Abstract
The hybrid nature of lager-brewing yeast strains has been known for 25 years; however, yeast hybrids have only recently been described in cider and wine fermentations. In this study, we characterized the hybrid genomes and the relatedness of the Eg8 industrial yeast strain and of 24 Saccharomyces cerevisiae/Saccharomyces kudriavzevii hybrid yeast strains used for wine making in France (Alsace), Germany, Hungary, and the United States. An array-based comparative genome hybridization (aCGH) profile of the Eg8 genome revealed a typical chimeric profile. Measurement of hybrids DNA content per cell by flow cytometry revealed multiple ploidy levels (2n, 3n, or 4n), and restriction fragment length polymorphism analysis of 22 genes indicated variable amounts of S. kudriavzevii genetic content in three representative strains. We developed microsatellite markers for S. kudriavzevii and used them to analyze the diversity of a population isolated from oaks in Ardèche (France). This analysis revealed new insights into the diversity of this species. We then analyzed the diversity of the wine hybrids for 12 S. cerevisiae and 7 S. kudriavzevii microsatellite loci and found that these strains are the products of multiple hybridization events between several S. cerevisiae wine yeast isolates and various S. kudriavzevii strains. The Eg8 lineage appeared remarkable, since it harbors strains found over a wide geographic area, and the interstrain divergence measured with a (δμ)2 genetic distance indicates an ancient origin. These findings reflect the specific adaptations made by S. cerevisiae/S. kudriavzevii cryophilic hybrids to winery environments in cool climates.
INTRODUCTION
The production of alcoholic beverages is very likely one of the most ancient food traditions. Indeed, traces of fermented beverages have been found on 9,000-year-old Chinese pottery and in 3,000-year-old sealed bronze vessels of the Shang and Western Zhou Dynasties (41). Human cultures have continually sought to control beverage fermentation by progressively selecting specific yeast strains adapted to their needs. The selection of strains able to complete fermentation at low temperatures is very likely one of the milestones in the development of lager brewing technology. Two such yeast species used for beer fermentation have been characterized: Saccharomyces pastorianus and Saccharomyces bayanus, with the second species including type strain CBS380 (NRC1948) (51). Chromosomal transfer experiments suggested that S. pastorianus strains are hybrids between S. cerevisiae and a second Saccharomyces species. This was further demonstrated by a dual restriction fragment length polymorphism (RFLP) pattern typical of hybrids (47). Considering their genomic make-up, lager strains (e.g., S. pastorianus) can be gathered into two groups (14, 36). The first group contains strain CBS1515, also known as the Saccharomyces carlsbergensis type strain. Almost all of these strains are diploid, and most have lost a significant part of the S. cerevisiae genome. The second group includes the S. pastorianus strain Weihenstephan 34/70 and most of the modern lager strains. These strains are triploid and have virtually complete S. cerevisiae diploid genomes.
The second parental species for all of these beer strains was originally thought to be S. bayanus var. bayanus, but the actual species, Saccharomyces eubayanus, has been only recently isolated and described (34). S. bayanus var. bayanus strains have been characterized as other multiple hybrids between Saccharomyces uvarum (controversially classified as S. bayanus var. uvarum), S. cerevisiae, and the recently characterized species S. eubayanus (34, 45). Other interspecific hybrids between S. cerevisiae and S. uvarum have been isolated from cider or wine fermentations (9, 40). More recently, several strains involved in wine making (7, 20) or beer brewing (21), which were assumed to be S. cerevisiae, were found to be hybrids between S. cerevisiae and S. kudriavzevii. Strikingly, S. kudriavzevii yeast strains have never been isolated from wine fermentation but instead have been isolated from decaying leaves in Japan (44) and from oak bark in Portugal (52). It can only be speculated where and when S. cerevisiae and S. kudriavzevii hybridization took place. The resulting hybrids exhibit the best properties of both parental species, such as the low-temperature fermentation abilities of S. kudriavzevii and the high ethanol resistance of S. cerevisiae (1, 2, 17). Like those of lager yeasts, the genomes of S. cerevisiae/S. kudriavzevii hybrid strains display a mosaic structure that very likely resulted from selective pressures experienced over time (50).
Recently, we found that the Eg8 industrial strain isolated in our laboratory 30 years ago and now distributed under the brands ALS and Uvaferm CS2 is, in fact, an S. cerevisiae/S. kudriavzevii hybrid. We characterized the genomic structure of this hybrid strain, as well as that of 24 other hybrids isolated in Hungary, Germany, France (Alsace), and the United States, several of which are related to Eg8. We measured the DNA content per cell by flow cytometry to assess the ploidy of the different strains. Hybrid diversity was evaluated through a multilocus microsatellite analysis for the S. kudriavzevii and S. cerevisiae moieties of the genome, and we compared these results to those previously reported for hybrid strains isolated from wine (20) and beer (21). Our analyses revealed that these hybrids resulted from different hybridization events and that some of them have been dispersed widely, suggesting that they exhibit specific adaptations to wine making in northern European vineyards.
MATERIALS AND METHODS
Strains.
A total of 25 S. cerevisiae/S. kudriavzevii hybrid yeast strains were initially obtained from different collections and were isolated from spontaneously fermenting vats. Seven strains identified as Eg (plus the isolate number) were isolated in 1978 from three vats fermenting at ca. 15°C in the same winery in Eguisheim (Alsace, France). EL1D4 was isolated in 2002 in Bergheim (Alsace, France). Six strains named UHA1 to UHA6 were isolated in 1997 in Turckheim (Alsace, France). Strain 1T1a was isolated in 1996 at the INRA Colmar winery (Alsace, France). The four strains (GEI 5, 7, 10, and 12) and the industrial strain SIHAD4 were isolated in a winery near Geisenheim (Germany). Three strains H10418, H10422, and H10423, were isolated in a Hungarian winery. The UCD505 and UCD580 strains were isolated in California wineries. Swiss hybrids were obtained from the Swiss Federal Research Station for Fruit-Growing, Viticulture, and Horticulture Wadenswill yeast collection, and commercial yeast samples were obtained from Lallemand, Inc. (Montreal, Canada) (see Table S1 in the supplemental material). The Vin7 industrial strain was provided to Anchor Yeast, Ltd., by the ARC-Infruitec/Nietvoorbij yeast collection (Stellenbosch, South Africa) and was isolated in Alsace. With the exception of Vin7, EL1D4, and the Swiss hybrids, all of these strains have been previously genotyped for the S. cerevisiae moiety of their genomes (32).
The 20 S. kudriavzevii strains were isolated from five different bark samples collected at sampling distances of 500 m in October 2007 from a small oak forest near Annonay, Ardèche (France). The strains were isolated after 3 weeks of incubation at 8°C using procedures previously described (52). S. kudriavzevii strains from Portuguese oak were provided by J. P. Sampaio. The S. kudriavzevii-type strain isolated from forest litter in Japan (44) was provided by H. V. Nguyen.
Strain authentication, RFLP analysis, and DNA sequencing.
