Abstract
Microbial fuel cells often produce more electrical power with mixed cultures than with pure cultures. Here, we show that a coculture of a nonexoelectrogen (Escherichia coli) and Geobacter sulfurreducens improved system performance relative to that of a pure culture of the exoelectrogen due to the consumption of oxygen leaking into the reactor.
TEXT
Microbial communities that develop in microbial fuel cells (MFCs) vary considerably and can depend on the system architecture, inoculum, catholyte, and fuel (organic substrate) (18, 20). Some early studies indicated very diverse anode communities (14), although the MFCs used had very high internal resistances which limited power production. Early MFC studies indicated low coulombic efficiencies (30%) (24) or did not report data on this (14, 25), so that a substantial amount of substrate may have been lost to processes that did not generate current, such as methanogenesis and aerobic substrate utilization sustained by oxygen leaking through the cathode (6, 7, 16, 17).
More recently, it has been shown with a variety of substrates that high power generation is usually associated with a large proportion of bacteria most similar to various Geobacter spp. (11–13). Geobacter sulfurreducens is usually (2) but not always (12) the predominant microbe in acetate-fed MFCs based on 16S rRNA clone libraries, and the proportion of Geobacter appears to increase when anodes in MFCs are shifted to operation as microbial electrolysis cells (MECs) where oxygen is not used at the cathode (11). The diversity of substrates that can be used by G. sulfurreducens PCA is larger than previously reported (2) and now includes lactate and pyruvate, in addition to acetate (3, 26) and the syntrophic use of formate converted to acetate (27).
G. sulfurreducens was originally considered to be a strict anaerobe (2), but it is now well established that it can grow under low dissolved oxygen conditions (10% or less) and that it is inactivated (15) or killed at higher concentrations (22). We hypothesized that oxygen leakage into a reactor was the reason that the amount of power generated by mixed cultures is often larger than that generated by a pure culture of G. sulfurreducens. The maximum power produced in a single-chamber, bottle-type, air cathode reactor was 576 ± 25 (mean standard ± deviation) mW/m2, compared to 461 ± 8 mW/m2 by a pure culture of G. sulfurreducens (10). Nevin et al. (21) found that the maximum amount of power produced by G. sulfurreducens in a two-chamber MFC with a membrane and no dissolved oxygen (ferricyanide catholyte; 1,900 mW/m2) was larger than that of a mixed culture (1,600 mW/m2) in the same reactor. They also noted that power was increased when the reactor was placed in a glove box and suggested that oxygen diffusion into the system was reducing power generation. There are other findings which similarly suggest that oxygen leakage into an MFC reduces power generation by pure cultures. Choi et al. (8) found that power generation by G. sulfurreducens was enhanced when l-cystine (a chemical oxygen scavenger) was added into a microsized MFC (ferricyanide catholyte). While oxygen leakage through the cathode is well known (6, 7, 16, 17), Yang et al. (30) have shown that there is substantial oxygen leakage through the gaskets and septa used in these single-chamber, cube-type MFCs used in many studies (11, 12, 16, 17, 28). The results of these studies suggested that nonexoelectrogenic bacteria are important to the microbial ecology of an MFC because they can scavenge dissolved oxygen to low levels, allowing greater current generation and higher power production by G. sulfurreducens in a mixed culture than those possible by the pure culture (21, 29).
In order to directly demonstrate the importance of oxygen scavenging by microorganisms to enable power generation by G. sulfurreducens, we examined growth, yields, and power generation using this microbe in MFCs in the presence and absence of a nonexoelectrogen, Escherichia coli. Although one study showed that strain evolution eventually resulted in current generation by E. coli (31), most studies have failed to show that E. coli can generate appreciable current, and this species has previously been used as a negative control for current production (5).
