Abstract
Establishment and maintenance of cell polarity in eukaryotes depends upon the regulation of Rho GTPases. In Saccharomyces cerevisiae, the Rho GTPase activating protein (RhoGAP) Rgd1p stimulates the GTPase activities of Rho3p and Rho4p, which are involved in bud growth and cytokinesis, respectively. Consistent with the distribution of Rho3p and Rho4p, Rgd1p is found mostly in areas of polarized growth during cell cycle progression. Rgd1p was mislocalized in mutants specifically altered for Golgi apparatus-based phosphatidylinositol 4-P [PtdIns(4)P] synthesis and for PtdIns(4,5)P2 production at the plasma membrane. Analysis of Rgd1p distribution in different membrane-trafficking mutants suggested that Rgd1p was delivered to growth sites via the secretory pathway. Rgd1p may associate with post-Golgi vesicles by binding to PtdIns(4)P and then be transported by secretory vesicles to the plasma membrane. In agreement, we show that Rgd1p coimmunoprecipitated and localized with markers specific to secretory vesicles and cofractionated with a plasma membrane marker. Moreover, in vivo imaging revealed that Rgd1p was transported in an anterograde manner from the mother cell to the daughter cell in a vectoral manner. Our data indicate that secretory vesicles are involved in the delivery of RhoGAP Rgd1p to the bud tip and bud neck.
INTRODUCTION
Cell polarity is an essential phenomenon in eukaryotes and is necessary for cell division, morphogenesis, and motility (51). Polarized growth requires the delivery of material to a specific cellular site and is determined by dynamic control of the cytoskeleton and trafficking (12). Many factors control cell polarity; however, Rho proteins belonging to the Ras superfamily have emerged as key polarity determinants (4, 25). Members of the Rho family are involved in actin cytoskeleton polarization, intracellular trafficking, cytokinesis, and cell cycle control (31). Misfunction of Rho proteins can result in cell cycle abnormalities that contribute to tumor progression (36). In addition, defects in Rho activity result in aberrant synaptic secretion, altered dendritic morphology, and mental retardation (20, 77). Rho GTPases usually exist in two states: an active GTP-bound form anchored in the membrane and an inactive GDP-bound form. GTPases cycle between these two states in response to various stimuli, a property that has led to the notion of Rho GTPases as “molecular switches.” Switching between the active and inactive forms is regulated by two families of regulatory proteins: the RhoGEFs (Rho guanine nucleotide exchange factors), which facilitate the exchange of GDP by GTP and thus activate the GTPase, and RhoGAPs (Rho GTPase-activating proteins), which negatively regulate the Rho proteins by stimulating the intrinsic activity of GTP hydrolysis by Rho GTPases.
In the yeast Saccharomyces cerevisiae, polarized growth is initiated when a new cell, called the bud, emerges from a mother cell. Following bud emergence, the bud continues to grow in a polarized fashion by apical extension at the bud tip. After spindle pole body duplication, cytoplasmic microtubules orient the nucleus, which is pulled into the bud. During cytokinesis, an actomyosin ring is generated at the bud neck to allow separation of the daughter cell from its mother (67, 84). Transport of secretory vesicles and many intracellular organelles is dependent on the presence of polarized actin cables that radiate from the bud cortex and the neck, extending into the mother cell (29, 57, 58). In S. cerevisiae, six Rho proteins have been identified: Cdc42p and Rho1p through Rho5p. Previously, we demonstrated that Rho3p and Rho4p are regulated by the same RhoGAP, Rgd1p (18). Rho3p is involved in actin cytoskeleton organization by activating the formin Bni1p, which nucleates actin filaments, thus directing the assembly of actin cables (19). Rho3p also acts in the late steps of exocytosis by controlling vesicle docking at the bud tip through an interaction with the exocyst subunit Exo70p (60). Rho3p was localized to the growing bud by immunofluorescence microscopy (60, 82), and we corroborated this observation after tagging Rho3p with green fluorescent protein (GFP). The Rho4p GTPase controls actomyosin ring formation via interaction with the formin Bnr1p and regulates the interaction between Bnr1p and Hof1p, two proteins involved in cytokinesis in a GTP-Rho4p-dependent manner (35). Consistently, Rho4p was localized at the bud neck (74).
To investigate RhoGAP signaling to these GTPases, it is important to know the elements modulating Rgd1p function. When using GFP-tagged Rgd1p, a dynamic localization of Rgd1p was observed during different phases of the cell cycle, related to the cell localization of Rho3p and Rho4p. During G1 and S phases, Rgd1p is localized at the bud tip, and it is localized at the bud neck during M phase (41, 56). In addition, some phosphoinositides can affect the cellular distribution as well as the RhoGAP activity of Rgd1p in S. cerevisiae (56). We have demonstrated a role for phosphatidylinositol 4-P [PtdIns(4)P] and PtdIns(4,5)P2 in the apical localization of Rgd1p (56). The localization of Rgd1p-3×GFP was abnormal in the pik1ts-83 mutant, while this localization was not affected by inactivation of the gene encoding the other PtdIns 4-kinase, Stt4p. Both PtdIns 4-kinases have a distinct function and distinct localization in yeast. Pik1p is localized within the nucleus and at the Golgi apparatus (5), while Stt4p localizes to the plasma membrane (16, 23). Thus, while two pools of PtdIns(4)P are synthesized in yeast (80), the pool derived from Pik1p is essential for normal secretion from the Golgi apparatus (5). Rgd1p is also drastically mislocalized in the mss4-102 mutant, which has a very low level of PtdIns(4,5)P2 in the plasma membrane, indicating that PtdIns(4,5)P2 is essential for the correct localization of Rgd1p at the bud tip. These data led us to propose that Rgd1p could be delivered to the plasma membrane via intracellular trafficking by interacting with PtdIns(4)P present on the cytosolic face of secretory vesicles (41, 56). In this work, we demonstrate the association of the RhoGAP Rgd1p with post-Golgi secretory vesicles and the involvement of the secretory pathway in the proper localization of the RhoGAP to sites of polarized growth.
MATERIALS AND METHODS
Yeast strains and media.
The S. cerevisiae strains used in this study are listed in Table 1. Standard techniques were used, and the composition of rich (YPD) and synthetic (SC) media for yeast growth has been reported elsewhere (61). Yeast strains were usually grown at 30°C, with the exception of temperature-sensitive mutants. The temperature-sensitive (sec4-2, sec6-4, sec14-1, and sec61-3) strains and the respective control strains were grown overnight at the permissive temperature (26°C) to an optical density at 600 nm (OD600) of 0.2. The strains were shifted to the nonpermissive temperature (38°C) for 3 h, and cells were observed by fluorescence microscopy. The viability of the cells shifted to the nonpermissive temperature was assessed by methylene blue staining (17).
Table 1.
Yeast strains used in this study
| Strain and/or description | Genotype | Source or reference |
|---|---|---|
| BY4742 | MATα his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 | Euroscarf |
| vps34Δ mutant | BY4742, vps34::kanMX4 | Euroscarf |
| fab1Δ mutant | BY4742, fab1::kanMX4 | Euroscarf |
| sec22Δ mutant | BY4742, sec22::kanMX4 | Euroscarf |
| erd1Δ mutant | BY4742, erd1::kanMX4 | Euroscarf |
| end3Δ mutant | BY4742, end3::kanMX4 | Euroscarf |
| CTY-182 | MATaura3-52 his3Δ200 lys2-801 SEC14 | 7 |
| CTY-1A | MATaura3-52 his3Δ200 lys2-801 sec14-1 | 7 |
| X2180-1A | MATamal mel gal2 CUP1 SUC2 | 48 |
| sec4-2 mutant | MATamal mel gal2 CUP1 SUC2 ura3-52 sec4-2 | 48 |
| sec6-4 mutant | MATamal mel gal2 CUP1 SUC2 ura3-52 sec6-4 | 48 |
| RSY257 | MATα leu2-3,112 ura3-52 SEC61 | 69 |
| sec61-3 mutant | MATα leu2-3,112 ura3-52 trp1-1 sec61-3 | 69 |
| BY4742, Rgd1p-6×HA | BY4742, RGD1::RGD1-6×HA | This work |
| BY4742, Rgd1p-3×GFP, Sec2p-tdimer | BY4742, RGD1::RGD1-3×GFP SEC2::SEC2-tdimer(2)12 | This work |
| sec6-4 mutant; Rgd1p-6×HA, GFP-Sec4p (7165) | MATasec6-4 RGD1::RGD1-6×HA pUG36-GFP-SEC4 (URA3) | This work |
| sec6-4 mutant; GFP-Sec4p (7171) | MATasec6-4 pUG36-GFP-SEC4 (URA3) | This work |
| sec6-4 mutant; Rgd1p-6×HA (7161) | MATasec6-4 RGD1::RGD1-6×HA | This work |
| SEY6210 | MATaleu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 | 5 |
| mss4-102 mutant | SEY6210, mss4::HIS3 with pYCplac111-mss4-102ts (LEU2) | 68 |
Plasmid constructs.
