC45 (cell division cycle 45)-like protein, DNA replication initiation | 50273 | 11.7 |
| L: origin recognition complex, subunit 4 | 46745 | 8.2 |
| C: mitochondrial carnitine-acylcarnitine carrier protein, adenine nucleotide translocator 1 | 58334 | 17.4 |
| D: halotolerance protein HAL3 (contains flavoprotein domain), peptidyl-prolyl cis-trans isomerase activity | 113841 | 26.5 |
| D: halotolerance protein HAL3 (contains flavoprotein domain), phosphopantothenoylcysteine decarboxylase | 15072 | 24.3 |
| D: Mis12, chromosome, pericentric region | 14071 | 15.9 |
| D: mitotic spindle checkpoint protein BUB3, WD repeat superfamily, G-protein beta WD-40 repeat | 86026 | 5.5 |
| E: oxoprolinase, hydantoinase/oxoprolinase, glutathione metabolism | 82383 | 7.3 |
| E: serine carboxypeptidases (lysosomal cathepsin A), peptidase S10, serine carboxypeptidase | 40585 | 5.8 |
| E: Xaa-Pro aminopeptidase, peptidase M24 | 58445 | 5.2 |
| G: glucose-6-phosphate/phosphate and phosphoenolpyruvate/phosphate antiporter | 36941 | 22.9 |
| G: general substrate transporter, sugar transporter superfamily | 66123 | 14.4 |
| G: esterase, poly-β-hydroxybutyrate depolymerase, esterase/lipase/thioesterase, carbohydrate esterase family 1 protein | 47380 | 12.1 |
| G: inositol monophosphatase | 47747 | 7.7 |
| G: beta-1,6-N-acetylglucosaminyltransferase, contains WSC domain | 110551c | 7.5 |
| G: glycoside hydrolase, family 43 | 232782 | 6.6 |
| I: acyl coenzyme A:diacylglycerol acyltransferase (DGAT) | 53861 | 21.1 |
| I: S-adenosylmethionine-dependent methyltransferases, generic methyltransferase | 14559 | 13.5 |
| I: cytochrome P450 CYP4/CYP19/CYP26 subfamilies, E-class P450, group I, gamma-hexachlorocyclohexane degradation, ascorbate and aldarate metabolism | 233430 | 11.6 |
| I: cytochrome P450 CYP4/CYP19/CYP26 subfamilies, unspecific monooxygenase, tryptophan metabolism, fatty acid metabolism | 76871 | 6.0 |
| P: Ca2+/H+ antiporter VCX1 and related proteins, sodium/calcium exchanger membrane region, calcium/proton exchanger | 27729 | 9.4 |
| P: Ca2+ transporting ATPase, potassium/sodium efflux P-type ATPase, fungal type | 53464 | 5.4 |
| Q: multidrug/pheromone exporter, ABC superfamily | 113902 | 53.4 |
| Q: multidrug/pheromone exporter, ABC superfamily | 258386 | 5.0 |
| R: Zn finger, C2H2 type, similar to vegetative cell wall protein gp1 precursor (hydroxyproline-rich glycoprotein 1) | 113591 | 19.2 |
| R: WD-40 repeat-containing protein | 84316 | 9.0 |
| R: Zn finger, cytochrome c heme-binding site, electron transport activity | 233610 | 5.7 |
| S: UbiA prenyltransferase, prenyltransferase activity | 238827 | 23.8 |
| S: chloroperoxidase, peroxidase activity, electron transport | 57566 | 21.7 |
| S: cyclin-like F-box domain | 111277 | 18.4 |
| S: cyclin-like F box, Zn finger, C2H2 type | 104797 | 8.7 |
| S: glutathione S-transferase, N terminal | 111982 | 8.3 |
| S: bucentaur or craniofacial development | 112020 | 8.2 |
| S: protein binding, BTB/POZ domain | 236141 | 7.8 |
| S: bacterial extracellular solute-binding protein, family 3, transporter activity | 105400 | 7.7 |
| S: predicted membrane protein | 108226 | 7.7 |
| S: Zn finger, MYND type | 231200 | 7.1 |
| S: target SNARE coiled-coil region | 56068 | 6.5 |
| S: cyclin-like F box, Zn finger, C2H2 type | 234065 | 6.3 |
| S: Zn finger, CCHC type | 237097 | 6.0 |
| S: notchless-like WD-40 repeat-containing protein, 2-acetyl-1-alkylglycerophosphocholine esterase, G-protein beta WD-40 repeat | 37220 | 5.2 |
| Downregulated | ||
| O: alkyl hydroperoxide reductase/peroxiredoxin | 56996 | −20.1 |
| O: E3 ubiquitin ligase interacting with arginine methyltransferase, Zn finger, CCHC type | 83759 | −19.5 |
| O: peptidase M28, transferrin receptor and related proteins containing the protease-associated (PA) domain | 17256 | −11.3 |
| T: serine/threonine protein kinase | 108101 | −8.5 |
| T: (brl3) fungal pheromone STE3 6-protein-coupled receptor, mating-type alpha-factor pheromone receptor activity | 258344 | −5.6 |
| T: (bbp2) pheromone precursor | 12028 | −14.2 |
| U: nuclear pore complex, Nup98 component (sc Nup145/Nup100/Nup116), ribosomal protein L9 N-terminal like | 112737 | −5.9 |
| U: clathrin adaptor complex, medium chain, adaptor complexes medium subunit family | 61803 | −5.2 |
| K: fungal transcriptional regulatory protein, N-terminal, fungus-specific transcription factor, DNA binding, zinc ion binding | 65707 | −12.8 |
| K: transcription factor activity, similar to Cu-dependent DNA-binding protein, copper fist DNA binding | 61956 | −9.4 |