Yeast cells were cultivated in 10 ml of yeast extract-peptone-dextrose (YPD) medium (36 h, 28°C, 160 rpm), and genomic DNA was isolated using classical techniques consisting of grinding the yeast with glass beads, phenol-chloroform extraction, and isopropanol precipitation as previously described (31).
Strain species were identified using PCR amplification and RFLP analysis of the 5.8S-ITS region (15). Crude PCR products were digested with HaeIII (MBI Fermentas, Lithuania), and the ITS sequences were determined from the digested fragments. The sequence of ARD6.1 has been submitted to GenBank database under accession number HE580229.
To detect the S. kudriavzevii content in the genome of the hybrid strains, we selected 22 S. cerevisiae genes for which we could also obtain a S. kudriavzevii ortholog from the Saccharomyces Genome Database (SGD; http://www.yeastgenome.org/). In the regions containing the open reading frame (ORF) and the adjacent 500 bp upstream and 500 bp downstream for each gene, we searched for polymorphic restriction patterns using Serial Cloner 2.1 software (http://serialbasics.free.fr/Serial_Cloner.html) and designed primer pairs using Eprimer3 software. These genes were also selected to analyze the 16 yeast chromosomes, with one marker close to the centromere and a second marker (if available) on the other chromosomal arm.
The ALD4, ALD5, and ALD6 genes of strain Eg8 were amplified and sequenced after cloning in the pGEM-T cloning vector (Promega). The S. kudriavzevii alleles have been submitted to the GenBank database under accession numbers HE585129 to HE585131.
Flow cytometry.
After a first overnight preculture in YPD liquid medium at 28°C with shaking, each strain was cultured a second time by inoculating the primary culture into 10 ml of YPD to an optical density at 600 nm of 0.05, followed by growth for 5 h at 28°C with shaking. The yeast cells from 2 ml of the secondary culture were harvested by centrifugation and, after careful elimination of the remaining medium, the pellets were resuspended in 1 ml of water and left at room temperature for 1 h to block cells in the G0/G1 phase. These cell suspensions contained approximately 2 × 107 cells and were added dropwise into 8 ml of 70% ethanol for fixation for at least 16 h at 4°C. Fixed cells were prepared for flow cytometry as previously described (22). Briefly, the cells were washed once with phosphate-buffered saline (PBS), treated first with RNase A and then with pepsin, before being resuspended in PBS and dispersed by using ultrasound. Approximately 106 cells were stained with Sytox green (1 μM final concentration). The DNA content was determined on a C6 Accuri (Ann Arbor, MI) spectrophotometer with an excitation wavelength of 488 nm and an emission wavelength of 530 ± 15 nm. Acquisition was performed on 20,000 events observed with a gating on forward scatter/side scatter signal. The flow rate was set to approximately 2,000 events per second (medium flow, 35 μl/min; core, 16 μm). Doublet cells were eliminated by gating for fluorescence area versus height on a linear scale. Finally, the median fluorescence of the G0/G1 peak was recorded.
Microsatellite loci selection and analysis.
The different trinucleotide loci were obtained from the genome sequence of S. kudriavzevii IFO1802 using the Fungal BLAST (Basic Local Alignment Search Tool) tools available at the SGD website. On the basis of the BLAST results, sites showing the largest number of repeats were retained. Primers were designed using the Eprimer3 software from the Emboss package to obtain a minimum melting temperature of 58°C. All primers sequences are listed in Table 1.
Table 1.
Characteristics of the seven S. kudriavzevii microsatellite loci
| Locus | Closest ORF in S. cerevisiae | No. of repeats in IFO1802 | No. of alleles detected | Size range (bases) | Primer |
|
|---|---|---|---|---|---|---|
| Orientationa | Sequence (5′–3′) | |||||
| SKC4 | YOL109W (ZEO1) | (TAA)17 | 4 | 230–242 | F | AAGAATGGTGGAAAGATGCTGT |
| R | TCTTTTTCTCTCCTGCGTCAGA | |||||
| SKTAA1 | YGL035c (MIG1) | (TAA)20 + (TAA)10 | 12 | 284–374 | F | AGAAGCGGAAACACTACCGCCTAT |
| R | TGTATGTTTGTCAAAAGCATCCAC | |||||
| SKYPL009c | YPL009c | (CTT)16 | 8 | 162–258 | F | TGCGACACCATCCGCATTTTC |
| R | ATGGAAAGTGAAGACAGGTGA | |||||
| SKCTT2 | YJL123c (MTC1) | (CTT)11 | 4 | 267–285 | F | TAGCATGGCGAATGGAGATC |
| R | ACGAGGCCGACGGCAGCTCCT | |||||
| SKCTT3 | YKL201c (MNN4) | Uneven 7(CTT)3 | 12 | 299–445 | F | CATCTTCTTGTCTGTTTTTGC |
| R | AAGATTATGCGTATGGGAAA | |||||
| SKGTT1 | YJL206c | (GTT)17 | 5 | 116–173 | F | TGGACCGGAGACTTGTGGCT |
| R | TTTGACGGTCCAACAGCAA | |||||
| SKGTT2 | YCR093w (CDC39) | (GTT)16 | 9 | 188–286 | F | CCGAACAAATAAGAGCTCAA |
| R | GCTGATTGAGGAGGTTGAT | |||||
F, forward; R, reverse.
S. cerevisiae microsatellite loci were amplified as described previously (32). S. kudriavzevii microsatellite loci were amplified individually in 25-μl PCRs containing 50 to 250 ng of yeast DNA, 0.5 μM concentrations of each oligonucleotide primer, 200 μM deoxynucleoside triphosphates, 1.25 U of Taq DNA polymerase (MP Biomedicals, France), 10 mM Tris (pH 9.0), 50 mM KCl, 0.1% Triton X-100, 0.2 mg of gelatin/ml, and 1.5 mM MgCl2. The buffer was provided by the Taq supplier. For capillary gel electrophoresis, loci were amplified in the same manner, but each reverse primer was labeled with a fluorescent compound: 6-carboxyfluorescein (FAM), hexachlorofluorescein (HEX), or benzofluorotrichlorocarboxy-fluorescein (NED) (Applied Biosystems, Foster City, CA). Amplifications were performed in a Stratagene (Amsterdam, Netherlands) thermal cycler with a three-phase temperature program: phase 1 (one cycle of 95°C for 4 min), phase 2 (34 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 1 min), and phase 3 (one cycle of 72°C for 10 min). For the SKTAA1 locus, 1% dimethyl sulfoxide was added to the reaction mixture to limit primer-dimer formation. the PCR products were analyzed as described previously for S. cerevisiae (33). If no amplification was detected for a particular locus, these data were considered to be missing.
Array-based comparative genome hybridization (aCGH) analyses.