For the localization of Rgd1p in vivo, we generated various constructs tagged at the C terminus with green fluorescent protein. As previously described, we used, depending on the selection marker, the integrative yeast vectors pRS304-, pRS305-, and pRS306-3×GFP, which contain three tandem copies of the GFP gene (56). This construct was integrated at the RGD1 locus in phosphatidylinositol kinase and secretory pathway mutants and in control strains. An 800-bp fragment of RGD1 preceding the stop codon was cloned into different plasmids, pRS304-, pRS305-, and pRS306-3×GFP, and targeted for integration at the RGD1 locus. The resulting RGD1 plasmids were verified by sequencing, while integration at the RGD1 locus was checked by PCR. Using the sensitivity of rgd1Δ mutant cells to low pH (21), we also verified the functionality of the GFP-tagged construct. Adding 3× GFP to the carboxy terminus of Rgd1p in control strains did not change the functional behavior compared with that of the untagged protein, and full-length tagged Rgd1p rescued the rgd1Δ phenotype.
Sec2p was tagged at the C terminus with a red variant of DsRed, using the integrative yeast vector pRS306-tdimer2 (12) (generously provided by Isabelle Sagot). We inserted the last 1,200 bp from SEC2 before the stop codon into this plasmid and targeted integration of the construct to the SEC2 locus.
For subcellular fractionation and immunoprecipitation of secretory vesicles, Rgd1p was tagged with 6× hemagglutinin (HA) at the C terminus using pYM14 from the PCR Toolbox kit (32). The GFP-Sec4p protein used for immunoprecipitation of secretory vesicles was produced from the pUG36-GFP-Sec4 plasmid (15). The GFP-SEC4 construct was expressed from the MET25 promoter.
Sucrose gradient fractionation.
A BY4742 strain expressing Rgd1p tagged with 6× HA was grown at 30°C in YPD medium up to an OD600 of 0.6. Cell lysate preparation and sucrose gradient fractionation were performed as previously described (10). Briefly, cells were harvested, spheroplasted, and broken by Dounce homogenization in lysis buffer (20 mM triethanolamine [pH 7.2], 0.8 M sorbitol, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride [PMSF] supplemented with a cocktail of protease inhibitors [Sigma-Aldrich]), and remnants were discarded by centrifugation at 500 × g for 5 min. The pellet obtained after 10 min of centrifugation at 13,000 × g was resuspended in 2 ml 55% sucrose, 10 mM morpholineethanesulfonic acid (MES) (pH 6.5) and loaded at the bottom of a 30 to 55% sucrose gradient. The gradient was centrifuged for 16 h at 170,000 × g, 4°C (TH-641 rotor; Sorvall). After spinning at equilibrium, the gradient was aliquoted into 20 fractions of 500 μl. Fraction 1 corresponded to the gradient pellet, and fraction 20 was the top of the gradient. Rgd1p was detected in fractions by Western blotting with anti-HA antibodies. To monitor the different cellular compartments in the sucrose gradient, we tested the vanadate-sensitive ATPase activity of Pma1p, which is specific to the plasma membrane (9, 10), a Ca2+-dependent GDPase activity specific for the cis-Golgi network (1), and a cytochrome c reductase activity specific to the endoplasmic reticulum (ER) (10).
Immunoprecipitation of post-Golgi secretory vesicles.
The first part of this protocol is derived from the work of Klemm et al., (38), maximizing the preservation of intact secretory vesicles. The sec6-4 strain expressing the GFP-Sec4p and Rgd1p-6×HA proteins (7165) or GFP-Sec4p alone (7171) was cultured at 25°C in 500 ml SC medium minus uracil, and the sec6-4 strain expressing only Rgd1p-6×HA (7161) was cultured on SC medium to 1 OD600. For simultaneous GFP-Sec4p expression and vesicle accumulation, cells from the 7165 and 7171 strains were shifted into 250 ml SC medium minus uracil and methionine at 38°C for 45 min; the 7161 strain was shifted into SC medium minus methionine. To stop membrane trafficking after 45 min of incubation, the culture was supplemented with 10 mM NaN3 and incubated at 38°C for 20 min. Cells were harvested and resuspended in 3 ml lysis buffer (0.8 M sorbitol, 1 mM EDTA, 10 mM triethanolamine [pH 7.4], 1 mM PMSF, 10 mM NaN3, and a cocktail of protease inhibitors [Sigma-Aldrich]). An equivalent volume of glass beads (diameter, 0.45 mm; Biospec Products) was added, and cells were broken by vortexing. The supernatant of a centrifugation at 2,000 × g for 10 min at 4°C was collected and centrifuged for a further 30 min at 20,000 × g, at 4°C. One milliliter of 20,000 × g supernatant (S20) was used for immunoprecipitation of secretory vesicles with an anti-GFP antibody (sc-9996 from Santa Cruz Technology, Inc.) and magnetic beads coupled to protein G (Dynabeads, Invitrogen). The immunoprecipitate was washed three times with lysis buffer supplemented with 100 mM NaCl and released with 50 μl of Laemmli buffer, concentrating the extract 20-fold compared to S20. GFP-Sec4p, Rgd1p-6×HA, Pgk1p, and Snc1p/Snc2p were detected using anti-GFP, anti-HA (12CA5), anti-Pgk1p (22C5D8; Invitrogen), and anti-Snc1p/Snc2p (a generous gift from P. Brennwald) antibodies, respectively.
Fluorescence microscopy.
Cells were grown in synthetic complete medium (SC) minus the appropriate metabolites. Aliquots of exponentially growing yeast cells (0.2 to 0.4 OD600 unit) were centrifuged, and the pellet was resuspended in DABCO solution (218 nM diazabicyclo-2-2-2 octane [Sigma], 25% [vol/vol] phosphate-buffered saline buffer, and 75% [vol/vol] glycerol). Rgd1p localization was observed in cells by using an epifluorescence microscope (Zeiss AxioSkop 2 plus, with a 100× oil objective and a GFP band-pass filter).
Colocalization between Sec2p-tdimer2 (12) and Rgd1p-3×GFP was tested using an imaging system comprising an Axiovert 200M (Zeiss) microscope chassis with a 100× (1.4-numerical-aperture) Plan Apochromat oil objective, an Evolve electron-multiplying charge-coupled device (EM-CCD) camera (Photometrics), and a dual-view DV2 beam-splitter (Photometrics) with appropriate filters to enable the simultaneous acquisition of GFP and tdimer images. GFP and tdimer were illuminated using a mercury lamp. The system was controlled by MetaMorph software (Molecular Devices). The data presented in Fig. 5B and C were generated by acquiring images in a single z plane at 30-ms intervals.
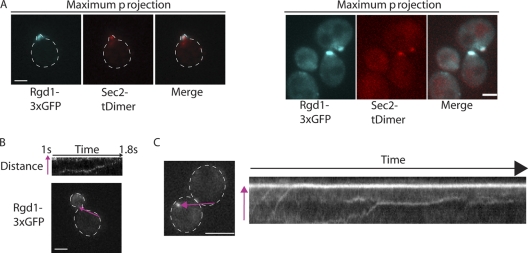
Fig 5.