| K: fungus-specific transcription factor, DNA binding, zinc ion binding | 35685 | −7.3 |
| K: peptidase M, neutral zinc metallopeptidases, zinc-binding site, selective LIM domain binding factor | 114395 | −6.9 |
| K: GATA-4/5/6 transcription factors, Zn finger, GATA type | 256713 | −6.2 |
| C: aldehyde dehydrogenase, tyrosine metabolism, glycolysis/gluconeogenesis | 258124 | −7.7 |
| C: kynurenine 3-monooxygenase and related flavoprotein monooxygenases, salicylate 1-monooxygenase | 238637 | −6.5 |
| G: gluconate transport-inducing protein | 108884 | −25.9 |
| G: predicted short-chain-type dehydrogenase, glucose/ribitol dehydrogenase | 236244 | −20.5 |
| G: predicted short-chain-type dehydrogenase, glucose/ribitol dehydrogenase | 110470 | −10.0 |
| G: general substrate transporter, permease of the major facilitator superfamily | 55688 | −9.6 |
| G: dTDP-glucose 4-6-dehydratase/UDP-glucuronic acid decarboxylase, erythromycin biosynthesis | 13089 | −6.8 |
| G: permease of the major facilitator superfamily | 13397 | −6.0 |
| G: glycoside hydrolase family 23 protein, candidate beta-glycosidase distantly related to N-acetylmuramidases | 31488 | −5.4 |
| G: alpha-amylase | 70398 | −5.2 |
| G: predicted transporter (major facilitator superfamily), sugar:hydrogen symporter activity | 109961 | −5.1 |
| I: esterase/lipase/thioesterase, hormone-sensitive lipase (HSL) | 85341 | −8.1 |
| I: fatty acid desaturase, Cytochrome b5 | 43089 | −5.1 |
| Q: laccase, multicopper oxidase, type 1 | 111478 | −5.2 |
| R: Zn finger, C2H2 type, cytochrome c heme-binding site, electron transport | 111555 | −19.5 |
| R: transposon-encoded proteins with TyA, reverse transcriptase, integrase domains in various combinations | 41205 | −11.2 |
| R: oxidoreductase, electron transport | 16666 | −7.5 |
| R: transposon-encoded proteins with TyA, reverse transcriptase, integrase domains in various combinations | 40072 | −7.4 |
| R: reductases with broad range of substrate specificities, oxidoreductase activity | 61612 | −6.5 |
| R: O-methyltransferase, family 2, hydroxyindole-O-methyltransferase, S-adenosylmethionine-dependent methyltransferase (SAM) activity | 238432c | −5.5 |
| R: monodehydroascorbate/ferredoxin reductase, flavin adenine dinucleotide-dependent pyridine nucleotide-disulfide oxidoreductase, electron transport | 62520 | −5.1 |
| S: pyridoxamine 5′-phosphate oxidase-related, flavin mononucleotide binding | 42417 | −17.7 |
| S: S-adenosylmethionine-dependent methyltransferase, S-adenosylmethionine (and some other nucleotide) binding motif | 80619 | −9.9 |
| S: d-alanyl–d-alanine endopeptidase activity, peptidase A22B, minor histocompatibility antigen H13, aspartic-type endopeptidase activity | 58664 | −8.5 |
| S: cell growth regulatory protein CGR11 | 238935 | −7.9 |
| S: cyclin-like F box | 110269 | −7.7 |
| S: thaumatin, pathogenesis related | 111995 | −6.3 |
| S: variant SH3 domain | 65138 | −6.1 |
| S: cyclin-like F box | 114268 | −5.7 |
KOG groups are defined in the legend to Fig. 10.
Additionally downregulated in thin phenotype.
Additionally upregulated in thin phenotype.
Intracellular modification of pheromone signaling.
To obtain more information on cellular pathways involved in sexual development, we investigated several mutant strains affected in mating interactions. The influence of Thn1 was examined by the expression profiling of a homokaryotic S. commune strain expressing a thin phenotype. The 114 regulated genes (72 up, 42 down) (Fig. 10; see Tables S7 and S8 in the supplemental material) showed high overlap with cellular responses, in accordance with the known RGS function of Thn1 as a repressor of G-protein-coupled signaling. Upregulated genes included those influencing processing and posttranslational modification or genes with a stress-related protein function. Proteins involved in biogenesis of the cell wall or membrane turnover were identified among both the up- and downregulated genes, suggesting reprogramming of the cells via genetic means (Table 4; see also Tables S7 and S8 in the supplemental material). Among the upregulated genes, we also found several candidates for chitinases (IDs 85084, 85210, 46134, 79630) and other glycoside hydrolases (family 61, IDs 60863, 16233, 41145; family 5, ID 16928; others, ID 234329), suggesting functions associated with a reorganization of the cell wall. The expression of thn1 itself was highly downregulated in a loss-of-function thn mutant compared to a wild-type homokaryon (Table 4).
Table 4.