The total genomic DNA of Eg8 and S288C were prepared from cultures grown on YPD. The genomic DNA was labeled and hybridized against GeneChip Yeast Genome 2.0 Array from Affymetrix (Santa Clara, CA), which covers all S. cerevisiae S288C genes (56). Labeled fragments were prepared from 200 to 500 ng of genomic DNA using the BioPrime DNA labeling system (Invitrogen). The hybridization and detection steps were performed using the IGBMC microarray and sequencing platform (Illkirch, France). Two arrays were used for each strain. The data were analyzed using the RMA package under R. In the graphs, for smoothing purposes, the log ratios were averaged in a sliding window containing three ORFs. The full data set has been deposited at the NCBI Gene Expression Omnibus (GEO) under accession number GSE31703 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE31703).
Statistical data analysis.
Genetic distances between the individual S. kudriavzevii strains were computed with MicrosatAnalyzer (10), and the linkage disequilibrium was tested using Genepop software (http://genepop.curtin.edu.au/). Distances based on the allelic variation observed at S. cerevisiae microsatellite loci were analyzed using methods we described previously (32). Mantel tests for the comparison of matrix distances were performed using Genetix 4.05 (http://www.genetix.univ-montp2.fr/genetix/intro.htm). Structure analysis of the population was performed using the Structure program (49) after inferring the best partition coefficient K using the Structurama program (27).
RESULTS
Characterization of the Eg8 strain as a S. cerevisiae/S. kudriavzevii hybrid.
While searching for the genetic basis of the Eg8 strain's high production of acetic acid during alcoholic fermentation, we detected two alleles for each of the ALD4, ALD5, and ALD6 genes. Sequencing revealed that these alleles corresponded to one S. cerevisiae and one S. kudriavzevii copy of these genes, suggesting that Eg8 was a hybrid strain between these two species. We evaluated the extent of S. kudriavzevii genetic material in this genome using the amplification and restriction profiles of 22 genes located on the 16 chromosomes and the amplification profiles of seven microsatellite loci. Typical hybrid profiles resulting from overlays of the S. cerevisiae and S. kudriavzevii RFLP patterns were detected for 15 of the 16 chromosomes (Table 2). For two loci, one on chromosome III (left arm) and one on chromosome VIII (right arm), we could not detect the S. kudriavzevii copies of the genes but were able to amplify another S. kudriavzevii marker on the other arm of the respective chromosome.
Table 2.
Detection of S. kudriavzevii DNA in the genomes of three S. cerevisiae/S. kudriavzevii hybrids
| Chromosome | Locusa (accession no.) | Enzyme | Profileb |
||
|---|---|---|---|---|---|
| Eg8/136 | El1d4 | Uvaferm CEG | |||
| 1 | YAL008W | DraI or HindIII | Sc+Sk | Sc+Sk | Sc |
| 2 | YBL021C | ClaI or PstI | Sc+Sk | Sc+Sk | Sc+Sk |
| 3 | SK_YCR093w | Sk | Sk | ND | |
| YCL014w | EcoRI | Sc | Sc+Sk | Sc | |
| 4 | YDL003W | ClaI or SmaI | Sc+Sk | Sc+Sk | Sc |
| YDR337fw | DraI | Sc+Sk | Sc+Sk | Sc | |
| 5 | YEL061wfw | HaeIII | Sc+Sk | Sk+Sc | Sc |
| YER073w (HE585129) | Sc+Sk | ||||
| 6 | YFR030W | HindIII or BamHI | Sc+Sk | Sc+Sk | Sc+Sk |
| 7 | SK_YGL035 | Sk | Sk | ND | |
| YGL162W | HindIII | Sc+Sk | Sc+Sk | Sc | |
| 8 | YHL038c | HaeIII | Sc+Sk | Sc+Sk | Sc |
| YHR043C | SacI or HindIII | Sc | Sc+Sk | Sc | |
| 9 | YIL013C | SacI or BamHI | Sc+Sk | Sc+Sk | Sc+Sk |
| 10 | SK_YJL206c | Sk | Sk | ND | |
| SK_YJL123c | Sk | Sk | ND | ||
| YJL052W | ClaI | Sc+Sk | Sc+Sk | Sc | |
| YJR139c | DraI | Sc+Sk | Sc+Sk | ND | |
| 11 | YKL004W | HindIII | Sc+Sk | Sc | Sc |
| 12 | YLR001C | EcoRI | Sc+Sk | Sc+Sk | Sc |
| 13 | YMR001c | BamHI | Sc+Sk | Sc+Sk | Sc+Sk |
| YMR303w | HaeIII | Sc+Sk | Sc+Sk | Sc | |
| 14 | YNL291c | HindIII or HinfI | Sc+Sk | Sc+Sk | Sc |
| YNR001C | HindIII | Sc+Sk | Ndet | Sc | |
| 15 | SK_YOL109w | Sk | Sk | ND | |
| YOL002C | SacI | Sc+Sk | Sc+Sk | Sc | |
| YOR374w (HE585131) | Sc+Sk | ||||
| 16 | SK_YPL009w | Sk | Sk | ND | |
| YPL001W | EcoRV | Sc+Sk | Sc+Sk | Sc | |
| YPL061w (HE585130) | Sc+Sk | ||||
| YPR140 | HinfI | Sc+Sk | Sc+Sk | Sc | |
S. kudriavzevii microsatellite loci are indicated by an “SK_” prefix. For the locus YCL014w, ARD6.1 (a French isolate) was used in place of IFO1802 as a reference.
Sk, Saccharomyces kudriavzevii; Sc, Saccharomyces cerevisiae; ND, not detected; Ndet, not determined.
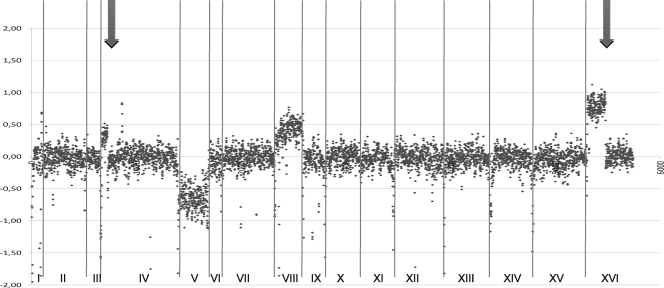
The presence of chimeric genomes is another characteristic of hybrids that has been described for lager yeasts (5, 30). To more fully characterize the organization of the Eg8 genome, we hybridized Eg8 genomic DNA to the Yeast Genome 2.0 Affymetrix microarray (Fig. 1). We found two S. cerevisiae copies for most of the chromosomes but only one copy of S. cerevisiae chromosome V and three copies of S. cerevisiae chromosome VIII. Moreover, we found higher hybridization signals for parts of chromosomes IV and XVI, indicating regions of duplication or translocation. A translocation between chromosome VIII and chromosome XVI leading to the amplification of the SSU1 gene and an increase in sulfite resistance has been described previously (48). We confirmed this translocation by PCR, whereas the hybridization signal for this region indicated that this translocation was present in two copies. Another translocation or duplication event involved the region between YDL186w and YDL244w. The CBS369 strain was found to have a similar mosaic genome after sequencing (25).
Fig 1.
