Abstract
The species Babesia microti, commonly found in rodents, demonstrates a high degree of genetic diversity. Three lineages, U.S., Kobe, and Hobetsu, are known to have zoonotic potential, but their tick vector(s) in Japan remains to be elucidated. We conducted a field investigation at Nemuro on Hokkaido Island and at Sumoto on Awaji Island, where up to two of the three lineages occur with similar frequencies in reservoirs. By flagging vegetation at these spots and surrounding areas, 4,010 ticks, comprising six species, were collected. A nested PCR that detects the 18S rRNA gene of Babesia species revealed that Ixodes ovatus and I. persulcatus alone were positive. Lineage-specific PCR for rRNA-positive samples demonstrated that I. ovatus and I. persulcatus carried, respectively, the Hobetsu and U.S. parasites. No Kobe-specific DNA was detected. Infected I. ovatus ticks were found at multiple sites, including Nemuro and Sumoto, with minimum infection rates (MIR) of ∼12.3%. However, all I. persulcatus ticks collected within the same regions, a total of 535, were negative for the Hobetsu lineage, indicating that I. ovatus, but not I. persulcatus, was the vector for the lineage. At Nemuro, U.S. lineage was detected in 2 of 139 adult I. persulcatus ticks (MIR, 1.4%), for the first time, while 48 of I. ovatus ticks were negative for that lineage. Laboratory experiments confirmed the transmission of Hobetsu and U.S. parasites to hamsters via I. ovatus and I. persulcatus, respectively. Differences in vector capacity shown by MIRs at Nemuro, where the two species were equally likely to acquire either lineage of parasite, may explain the difference in distribution of Hobetsu throughout Japan and U.S. taxa in Nemuro. These findings are of importance in the assessment of the regional risk for babesiosis in humans.
INTRODUCTION
Babesia microti, which has long been considered a single species, demonstrates a high degree of genetic diversity (the so-called “Babesia microti group”) of rodent parasites. These parasites comprise several phylogenetically diverse clusters, each of which represents a monophyletic branch on phylogenetic trees of the β-tubulin and CCT7 genes and, only to some extent, on the slowly evolving 18S rRNA gene tree (9, 14, 22, 31, 37, 38). Three of these clusters, the Hobetsu, the U.S. (also referred as B. microti sensu stricto), and the Kobe taxa, have been associated with human infection. Other taxa, such as the Munich taxon in Europe and B. microti in Alaskan voles, are believed to be maintained only in wild animals. All of these lineages are found naturally parasitizing a variety of wild mammalian species in the rodent subfamilies of the Murinae, Arvicolinae, and Sigmodontinae and, occasionally, in the Soricidae shrew (9, 11, 12, 26, 30, 32, 34, 37, 38).
The frequencies of disease occurrence in humans and the patterns of geographic distribution differ greatly regardless of the wider and overlapping host ranges of the Babesia microti group. Symptomatic human cases of the U.S. parasite have been reported almost exclusively in the northeastern and upper midwestern United States (11, 12, 30), while in Europe only a single autochthonous case caused by the U.S. parasite has been described (10). No human case with this parasite lineage has been described in Asia. The Kobe lineage has been reported in a single case of transfusion-associated human babesiosis in Japan (22, 34). No symptomatic infection of the Hobetsu parasites has been reported, though subclinical human infection alone has been documented by serology, incidentally performed, at a rate of 1.05% (14/1,335) in residents of the rural Boso Peninsula, Chiba Prefecture, Japan, where tick-borne rickettsial disease (Japanese spotted fever) is endemic (1).