Identification of genes differentially expressed in RGS signaling with thin phenotype ordered by KOG group
| Regulation, KOG groupa: (gene name) protein function/biological process/cellular component | Protein ID | Fold change in expression compared to: |
|
|---|---|---|---|
| Mon1 | Mon2 | ||
| Upregulated | |||
| M: chitinase, glycoside hydrolase, family 18 | 85210 | 48.5 | 26.2 |
| M: chitinase, glycoside hydrolase, family 18 | 46134 | 8.9 | 8.5 |
| O: glutathione S-transferase | 61450 | 78.0 | 143.0 |
| O: molecular chaperones GRP170/SIL1, HSP70 superfamily, heat shock protein Hsp70 | 78951 | 10.2 | 10.2 |
| O: predicted E3 ubiquitin ligase, Zn finger, CCHC type | 112446 | 7.5 | 7.5 |
| O: metalloendopeptidase family, mitochondrial intermediate peptidase, mitochondrial intermediate peptidase. | 13586 | 5.9 | 5.2 |
| T: serine/threonine protein kinase, fungal transcriptional regulatory protein, N-terminal, coenzyme F420-0 gamma-glutamyl ligase activity | 111022 | 19.3 | 22.8 |
| T: (aay4) large RNA-binding protein (RRM superfamily), A-alpha-Y mating type-dependent binding region, A-alpha-Y4 protein; HD2 | 231556 | 6.1 | 7.9 |
| U: cytosolic sorting protein GGA2/TOM1, intracellular protein transport | 62583 | 18.4 | 15.3 |
| V: inositol-1,4,5-trisphosphate 5-phosphatase | 232626 | 27.9 | 44.9 |
| V: cyclin-dependent protein kinase activity | 230362 | 9.2 | 8.3 |
| Y: nucleolar GTPase/ATPase p130 | 112837 | 9.4 | 8.5 |
| Z: myosin class II heavy chain | 67570 | 17.0 | 13.3 |
| A: splicing coactivator SRm160/300, subunit SRm160 (contains PWI domain), nucleic acid-directed DNA polymerase | 237177 | 9.5 | 13.1 |
| K: transcription initiation factor TFIID, subunit BDF1 and related bromodomain proteins | 78700 | 37.1 | 20.4 |
| L: tyrosyl-DNA phosphodiesterase, phosphodiesterase I | 47163 | 22.5 | 15.1 |
| C: dihydrolipoamide acetyltransferase, biotin/lipoyl attachment | 75006 | 15.8 | 15.6 |
| C: acyl carrier protein/NADH-ubiquinone oxidoreductase, NDUFAB1/SDAP subunit, fatty acid biosynthetic process | 64587 | 15.6 | 16.5 |
| C: flavin adenine dinucleotide-linked oxidase, N terminal | 104138 | 9.7 | 6.1 |
| D: putative transcription factor HALR/MLL3, involved in embryonic development | 104983 | 8.2 | 8.6 |
| E: kynurenine hydrolase, tryptophan metabolism | 232621 | 119.0 | 154.0 |
| E: indoleamine 2,3-dioxygenase, similar to tryptophan 2,3-dioxygenase, putative | 113210 | 74.7 | 109.0 |
| E: beta-lactamase | 62798 | 14.3 | 8.8 |
| F: IMP dehydrogenase/GMP reductase, purine metabolism | 14863 | 5.7 | 5.1 |
| G: glycoside hydrolase, chitinase active site | 234329 | 62.4 | 44.5 |
| G: alpha-amylase | 107514 | 29.3 | 17.3 |
| G: polygalacturonase | 233414 | 20.7 | 12.6 |
| G: glycoside hydrolase, family 61 (endoglucanase) | 41145 | 13.9 | 8.4 |
| G: cellulose-binding region, fungal, esterase, poly-β-hydroxybutyrate depolymerase, esterase/lipase/thioesterase | 236921 | 10.2 | 8.0 |
| G: chitinase, glycoside hydrolase, family 18 | 79630 | 10.0 | 5.9 |
| G: glycoside hydrolase, family 5 (mannan) | 16928 | 9.6 | 5.2 |
| G: beta-1,6-N-acetylglucosaminyltransferase, contains WSC domain | 110551 | 9.2 | 9.2 |
| G: glycoside hydrolase, family 61 | 60836 | 8.7 | 15.8 |
| G: ricin B lectin | 29538 | 8.6 | 5.4 |
| G: glycoside hydrolase, family 61 (lignocellulose) | 16233 | 7.0 | 5.3 |
| G: predicted transporter (major facilitator superfamily), sugar transporter family | 56704 | 6.1 | 5.9 |
| I: acyl coenzyme A synthetase, flavonoids, stilbene and lignin biosynthesis | 49211 | 16.1 | 12.8 |
| I: S-adenosylmethionine-dependent methyltransferases, generic methyltransferase | 14559 | 12.1 | 8.2 |
| I: very-long-chain acyl coenzyme A dehydrogenase, acyl coenzyme A dehydrogenase, C terminal | 233523 | 12.0 | 14.7 |
| I: fatty acid desaturase, cytochrome b5 | 78094 | 5.7 | 6.7 |
| I: predicted lipase | 61780 | 5.1 | 6.6 |
| R: synaptic vesicle transporter SVOP and related transporters (major facilitator superfamily) | 237837 | 30.2 | 50.5 |
| R: predicted dehydrogenase, steroid dehydrogenase activity, acting on the CH-OH group of donors, NAD or NADP as acceptor | 84264 | 28.6 | 19.9 |
| R: glucose dehydrogenase/choline dehydrogenase/mandelonitrile lyase (GMC oxidoreductase family) | 256423 | 20.0 | 14.1 |
| R: Zn finger, C2H2 type | 113495 | 9.9 | 15.2 |
| R: PPR repeat (pentatricopeptide, RNA binding, eventually regulation in mitochondria) | 230806 | 9.1 | 5.0 |
| R: O-methyltransferase, family 2, hydroxyindole-O-methyltransferase, S-adenosylmethionine-dependent methyltransferase (SAM) activity | 238432 | 8.7 | 5.3 |
| S: cyclin-like F box | 54225 | 14.9 | 19.2 |
| S: Zn finger, CCHC type | 104352 | 8.4 | 9.3 |
| S: rare lipoprotein A | 38806 | 7.7 | 9.0 |
| S: flank to A genes, hypothetical proteins in Laccaria bicolor (NCBI accession no. XP_001886997.1) and C. cinerea (NCBI accession no. XP_001840121.2) by BLASTp analysis | 241485 | 5.9 | 6.7 |
| Downregulated | |||
| M: (sc4) fungal hydrophobin SC4 | 73533 | −151.9 | −156.7 |
| M: chitinase | 85084 | −29.9 | −20.9 |
| M: (sc1) fungal hydrophobin SC1 | 49129 | −11.0 | −12.8 |
| O: Zn finger, MYND type, ubiquitin carboxyl-terminal hydrolase | 17275 | −8.2 | −5.8 |
| T: (thn1) regulator of G protein, Thn1 | 83983 | −92.9 | −71.9 |
| U: vacuolar sorting protein VPS1, dynamin, and related proteins, GTPase activity | 61420 | −9.8 | −6.0 |
| W: collagens (type IV and type XIII) and related proteins | 84580 | −9.6 | −5.1 |
| J: ribosomal protein L15 (large subunit) | 85903 | −8.6 | −15.7 |
| E: d-aspartate oxidase | 55341 | −29.1 | −26.0 |
| E: methionine synthase, vitamin-B12 independent | 57220 | −6.9 | −9.7 |
| F: thymidylate kinase/adenylate kinase | 233884 | −14.4 | −8.4 |
| G: beta-1,6-N-acetylglucosaminyltransferase | 233751 | −31.1 | −16.7 |
| G: beta-1,6-N-acetylglucosaminyltransferase, contains WSC domain | 103498 | −7.2 | −11.6 |
| I: esterase/lipase/thioesterase, hormone-sensitive lipase HSL | 85341 | −9.6 | −8.6 |
| I: esterase/lipase/thioesterase | 69185 | −7.2 | −5.1 |
| Q: dehydrogenase activity, related to short-chain alcohol dehydrogenases | 234748 | −7.1 | −9.4 |
| Q: cytochrome P450 CYP2 subfamily, unspecific monooxygenase, tryptophan metabolism | 111260 | −7.0 | −7.9 |
| R: predicted transporter (major facilitator superfamily), tetracycline resistance protein TetB, similar to Mfs1.1 | 233429 | −82.1 | −87.4 |
| R: predicted hydrolases or acyltransferases, esterase/lipase/thioesterase | 67288 | −11.9 | −6.7 |
| R: Zn finger, C2H2 type, predicted hydrolase (HIT domain-containing family) | 27513 | −5.4 | −8.3 |
| S: mitochondrial carrier domain | 258521 | −9.6 | −5.9 |
| S: Zn finger, C-X8-C-X5-C-X3-H type, nucleic acid binding | 63596 | −9.3 | −6.7 |
| S: GCN5-related N-acetyltransferase | 80737 | −8.9 | −5.3 |
KOG groups are defined in the legend to Fig. 10.