Comparative genome hybridization of Eg8 DNA on an Affymetrix Yeast Genome 2 microarray compared to S288C DNA hybridization. The concatenated chromosomes are displayed on the x axis (the number of each chromosome is indicated in Roman numerals), and the gene order is shown in Arabic numerals (ranked from 0 to 5750 according to their coordinates on the S288C genome). The y axis gives the log of the signal of the two arrays averaged on a sliding window of three genes. The two black arrows indicate the sites of translocation.
Our analyses revealed that the Eg8 strain is a hybrid between S. cerevisiae and S. kudriavzevii, with an aneuploid genome constituted of one to four copies of the S. cerevisiae chromosomes and single copies of the S. kudriavzevii chromosomes.
Detection and characterization of S. cerevisiae/S. kudriavzevii hybrids related to Eg8.
To identify other hybrids related to Eg8, we investigated 38 strains: 8 strains were isolated from the same winery from which Eg8 was isolated, and 30 strains were isolated from other wineries in Alsace, Germany, Hungary, and the United States and were known to be related to Eg8 on the basis of previously determined S. cerevisiae microsatellite profiles (32). To determine whether S. kudriavzevii DNA was present in these strains, we amplified a 1,896-bp fragment of the HAP3 gene on chromosome II using genomic DNA from each strain. Restriction analysis of these fragments with PstI and ClaI revealed a hybrid pattern for 23 of the strains. In addition, one strain (EL1D4) isolated from another Alsatian winery during another experiment was found to be a hybrid when the D1-D2 region was sequenced.
To assess the ploidy of these hybrid strains, we analyzed the cellular DNA content of 18 of the 24 hybrids and several reference strains using flow cytometry (Table 3). The hybrids related to Eg8 (indicated in boldface in the table) presented similar ploidy levels between 2.7n and 3n, which were consistent with the organization of the Eg8 genome. Similar ploidy levels were also observed for most of the Swiss hybrids. However, the HWD231 and Uvaferm CEG strains presented ploidy levels close to 2n and El1d4 presented a ploidy level of 4n. We also analyzed the extent of S. kudriavzevii DNA in the genomes of the El1d4 strain and the Uvaferm CEG industrial strain. We found that EL1D4 contains genes from 15 S. kudriavzevii chromosomes (Table 2) (chromosome XI is entirely of S. cerevisiae origin). In contrast, hybrid profiles were obtained for only four Uvaferm CEG chromosomes (II, VI, IX, and XIII), indicating that this strain contains only a small proportion of S. kudriavzevii genes.
Table 3.
DNA ploidy of different hybrids as measured by flow cytometry
| Region | Straina | DNA ploidy | CVb (%) |
|---|---|---|---|
| Not known | S288C (S. cerevisiae) | 1.0 | |
| BY4743 (S. cerevisiae) | 2.0 | 17 | |
| France (Ardèche) | Ard6,1 (S. kudriavzevii) | 2.0 | 16 |
| France (Alsace) | 1T1a | 3.1 | 15 |
| Eg8 | 3.0 | 7 | |
| Eg2 | 3.0 | 6 | |
| Vin7 | 3.0 | 6 | |
| UHA5 | 3.0 | 11 | |
| UCD505 | 2.9 | 14 | |
| Eg12 | 2.9 | 6 | |
| Eg24 | 2.9 | 6 | |
| Eg17 | 2.9 | 7 | |
| Eg6 | 2.8 | 6 | |
| UHA6 | 2.8 | 11 | |
| UHA4 | 2.8 | 13 | |
| UHA3 | 2.7 | 14 | |
| UHA2 | 2.7 | 13 | |
| Eg1 | 2.7 | 8 | |
| EL1D4 | 4.0 | 6 | |
| France (Champagne) | Uvaferm CEG | 1.9 | 11 |
| Hungary | H10418 | 3.0 | 16 |
| H10423 | 3.1 | 16 | |
| Switzerland | HWD441 | 3.0 | 10 |
| HWD77 (=WD27) | 3.0 | 8 | |
| HWD278 | 3,0 | 9 | |
| HWD78 | 3.0 | 10 | |
| HWD205 | 3.0 | 8 | |
| HWD126 | 3.1 | 15 | |
| HWD319 | 3.0 | 13 | |
| HWD231 | 1.7 | 9 |
Strains of the Eg8 group are indicated in boldface.
CV, coefficient of variation.
We found the ploidy levels of these hybrids to be significantly higher than those reported previously (3). These differences may have been due to the higher resolution of our flow cytometer, an improved cell preparation protocol or to the different fluorescent dyes used (22). Indeed, we obtained lower and more variable ploidy levels from stationary phase cells. Our results have been confirmed for the HWD77 (= WD27) and VIN7 strains by sequencing (6, 26).
Characterization of the S. kudriavzevii population structure. (i) Selection of seven microsatellite loci for S. kudriavzevii characterization.
Microsatellite loci are more efficient markers than single nucleotide polymorphisms for comparing recently evolved populations (23). To identify the phylogenic relationships among our hybrid strains and to analyze the S. kudriavzevii population structure, we searched for repeated motifs in the S. kudriavzevii genome sequence. Seven loci were retained for analysis (Table 1). Six of these loci are trinucleotide repeats and the seventh locus (SKCTT3) is a complex region containing several CTT repeats. Two loci (SKC4 and SKYPL009) were homologous to the highly polymorphic C4 and YPL009c loci used for S. cerevisiae typing (33).
These microsatellite loci were used to genotype the 25 new hybrids, 11 hybrids characterized previously (20, 21, 24), and 24 S. kudriavzevii strains isolated in Ardèche (France), Japan, and Portugal. A total of 56 strains exhibited between 4 and 15 alleles per locus, with the SKCTT3 locus presenting the highest variability. The combination of these seven markers allowed us to differentiate 40 genotypes: 16 S. kudriavzevii strains and 24 hybrid strains. For four hybrids (Uvaferm CEG, Hassmanhausen, HWD231, and UCD580), we were unable to amplify sufficient numbers of loci for the strains to be included in the tree.
(ii) S. kudriavzevii population structure.
Since little is known of the S. kudriavzevii population structure, we first searched for a natural population of S. kudriavzevii originating from the area where our hybrids strains were isolated. Although the first trials we performed for Alsace were unsuccessful, we subsequently isolated a set of 20 strains obtained from the decaying bark of oak trees growing in a small oak forest located in Ardèche (France). This set was resolved into 12 genotypes. These genotypes shared only one allele with the type strain IFO1802 and the two Portuguese isolates.
In the Ardèche population, all isolates were homozygous at all loci, and as a consequence the population alleles deviated significantly from the Hardy-Weinberg equilibrium (P < 0.001). However, linkage disequilibrium was not significant for one-third of the locus pairs. This indicates that recombination had occurred between alleles at several loci as a consequence of sexual reproduction. Since the Fis value was 1 at all loci in this natural population of S. kudriavzevii, inbreeding is almost the exclusive reproduction mode of this species.
Diversity of the S. kudriavzevii moiety of the genome of hybrid strains.