The MFCs used in these experiments were single-chamber, air cathode reactors consisting of a single chamber 3 cm in diameter and 5 cm long (28-ml working volume) (17). Heat-treated graphite fiber brushes (28) were used as anodes. Carbon cloth (30% wet proofed; BASF) with 0.35 mg/cm2 of Pt on the water side and four polytetrafluoroethylene (PTFE) diffusion layers on the air side (6) were used for the cathodes (projected surface area, 7 cm2), and a membrane filter (0.2-μm pore diameter, Supor-200; Millipore) was placed on the air side of the cathode to avoid bacterial contamination. The two strains used were G. sulfurreducens strain PCA (DSM 12127) and Escherichia coli strain BL21. G. sulfurreducens was cultured using ATCC medium 1957 as previously described (4). E. coli was initially incubated in Luria-Bertani medium containing (per liter) 10 g tryptone, 5 g yeast extract, and 10 g NaCl, pH 7.0. Cells were grown overnight at 37°C with shaking, harvested by centrifugation at 10,000 × g for 2 min, resuspended in 50 mM phosphate buffer solution (PBS) containing (per liter) 0.13 g KCl, 0.31 g NH4Cl, 2.77 g NaH2PO4 · 2H2O, 11.54 g Na2HPO4 · 12H2O, and mixed (by vortexing) prior to inoculation. MFCs were supplied with acetate (1 g/liter) in 50 mM PBS with 10 ml vitamins and 10 ml minerals per liter (pH 6.9, and conductivity, 8.1 mS/cm) (4). MFCs were inoculated with G. sulfurreducens, E. coli (1:1, ∼2 × 108 CFU), or both strains, at a fixed resistance (1,000 Ω) or in open-circuit mode (control). Coulombic efficiencies (CEs) and removal of substrate based on chemical oxygen demand (COD) were calculated as previously described (19). Polarization data were obtained by varying the external resistors from 5,000 to 25 Ω.
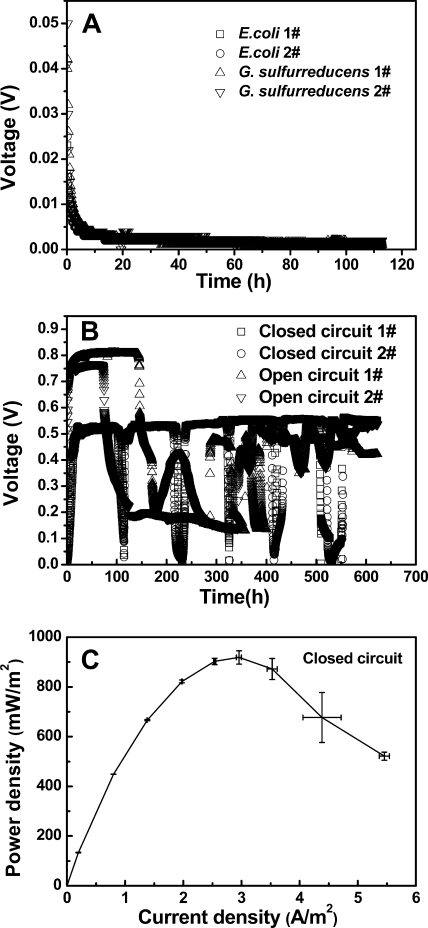
When the MFCs were inoculated with a single strain, there was little voltage generation. Pure cultures of G. sulfurreducens or E. coli initially produced ∼0.05 V, but within 24 h and thereafter the voltage decreased to <0.002 V (Fig. 1A). However, voltage was rapidly generated by a coculture of G. sulfurreducens and E. coli in closed- and open-circuit MFCs. The voltage increased to 0.5 V within 20 h after inoculation and was maintained at 0.51 ± 0.02 V over the next 85 h (Fig. 1B). Based on polarization data (Fig. 1C), the maximum power densities produced by the coculture reactors were 918 ± 27 mW/m2. COD removal in closed-circuit reactors was 85% ± 2%, with a CE of 85% ± 3%. This CE is comparable to that obtained using pure cultures of G. sulfurreducens in an MEC (82%±8%) (4). When the coculture reactors operated in open circuit mode, the voltage reached ∼0.8 V, but it was not stable and decreased over time (Fig. 1B). The COD removal in the open-circuit MFCs was only 45% ± 4%, indicating that the voltage decline was not a result of substrate depletion.
Fig 1.
Voltage generation by pure cultures (A) and coculture MFCs (B) (1,000 Ω; numbers indicate duplicate reactors). (C) Power density curve for the coculture MFC.