Colocalization of Rgd1p with Sec2p, a secretory vesicle marker, and Rgd1p dynamics. Rgd1p-3×GFP colocalizes with a subset of Sec2p-tdimer2, a post-Golgi vesicle marker. Images show maximum projections of separate z planes. Scale bars, 2 μm. (B and C) Rgd1p-3×GFP localized to puncta that were transported to growth sites in a vectoral manner. The movement of Rgd1p-3×GFP is presented as a kymograph (top panel [B] and right panel [C]). To generate the kymograph, a line was drawn along the path that an Rgd1p-3×GFP punctum followed during a movie. At each time point of the movie, the fluorescence along the line was projected vertically in the image. The line used to generate the kymograph is shown in purple in the bottom panel (B) and left panel (C), while the direction of the vesicle movement is shown by an arrowhead. In the kymograph showing Rgd1p dynamics in a large budded cell (C), two Rgd1p puncta near the beginning of the movie move toward the polarized Rgd1p patch. Then, a third punctum moves to the bud neck, pauses, and then continues its movement.
RESULTS
PtdIns(3)P and PtdIns(3,5)P2 have no influence in Rgd1p distribution.
Given the localization defects of Rgd1p in pik1 and mss4 mutants, we proposed that Rgd1p initially binds to PtdIns(4)P on the cytosolic face of Golgi membranes before being transported via the secretory pathway to the plasma membrane, where Rgd1p would interact with PtdIns(4,5)P2 (56). However, Rgd1p was also shown to interact in vitro with other phosphoinositides [PtdIns(3)P, PtdIns(5)P, PtdIns(3,5)P2, PtdIns(3,4)P2, and PtdIns(3,4,5)P3] via its F-BAR domain (56). To determine whether the interaction between Rgd1p and phosphoinositides is functionally important in vivo, we examined the localization of Rgd1p in mutants altered for phosphoinositides biosynthesis. Since PtdIns(5)P, PtdIns(3,4)P2, and PtdIns(3,4,5)P3 have not been detected in S. cerevisiae (71), we studied Rgd1p localization in mutants defective in PtdIns(3)P and PtdIns(3,5)P2 synthesis.
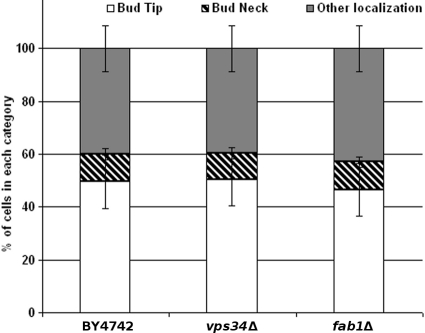
While PtdIns(4)P and PtdIns(4,5)P2 phospholipids are involved in anterograde transport from the Golgi apparatus to the plasma membrane (71, 84), PtdIns(3)P and PtdIns(3,5)P2 are involved in endocytosis, recycling, and degradation in the vacuole (13, 39). The localization of Rgd1p-3×GFP was tested in the vps34Δ and fab1Δ mutant strains. The VPS34 gene encodes the only PtdIns 3-kinase, and the FAB1 gene encodes the only yeast PtdIns(3)P-5 kinase in the budding yeast. The localization of Rgd1p was not defective in these mutants compared to that in a control strain (Fig. 1). Thus, PtdIns(3)P and PtdIns(3,5)P2 do not appear to play a role in Rgd1p localization. This observation suggested that neither the endocytic pathway nor vacuolar trafficking is required for Rgd1p localization. In agreement with this interpretation, localization of Rgd1p-3×GFP was not affected in the end3Δ mutant (Fig. 2). The End3p protein interacts with Las17p, an activator of the Arp2/3p complex, which is required for normal actin patch dynamics; End3p promotes early endocytic events associated with actin patch function (34). Together, these results indicate that only PtdIns(4)P synthesized by Pik1p at the Golgi apparatus and PtdIns(4,5)P2 synthesized by Mss4p at the plasma membrane have a role in Rgd1p localization.
Fig 1.
Rgd1p distribution in mutants impaired in the biosynthesis of PtdIns(3)P and PtdIns(3,5)P2. The control strain (BY4742) and mutant (vps34Δ and fab1Δ) cells expressing Rgd1p-3×GFP were cultured at 30°C up to 0.6 OD600, and Rgd1p localization was examined by GFP fluorescence. The vps34Δ mutant has smaller pools of PtdIns(3)P, whereas the amount of PtdIns(3,5)P2 is much smaller in the fab1Δ mutant than in a wild-type strain. Cells are assigned to three categories according to Rgd1p distribution: “bud tip,” “bud neck,” or “other localization” (not present in either the bud tip or the bud neck). For each strain, three independent clones were observed, and 100 or more cells were counted for each clone. Bars indicate the standard deviation of three measurements for each strain.
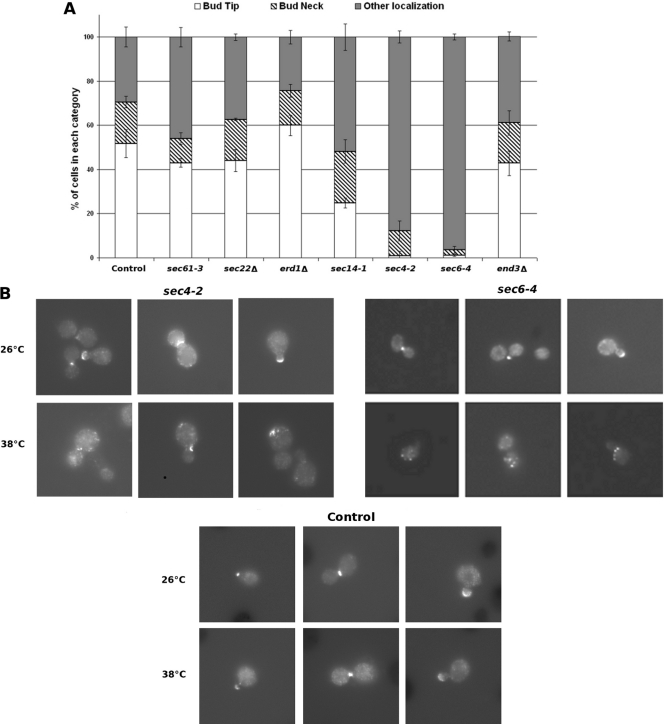
Fig 2.
Rgd1p distribution in mutants impaired in different trafficking steps. The sec mutants, the end3Δ and erd1Δ mutants, and the respective control strains were tagged with a Rgd1p-3×GFP construct integrated at the RGD1 locus. sec61-3, sec14-1, sec4-2, and sec6-4 cells and a control strain were grown on selective SC at the permissive temperature of 26°C and then shifted to the restrictive temperature of 38°C for 3 h. sec22Δ, erd1Δ, and end3Δ cells were grown at 30°C. (A) Cells are assigned to three categories according to Rgd1p distribution: “bud tip,” “bud neck,” or “other localization.” The category “other localization”' was defined by cells with several spots not present in either the bud tip or the bud neck. For each strain, three independent clones were observed and 100 or more cells were counted for each clone. The control is representative of results obtained from the different control strains. Bars indicate the standard deviations of three measurements for each strain. (B) Localization of Rgd1p in mutants for late steps of the secretory pathway. sec4-2 and sec6-4 mutants, as well as the control strain, were cultured at the permissive temperature (26°C) up to a cell density of 0.5 OD600. One sample was shifted to 38°C for 3 h. Rgd1p-3×GFP distribution was observed by fluorescence microscopy. The images show Rgd1p mislocalization in mutant cells grown at restrictive temperature.
Involvement of the secretory pathway in Rgd1p localization.
To investigate the role of secretion in Rgd1p localization, we used mutants blocked at different steps in the secretory pathway. Testing the localization of Rgd1p in secretory pathway mutants blocked at different steps of secretion may identify the trafficking route followed by Rgd1p. We selected mutants with the following genotypes: sec61-3 (endoplasmic reticulum block [(81]), sec22Δ (ER-to-Golgi apparatus block [55]), erd1Δ (Golgi apparatus-to-ER block [26]), sec14-1 (Golgi apparatus-to-plasma-membrane block [(47]), and sec4-2 and sec6-4. The sec4-2 and sec6-4 mutants are defective in exocytosis and accumulate exocytic vesicles in the bud at the nonpermissive temperature (60, 79).