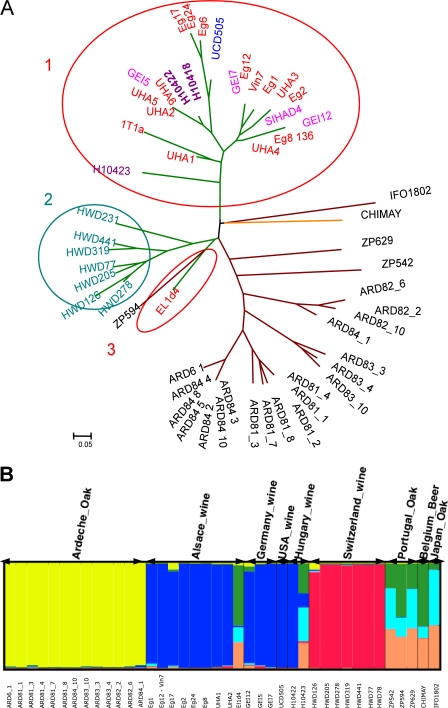
Microsatellite analysis of the polymorphisms of seven S. kudriavzevii loci allowed us to gain insights about the S. kudriavzevii moieties in the genomes of the hybrid strains. We did not observe any heterozygosity in the S. kudriavzevii moieties of the hybrid strains, suggesting that these moieties were present as single-copy chromosomes in the hybrids. We investigated the relationships between the different hybrid strains by building a dendrogram from the DC chord distances (8a) (Fig. 2A), which revealed different clusters of hybrids that corresponded to the different origins of the isolates. We tried to confirm this structure using a Bayesian population analysis method (49) extended by Huelsenbeck and Andolfatto (27), which aimed at detecting the most probable number of subpopulations. We found the optimal subpopulation number to be 7 and that the structure output (Fig. 2B) delineated different clusters, one for the Ardèche oak isolates (yellow bars) and two containing the hybrid strains. The first hybrid strain cluster included hybrid strains from Alsace, Germany, and Hungary (blue bars), and the second cluster contained very similar strains that all originated from Switzerland (red bars). The remaining strains included the El1d4 hybrid strain, the beer isolate CHIMAY, and the wild S. kudriavzevii isolates from Japan or Portugal. These results suggest that at least three different hybridization events were at the origins of these hybrid strains.
Fig 2.
(A) Dendrogram presenting the diversity of the yeast specimens based on seven S. kudriavzevii-specific microsatellite loci. A total of 24 S. kudriavzevii strains isolated from oak trees in Japan, Portugal, or France (Ardèche) are compared to 32 S. kudriavzevii/S. cerevisiae hybrids isolated from wine (green lines) or beer (orange line) from France (red letters), Switzerland (blue-green letters), Germany (pink letters), or Hungary (purple letters). The clusters found for the different S. cerevisiae/S. kudriavzevii hybrids are highlighted by circles. (B) Assignment of the different strains to seven clusters inferred using the Structure software program. Identical clones were removed before the analysis. The names of the strains are shown below the figure, and the isolation sources are listed above the figure.
It is also noteworthy that the reason we could not amplify sufficient loci from some of the strains (HWD231, Uvaferm CEG, UCD580, and Assmannshausen) to include them in the phylogenic analysis was very likely due to the small amount of S. kudriavzevii moieties in their genome. In agreement with this, HWD172 and Uvaferm CEG presented relatively low ploidy levels close to 2n. Thus, these strains have been produced by additional hybridization events.
Analysis of the S. cerevisiae moiety of the genome of the hybrids strains.
We compared the polymorphisms of the S. cerevisiae moieties in the genomes of 32 hybrids strains we genotyped at 12 microsatellite loci to the polymorphisms of the S. kudriavzevii markers. The results obtained for each set of species-specific markers were highly correlative (P < 10−4 for a Mantel test calculated after 10,000 permutations), and the Pearson coefficient of 0.715 indicated that 51% of one matrix variation is explained by the other.
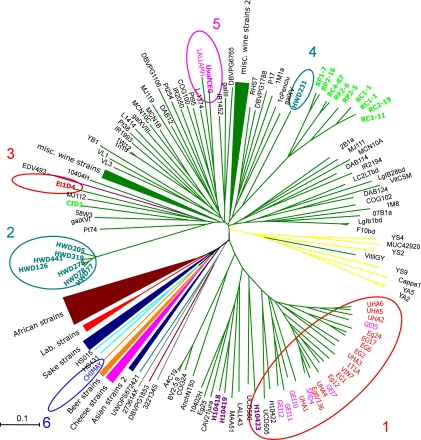
We then compared the S. cerevisiae genotypes of these hybrids to the genotypes of 175 S. cerevisiae strains from various origins. As expected, a dendrogram built from the DC chord distances (Fig. 3) separated the wine yeast strains well apart from strains of other origins (sake, African, cheese, or beer strains). In this dendrogram, the wine hybrids were spread into five groups, and the beer hybrid (CHIMAY, group 6) is well separated from the wine strains. Three of the wine groups corresponded to those obtained from S. kudriavzevii genotyping, but HWD231 was separated from the other Swiss hybrids, resulting in a fourth group. Finally, a fifth group included two other strains (Uvaferm CEG and Assmannshausen), which contain less S. kudriavzevii genetic material in their genome than the other hybrids, such that it was not possible to genotype them using the S. kudriavzevii loci. The first group of strains is remarkable due to its size. It includes 25 strains, 23 of which originated from Hungary, Germany, or Alsace, which implies that this group has expanded on a wide geographic scale. Further subclustering could be also detected within this group, which corresponded to the phylogenic structure obtained using the S. kudriavzevii genome markers. Hungarian strains appear to be the most basal, in contrast to the intertwined German and Alsace strains, suggesting that these Hungarian strains might be more closely related to the original hybrid ancestor shared by all of the strains from this group. The average divergence time for the strains of the Eg8 group measured on a tree built with (δμ)2 genetic distance (18) is approximately half of that calculated for all of the wine strains, and 25 times more than what we obtained for a group of nine lager beer strains (32). However, since (δμ)2 presents a high variance, these ratios are only rough estimates, but they are consistent with divergences already known to be ancient.
Fig 3.
Dendrogram presenting the diversity obtained at 12 S. cerevisiae-specific microsatellite loci for 157 S. cerevisiae strains and 35 S. cerevisiae/S. kudriavzevii hybrids isolated from wineries in France (red letters; groups 1 and 3), Switzerland (blue-green; group 2), Germany (pink), and Hungary (purple). Ten S. cerevisiae/S. uvarum hybrids were included in the analysis (light green). The clusters formed by the different S. cerevisiae/S. kudriavzevii hybrids are highlighted by circles.
Altogether, the S. kudriavzevii and S. cerevisiae microsatellite analyses indicate that strains originating from Switzerland can be separated into two groups that probably resulted from two different hybridization events in which the same S. kudriavzevii strain hybridized with two different S. cerevisiae strains.