The truncation of the intracellular C terminus of the receptor would be predicted to interfere with some intracellular protein-protein interactions upon pheromone stimulation. Only 25 genes with predicted function (46 in total) were altered in expression at least 5-fold (Fig. 10; Table 5; see also Tables S9 and S10 in the supplemental material). A strong effect on genes involved in cellular processes and signaling was observed, with changes ranging from 10- to 60-fold in magnitude. Of specific interest was the finding that a small GTPase involved in nuclear protein import (ID 43735) was downregulated more than 1,000-fold (Table 5).
Table 5.
Identification of genes differentially expressed with truncated receptor ordered by KOG group
| Regulation, KOG groupa: (gene name) protein function/biological process/cellular component | Protein ID | Fold change in expression compared to: |
|
|---|---|---|---|
| Dik | Vbar2f | ||
| Upregulated | |||
| T: protein-tyrosine-phosphatase | 105372 | 8.8 | 8.4 |
| V: inositol-1,4,5-trisphosphate 5-phosphatase | 232626b | 28.7 | 14.8 |
| V: stress responsive protein | 112004 | 9.2 | 14.7 |
| A: splicing factor 3a, subunit 3 | 83890 | 14.7 | 7.7 |
| D: protein with predicted involvement in meiosis (GSG1) | 103874 | 5.6 | 7.8 |
| G: monocarboxylate transporter | 53388 | 6.3 | 9.2 |
| G: glycoside hydrolase, family 10 | 15936 | 5.5 | 7.4 |
| Downregulated | |||
| M: (sc1) fungal hydrophobin SC1 | 49129 | −23.9 | −14.4 |
| O: ubiquitin carboxyl-terminal hydrolase | 113792 | −45.0 | −42.5 |
| T: GTP-binding protein CRFG/NOG1 (ODN superfamily) | 69329 | −58.8 | −37.4 |
| T: serine-threonine phosphatase 2B, catalytic subunit | 70929 | −34.4 | −25.8 |
| T: ankyrin repeat protein | 112035 | −11.6 | −7.2 |
| T: regulator of chromosome condensation, ankyrin and BTB/POZ domains | 256996 | −5.4 | −7.2 |
| U: predicted small GTPase involved in nuclear protein import | 43735 | −1196.1 | −719.6 |
| V: heme peroxidase, response to oxidative stress | 112999 | −18.1 | −9.5 |
| Z: beta-tubulin | 237626 | −7.8 | −5.7 |
| A: peptidase, splicing coactivator SRm160/300, subunit SRm300 | 102823 | −9.6 | −10.1 |
| J: ribosomal protein L15 (large subunit) | 85903 | −6.6 | −7.9 |
| E: peptidase S33, prolyl aminopeptidase; esterase/lipase/thioesterase | 231359 | −6.2 | −5.2 |
| F: atrazine chlorohydrolase/guanine deaminase | 81437 | −18.3 | −10.8 |
| G: glycoside hydrolase, family 43 | 109664 | −14.9 | −28.9 |
| G: beta-1,6-N-acetylglucosaminyltransferase, glycoside hydrolase, family 71 | 53341 | −11.8 | −9.4 |
| R: predicted 3′-5′ exonuclease | 103723 | −60.0 | −30.9 |
| R: FOG, Zn finger, nucleic acid binding | 109000 | −20.3 | −11.5 |
| R: cyclin | 236990 | −11.2 | −6.0 |
KOG groups are defined in the legend to Fig. 10.
Additionally upregulated in thin phenotype.
DISCUSSION
The localization of the pheromone receptor protein in hyphae of the fungus S. commune provides an important link between pheromone perception and the cellular responses to that signal (55). In this study, the pheromone receptor was visualized at the cell periphery, consistent with a localization in the plasma membrane. No evidence of nuclear membrane localization, as has been proposed in some previous hypotheses, was found (5, 60). Another prediction of these models was that pheromone receptors would be expected to be localized in the plasma membrane close to the encoding nucleus by virtue of localized expression and incorporation. We did not observe an increased occurrence of fluorescence label in areas close to one of the nuclei in the dikaryotic cells. The involvement of pheromone activity in nuclear pairing could not be verified by receptor localization in vivo and in situ.
Prior unsuccessful attempts to localize the pheromone receptors in mushroom-forming basidiomycetes with Gfp labeling were attributed to the low level of expression of the B genes, also confirmed in this study. For these reasons, it seemed of vital importance to determine the critical parameters necessary for high receptor expression under natural conditions. This goal was greatly facilitated by the induction of mating in many hyphal compartments at the same time through the setup consisting of a top-to-top sandwich formed from two pregrown mycelia. This experimental system allowed us to define the period of highest expression to a time between 6 and 12 h in mating interactions of compatible strains. To the best of our knowledge, this is the first time that a pheromone receptor has been localized in the hyphae of a mushroom-forming fungus.