The distribution of Rgd1p-3×GFP was analyzed in these mutants by fluorescence microscopy (Fig. 2). Rgd1p-3×GFP was expressed from the RGD1 locus in the sec61-3, sec22Δ, erd1Δ, sec14-1, sec4-2, and sec6-4 mutants and in the respective control strains. The sec22Δ and erd1Δ deletion mutants were examined after culture at 30°C. The temperature-sensitive mutants (sec61-3, sec14-1, sec4-2, and sec6-4) were grown at permissive temperature (26°C) and shifted for 3 h to the nonpermissive temperature (38°C). The thermosensitive mutants displayed normal Rgd1p localization at the permissive temperature (see Fig. 2B). Cell microscopic examination in mutant strains revealed Rgd1p fluorescence levels comparable to those observed in the control strain. Cell mortality of temperature-sensitive (ts) mutants was determined at the restrictive temperature and was below 5%; under the same conditions, 2% of control cells died. This very low mortality rate ruled out the possibility of a defect in cell viability accounting for the different distribution of Rgd1p in temperature-sensitive secretory pathway mutants.
The sec22Δ and erd1Δ mutants and the sec61-3 mutant grown at the restrictive temperature showed a distribution of Rgd1p-3×GFP comparable to that of control strains. In contrast, inactivation of the sec14-1, sec4-2, and sec6-4 strains after shifting to nonpermissive temperature resulted in major changes in Rgd1p distribution. In the sec14-1 mutant, the proportion of cells with Rgd1p-3×GFP localized at the bud tip decreased to 25% ± 3%, while the proportion of cells with Rgd1p exhibiting other localization increased to about 53% ± 5% (Fig. 2A). Interestingly, the percentage of sec14-1 cells with Rgd1p located at the neck (23% ± 5%) was similar to that for the control (19% ± 2%).
In the sec4-2 mutant, the localization of Rgd1p-3×GFP at the bud tip was strongly disrupted (Fig. 2B). We observed that more than 85% of cells belonged to the class termed “other localization” (Fig. 2A). In this class, many intense spots spread throughout the cells were observed. The proportion of cells with Rgd1p localized at the bud neck seemed slightly lower (11% ± 5% of sec4-2 cells versus 19% ± 2% in the control strain). At 38°C, the sec6-4 mutation resulted in the complete depolarization of Rgd1p localization (Fig. 2). Very few cells showed polarized Rgd1p at the bud tip or neck. The Rgd1p level in sec4-2 and sec6-4 mutants after 3 h of transfer to the restrictive temperature was similar to that of the control as shown by Western blotting (see Fig. S1 in the supplemental material).
These data demonstrate a functional link between the secretory pathway and Rgd1p distribution in yeast cells. Our results show that the localization of Rgd1p was first disrupted from the step controlled by Sec14p at the trans-Golgi network to the last steps of the secretory pathway controlled by Sec4p and Sec6p. These results support the idea that Rgd1p requires ongoing post-Golgi trafficking for its transport to polarized growth sites, where the RhoGAP would interact with PtdIns(4,5)P2 synthesized by Mss4p. Yakir-Tamang and Gerst (83) described a significant reduction in the amount of PtdIns(4,5)P2 at the plasma membrane after transfer of a sec6-4 mutant at the restrictive temperature of 37°C for 1 h in synthetic medium. These data suggest that Rgd1p could be mislocalized due to a lack of binding to PtdIns(4,5)P2 following its unavailability at the plasma membrane. However, we found that a sec6-4 mutant expressing the same GFP-2×PH (PLCδ) probe (42) from the URA3 locus showed 53% of cells with membrane-localized PtdIns(4,5)P2after 3 h of incubation at the nonpermissive temperature (see Fig. S2 in the supplemental material). The difference in the reporter expression level could explain this difference in PtdIns(4,5)P2 distribution. Thus, the reduction in Rgd1p localization in the sec6-4 mutant is unlikely to be an indirect consequence of reduced PtdIns(4,5)P2 at the plasma membrane. Altogether, our results suggest that Rgd1p might be routed to the bud tip and bud neck by vesicles emanating from the trans-Golgi network; neither vesicle trafficking from the ER to the Golgi apparatus nor retrograde transport from the Golgi apparatus to the ER was required for Rgd1p localization.
Rgd1p is associated with the plasma membrane fraction.
We have highlighted the role of PtdIns(4)P synthesized at the Golgi apparatus and PtdIns(4,5)P2 synthesized at the plasma membrane in the apical localization of Rgd1p (56). We have also shown that the late steps of the secretory pathway play a crucial role in localizing Rgd1p. These data are consistent with dynamic transport of Rgd1p from the Golgi apparatus to the plasma membrane.
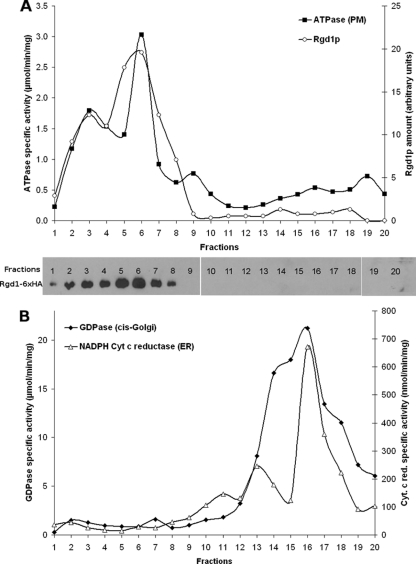
To biochemically explore Rgd1p localization during the secretion process, we fractionated yeast lysate on a density gradient and assayed fractions for activities specific to different compartments of the secretory pathway. Rgd1p was tagged with the 6× HA epitope at its C terminus, and the corresponding gene was integrated at its own locus in the control strain. The harvested cells were lysed, and after clarification of the lysate at 500 × g, the supernatant was centrifuged at 13,000 × g. The P13 pellet contains the plasma membrane, vacuole, mitochondria, ER, and cis-Golgi network; secretory vesicles and late Golgi vesicles are present in the supernatant (10, 59).
The P13 pellet was then centrifuged on a sucrose gradient (30 to 55%) at 100,000 × g for 16 h as previously reported (10). From each fraction, activities specific to different cellular compartments were tested; in parallel, an aliquot of each fraction was analyzed by Western blotting to detect Rgd1p-6×HA. The plasma membrane was followed by measuring the vanadate-sensitive ATPase activity of Pma1p, the cis-Golgi network was followed by measuring Ca2+ dependent GDPase activity, and the ER was followed by measuring cytochrome c reductase (10). The GDPase activity, which is characteristic of the cis-Golgi network, and cytochrome c reductase activity, specific to the ER, were present in the lowest-density fractions from the gradient, while Pma1p activity was recovered at the bottom of the gradient (Fig. 3). The peaks of GDPase and cytochrome c reductase activities were superimposed over the various experiments but distinct from those of Pma1p activity. The curve of vanadate-sensitive ATPase activity had a broad peak from fractions 2 to 9. Rgd1p was detected by Western blotting in fractions 1 to 8, with a shoulder corresponding to more dense biological elements (Fig. 3A). Moreover, Rgd1p was never detected in fractions corresponding to the ER and cis-Golgi compartments (Fig. 3B). Sucrose gradient analyses unambiguously demonstrated that Rgd1p was not associated with the ER and cis-Golgi network, two compartments involved in the first steps of the secretory pathway. Cofractionation of Rgd1p with dense fractions containing Pma1p activity is consistent with RhoGAP association with the plasma membrane.
Fig 3.
Fractionation of Rgd1p and organelle-specific enzyme marker activities. Exponentially growing BY4742 cells were lysed, and the resuspended 13,000 × g pellet was loaded at the bottom of a 30 to 55% sucrose gradient. The gradient was spun until equilibrium and fractionated into 20 fractions of 500 μl, and aliquots of each fraction were assayed for Pma1p ATPase, cytochrome c reductase, GDPase, protein concentration, and Rgd1p abundance. The quality of the gradient was verified by determining the sucrose density. Fraction 1 corresponded to the bottom, and fraction 20 was the top of the gradient. (A) Plasma membrane vanadate-sensitive Pma1p ATPase activity was determined, and Rgd1p was detected by Western blotting. ATPase activity was determined by measuring the release of inorganic phosphate for 10 min at 37°C, and the result was expressed as μmoles of liberated phosphate per min per mg of protein. Rgd1p was quantitated from Western blots by determining the relative intensity of the signal with the Fluor-S MAX Multimager apparatus (Bio-Rad), and the corresponding values are indicated in the figure. (B) Distribution of cytochrome c reductase and GDPase activities within the gradient. Cytochrome c reductase was measured as the rate of increase of absorbance at 550 nm and expressed in nmol of reduced cytochrome c per min per mg of protein. The GDPase activity was expressed as μmoles of liberated phosphate per min per mg of protein.