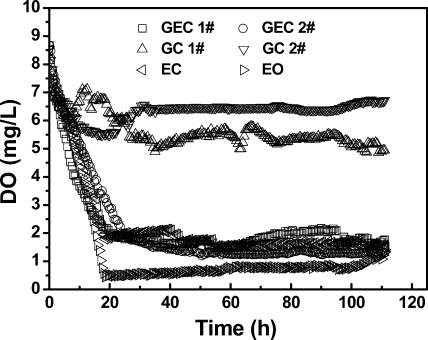
Dissolved oxygen (DO) concentrations in the anode chamber medium were monitored during the first cycle in closed-circuit MFCs and, after 110 h, in open-circuit MFCs using a nonconsumptive fiberoptic DO probe (FOXY; Ocean Optics, Inc., Dunedin, FL). In the MFC inoculated only with G. sulfurreducens, the DO was ∼6 mg/liter (Fig. 2). Oxygen diffused into the solution primarily through the cathode and was not effectively removed by G. sulfurreducens. Oxygen has been shown to adversely affect current generation by other exoelectrogens (29). When the coculture reactors were operated with a closed circuit, the DO in the bulk solution rapidly decreased to <2 mg/liter (Fig. 2) and the voltage increased to 0.5 V within 20 h. Both open- and closed-circuit MFCs containing E. coli also had <2 mg/liter of DO (Fig. 2). These results show that oxygen consumption by E. coli created sufficiently anaerobic conditions for current generation by G. sulfurreducens.
Fig 2.
Dissolved oxygen (DO) concentrations measured in the bulk solution in pure or coculture MFCs (1,000 Ω). In the key, the numbers indicate the duplicate reactors, GEC indicates G. sulfurreducens and E. coli in closed-circuit MFCs, GC indicates G. sulfurreducens in closed-circuit MFCs, EC indicates E. coli in closed-circuit MFCs, and EO indicates E. coli in open-circuit MFCs.
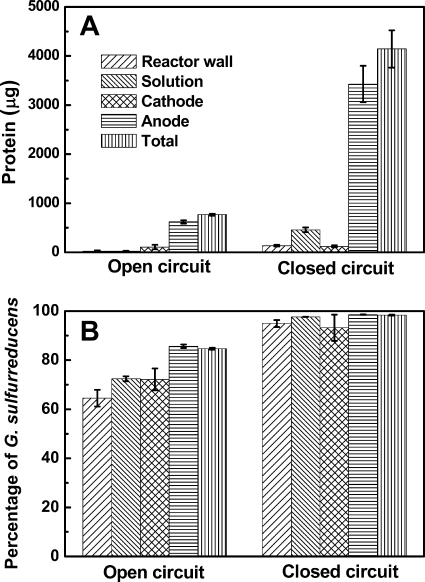
The growth of the two strains in the MFCs was evaluated on the basis of protein amounts using the bicinchoninic acid (BCA) method (1). The total biomass after 620 h in closed-circuit MFCs (4,142 ± 379 μg) was ∼5 times higher than that in open-circuit MFCs (768 ± 21 μg) (Fig. 3A). The relative abundance of the bacteria was obtained using real-time PCR using two pairs of specific primers designed for specific 16S rRNA genes for these strains (E394F, GCCATGCCGCGTGTATGAA, and E553R, TTCATACACGCGGCATGGC, for E. coli, and G995F, TGACATCCACGGAACCCTC, and G1137R, TCAGAGTGCCCAACTTAATG, for G. sulfurreducens). Based on these results, G. sulfurreducens was the dominant population in coculture reactors, comprising 98.4% ± 0.2% of all cells in closed-circuit reactors and 84.7% ± 0.4% in open-circuit reactors (Fig. 3B). Therefore, not only was there more biomass produced in the closed-circuit reactors, but proportionally more cells were produced by growth of G. sulfurreducens through acetate oxidation and current generation (1). The anode contained most of the biomass, with 83% ± 2% of protein in closed circuit MFCs and 80% ± 8% in open circuit reactors (Fig. 3A). This shows that most cells could be found on the anode, probably due to its relatively high surface area, both in the presence and absence of current generation. Based on these percentages and protein measurements, the total mass of G. sulfurreducens increased (from 268 μg to 660 μg in open-circuit reactors and 4,073 μg in closed-circuit reactors) and that of E. coli decreased (from 196 μg to 120 μg in open-circuit reactors and 67 μg in closed-circuit reactors) in the MFCs. Thus, there was appreciable growth of G. sulfurreducens but not of E. coli with acetate (9, 23) under both open- and closed-circuit conditions.