Because the B genes regulate both nuclear migration and the fusion of clamp cells, pheromone activity could be envisioned to play a key role in the upregulation of pheromone expression in as yet unfused clamps. It follows that pheromone receptors would be expected to be localized to the plasma membrane of pseudoclamps and to the hyphal cell close to the fusion site. However, localization studies were hampered by both the low expression level of the pheromone receptors and the transient nature of hook cell formation, which is normally completed along with a fused clamp connection within seconds (59). The unexpected finding that clamp cell fusion was inhibited in mated mycelium of receptor transformants carrying a C-terminally truncated receptor gene enabled us to detect a fluorescence signal predominantly in unfused pseudoclamps. The Gfp-tagged receptor could be clearly localized at the cell periphery in association with the plasma membrane. In addition, septa of the clamped mycelia gave a strong signal. Mislocalization of the tagged receptor seems unlikely because transformants of the Bnull mutant integrating this construct exhibited the normal self- versus non-self-recognition phenotype characteristic of wild-type cells. Clamp connections were formed more slowly than wild-type mating interactions, but this was also observed in nontagged truncated and full-length receptor transformants. While the transformants did contain mostly one copy of the respective gene (but in one case the transformant contained up to five copies of the respective gene), a difference in phenotype was not connected to copy number, neither for number of pseudoclamps formed or fruit body formation nor for formation of anucleate spores.
In our receptor transformants of the Bnull recipient strain, the distribution of nuclei in mated mycelia was disturbed, with one nucleus being trapped in the unfused clamp cell and the other being trapped in the main hyphal compartment. Thus, two monokaryotic compartments were formed, resulting from the failure to achieve the dikaryotic condition after the initiation of mitosis and clamp formation. The strong fluorescence visible in pseudoclamps is an indication for mating competence associated with higher levels of receptor within that cellular structure. A fluorescent signal would be visible only in those unfused pseudoclamps trapping a transformed, Gfp-receptor-encoding nucleus and not those trapping an untransformed nucleus. Given that we did not observe fluorescence in every unfused pseudoclamp that had trapped a nucleus, this observation supports the idea of positioning of either nucleus within the newly formed clamp. However, we did not observe a regular, alternating distribution for the nuclei going into the hook cells of S. commune, as has been demonstrated for C. cinerea (24). Rather, nuclear distribution is mixed in accordance with the possibility of nuclei randomly passing each other within a hyphal compartment upon nuclear migration (11, 36, 37).
Endocytosis and replacement of receptor molecules or, alternatively, the strong expression and membrane incorporation of new receptor molecules are needed to allow the localization in situ and in vivo in pseudoclamps without diffusion of the label into adjacent cells. In our study with the truncated Gfp-labeled pheromone receptor, we could detect the receptor in vesicles and endosomes, cellular structures known to be involved in receptor internalization and degradation after induction with pheromone in S. cerevisiae (34). In S. cerevisiae, Ste3 pheromone receptor protein is short-lived and is rapidly removed from the cell surface via endocytosis (10, 57). The recycling of pheromone receptor via constitutive endocytosis has also been described for U. maydis, where the tagged pheromone receptor Pra1::Gfp is internalized by early endosomes and localized in vacuoles (17).
To investigate processes under the respective control of A and B mating type genes, microarray analyses were performed. Since different specificities are reflected in the sequence divergence between alleles of the mating type genes, detection of these genes was limited to the specificity of the sequenced strain H4-8 (A4,6 B3,2). The increased expression of homeodomain protein-encoding gene aay4 was seen in a semicompatible mating interaction of strains differing in A specificity. Accompanying this interaction, we also detected a strong activation of the gene clp3, which encodes a protein similar to Clp1 of C. cinerea. The Clp1 protein promotes hook cell formation in that fungus, and a loss-of-function mutant strain exhibited a clampless phenotype (23). A similar protein in U. maydis, Clp1, is required for the formation of dikaryotic hyphae in planta and also triggers the development of clamp-like structures (58). Within the S. commune genome, five Clp1-like proteins were identified, but only the expression of clp3 is regulated under all tested conditions, making this the most likely candidate for transcriptional regulation during A-specific development. During this process, we also detected an upregulation of both a catalytic subunit of cAMP-dependent protein kinase (PKA) and dystonin, which is a growth-arrest-specific protein. Although not expected for S. commune, cell cycle arrest is a specific pheromone response known to occur in both S. cerevisiae and U. maydis (33, 58). Thus, transcriptome analysis can sometimes support evolutionary conservation of pathways not obvious from phenotypic observation. However, it can be inferred that during the initial stages of mating and before ongoing nuclear migration ensues, a cell cycle arrest would occur in order to synchronize the two different nuclei involved in mating.
Transcriptional regulation associated with the B pathway revealed a massive reorganization of metabolism and induction of several signaling pathways, even at the stringent cutoff value of 5-fold changes in expression. The downregulated gene brl3, which is located next to the known pheromone receptor genes at Bα, was shown before to be highly expressed in monokaryon and decreased in mating interactions (41). The downregulation of brl3 in pheromone response hints to a function of this gene as a repressor under homokaryotic, nonmated conditions. From the genome, the G-protein-associated downstream pathway in sexual development and involvement of Gβγ signaling have been predicted (55). The transcriptional induction of a predicted Gβ subunit of a heterotrimeric G protein under Bon conditions hints to an activating role of this Gβ in pheromone response.