The heterozygosity observed at the S. cerevisiae loci is another indication of the ploidy levels of these strains. The strains of the Eg8 group were heterozygous at between 4 and 10 loci out of 12, and the Swiss isolates were heterozygous at either 4 or 5 loci out of 12. These data indicate that these hybrids have at least two copies of their S. cerevisiae loci. In contrast, the EL1D4 strain had no heterozygous loci, which was similar to the findings for the other known S. cerevisiae/S. uvarum hybrid strains analyzed here, such as CID1 (one heterozygous locus), RP2-5, RP2-6, RC1-1, RC1-11, RC2-19, and RC4-87 (26). This suggests that EL1D4 underwent an autotetraploidization step following mating.
DISCUSSION
Prior to this study, S. kudriavzevii had not been encountered in studies of wine-making yeast strains. Given this absence, it is rather puzzling to find a high frequency of S. kudriavzevii/S. cerevisiae hybrids being used for wine making. Nevertheless, this situation is reminiscent of the many hybrids that are present only in lager beer fermentation. Since the hybrid nature of S. pastorianus strains was first discovered, many hybrids have been detected in various fermentation environments, including cider making (40), wine making (7, 20, 33a, 37), and beer fermentation (21; reviewed in reference 53). As a consequence, it was not surprising to find that the Eg8 industrial strain is a hybrid. Indeed, this strain was isolated in 1979 from a vat fermenting at low temperature (approximately 15°C) and is known for its ability to ferment at low temperature (http://www.vignevin.com/outils-en-ligne/fiches-levures or Lallemand website). This strain, which has been produced industrially as a yeast starter since 1981, is also famous for its ability to complete alcoholic fermentations. The Eg8 S. cerevisiae genome moiety is related to Jura flor yeast and to EC1118, which are both well known for their resistance to ethanol (8). Our results show that this strain is indeed a triploid S. cerevisiae/S. kudriavzevii hybrid with almost two copies of the S. cerevisiae chromosomes and one copy of all of the S. kudriavzevii chromosomes. In addition, it very likely has a chimeric genome with one copy of chromosome V and three copies of S. cerevisiae chromosome VIII. Finally, we could detect two translocation events, including the classical SSU1 translocation (48) and a translocation on chromosome IV.
The finding that other strains isolated from the same winery (Eg strains) are also hybrids is expected; however, considering their position in the tree, these hybrids are already quite different from the original Eg8 strain. Our finding that many of the hybrids strains isolated in a large area covering Hungary, Germany, and France (Alsace) are clearly related to Eg8 was unexpected and especially remarkable. This indicates that these wine strains have been dispersed on a wide scale from one vineyard to another. This migration or separation of the Hungarian strains from the other strains is apparently more ancient than the separation of the German strains from the Alsatian strains because the latter two groups are intertwined in the microsatellite tree. The split between the Hungarian and other strains from Alsace and Germany also appears to be rather ancient and much older than what we could estimate for the separations between lager strains (which happened approximately at the end of 19th century). The mosaic character of the Eg8 genome indicated by the aCGH array can be interpreted as the result of the complex forces that have shaped the genomes of these hybrids. Such mosaic genome has also been described for lager yeast (4, 43) and for the CBS 679 hybrid strain (25).
In addition to the Eg8 hybrid strain group, we have shown that other hybrids can be distinguished by their genotypic profiles and ploidy levels. The ploidy level differences of the different hybrid strains correspond to the groups characterized with S. kudriavzevii or S. cerevisiae microsatellite loci and likely resulted from different mechanisms. The second group of Swiss hybrids are mostly triploid (HWD77, HWD78, HWD126, HWD205, HWD278, and HWD319), with two diploid strains (Uvaferm CEG and HWD231) containing a limited amount of S. kudriavzevii genome and one tetraploid strain containing a complete S. kudriavzevii diploid genome (EL1D4).
It has been proposed for one group of lager beer strains, almost all of which are triploid hybrids, that they may have resulted from hybridization between a diploid S. cerevisiae and a haploid S. bayanus (14). Although this type of hybridization may be rare, the same mechanism may have created the S. cerevisiae/S. kudriavzevii triploids studied here. Since their genotypes indicate a wine fermentation origin, the diploid spores necessary to form such hybrids would have been produced by a tetraploid wine strain. Indeed, tetraploid as well as triploid strains have been isolated from nature (16), and we identified several such strains in Alsatian wineries. Alternatively, diploids could have become homozygous for MATα or MATa as a consequence of a mitotic recombination at the mating-type locus on chromosome III (54).
For strains with limited S. kudriavzevii content, we can speculate whether this content arose from a horizontal transfer event or by classical hybridization. The meiotic chromosome segregation of triploids (54) could offer an attractive explanation to this uneven S. kudriavzevii content. Since these strains are diploid, it is likely that such S. kudriavzevii introgressions are similar to the numerous S. paradoxus introgressions that have been described for S. cerevisiae (42) or that have been described for the trace S. cerevisiae introgressions in the genome of European S. paradoxus natural isolates (13, 35, 55). A monotonic relation between diversity and reproductive isolation has been reported for the Saccharomyces group (35). This is similar to what has been described for pea aphids (46) and suggests a genetic continuum inside the Saccharomyces genus and that these species may have “fuzzy boundaries.” Such blurred boundaries are exemplified by the S. bayanus var. bayanus group that has resulted from multiple crosses between three varieties: S. uvarum, S. cerevisiae, and S. eubayanus. This very likely occurred in relation to the development of yeast able to ferment malt at low temperature, suggesting that hybridization is a key phenomenon for adapting yeast to new ecological niches.
The production of hybrids in the Saccharomyces genus can be compared to those that occur in the plant kingdom, which are quite frequent, and that lead to the production of individuals having the phenotypic properties (38, 39) necessary to colonize new ecological niches. Two types of hybridization have been described for plants, homoploid and alloploid, and both of these can lead to speciation as a result of genetic isolation. Our results show that both types of hybridization have taken place in the Saccharomyces genus.
The question remains as to whether these hybridizations rendered adaptive advantages to these wine strains which then favored their expansion. In these Northern European vineyards, grapes are frequently harvested in autumn at cool temperatures, which produce cold must when pressed and may lead to the selection of cryophilic yeast. Since S. uvarum and S. kudriavzevii have been described as more cryophilic than S. cerevisiae and S. paradoxus (1, 19, 52), we can expect that these cryophilic properties have been combined with the fermentation efficiency of S. cerevisiae in the hybrids. S. cerevisiae/S. uvarum hybrids have already been isolated in an Alsatian winery (9, 33a), and we have isolated others in other Alsatian wineries (unpublished data). In agreement with this, strains of the Eg8 group were isolated in 1979 from three vats fermenting at low temperature, and Eg8 is considered one of the most cryophilic of the industrial yeasts used for wine making. We have also observed that three of the five strains of the Eg8 group showed visible growth after 5 weeks of incubation at 6°C, whereas the S. cerevisiae strains did not (data not shown). In addition to being a cryophile, Eg8 displays the high alcohol resistance also found in flor strains (8) and in strains involved in the refermentation of botrytized wine (12), which is a characteristic related to the S. cerevisiae moiety of its genome. The remarkable ecological success of this family of hybrids isolated from cellars in Hungary, Germany, and France (Alsace) may be considered another example of yeast domestication.