Rgd1p is associated with post-Golgi secretory vesicles.
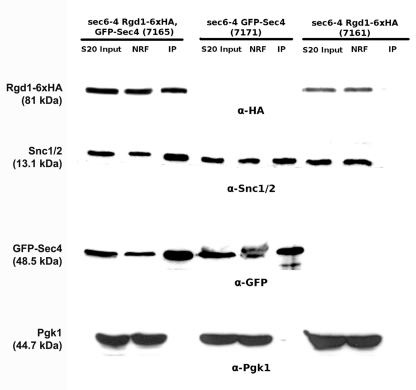
We hypothesized that Rgd1p could be transported from the Golgi apparatus to the plasma membrane on post-Golgi secretory vesicles. We therefore tested whether Rgd1p cofractionated with post-Golgi secretory vesicles. Relatively few secretory vesicles are found in wild-type cells, presumably because transport and fusion with the plasma membrane are rapid (49). We therefore tagged Rgd1p with 6× HA in the sec6-4 mutant, which accumulates many secretory vesicles when SEC6 is impaired (24, 27, 78). A strain (7165) with the sec6-4 mutation expressing Rgd1p-6×HA from its chromosomal locus and containing the pUG36-GFP-SEC4 plasmid expressing GFP-Sec4p from the MET25 promoter was constructed. These tagged proteins provided molecular markers for the RhoGAP and post-Golgi secretory vesicles (22, 78). Immunoprecipitation of GFP-Sec4p enabled the isolation of secretory vesicles. For this purpose, the 7165 strain was grown at the nonpermissive temperature (38°C) during 45 min in synthetic medium lacking uracil and methionine, conditions under which vesicles accumulate (38). Secretory vesicles were prepared using a previously established protocol (38) and immunoprecipitated from a supernatant at 20,000 × g (S20) using anti-GFP antibodies. Western blot analysis using anti-HA (Rgd1p) and anti-GFP (Sec4p) was performed with the S20 input, nonretained, and immunoprecipitated fractions (Fig. 4). This analysis revealed the presence of Rgd1p and Sec4p in all fractions. Coimmunoprecipitation of Rgd1p with Sec4p suggests a physical interaction between Rgd1p and secretory vesicles. Two control strains were used to validate the results from immunoprecipitation: a sec6-4 strain (7171) expressing only GFP-Sec4p (lacking Rgd1p-6×HA) and a sec6-4 strain (7161) expressing only Rgd1p-6×HA without GFP-Sec4p. Secretory vesicles were prepared from these control strains, immunoprecipitation was performed with anti-GFP antibodies, and Rgd1p and Sec4p were probed by Western blotting (Fig. 4). With the 7171 strain, no HA signal was detected in any fraction, indicating that the HA signal detected in the 7165 strain originated with Rgd1p-6×HA. For an unknown reason, the Rgd1p signal was slightly lower in the 7161 strain than in the 7165 strain. No GFP signal was observed in the 7161 strain, as expected, demonstrating the specificity of the GFP antibody for GFP-Sec4p. Finally, the specificity of Rgd1p binding to post-Golgi vesicles was checked following fractionation of the Pgk1p, Snc1p, and Snc2p proteins. The 3-phosphoglycerate kinase Pgk1p is a cytosolic protein, whereas Snc1p/Snc2p are vesicle membrane receptor proteins (v-SNAREs) involved in the fusion of Golgi apparatus-derived secretory vesicles and the plasma membrane (37). As expected, no anti-Pgk1p signal was detected in the fraction containing vesicles. Consistently, the Snc1p/Snc2p signal was not detected in the 7161 strain when secretory vesicles were not immunoprecipitated with GFP-Sec4p. Together these results demonstrate that the presence of Rgd1p in the immunoprecipitated fraction was linked to the presence of secretory vesicles and support the notion that this RhoGAP associates with vesicles.
Fig 4.
Detection of Rgd1p in a post-Golgi secretory vesicle fraction. Cells from strains 7165 (sec6-4, Rgd1p-6×HA, GFP-Sec4p), 7171 (sec6-4, GFP-Sec4p), and 7161 (sec6-4, Rgd1p-6×HA) were incubated and lysed with a buffer preserving the integrity of secretory vesicles. Post-Golgi secretory vesicles were immunoprecipitated from the 20,000 × g supernatant using anti-GFP antibody (see Materials and Methods). Ten microliters of the 20,000 × g (S20 Input) supernatant, nonretained fraction (NRF), and immunoprecipitated vesicles (IP) were analyzed by SDS-PAGE. The IP fraction was concentrated 20-fold compared to the S20 input (for details, see Materials and Methods). The different fractions were followed by Western blotting. Secretory vesicles were detected with anti-Snc1p/2p and anti-GFP antibodies; Rgd1p and Pgk1p were detected using anti-HA and anti-Pgk1p antibodies, respectively. This figure is representative of the results obtained from different experiments (n = 3).
Rgd1p colocalizes with a subset of the post-Golgi marker Sec2p and is transported to the bud tip in a vectoral manner.
By tagging Rgd1p with three copies of GFP in tandem, Rgd1p was present mostly in areas of polarized growth during the cell cycle (56). Rgd1p was found to be polarized at the cortex during G1 and at the bud tip during S and G2 phases. During isotropic bud growth, Rgd1p was localized to dense patches resembling intensely fluorescent crescents under the cortex at the bud tip.
We tested if Rgd1p colocalized with secretory vesicles. To do so, a strain was constructed in which Rgd1p was tagged with three copies of GFP and the post-Golgi marker Sec2p was tagged with the DsRed variant tdimer (2). Sec2p is the GEF for the Rab GTPase Sec4p and thus serves as a secretory vesicles marker (52). The localization of Rgd1p in this strain was comparable to that already observed (56); Rgd1p was polarized to growth sites in nearly 75% of cells. Simultaneous acquisition of Rgd1p-3×GFP and Sec2p-tdimer (2) revealed that Rgd1p-3×GFP colocalized with a subset of Sec2p-tdimer in the bud (Fig. 5A).
High-speed imaging was used to determine the temporal dynamics of Rgd1p-3×GFP. Rgd1p-3×GFP was imaged every 30 ms, and the protein moved in discrete puncta from the mother to the bud (Fig. 5B and C). Kymographs were then generated in which the horizontal axis shows time while the vertical axis shows the position along the purple line shown in Fig. 5B and C. Rgd1p-3×GFP moved at a velocity of around 1 μm per second from the mother to the bud, consistent with transport of the protein along actin cables (3). In the kymograph presented in Fig. 5C, we observed two Rgd1p puncta near the beginning of the movie moving toward the polarized Rgd1p patch. Subsequently, a third punctum moves to the bud neck, pauses, and then continues toward the bud tip.
DISCUSSION
Phosphoinositides PtdIns(4)P and PtdIns(4,5)P2 but not PtdIns(3)P and PtdIns(3,5)P2 influence Rgd1p localization in S. cerevisiae. A previous study demonstrated Rgd1p mislocalization in the pik1ts-83 mutant but not in the stt4ts-4 mutant, indicating the involvement of a specific Golgi pool of PtdIns(4)P in Rgd1p localization. This indicates that PtdIns(4)P itself is functionally relevant and is not important merely as a precursor of PtdIns(4,5)P2. Pik1p is associated with the trans-Golgi network (70), and PtdIns(4)P is enriched at the late Golgi compartment and the trans-Golgi network (64). The phenotype of a pik1 mutant is consistent with an essential role for PtdIns(4)P in trafficking from the Golgi apparatus to the plasma membrane in yeast (23, 43). It is thought that Pik1-generated PtdIns(4)P serves as a specific docking epitope on the Golgi membrane for recruitment of other proteins that are involved in vesicle formation and trafficking. For example, Sec2p, the GEF for Sec4p, binds PtdIns(4)P and is necessary for Sec2p localization (46). PtdIns(4,5)P2 is synthesized in the plasma membrane by the PtdIns(4)P 5-kinase encoded by MSS4; reduction of Mss4p activity also led to Rgd1p mislocalization. Altogether, these results suggest that the secretory pathway is necessary for the correct localization of Rgd1p to growth sites.