Similarly, the geographical distribution of the parasites varies greatly by lineage. The U.S. taxon is widely distributed throughout the Holarctic (both the Nearctic and Palearctic) temperate regions comprising the United States, Germany, Russia, China, South Korea, and Japan, where the parasites are harbored by various small mammals such as Peromyscus and Microtus spp. (rodents) and Blarina spp. (shrew) in North America and Apodemus, Myodes (formerly Clethrionomys), Microtus spp. (rodents), and Sorex spp. (shrew) in Eurasia, including Japan (2, 4, 9, 21, 37, 38). The two regional taxa of the Hobetsu and Munich, biologically inseparable from U.S. parasites but distinctive phylogenetically by the β-tubulin and CCT7 gene analyses, are common in Japan and Europe, respectively. In these regions either of the two taxa overlaps in geographic distribution with the U.S. taxon by adaptation to the same or similar reservoir hosts (2, 37). The Kobe lineage, also found in Apodemus and Ruttus spp., occurs only locally in a few narrowly defined areas such as Mikura Island in the Izu-Ogasawara (Izu-Bonin) Trench, Awaji Island near Osaka, and Daito-town on the western tip of the main island of Japan (26, 34), where the Hobetsu parasites have been shown to coexist in similar reservoir hosts. (On Mikura Island, the Kobe parasite was detected by chance from a wild rat despite the fact that local surveillance data have not been reported.)
In the United States, Ixodes scapularis (also been known as I. dammini) has been identified as the primary vector for B. microti parasites based on intensive field ecologic and laboratory studies, particularly on Nantucket Island, MA, where the mammalian and tick fauna are both scant (17–19, 24). Little is known regarding tick vectors elsewhere in the world. Inevitably, this raises the question of whether or not the regional ixodid species are equally competent as vectors for the regional lineages of B. microti parasites, particularly when two lineages, each with nearly identical rRNA gene sequences, co-occur. In selected areas in Japan, the U.S. and Kobe taxa are minimally sympatric with the widely distributed Hobetsu taxon (31, 38).
The present study was undertaken in areas of sympatry at Nemuro on Hokkaido Island and at Sumoto on Awaji Island, to assess the lineage-specific prevalence in ticks in order to ascertain which species of ticks are competent enough to be predominantly involved in the natural transmission of individual lineages of B. microti.
MATERIALS AND METHODS
Field collections.
Unfed, host-seeking ticks were collected in forests by flagging vegetation during March and September for 4 years (2000, 2001, 2002, and 2003) (Table 1). The survey areas included Sumoto on Awaji Island and Nemuro, Horonobe, Kiyosato, Shimokawa, Aibetsu, Furano, Hobetsu, Ebetsu, and Chitose on Hokkaido Island. The locations of the study areas are shown in Fig. 1. The areas where B. microti parasites were isolated repeatedly from rodents in previous reports are also listed in Table 1 (31, 34, 37). The investigations of rodent reservoirs were performed from 2000 to 2003. The species identification of ticks was determined by examination under microscopy of the collected tick morphological key characters of mouth parts, coxal shape, short proximal base-jointed segment of the leg, and adjacent structures as described by Takada (27) and Ehara (5). Ticks of the same species collected in the same area were pooled into groups (several ticks/tube) and frozen at −30°C until DNAs were extracted. The number of ticks in each pool is listed in Table 2. (At the beginning of the field survey only a few ticks were pooled; thereafter we pooled the collected ticks in groups of five to deal with as large a number of ticks as possible.)
Table 1.