A Bnull strain (16) which carries a large genomic deletion and has never been observed to respond to a potential mating partner has previously been used to study the function of ectopically integrated receptor genes expressed under the control of their native promoters (approximately 300 bp). In these transformants, receptor expression was lower and delayed compared to wild type. This is consistent with the delay in mating response of these receptor transformants to a compatible tester strain. On the other hand, the comparatively lower expression levels might also be the consequence of the fact that no pheromone is produced by these Bnull receptor transformants to trigger nuclear migration in the wild-type mating partner. This results in a unilateral mating (30) where only half the cells in the sandwich overlay are in fact activated for B-regulated development. The formation of pseudoclamps is a phenomenon which has been observed earlier in semicompatible mating interactions (A≠B=), where nuclei enter the hyphae of the mating partner only at the fusion point. This results in the formation of some pseudoclamps and the death of hyphae in the fusion area (53). In contrast to these bona fide semicompatible mating interactions, the formation of pseudoclamps in mated bar2f and bar2t receptor transformants occurs throughout the colony, with higher occurrence in the latter. This phenotype remained stable, and the mated mycelium was able to develop fruiting bodies and produced spores in both types of receptor transformant matings. Nevertheless, more basidiospores arising from those matings involving transformants integrating the truncated receptor were observed to be anucleate. Thus, a role of the C terminus in nuclear migration toward the spore can also be postulated.
The intracellular regions of S. commune pheromone receptors are considerably longer than those found in other fungi (3, 35, 69). No influence of C-terminal truncation of S. commune receptors on ligand binding and pheromone discrimination like that found for C-terminal truncations of the Ste2 pheromone receptor of S. cerevisiae (56) has been observed (19, 20, 22). In our study, we found that the truncated bar2t receptor of 518 amino acids still induces B-regulated development, as does the 636-amino-acid full-length bar2f receptor. However, the downstream signal cascade seems to be affected by the truncation, the result of which is a change in the expression of downstream genes. An example of this is the small GTPase regulator (RanGEF) commonly involved in nuclear protein import, whose expression is drastically reduced in the truncated receptor transformants.
The pheromone receptors in S. commune are expected to interact with the RGS protein Thn1, a homolog to the S. cerevisiae protein Sst2. Deletion of Sst2 (Δsst2) results in hypersensitivity to pheromone (2, 28, 34, 56). The deletion of thn1 in S. commune also resulted in an increase of pheromone sensitivity (13). In wild-type cells, Thn1 is expected to bind to the unphosphorylated receptor protein and inhibit pheromone response prior to mating, while after ligand binding, internalization is enabled and/or turnover is increased.
We determined that the expression of thn1 is strongly downregulated within the mutant with the thn mutation, which affects both A- and B-regulated signaling. An influence on the redox status, possibly related to the characteristic odor of the thn mutant strains by release of reduced molecules, is reflected by an upregulation of glutathione S-transferase and downregulation of cytochrome monooxygenase P450. The results of the present study confirm earlier observations that link cell integrity as well as cell wall and membrane synthesis to B-regulated development (47). The strong reduction of aerial mycelium formation seen in Bon as well as in thn mutants correlated, in both cases, with the absence of hydrophobin expression, as evidenced by the results of our microarray experiments.
With our study, we could localize the pheromone receptor to the cell periphery, with higher expression in unfused pseudoclamps. B pathway activation and its function in clamp fusion could be linked to the intracellular C terminus, where RGS binding impacts development of sexual structures and the formation of clamp connections as well as nucleated spores. Using mushroom-forming S. commune, pheromone signaling in a tetrapolar mating system was linked to cell biology which previously had not been apparent from investigation of other fungal systems, including the yeast S. cerevisiae. The transcriptome analysis could show an extensive set of genes being regulated. The phenotype reflects this fact, with extended growth of mycelia after semicompatible induction of the B pheromone response pathway. The study on genes regulated by mating interactions allowed us to identify processes and genes, including clp3, involved in regulation of mating type-dependent pathways.
Supplementary Material
ACKNOWLEDGMENTS
Alexander Gehrke and Susanne Nolden are thanked for their help. We thank J. Stephen Horton for his help with editing.
We thank ILRS and JSMC for financial support.
Footnotes
Published ahead of print 30 December 2011
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1. Attwood TK, Findlay JBC. 1994. Fingerprinting G-protein-coupled receptors. Protein Eng. 7:195–203 [DOI] [PubMed] [Google Scholar]
- 2. Ballon DR, et al. 2006. DEP-domain-mediated regulation of GPCR signaling responses. Cell 126:1079–1093 [DOI] [PubMed] [Google Scholar]
- 3. Bölker M, Urban M, Kahmann R. 1992. The A mating type locus of U. maydis specifies cell signaling components. Cell 68:441–450 [DOI] [PubMed] [Google Scholar]
- 4. Casselton LA, Olesnicky NS. 1998. Molecular genetics of mating recognition in basidiomycete fungi. Microbiol. Mol. Biol. Rev. 62:55–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Debuchy R. 1999. Internuclear recognition: a possible connection between euascomycetes and homobasidiomycetes. Fungal Genet. Biol. 27:218–223 [DOI] [PubMed] [Google Scholar]
- 6. De Vries L, Zheng B, Fischer T, Elenko E, Farquhar MG. 2000. The regulator of G protein signaling family. Annu. Rev. Pharmacol. Toxicol. 40:235–271 [DOI] [PubMed] [Google Scholar]