Consistent with our hypothesis, Rgd1p distribution was impaired in the sec14-1, sec4-2, and sec6-4 mutants, three secretory pathway mutants. In S. cerevisiae, Sec14p, a member of phosphatidylinositol/phosphatidylcholine transfer proteins, is essential for the biogenesis of secretory vesicles from the trans-Golgi network (6). Sec14p plays a role in Golgi function by acting on PtdIns(4)P synthesis, on maintenance of the di-acyl-glycerol pool, and on reduction of the phosphatidylcholine pool (23). Functional links between Sec14p and Pik1p have been proposed, and Sec14p may facilitate the presentation of PtdIns to Pik1p (8, 65). Given this functional connection, Rgd1p mislocalization in the sec14-1 mutant was expected and was in agreement with our previous data from the pik1ts-83 mutant. However, the number of cells with mislocalized Rgd1p at the bud tip was lower in the sec14-1 mutant than in the pik1 mutant. The phosphoinositide binding protein Kes1p was also reported to display a differential mislocalization in these mutants (43). This differential effect is consistent with the respective roles of Sec14p and Pik1p. Pik1p plays a major role in activation at the Golgi apparatus, whereas Sec14p facilitates Pik1p action (23).
Sec4p, a RabGTPase, and Sec6p, a component of the exocyst vesicle tethering complex, are two proteins involved in exocytosis. The presence of GTP-bound Sec4p on vesicles is necessary for subsequent vesicle tethering to specific sites on the plasma membrane (11, 44, 63). Sec6p is also required for exocytosis, playing a role in the fusion of secretory vesicles at the plasma membrane (28, 73). In the yeast S. cerevisiae, some cell polarity components are bound to secretory vesicles, and their localization is dependent on the secretory pathway. The GTPase Rho1p was found to be localized to the Golgi apparatus and post-Golgi vesicles, and its localization is disrupted in mutants acting at late steps of the secretory pathway, including sec6, sec2, sec3, sec4, sec8, sec15, and sec1 mutants but not in sec18 and sec7 mutants, whose mutations are involved in ER-to-Golgi traffic (45). Cdc42p GTPase localization at the bud tip is also dependent on a functional exocyst, since Cdc42p localization is disrupted in sec4-8, sec6-4, and sec5-24 mutants (85). The yeast protein Aip3p/Bud6p localizes to the bud tip and bud neck in a cell cycle-specific fashion (66) similarly to Rgd1p and also shows altered localization in mutants defective in the late steps of the secretory pathway (33). In mammals, Cdc42p and its GEF activator βPIX/ARHGEF7 are involved in cell polarity and are located at growth sites in a manner dependent upon secretory vesicles (53).
In agreement with the involvement of the secretory pathway in the proper localization of Rgd1p, coimmunoprecipitation of Rgd1p with secretory vesicles demonstrated the association of the RhoGAP with post-Golgi vesicles. In addition, Rgd1p colocalized with the post-Golgi vesicle marker Sec2p. Moreover, kymographs revealed the dynamics of Rgd1p being transported from the mother cell to the bud in a vectoral manner, in agreement with the known transport properties of secretory vesicles (40). Consistent with this, Rgd1p was also found in fractions containing the plasma membrane, where Rho3p and Rho4p are thought to act on effectors. In conclusion, we propose that at least a part of Rgd1p is transported to the plasma membrane by the late steps of the secretory pathway during bud growth. Rgd1p would associate with post-Golgi vesicles, and then Rgd1p would be transported to the plasma membrane, where the RhoGAP would interact with PtdIns(4,5)P2 synthesized by Mss4p. In Arabidopsis thaliana, a comparable situation was reported for the RhoGAP REN1 (30). REN1 is a RhoGAP for ROP1 involved in the regulation of pollen tube elongation, and it localizes to exocytic vesicles accumulated in the pollen tube apex and to the apical plasma membrane at the site of ROP1 regulation. The apical localization of this RhoGAP is compromised by interrupting vesicular trafficking using brefeldin A. For this protein, the determination of protein domains indicates the presence of a coiled-coil domain at the C terminus, which shares some structural homology with BAR domains (30).
Rgd1p localization at the bud neck was also altered in sec mutants. This is observed for mutants impaired in exocytosis, particularly the sec6-4 mutant. Thus, Sec4p and Sec6p play a role in localizing Rgd1p at both the bud tip and the bud neck. These data suggest a role for trans-Golgi vesicles in the apical localization of Rgd1p but not for its localization to the bud neck, whereas exocytosis would be necessary to bring Rgd1p to both locations. A role for the exocyst complex was already reported in the bud neck localization of the chitin synthases Chs2p and Chs3p (75, 76). Post-Golgi vesicles, as well as the exocyst, localize to the bud neck at a precise time, just before spindle assembly and actomyosin ring contraction (76). In conjunction with membrane addition at the cleavage furrow, targeted vesicle fusion in the cleavage furrow may provide the timely delivery of factors required for cytokinesis. Indeed, Rho4p regulates the interaction between Hof1p and Bnr1p, two proteins involved in cytokinesis and localized at the bud neck, in a GTP-Rho4p dependent manner (35). The transport of Rgd1p could help to control this interaction and the progression of cytokinesis.
Rgd1p presents a specific organization with an F-BAR domain at the N terminus and the RhoGAP domain at the C terminus. F-BAR domains have been shown to generate and bind to tubular membrane structures (72). Deletion of the F-BAR region led to mislocalization of Rgd1p throughout the cell cycle, demonstrating the importance of this domain in controlling the distribution of Rgd1p in S. cerevisiae. The F-BAR domain was shown to interact with phosphoinositides by protein-lipid overlay assays (56). These data led us to postulate a key role for this domain in interaction with PtdIns(4)P during secretory transport, and analysis of mutations of positively charged amino acids within the F-BAR domain would help in defining the interaction sequence. However, the regulation of Rgd1p trafficking might be more subtle. It is known that the GTP-bound form of Rho3p interacts with the exocyst subunit Exo70p (60). A specific mutation in the Rho3p effector domain (rho3E51V), which no longer interacts with Exo70p, causes the accumulation of post-Golgi vesicles in yeast (2). This raises the possibility of cross talk between the secretory pathway and the Rgd1p RhoGAP through Rho3p: Rgd1p depends on traffic for its apical localization, and trafficking may utilize Rgd1p via the Rho3p/Exo70p module.
Moreover, PtdIns(4,5)P2 enhances the RhoGAP activity of Rgd1p toward the Rho4p GTPase (56). Recently, we demonstrated that the binding of PtdIns(4,5)P2 to the RhoGAP domain of Rgd1p modified the structural dynamics of the protein (50). PtdIns(4)P and PtdIns(4,5)P2 signaling, and exocytosis are closely linked to the regulation of Rho GTPases. This is also the case for the GTPase Cdc42p, a key regulator for cell polarity, which regulates the actin cytoskeleton and secretory pathway via exocyst regulation, so fine-tuning of Cdc42p activity must be regulated by distinct RhoGAPs (14, 62).
Rho GTPases function as key signaling molecules in polarity development, and there is a remarkable conservation of these GTPases from yeast to humans at both the structural and functional levels (54). Exploring the regulatory hub between trafficking, membrane dynamics, Rho function, and RhoGAP activation represents a future challenge in our efforts to understand the role of these actors in cell polarity.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from University Bordeaux Segalen and CNRS. DNA sequencing was performed at the Genotyping and Sequencing Facility of the University of Bordeaux (supported by Conseil Regional d'Aquitaine grants 20030304002FA and 20040305003FA). Work in D.M.'s lab is funded by FP7 Marie Curie grant IRG249298/Growth and Division, ANR grant 2010 JCJC 1210 01, Fondation pour la Recherche Medicale (FRM grant INE20100518678), CNRS, Université Bordeaux Segalen, and Conseil Régional d'Aquitaine Volet Recherche, 20091301015.
The GFP-2×PH (PLCδ) probe was given by Tim Levine (University College London). The pUG36-GFP-Sec4 plasmid was a generous gift from Marie-Hélène Cuif (University Paris-Orsay). The Snc1p/Snc2p antibodies were kindly provided by Patrick Brennwald (University of North Carolina).