Number, species, and stages of field-collected ticks
| Survey area | No. of ticks by species and stage |
Parasite lineage(s) in small mammalsa | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
I. ovatus |
I. persulcatus |
I. turdus |
I. tanuki |
H. flava |
H. douglasi |
Total | ||||||||
| Adult | Nymph | Adult | Nymph | Adult | Nymph | Adult | Nymph | Adult | Nymph | Adult | Nymph | |||
| Hokkaido Island | ||||||||||||||
| Nemuro | 137 | 0 | 174 | 298 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 610 | Hobetsu, U.S. |
| Horonobe | 4 | 0 | 43 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 49 | —b |
| Kiyosato | 171 | 0 | 275 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 3 | 471 | Hobetsu |
| Shimokawa | 270 | 0 | 35 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 305 | NDc |
| Aibetsu | 167 | 0 | 178 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 345 | ND |
| Furano | 265 | 0 | 83 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 348 | ND |
| Hobetsu | 1,252 | 6 | 99 | 60 | 0 | 0 | 0 | 0 | 0 | 2 | 5 | 27 | 1,451 | Hobetsu |
| Ebetsu | 36 | 0 | 15 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 52 | — |
| Chitose | 76 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 77 | ND |
| Subtotal | 2,738 | 0 | 903 | 371 | 2 | 0 | 1 | 0 | 0 | 2 | 15 | 30 | 3,708 | |
| Awaji Island | ||||||||||||||
| Sumoto | 190 | 0 | 0 | 0 | 11 | 12 | 0 | 0 | 14 | 75 | 0 | 0 | 302 | Hobetsu, Kobe |
| Total | 2,568 | 6 | 903 | 371 | 13 | 12 | 1 | 0 | 14 | 77 | 15 | 30 | 4,010 | |
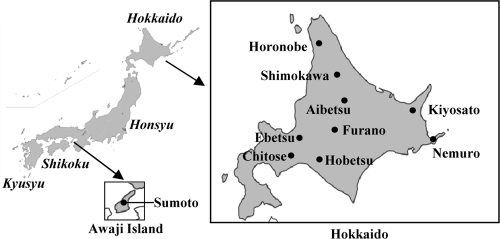
Fig 1.
Map of Japan showing the locations of field survey areas.
Table 2.
Detection of B. microti group-specific rRNA gene by PCR in the field-collected ticks
| Tick species | Survey area | Tick stage | No. of ticks examined | No. of ticks pooled | No. of ticks tested | No. of PCR-positive samples | Minimum infection rate (%)a |
|---|---|---|---|---|---|---|---|
| I. ovatus | Hokkaido Island | ||||||
| Nemuro | Adult | 48 | 2 to 3 | 19 | 4 | 8.3b | |
| Kiyosato | Adult | 65 | 5 | 13 | 8 | 12.3 | |
| Shimokawa | Adult | 100 | 5 | 20 | 2 | 2.0 | |
| Aibetsu | Adult | 85 | 5 | 17 | 0 | 0 | |
| Furano | Adult | 115 | 5 | 23 | 3 | 2.6 | |
| Hobetsu | Adult | 140 | 5 | 28 | 10 | 7.1 | |
| Ebetsu | Adult | 36 | 3 | 12 | 0 | 0 | |
| Chitose | Adult | 68 | 4 to 5 | 15 | 0 | 0 | |
| Awaji Island | |||||||
| Sumoto | Adult | 180 | 5 | 36 | 11 | 6.1 | |
| Subtotal | 789 | 183 | 38 | ||||
| I. persulcatus | Hokkaido Island | ||||||
| Nemuro | Adult | 139 | 3 to 5 | 33 | 2 | 1.4 | |
| Nymph | 196 | 1 | 196 | 0 | 0 | ||
| Horonobe | Adult | 42 | 3 | 14 | 0 | 0 | |
| Kiyosato | Adult | 105 | 5 | 21 | 0 | 0 | |
| Shimokawa | Adult | 15 | 5 | 3 | 0 | 0 | |
| Aibetsu | Adult | 85 | 5 | 17 | 0 | 0 | |
| Furano | Adult | 44 | 5 | 15 | 0 | 0 | |
| Hobetsu | Adult | 36 | 33 | 13 | 0 | 0 | |
| Ebetsu | Adult | 15 | 5 | 3 | 0 | 0 | |
| Subtotal | 677 | 315 | 2 | 0.3 | |||
| Other tick species | Six areasc | Adult | 162 | 162 | 0 | 0 | |
| Total | 1,656 | 542 | 40 | 2.4 |
Values (%) were calculated by comparing the number of pools that were PCR positive for the B. microti group to the total number of ticks examined. The calculation was based on the assumption that each PCR-positive pool contains at least one tick with a detectable B. microti group parasite(s).