Our results also provide insights into S. kudriavzevii diversity. Since the description of the S. kudriavzevii (44), little has been learned about its diversity. The characterization of the seven genes of the GAL pathway in the type strain IFO1902 isolated in Japan revealed the ancient degeneration of the entire pathway (26). Since this pathway is fully functional in a population of Portuguese isolates, this suggests the existence of a balanced unlinked gene network polymorphism (25). One explanation for this phenomenon may be the existence of isolated populations in this species. We propose that the set of markers we used for our analysis can also be used to characterize S. kudriavzevii diversity. Our results obtained for a population of wild isolates and for these hybrid strains showed a clear population structure for this species since we could assign several clusters of strains to their isolation sources despite the close geographical proximity of these areas (Switzerland/Alsace). This may have resulted from the high selfing rate that we inferred from the homozygosity of the oak S. kudriavzevii isolates. Similar levels of homozygosity were observed in S. paradoxus oak populations (29) and in S. cerevisiae soil isolates (11), suggesting that these species share similar reproductive behavior.
Supplementary Material
ACKNOWLEDGMENTS
We thank L. Bisson, J. Gafner, M. Sipiczki, A. Julien (Lallemand), G. Reid (Anchor yeast), M. Grossman, H. V. Nguyen, and J. P. Sampaio for providing strains. We also thank E. Barrio for helpful suggestions and comments.
This study was supported in part by INRA (Department CEPIA) and SOFRALAB.
Footnotes
Published ahead of print 17 February 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Arroyo-López FN, Orlić S, Querol A, Barrio E. 2009. Effects of temperature, pH, and sugar concentration on the growth parameters of Saccharomyces cerevisiae, S. kudriavzevii, and their interspecific hybrid. Int. J. Food Microbiol. 131:120–127 [DOI] [PubMed] [Google Scholar]
- 2. Belloch C, Orlic S, Barrio E, Querol A. 2008. Fermentative stress adaptation of hybrids within the Saccharomyces sensu stricto complex. Int. J. Food Microbiol. 122:188–195 [DOI] [PubMed] [Google Scholar]
- 3. Belloch C, et al. 2009. Chimeric genomes of natural hybrids of Saccharomyces cerevisiae and Saccharomyces kudriavzevii. Appl. Environ. Microbiol. 75:2534–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bond U. 2009. The genomes of lager yeasts. Adv. Appl. Microbiol. 69:159–182 [DOI] [PubMed] [Google Scholar]
- 5. Bond U, Neal C, Donnelly D, James TC. 2004. Aneuploidy and copy number breakpoints in the genome of lager yeasts mapped by microarray hybridisation. Curr. Genet. 45:360–370 [DOI] [PubMed] [Google Scholar]
- 6. Borneman AR, et al. 2011. The genome sequence of the wine yeast VIN7 reveals an allotriploid hybrid genome with Saccharomyces cerevisiae and Saccharomyces kudriavzevii origins. FEMS Yeast Res. 12:88–96 [DOI] [PubMed] [Google Scholar]
- 7. Bradbury JE, et al. 2006. A homozygous diploid subset of commercial wine yeast strains. Antonie Van Leeuwenhoek 89:27–37 [DOI] [PubMed] [Google Scholar]
- 8. Budroni M, Zara S, Zara G, Pirino G, Mannazzu I. 2005. Peculiarities of flor strains adapted to Sardinian sherry-like wine ageing conditions. FEMS Yeast Res. 5:951–958 [DOI] [PubMed] [Google Scholar]
- 8a. Cavalli-Sforza LL, Edwards AWF. 1967. Phylogenetic analysis: models and estimation procedures. Evolution 21:550–570 [DOI] [PubMed] [Google Scholar]
- 9. Demuyter C, Lollier M, Legras JL, Le Jeune C. 2004. Predominance of Saccharomyces uvarum during spontaneous alcoholic fermentation, for three consecutive years, in an Alsatian winery. J. Appl. Microbiol. 97:1140–1148 [DOI] [PubMed] [Google Scholar]
- 10. Dieringer D, Schlotterer C. 2003. Microsatellite analyser (MSA): a platform independent analysis tool for large microsatellite data sets. Mol. Ecol. Notes 3:167–169 [Google Scholar]
- 11. Diezmann S, Dietrich FS. 2009. Saccharomyces cerevisiae: population divergence and resistance to oxidative stress in clinical, domesticated, and wild isolates. PLoS One 4:e5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Divol B, Miot-Sertier C, Lonvaud-Funel A. 2006. Genetic characterization of strains of Saccharomyces cerevisiae responsible for “refermentation” in botrytis-affected wines. J. Appl. Microbiol. 100:516–526 [DOI] [PubMed] [Google Scholar]
- 13. Doniger SW, et al. 2008. A catalog of neutral and deleterious polymorphism in yeast. PLoS Genet. 4:e1000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dunn B, Sherlock G. 2008. Reconstruction of the genome origins and evolution of the hybrid lager yeast Saccharomyces pastorianus. Genome Res. 18:1610–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Esteve-Zarzoso B, Belloch C, Uruburu F, Querol A. 1999. Identification of yeasts by RFLP analysis of the 5.8S rRNA gene and the two ribosomal internal transcribed spacers. Int. J. Syst. Bacteriol. 49(Pt 1):329–337 [DOI] [PubMed] [Google Scholar]
- 16. Ezov TK, et al. 2006. Molecular-genetic biodiversity in a natural population of the yeast Saccharomyces cerevisiae from “Evolution Canyon”: microsatellite polymorphism, ploidy, and controversial sexual status. Genetics 174:1455–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Giudici P, Caggia C, Pulvirenti A, Rainieri S. 1998. Karyotyping of Saccharomyces strains with different temperature profiles. J. Appl. Microbiol. 84:811–819 [DOI] [PubMed] [Google Scholar]
- 18. Goldstein DB, Linares AR, Cavalli-Sforza LL, Feldman MW. 1995. Genetic absolute dating based on microsatellites and the origin of modern humans. Proc. Natl. Acad. Sci. U. S. A. 92:6723–6727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gonçalves P, Valério E, Correia C, de Almeida JM, Sampaio JP. 2011. Evidence for divergent evolution of growth temperature preference in sympatric Saccharomyces species. PLoS One 6:e20739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. González SS, Barrio E, Gafner J, Querol A. 2006. Natural hybrids from Saccharomyces cerevisiae, Saccharomyces bayanus, and Saccharomyces kudriavzevii in wine fermentations. FEMS Yeast Res. 6:1221–1234 [DOI] [PubMed] [Google Scholar]
- 21. González SS, Barrio E, Querol A. 2008. Molecular characterization of new natural hybrids of Saccharomyces cerevisiae and S. kudriavzevii in brewing. Appl. Environ. Microbiol. 74:2314–2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haase SB, Reed SI. 2002. Improved flow cytometric analysis of the budding yeast cell cycle. Cell Cycle 1:132–136 [PubMed] [Google Scholar]
- 23. Haasl RJ, Payseur B. 2011. Multi-locus inference of population structure: a comparison between single nucleotide polymorphisms and microsatellites. Heredity 106:158–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heinrich AJ. 2006. Identification of genomic differences between laboratory and commercial strains of Saccharomyces cerevisiae. Ph.D. thesis University of Adelaide, Adelaide, Australia [Google Scholar]
- 25. Hittinger CT, et al. 2010. Remarkably ancient balanced polymorphisms in a multi-locus gene network. Nature 464:54–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hittinger CT, Rokas A, Carroll SB. 2004. Parallel inactivation of multiple GAL pathway genes and ecological diversification in yeasts. Proc. Natl. Acad. Sci. U. S. A. 101:14144–14149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huelsenbeck JP, Andolfatto P. 2007. Inference of population structure under a Dirichlet process model. Genetics 175:1787–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reference deleted.