Fig 3.
Distribution of protein (A) and percentages of cells that are G. sulfurreducens (B) (based on quantitative PCR) on the anode, cathode, reactor wall, and in solution (end of a cycle, after 6 cycles) in open- and closed-circuit MFCs. Error bars show standard deviations.
These results demonstrate the important role of microorganisms other than G. sulfurreducens to ensure efficient power generation in MFCs, even when these other microbes do not directly generate current. Without removal of bulk DO to low levels by E. coli, current generation was inhibited. Thus, while substrate can be lost to aerobic microorganisms, their presence is essential for ensuring exoelectrogenesis by the biofilm. Cell yields are typically higher for aerobic than for anaerobic microorganisms, and thus, the abundance of microbes does not directly translate to importance for current generation. However, in coculture tests described here, G. sulfurreducens remained the predominant microorganism in the presence and absence of current generation. This suggests that diverse populations may be present in reactors that have high current densities but that the primary exoelectrogenic species may still be predominant, depending on the substrates and extent of oxygen leakage into the system.
ACKNOWLEDGMENTS
We thank Xia Huang (Tsinghua University) for donating the G. sulfurreducens culture. The technical assistance of Yu Li (Harbin Institute of Technology) and Xia Huang is highly appreciated.
This research was supported by the State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology (grant 2009TS03), the National Innovation Team supported by the National Science Foundation of China (grant no. 50821002), the National Science Foundation for Distinguished Young Scholars of China (grant 51125033), and the King Abdullah University of Science and Technology (KAUST) (award KUS-I1-003-13).
Footnotes
Published ahead of print 17 February 2012
REFERENCES
- 1. Bond DR, Lovley DR. 2003. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 69:1548–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Caccavo F, et al. 1994. Geobacter sulfurreducens sp. nov., a hydrogen-oxidizing and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl. Environ. Microbiol. 60:3752–3759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Call DF, Logan BE. 2011. Lactate oxidation coupled to iron or electrode reduction by Geobacter sulfurreducens PCA. Appl. Environ. Microbiol. 77:8791–8794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Call DF, Wagner RC, Logan BE. 2009. Hydrogen production by Geobacter species and a mixed consortium in a microbial electrolysis cell. Appl. Environ. Microbiol. 75:7579–7587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cao XX, Huang X, Zhang XY, Liang P, Fan MZ. 2009. A mini-microbial fuel cell for voltage testing of exoelectrogenic bacteria. Front. Environ. Sci. Eng. 3:307–312 [Google Scholar]
- 6. Cheng S, Liu H, Logan BE. 2006. Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem. Commun. 8:489–494 [Google Scholar]
- 7. Cheng S, Liu H, Logan BE. 2006. Increased power generation in a continuous flow MFC with advective flow through the porous anode and reduced electrode spacing. Environ. Sci. Technol. 40:2426–2432 [DOI] [PubMed] [Google Scholar]
- 8. Choi S, et al. 2011. A μL-scale micromachined microbial fuel cell having high power density. Lab Chip 11:1110–1117 [DOI] [PubMed] [Google Scholar]
- 9. Glasky AJ, Rafelson ME. 1959. The utilization of acetate-C14 by Escherichia coli grown on acetate as the sole carbon source. J. Biol. Chem. 234:2118–2122 [PubMed] [Google Scholar]
- 10. Ishii S, Watanabe K, Yabuki S, Logan BE, Sekiguchi Y. 2008. Comparison of electrode reduction activities of Geobacter sulfurreducens and an enriched consortium in an air-cathode microbial fuel cell. Appl. Environ. Microbiol. 74:7348–7355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kiely PD, et al. 2011. Anode microbial communities produced by changing from microbial fuel cell to microbial electrolysis cell operation using two different wastewaters. Bioresour. Technol. 102:388–394 [DOI] [PubMed] [Google Scholar]