Footnotes
Published ahead of print 23 March 2012
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1. Abeijon C, Orlean P, Robbins PW, Hirschberg CB. 1989. Topography of glycosylation in yeast: characterization of GDPmannose transport and lumenal guanosine diphosphatase activities in Golgi-like vesicles. Proc. Natl. Acad. Sci. U. S. A. 86:6935–6939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adamo JE, Rossi G, Brennwald P. 1999. The Rho GTPase Rho3 has a direct role in exocytosis that is distinct from its role in actin polarity. Mol. Biol. Cell 10:4121–4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aghamohammadzadeh S, Ayscough KR. 2009. Differential requirements for actin during yeast and mammalian endocytosis. Nat. Cell Biol. 11:1039–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arkowitz RA, Iglesias PA. 2008. Basic principles of polarity establishment and maintenance. Conference on Mechanisms of Cell Polarity. EMBO Rep. 9:847–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Audhya A, Foti M, Emr SD. 2000. Distinct roles for the yeast phosphatidylinositol 4-kinases, Stt4p and Pik1p, in secretion, cell growth, and organelle membrane dynamics. Mol. Biol. Cell 11:2673–2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bankaitis VA, Aitken JR, Cleves AE, Dowhan W. 1990. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature 347:561–562 [DOI] [PubMed] [Google Scholar]
- 7. Bankaitis VA, Malehorn DE, Emr SD, Greene R. 1989. The Saccharomyces cerevisiae SEC14 gene encodes a cytosolic factor that is required for transport of secretory proteins from the yeast Golgi complex. J. Cell Biol. 108:1271–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bankaitis VA, Mousley CJ, Schaaf G. 2010. The Sec14 superfamily and mechanisms for crosstalk between lipid metabolism and lipid signaling. Trends Biochem. Sci. 35:150–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bowman BJ, Slayman CW. 1979. The effects of vanadate on the plasma membrane ATPase of Neurospora crassa. J. Biol. Chem. 254:2928–2934 [PubMed] [Google Scholar]
- 10. Bowser R, Novick P. 1991. Sec15 protein, an essential component of the exocytotic apparatus, is associated with the plasma membrane and with a soluble 19.5S particle. J. Cell Biol. 112:1117–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boyd C, Hughes T, Pypaert M, Novick P. 2004. Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. J. Cell Biol. 167:889–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bryant DM, Mostov KE. 2008. From cells to organs: building polarized tissue. Nat. Rev. Mol. Cell Biol. 9:887–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burda P, Padilla SM, Sarkar S, Emr SD. 2002. Retromer function in endosome-to-Golgi retrograde transport is regulated by the yeast Vps34 PtdIns 3-kinase. J. Cell Sci. 115:3889–3900 [DOI] [PubMed] [Google Scholar]
- 14. Caviston JP, Longtine M, Pringle JR, Bi E. 2003. The role of Cdc42p GTPase-activating proteins in assembly of the septin ring in yeast. Mol. Biol. Cell 14:4051–4066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chesneau L, et al. 2004. Gyp5p and Gyl1p are involved in the control of polarized exocytosis in budding yeast. J. Cell Sci. 117:4757–4767 [DOI] [PubMed] [Google Scholar]
- 16. D'Angelo G, Hotz AM, Todeschin P. 2008. Acute lymphoblastic leukemia with hypereosinophilia and 9p21 deletion: case report and review of the literature. Lab. Hematol. 14:7–9 [DOI] [PubMed] [Google Scholar]
- 17. de Bettignies G, et al. 1999. RGD1 genetically interacts with MID2 and SLG1, encoding two putative sensors for cell integrity signalling in Saccharomyces cerevisiae. Yeast 15:1719–1731 [DOI] [PubMed] [Google Scholar]
- 18. Doignon F, Weinachter C, Roumanie O, Crouzet M. 1999. The yeast Rgd1p is a GTPase activating protein of the Rho3 and Rho4 proteins. FEBS Lett. 459:458–462 [DOI] [PubMed] [Google Scholar]
- 19. Dong Y, Pruyne D, Bretscher A. 2003. Formin-dependent actin assembly is regulated by distinct modes of Rho signaling in yeast. J. Cell Biol. 161:1081–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Endris V, et al. 2002. The novel Rho-GTPase activating gene MEGAP/srGAP3 has a putative role in severe mental retardation. Proc. Natl. Acad. Sci. U. S. A. 99:11754–11759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gatti X, et al. 2005. RGD1, encoding a RhoGAP involved in low-pH survival, is an Msn2p/Msn4p regulated gene in Saccharomyces cerevisiae. Gene 351:159–169 [DOI] [PubMed] [Google Scholar]
- 22. Goud B, Salminen A, Walworth NC, Novick PJ. 1988. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell 53:753–768 [DOI] [PubMed] [Google Scholar]
- 23. Graham TR, Burd CG. 2011. Coordination of Golgi functions by phosphatidylinositol 4-kinases. Trends Cell Biol. 21:113–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guo W, Roth D, Walch-Solimena C, Novick P. 1999. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 18:1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hall A. 2005. Rho GTPases and the control of cell behaviour. Biochem. Soc. Trans. 33:891–895 [DOI] [PubMed] [Google Scholar]
- 26. Hardwick KG, Lewis MJ, Semenza J, Dean N, Pelham HR. 1990. ERD1, a yeast gene required for the retention of luminal endoplasmic reticulum proteins, affects glycoprotein processing in the Golgi apparatus. EMBO J. 9:623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harsay E, Bretscher A. 1995. Parallel secretory pathways to the cell surface in yeast. J. Cell Biol. 131:297–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. He B, Guo W. 2009. The exocyst complex in polarized exocytosis. Curr. Opin. Cell Biol. 21:537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Howell AS, et al. 2009. Singularity in polarization: rewiring yeast cells to make two buds. Cell 139:731–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hwang JU, Vernoud V, Szumlanski A, Nielsen E, Yang Z. 2008. A tip-localized RhoGAP controls cell polarity by globally inhibiting Rho GTPase at the cell apex. Curr. Biol. 18:1907–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iden S, Collard JG. 2008. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat. Rev. Mol. Cell Biol. 9:846–859 [DOI] [PubMed] [Google Scholar]
- 32. Janke C, et al. 2004. A versatile toolbox for PCR-based tagging of yeast genes: new fluorescent proteins, more markers and promoter substitution cassettes. Yeast 21:947–962 [DOI] [PubMed] [Google Scholar]
- 33. Jin H, Amberg DC. 2000. The secretory pathway mediates localization of the cell polarity regulator Aip3p/Bud6p. Mol. Biol. Cell 11:647–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kaksonen M, Toret CP, Drubin DG. 2005. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell 123:305–320 [DOI] [PubMed] [Google Scholar]
- 35. Kamei T, et al. 1998. Interaction of Bnr1p with a novel Src homology 3 domain-containing Hof1p. Implication in cytokinesis in Saccharomyces cerevisiae. J. Biol. Chem. 273:28341–28345 [DOI] [PubMed] [Google Scholar]
- 36. Karlsson R, Pedersen ED, Wang Z, Brakebusch C. 2009. Rho GTPase function in tumorigenesis. Biochim. Biophys. Acta 1796:91–98 [DOI] [PubMed] [Google Scholar]
- 37. Katz L, Hanson PI, Heuser JE, Brennwald P. 1998. Genetic and morphological analyses reveal a critical interaction between the C-termini of two SNARE proteins and a parallel four helical arrangement for the exocytic SNARE complex. EMBO J. 17:6200–6209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Klemm RW, et al. 2009. Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network. J. Cell Biol. 185:601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Krauss M, Haucke V. 2007. Phosphoinositide-metabolizing enzymes at the interface between membrane traffic and cell signalling. EMBO Rep. 8:241–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Layton AT, et al. 2011. Modeling vesicle traffic reveals unexpected consequences for Cdc42p-mediated polarity establishment. Curr. Biol. 21:184–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lefebvre F, et al. 2009. Through its F-BAR and RhoGAP domains, Rgd1p acts in different polarized growth processes in budding yeast. Commun. Integr. Biol. 2:120–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Levine TP, Munro S. 2002. Targeting of Golgi-specific pleckstrin homology domains involves both PtdIns 4-kinase-dependent and -independent components. Curr. Biol. 12:695–704 [DOI] [PubMed] [Google Scholar]