- 7. Dohlman HG, Apaniesk D, Chen Y, Song J, Nusskern D. 1995. Inhibition of G-protein signaling by dominant gain-of-function mutations in Sst2p, a pheromone desensitization factor in Saccharomyces cerevisiae. Mol. Biol. Cell 15:3635–3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dohlman HG, Song J, Ma D, Courchesne WE, Thorner J. 1996. Sst2, a negative regulator of pheromone signaling in the yeast Saccharomyces cerevisiae: expression, localization, and genetic interaction and physical association with Gpa1 (the G-protein alpha subunit). Mol. Cell. Biol. 16:5194–5209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Edgar R, Barrett T. 2006. NCBI GEO standards and services for microarray data. Nat. Biotechnol. 24:1471–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feng Y, Davis NG. 2000. Feedback phosphorylation of the yeast a-factor receptor requires activation of the downstream signaling pathway from G protein through mitogen-activated protein kinase. Mol. Cell. Biol. 20:563–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fischer R. 1999. Nuclear movement in filamentous fungi. FEMS Microbiol. Rev. 23:39–68 [DOI] [PubMed] [Google Scholar]
- 12. Fowler TJ, DeSimone SM, Mitton MF, Kurjan J, Raper CA. 1999. Multiple sex pheromones and receptors of a mushroom-producing fungus elicit mating in yeast. Mol. Biol. Cell 10:2559–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fowler TJ, Mitton MF. 2000. Scooter, a new active transposon in Schizophyllum commune, has disrupted two genes regulating signal transduction. Genetics 156:1585–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fowler TJ, Mitton MF, Raper CA. 1998. Gene mutations affecting specificity of pheromone/receptor mating interactions in Schizophyllum commune, p 130–134. In Griensven LJLDV, Nimegen JV. (ed), Proceedings of the Fourth Meeting on the Genetics and Cellular Biology of Basidiomycetes. Mushroom Experiment Station, Horst, The Netherlands [Google Scholar]
- 15. Fowler TJ, Mitton MF, Rees EI, Raper CA. 2004. Crossing the boundary between the B α and B β mating type loci in Schizophyllum commune. Fungal Genet. Biol. 41:89–101 [DOI] [PubMed] [Google Scholar]
- 16. Fowler TJ, Mitton MF, Vaillancourt LJ, Raper CA. 2001. Changes in mate recognition through alterations of pheromones and receptors in the multisexual mushroom fungus Schizophyllum commune. Genetics 158:1491–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fuchs U, Hause G, Schuchardt I, Steinberg G. 2006. Endocytosis is essential for pathogenic development in the corn smut fungus Ustilago maydis. Plant Cell 18:2066–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gentleman R, Carey V, Huber W, Irizarry R, Dudoit S. 2005. Bioinformatics and computational biology solutions using R and Bioconductor. In Statistics for biology and health, 1st ed. Springer, New York, NY [Google Scholar]
- 19. Gola S, Hegner J, Kothe E. 2000. Chimeric pheromone receptors in the basidiomycete Schizophyllum commune. Fungal Genet. Biol. 30:191–196 [DOI] [PubMed] [Google Scholar]
- 20. Gola S, Kothe E. 2003. The little difference: in vivo analysis of pheromone discrimination in Schizophyllum commune. Curr. Genet. 42:276–283 [DOI] [PubMed] [Google Scholar]
- 21. Gorfer M, et al. 2001. Characterization of small GTPases Cdc42 and Rac and the relationship between Cdc42 and actin cytoskeleton in vegetative and ectomycorrhizal hyphae of Suillus bovinus. Mol. Plant Microbe Interact. 14:135–144 [DOI] [PubMed] [Google Scholar]
- 22. Hegner J, Siebert-Bartholmei C, Kothe E. 1999. Ligand recognition in multiallelic pheromone receptors from the basidiomycete Schizophyllum commune studied in yeast. Fungal Genet. Biol. 26:190–197 [DOI] [PubMed] [Google Scholar]
- 23. Inada K, Morimoto Y, Arima T, Murata Y, Kamada T. 2001. The clp1 gene of the mushroom Coprinus cinereus is essential for A-regulated sexual development. Genetics 157:133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iwasa M, Tanabe S, Kamada T. 1998. The two nuclei in the dikaryon of the homobasidiomycete Coprinus cinereus change position after each conjugate division. Fungal Genet. Biol. 23:110–116 [DOI] [PubMed] [Google Scholar]
- 25. Kniep H. 1920. Über morphologische und physiologische Geschlechtsdifferenzierung: Untersuchungen an Basidiomyzeten. Verh. Phys.-Med. Gesell. 40:1–18 [Google Scholar]
- 26. Koltin Y, Flexer AS. 1969. Alteration of nuclear distribution in B-mutant strains of Schizophyllum commune. J. Cell Sci. 4:739–749 [DOI] [PubMed] [Google Scholar]
- 27. Koltin Y, Raper JR, Simchen G. 1967. Genetic structure of incompatibility factors of Schizophyllum commune—the B-factor. Proc. Natl. Acad. Sci. U. S. A. 57:55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Konopka JB, Jenness DD, Hartwell LH. 1988. The C-terminus of the S. cerevisiae α pheromone receptor mediates an adaptive response to pheromone. Cell 54:609–620 [DOI] [PubMed] [Google Scholar]
- 29. Kothe E. 1996. Tetrapolar fungal mating types: sexes by the thousands. FEMS Microbiol. Rev. 18:65–87 [DOI] [PubMed] [Google Scholar]
- 30. Kothe E. 2001. Mating-type genes for basidiomycete strain improvement in mushroom farming. Appl. Microbiol. Biotechnol. 56:602–612 [DOI] [PubMed] [Google Scholar]
- 31. Kothe E. 2008. Sexual attraction: on the role of fungal pheromone/receptor systems (a review). Acta Microbiol. Immunol. Hung. 55:125–143 [DOI] [PubMed] [Google Scholar]
- 32. Kronstad JW, Staben C. 1997. Mating type in filamentous fungi. Annu. Rev. Genet. 31:245–276 [DOI] [PubMed] [Google Scholar]
- 33. Kurjan J. 1993. The pheromone response pathway in Saccharomyces cerevisiae. Annu. Rev. Genet. 27:147–179 [DOI] [PubMed] [Google Scholar]
- 34. Moore TI, Chou CS, Nie Q, Jeon NL, Yi TM. 2008. Robust spatial sensing of mating pheromone gradients by yeast cells. PLoS One 3:e3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nakayama N, Miyajima A, Arai K. 1985. Nucleotide sequences of STE2 and STE3 cell type specific sterile genes from Saccharomyces cerevisiae. EMBO J. 4:2643–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Niederpruem DJ. 1980. Direct studies of dikaryotization in Schizophyllum commune. 1. Live inter-cellular nuclear migration patterns. Arch. Microbiol. 128:162–171 [DOI] [PubMed] [Google Scholar]