- 12. Kiely PD, Rader G, Regan JM, Logan BE. 2011. Long-term cathode performance and the microbial communities that develop in microbial fuel cells fed different fermentation endproducts. Bioresour. Technol. 102:361–366 [DOI] [PubMed] [Google Scholar]
- 13. Kiely PD, Regan JM, Logan BE. 2011. The electric picnic: synergistic requirements for exoelectrogenic microbial communities. Curr. Opin. Biotechnol. 22:378–385 [DOI] [PubMed] [Google Scholar]
- 14. Kim BH, et al. 2004. Enrichment of microbial community generating electricity using a fuel-cell-type electrochemical cell. Appl. Microbiol. Biotechnol. 63:672–681 [DOI] [PubMed] [Google Scholar]
- 15. Lin WC, Coppi MV, Lovley DR. 2004. Geobacter sulfurreducens can grow with oxygen as a terminal electron acceptor. Appl. Environ. Microbiol. 70:2525–2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu H, Cheng SA, Logan BE. 2005. Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environ. Sci. Technol. 39:5488–5493 [DOI] [PubMed] [Google Scholar]
- 17. Liu H, Logan BE. 2004. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ. Sci. Technol. 38:4040–4046 [DOI] [PubMed] [Google Scholar]
- 18. Logan BE. 2009. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 7:375–381 [DOI] [PubMed] [Google Scholar]
- 19. Logan BE, et al. 2006. Microbial fuel cells: methodology and technology. Environ. Sci. Technol. 40:5181–5192 [DOI] [PubMed] [Google Scholar]
- 20. Logan BE, Regan JM. 2006. Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol. 14:512–518 [DOI] [PubMed] [Google Scholar]
- 21. Nevin KP, et al. 2008. Power output and columbic efficiencies from biofilms of Geobacter sulfurreducens comparable to mixed community microbial fuel cells. Environ. Microbiol. 10:2505–2514 [DOI] [PubMed] [Google Scholar]
- 22. Nevin KP, Zhang P, Franks AE, Woodard TL, Lovley DR. 2011. Anaerobes unleashed: aerobic fuel cells of Geobacter sulfurreducens. J. Power Sources 196:7514–7518 [Google Scholar]
- 23. Oh MK, Rohlin L, Kao KC, Liao JC. 2002. Global expression profiling of acetate-grown Escherichia coli. J. Biol. Chem. 227:13175–13183 [DOI] [PubMed] [Google Scholar]
- 24. Pham TH, Jang JKHS, Moon Chang IS, Kim BH. 2005. Improved performance of microbial fuel cell using membrane-electrode assembly. J. Microbiol. Biotechnol. 15:438–441 [Google Scholar]
- 25. Phung NT, et al. 2004. Analysis of microbial diversity in oligotrophic microbial fuel cells using 16S rDNA sequences. FEMS Microbiol. Lett. 233:77–82 [DOI] [PubMed] [Google Scholar]
- 26. Speers AM, Reguera G. 2012. Electron donors supporting growth and electroactivity of Geobacter sulfurreducens anode biofilms. Appl. Environ. Microbiol. 78:437–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sun D, Call DF, Kiely PD, Wang A, Logan BE. 2012. Syntrophic interactions improve power production in formic acid fed MFCs operated with set anode potentials or fixed resistances. Biotechnol. Bioeng. 109:405–414 [DOI] [PubMed] [Google Scholar]
- 28. Wang X, et al. 2009. Use of carbon mesh anodes and the effect of different pretreatment methods on power production in microbial fuel cells. Environ. Sci. Technol. 43:6870–6874 [DOI] [PubMed] [Google Scholar]
- 29. Watson VJ, Logan BE. 2010. Power production in MFCs inoculated with Shewanella oneidensis MR-1 or mixed cultures. Biotechnol. Bioeng. 105:489–498 [DOI] [PubMed] [Google Scholar]
- 30. Yang Q, Feng YJ, Logan BE. 30 January 2012. Using cathode spacers to minimize reactor size in air cathode microbial fuel cells. Bioresour. Technol. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 31. Zhang T, et al. 25 April 2006. A novel mediatorless microbial fuel cell based on direct biocatalysis of Escherichia coli. Chem. Commun. (Camb.) 2006:2257–2259 [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]