- 43. Li X, et al. 2002. Analysis of oxysterol binding protein homologue Kes1p function in regulation of Sec14p-dependent protein transport from the yeast Golgi complex. J. Cell Biol. 157:63–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lipschutz JH, Mostov KE. 2002. Exocytosis: the many masters of the exocyst. Curr. Biol. 12:R212–R214 [DOI] [PubMed] [Google Scholar]
- 45. McCaffrey M, et al. 1991. The small GTP-binding protein Rho1p is localized on the Golgi apparatus and post-Golgi vesicles in Saccharomyces cerevisiae. J. Cell Biol. 115:309–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mizuno-Yamasaki E, Medkova M, Coleman J, Novick P. 2010. Phosphatidylinositol 4-phosphate controls both membrane recruitment and a regulatory switch of the Rab GEF Sec2p. Dev. Cell 18:828–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mousley CJ, Tyeryar KR, Vincent-Pope P, Bankaitis VA. 2007. The Sec14-superfamily and the regulatory interface between phospholipid metabolism and membrane trafficking. Biochim. Biophys. Acta 1771:727–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Novick P, Field C, Schekman R. 1980. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell 21:205–215 [DOI] [PubMed] [Google Scholar]
- 49. Novick P, Schekman R. 1979. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 76:1858–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Odaert B, et al. 2011. Evidence for specific interaction between the RhoGAP domain from the yeast Rgd1 protein and phosphoinositides. Biochem. Biophys. Res. Commun. 405:74–78 [DOI] [PubMed] [Google Scholar]
- 51. Orlando K, Guo W. 2009. Membrane organization and dynamics in cell polarity. Cold Spring Harb. Perspect. Biol. 1:a001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ortiz D, Medkova M, Walch-Solimena C, Novick P. 2002. Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J. Cell Biol. 157:1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Osmani N, Peglion F, Chavrier P, Etienne-Manneville S. 2010. Cdc42 localization and cell polarity depend on membrane traffic. J. Cell Biol. 191:1261–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Park HO, Bi E. 2007. Central roles of small GTPases in the development of cell polarity in yeast and beyond. Microbiol. Mol. Biol. Rev. 71:48–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Parlati F, et al. 2000. Topological restriction of SNARE-dependent membrane fusion. Nature 407:194–198 [DOI] [PubMed] [Google Scholar]
- 56. Prouzet-Mauleon V, Lefebvre F, Thoraval D, Crouzet M, Doignon F. 2008. Phosphoinositides affect both the cellular distribution and activity of the F-BAR-containing RhoGAP Rgd1p in yeast. J. Biol. Chem. 283:33249–33257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pruyne D, Legesse-Miller A, Gao L, Dong Y, Bretscher A. 2004. Mechanisms of polarized growth and organelle segregation in yeast. Annu. Rev. Cell Dev. Biol. 20:559–591 [DOI] [PubMed] [Google Scholar]
- 58. Pruyne DW, Schott DH, Bretscher A. 1998. Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. J. Cell Biol. 143:1931–1945 [DOI] [PubMed] [Google Scholar]
- 59. Rieder SE, Emr SD. 2001. Overview of subcellular fractionation procedures for the yeast Saccharomyces cerevisiae. Curr. Protoc. Cell Biol. 2001:3.7. [DOI] [PubMed] [Google Scholar]
- 60. Robinson NG, et al. 1999. Rho3 of Saccharomyces cerevisiae, which regulates the actin cytoskeleton and exocytosis, is a GTPase which interacts with Myo2 and Exo70. Mol. Cell. Biol. 19:3580–3587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rose MD, Winston F, Hieter P. 1990. Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 62. Saito K, et al. 2007. Transbilayer phospholipid flipping regulates Cdc42p signaling during polarized cell growth via Rga GTPase-activating proteins. Dev. Cell 13:743–751 [DOI] [PubMed] [Google Scholar]
- 63. Salminen A, Novick PJ. 1989. The Sec15 protein responds to the function of the GTP binding protein, Sec4, to control vesicular traffic in yeast. J. Cell Biol. 109:1023–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Santiago-Tirado FH, Legesse-Miller A, Schott D, Bretscher A. 2011. PI4P and Rab inputs collaborate in myosin-V-dependent transport of secretory compartments in yeast. Dev. Cell 20:47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schaaf G, et al. 2008. Functional anatomy of phospholipid binding and regulation of phosphoinositide homeostasis by proteins of the sec14 superfamily. Mol. Cell 29:191–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Segal M, Bloom K, Reed SI. 2000. Bud6 directs sequential microtubule interactions with the bud tip and bud neck during spindle morphogenesis in Saccharomyces cerevisiae. Mol. Biol. Cell 11:3689–3702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Slaughter BD, Smith SE, Li R. 2009. Symmetry breaking in the life cycle of the budding yeast. Cold Spring Harb. Perspect. Biol. 1:a003384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stefan CJ, Audhya A, Emr SD. 2002. The yeast synaptojanin-like proteins control the cellular distribution of phosphatidylinositol (4,5)-bisphosphate. Mol. Biol. Cell 13:542–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stirling CJ, Rothblatt J, Hosobuchi M, Deshaies R, Schekman R. 1992. Protein translocation mutants defective in the insertion of integral membrane proteins into the endoplasmic reticulum. Mol. Biol. Cell 3:129–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Strahl T, Hama H, DeWald DB, Thorner J. 2005. Yeast phosphatidylinositol 4-kinase, Pik1, has essential roles at the Golgi and in the nucleus. J. Cell Biol. 171:967–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Strahl T, Thorner J. 2007. Synthesis and function of membrane phosphoinositides in budding yeast, Saccharomyces cerevisiae. Biochim. Biophys. Acta 1771:353–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Takano K, Toyooka K, Suetsugu S. 2008. EFC/F-BAR proteins and the N-WASP-WIP complex induce membrane curvature-dependent actin polymerization. EMBO J. 27:2817–2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. TerBush DR, Novick P. 1995. Sec6, Sec8, and Sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae. J. Cell Biol. 130:299–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tiedje C, Sakwa I, Just U, Hofken T. 2008. The Rho GDI Rdi1 regulates Rho GTPases by distinct mechanisms. Mol. Biol. Cell 19:2885–2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Valdivia RH, Baggott D, Chuang JS, Schekman RW. 2002. The yeast clathrin adaptor protein complex 1 is required for the efficient retention of a subset of late Golgi membrane proteins. Dev. Cell 2:283–294 [DOI] [PubMed] [Google Scholar]
- 76. VerPlank L, Li R. 2005. Cell cycle-regulated trafficking of Chs2 controls actomyosin ring stability during cytokinesis. Mol. Biol. Cell 16:2529–2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Vogt DL, Gray CD, Young WS, III, Orellana SA, Malouf AT. 2007. ARHGAP4 is a novel RhoGAP that mediates inhibition of cell motility and axon outgrowth. Mol. Cell Neurosci. 36:332–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Walch-Solimena C, Collins RN, Novick PJ. 1997. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J. Cell Biol. 137:1495–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Walworth NC, Goud B, Kabcenell AK, Novick PJ. 1989. Mutational analysis of SEC4 suggests a cyclical mechanism for the regulation of vesicular traffic. EMBO J. 8:1685–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wera S, Bergsma JC, Thevelein JM. 2001. Phosphoinositides in yeast: genetically tractable signalling. FEMS Yeast Res. 1:9–13 [DOI] [PubMed] [Google Scholar]
- 81. Wilkinson BM, et al. 1997. Molecular architecture of the ER translocase probed by chemical crosslinking of Sss1p to complementary fragments of Sec61p. EMBO J. 16:4549–4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wu H, Brennwald P. 2010. The function of two Rho family GTPases is determined by distinct patterns of cell surface localization. Mol. Cell. Biol. 30:5207–5217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yakir-Tamang L, Gerst JE. 2009. A phosphatidylinositol-transfer protein and phosphatidylinositol-4-phosphate 5-kinase control Cdc42 to regulate the actin cytoskeleton and secretory pathway in yeast. Mol. Biol. Cell 20:3583–3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yakir-Tamang L, Gerst JE. 2009. Phosphoinositides, exocytosis and polarity in yeast: all about actin? Trends Cell Biol. 19:677–684 [DOI] [PubMed] [Google Scholar]
- 85. Zajac A, Sun X, Zhang J, Guo W. 2005. Cyclical regulation of the exocyst and cell polarity determinants for polarized cell growth. Mol. Biol. Cell 16:1500–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.