- 37. Niederpruem DJ. 1980. Direct studies of dikaryotization in Schizophyllum commune. 2. Behavior and fate of multikaryotic hyphae. Arch. Microbiol. 128:172–178 [DOI] [PubMed] [Google Scholar]
- 38. Niederpruem DJ. 1969. Direct studies of nuclear movements in Schizophyllum commune. Arch. Mikrobiol. 64:387–395 [DOI] [PubMed] [Google Scholar]
- 39. Niederpruem DJ, Jersild RA, Lane PL. 1971. Direct microscopic studies of clamp connection formation in growing hyphae of Schizophyllum commune. I. The dikaryon. Arch. Microbiol. 78:268–280 [DOI] [PubMed] [Google Scholar]
- 40. Niederpruem DJ, Wessels JGH. 1969. Cytodifferentiation and morphogenesis in Schizophyllum commune. Bacteriol. Rev. 33:505–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ohm RA, et al. 2010. Genome sequence of the model mushroom Schizophyllum commune. Nat. Biotechnol. 28:957–963 [DOI] [PubMed] [Google Scholar]
- 42. Palmer GE, Horton JS. 2006. Mushrooms by magic: making connections between signal transduction and fruiting body development in the basidiomycete fungus Schizophyllum commune. FEMS Microbiol. Lett. 262:1–8 [DOI] [PubMed] [Google Scholar]
- 43. Papazian HP. 1950. Physiology of the incompatibility factors in Schizophyllum commune. Bot. Gaz. 112:143–163 [Google Scholar]
- 44. Parag Y. 1962. Mutations in the B incompatibility factor of Schizophyllum commune. Genetics 48:743–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pfaffl MW. 2004. Real-time RT-PCR: Neue Ansätze zur exakten mRNA Quantifizierung. BIOspektrum 1:92–95 [Google Scholar]
- 46. Raper CA, Raper JR. 1966. Mutations modifying sexual morphogenesis in Schizophyllum. Genetics 54:1151–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Raper J. 1966. Sexuality of higher fungi. The Roland Press, New York, NY [Google Scholar]
- 48. Raper JR, Antonio JPS. 1954. Heterokaryotic mutagenesis in hymenomycetes. 1. Heterokaryosis in Schizophyllum commune. Am. J. Bot. 41:69–86 [Google Scholar]
- 49. Raper JR, Baxter MG, Ellingboe AH. 1960. The genetic structure of the incompatibility factors of Schizophyllum commune—the A-factor. Proc. Natl. Acad. Sci. U. S. A. 46:833–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Raper JR, Hoffman RM. 1974. Schizophyllum commune, p 597–626. In King RC. (ed), Handbook of genetics. Plenum Press, New York, NY [Google Scholar]
- 51. Raper JR, Krongelb GS, Baxter MG. 1958. The number and distribution of incompatibility factors in Schizophyllum. Am. Nat. 92:221–232 [Google Scholar]
- 52. Raper JR, Raper CA. 1973. Incompatibility factors—regulatory genes for sexual morphogenesis in higher fungi. Brookhaven Symp. Biol. 25:19–39 [Google Scholar]
- 53. Raper JR, Raudaskoski M. 1968. Secondary mutations at Bβ incompatibility locus of Schizophyllum. Heredity 23:109–117 [Google Scholar]
- 54. Raudaskoski M. 1998. The relationship between B-mating type genes and nuclear migration in Schizophyllum commune. Fungal Genet. Biol. 24:207–227 [DOI] [PubMed] [Google Scholar]
- 55. Raudaskoski M, Kothe E. 2010. Basidiomycete mating type genes and pheromone signaling. Eukaryot. Cell 9:847–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Reneke JE, Blumer KJ, Courchesne WE, Thorner J. 1988. The carboxy-terminal segment of the yeast α-factor receptor is a regulatory domain. Cell 55:221–234 [DOI] [PubMed] [Google Scholar]
- 57. Roth AF, Sullivan DM, Davis NG. 1998. A large PEST-like sequence directs the ubiquitination, endocytosis, and vacuolar degradation of the yeast a-factor receptor. J. Cell Biol. 142:949–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Scherer M, Heimel K, Starke V, Kamper J. 2006. The Clp1 protein is required for clamp formation and pathogenic development of Ustilago maydis. Plant Cell 18:2388–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schubert D, Raudaskoski M, Knabe N, Kothe E. 2006. Ras GTPase-activating protein Gap1 of the homobasidiomycete Schizophyllum commune regulates hyphal growth orientation and sexual development. Eukaryot. Cell 5:683–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schuurs TA, Dalstra HJ, Scheer JM, Wessels JG. 1998. Positioning of nuclei in the secondary mycelium of Schizophyllum commune in relation to differential gene expression. Fungal Genet. Biol. 23:150–161 [DOI] [PubMed] [Google Scholar]
- 61. Schwalb MN, Miles PG. 1967. Morphogenesis of Schizophyllum commune. I. Morphological variation and mating behavior of the thin mutation. Am. J. Bot. 54:440–446 [Google Scholar]
- 62. Schwalb MN, Miles PG. 1967. Morphogenesis of Schizophyllum commune. II. Effect of microaerobic growth. Mycologia 59:610–622 [PubMed] [Google Scholar]
- 63. Specht CA. 1995. Isolation of the Bα and Bβ mating type loci of Schizophyllum commune. Curr. Genet. 28:374–379 [DOI] [PubMed] [Google Scholar]
- 64. Spellig T, Bölker M, Lottspeich F, Frank RW, Kahmann R. 1994. Pheromones trigger filamentous growth in Ustilago maydis. EMBO J. 13:1620–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stamberg J, Koltin Y. 1974. Recombinational analysis of mutations at an incompatibility locus of Schizophyllum. Mol. Gen. Genet. 135:45–50 [Google Scholar]
- 66. Stankis MM, et al. 1992. The Aα mating locus of Schizophyllum commune encodes two dissimilar multiallelic homeodomain proteins. Proc. Natl. Acad. Sci. U. S. A. 89:7169–7173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vaillancourt LJ, Raudaskoski M, Specht CA, Raper CA. 1997. Multiple genes encoding pheromones and a pheromone receptor define the Bβ 1 mating type specificity in Schizophyllum commune. Genetics 146:541–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vandesompele J, et al. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wendland J, Kothe E. 1996. Allelic divergence at Bα 1 pheromone receptor genes of Schizophyllum commune. FEMS Microbiol. Lett. 145:451–455 [DOI] [PubMed] [Google Scholar]
- 70. Xue C, Hsueh YP, Heitman J. 2008. Magnificent seven: roles of G protein-coupled receptors in extracellular sensing in fungi. FEMS Microbiol. Rev. 32:1010–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.