- 29. Johnson LJ, et al. 2004. Population genetics of the wild yeast Saccharomyces paradoxus. Genetics 166:43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kodama Y, Kielland-Brandt M, Hansen J. 2006. Lager brewing yeast, p 145–164 In Sunnerhagen P, Piskur P. (ed), Topics in current genetics, vol 15 Springer, Berlin, Germany [Google Scholar]
- 31. Legras JL, Karst F. 2003. Optimisation of interdelta analysis for Saccharomyces cerevisiae strain characterisation. FEMS Microbiol. Lett. 221:249–255 [DOI] [PubMed] [Google Scholar]
- 32. Legras JL, Merdinoglu D, Cornuet J-M, Karst F. 2007. Bread, beer and wine: Saccharomyces cerevisiae diversity reflects human history. Mol. Ecol. 16:2091–2102 [DOI] [PubMed] [Google Scholar]
- 33. Legras JL, Ruh O, Merdinoglu D, Karst F. 2005. Selection of hypervariable microsatellite loci for the characterization of strains. Int. J. Food Microbiol. 102:73–83 [DOI] [PubMed] [Google Scholar]
- 33a. Le Jeune C, et al. 2007. Characterization of natural hybrids of Saccharomyces cerevisiae and Saccharomyces bayanus var. uvarum. FEMS Yeast Res. 7:540–549 [DOI] [PubMed] [Google Scholar]
- 34. Libkind D, et al. 2011. Microbe domestication and the identification of the wild genetic stock of lager-brewing yeast. Proc. Natl. Acad. Sci. U. S. A. 108:14539–14544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liti G, Barton DBH, Louis EJ. 2006. Sequence diversity, reproductive isolation, and species concepts in Saccharomyces. Genetics 174:839–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liti G, Peruffo A, James SA, Roberts IN, Louis EJ. 2005. Inferences of evolutionary relationships from a population survey of LTR-retrotransposons and telomeric-associated sequences in the Saccharomyces sensu stricto complex. Yeast 22:177–192 [DOI] [PubMed] [Google Scholar]
- 37. Lopandic K, et al. 2007. Genetically different wine yeasts isolated from Austrian vine-growing regions influence wine aroma differently and contain putative hybrids between Saccharomyces cerevisiae and Saccharomyces kudriavzevii. FEMS Yeast Res. 7:953–965 [DOI] [PubMed] [Google Scholar]
- 38. Mallet J. 2007. Hybrid speciation. Nature 446:279–283 [DOI] [PubMed] [Google Scholar]
- 39. Mallet J. 2008. Hybridization, ecological races, and the nature of species: empirical evidence for the ease of speciation. Philos. Trans. R. Soc. B 363:2971–2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Masneuf I, Hansen J, Groth C, Piskur J, Dubourdieu D. 1998. New hybrids between Saccharomyces sensu stricto yeast species found among wine and cider production strains. Appl. Environ. Microbiol. 64:3887–3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McGovern PE, et al. 2004. Fermented beverages of pre- and proto-historic China. Proc. Natl. Acad. Sci. U. S. A. 101:17593–17598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Muller LAH, McCusker JH. 2009. A multispecies-based taxonomic microarray reveals interspecies hybridization and introgression in Saccharomyces cerevisiae. FEMS Yeast Res. 9:143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nakao Y, et al. 2009. Genome sequence of the lager brewing yeast, an interspecies hybrid. DNA Res. 16:115–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Naumov GI, James SA, Naumova ES, Louis EJ, Roberts IN. 2000. Three new species in the Saccharomyces sensu stricto complex: Saccharomyces cariocanus, Saccharomyces kudriavzevii, and Saccharomyces mikatae. Int. J. Syst. Evol. Microbiol. 50(Pt 5):1931–1942 [DOI] [PubMed] [Google Scholar]
- 45. Nguyen H-V, Legras J-L, Neuvéglise C, Gaillardin C. 2011. Deciphering the hybridisation history leading to the lager lineage based on the mosaic genomes of Saccharomyces bayanus strains NBRC1948 and CBS380T. PLoS One 6:e25821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Peccoud J, Ollivier A, Plantegenest M, Simon J-C. 2009. A continuum of genetic divergence from sympatric host races to species in the pea aphid complex. Proc. Natl. Acad. Sci. U. S. A. 106:7495–7500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pedersen MB. 1985. DNA sequence polymorphisms in the genus Saccharomyces. II. Analysis of the genes RDN1, HIS4, LEU2 and Ty transposable elements in Carlsberg, Tuborg, and 22 Bavarian brewing strains. Carlsberg Res. Commun. 50:262–272 [Google Scholar]
- 48. Pérez-Ortín JE, Querol A, Puig S, Barrio E. 2002. Molecular characterization of a chromosomal rearrangement involved in the adaptive evolution of yeast strains. Genome Res. 12:1533–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pritchard JK, Stephens M, Donnelly P. 2000. Inference of population structure using multilocus genotype data. Genetics 155:945–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Querol A, Bond U. 2009. The complex and dynamic genomes of industrial yeasts. FEMS Microbiol. Lett. 293:1–10 [DOI] [PubMed] [Google Scholar]
- 51. Rainieri S, et al. 2006. Pure and mixed genetic lines of Saccharomyces bayanus and Saccharomyces pastorianus and their contribution to the lager brewing strain genome. Appl. Environ. Microbiol. 72:3968–3974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sampaio JP, Gonçalves P. 2008. Natural populations of Saccharomyces kudriavzevii in Portugal are associated with oak bark and are sympatric with S. cerevisiae and S. paradoxus. Appl. Environ. Microbiol. 74:2144–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sipiczki M. 2008. Interspecies hybridization and recombination in Saccharomyces wine yeasts. FEMS Yeast Res. 8:996–1007 [DOI] [PubMed] [Google Scholar]
- 54. St Charles J, Hamilton ML, Petes TD. 2010. Meiotic chromosome segregation in triploid strains of Saccharomyces cerevisiae. Genetics 186:537–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wei W, et al. 2007. Genome sequencing and comparative analysis of Saccharomyces cerevisiae strain YJM789. Proc. Natl. Acad. Sci. U. S. A. 104:12825–12830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Winzeler EA. 2008. Direct allelic variation scanning of the yeast genome. Science 281:1194–1197 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.