Considering the impact of the comparison of MIRs, the possible MIR would be between 2 and 8.3% when 5 ticks were pooled.
Nemuro, Horonobe, Kiyosato, Hobetsu, Ebetsu, and Sumoto were included.
Experimental animals.
Specific-pathogen-free (SPF) golden hamsters (Std: Syrian) at 8 weeks of age were purchased from SLC Inc. (Shizuoka, Japan). They were housed in vinyl-film isolators at a temperature of between 22 and 25°C and were provided with a gamma-irradiated pellet diet and autoclaved tap water. Animal experimentation was carried out according to the Laboratory Animal Control Guidelines of Rakuno-Gakuen University.
Extraction of DNA from ticks.
Ticks were processed by pestle homogenizer (Scientific Specialties, Inc., Lodi, CA) and suspended in TNE buffer (10 mM Tris-HCl, 150 mM NaCl, and 100 mM EDTA, pH 8.0) containing 1% sodium dodecyl sulfate (SDS). The suspensions were digested with 100 μg/ml proteinase K at 55°C overnight. DNAs were purified by phenol extraction followed by ethanol precipitations. Pellets were dissolved in 100 μl of TE buffer (10 mM Tris-HCl and 1 mM EDTA, pH 7.5).
PCR assay.
To amplify the 18S rRNA genes of all lineages of the B. microti group, we carried out nested PCRs using primer sets Bab1A and Bab4A for the first round and Bab2A and Bab3A for the second round (38). For the first-round PCR, approximately 1 μl of each of the final DNA preparations described above was used as a template in a 20-μl volume. For the second round, 1 μl of the first PCR product was used. Two universal primer sets for nested PCR, BmTubu93F and BmTubu897R for the first round and BmTubu192F and BmTubu783R for the second round, were used to detect β-tubulin genes of all B. microti lineages. Detailed procedures are described by Zamoto et al. (38). To prevent cross-contamination, all DNA preparations and PCRs were performed using filter tips in an isolated room ventilated with filtered air.
Data collected by PCR testing were used to provide an estimate of the infection prevalence of tick species. The minimum infection rate (MIR) was calculated based on the assumption that a PCR-positive pool contains only one positive tick (Table 2).
Lineage-specific PCR.
Lineage-specific PCR based on the β-tubulin gene, as described previously by Zamoto et al. (38), was performed, by which B. microti parasites could be classified into distinct lineages as Hobetsu, U.S., and Kobe. Briefly, a set of primers, BmTubu93F and BmTubu897R, were used for the first-round PCR to amplify all three lineages of β-tubulin gene sequences. Three other sets of primers, Tubu-US5′ and Tubu-US3′, Tubu-Ho5′ and Tubu-Ho3′, and Tubu-Ko5′ and Tubu-Ko3′, previously designed specifically to amplify the U.S., Hobetsu, and Kobe lineages, respectively, were used for second-round PCR as described previously (38).
Activation, preparation, and experimental transmission of Hobetsu sporozoites into hamsters.
In areas where the Hobetsu parasites are solely distributed, Hobetsu and Shimokawa in Hokkaido Island, 77 I. ovatus females were collected. They fed on four naive hamsters for 4 days to activate quiescent salivary gland sporozoites into a state of readiness for infection. Thereafter, the partially engorged ticks were removed manually. The salivary glands were dissected from the ticks under a stereomicroscope and were pooled into four groups corresponding to the hamsters used to feed. Each pool in 600 μl of cold phosphate-buffered saline (PBS) was homogenized by a tissue grinder (glass wall tissue grinder; Radnoti, Monrovia, CA). A total of four samples, 500 μl of each, were inoculated individually into four noninfected hamsters through the intraperitoneal route. The hamsters were monitored every 2 or 3 days for 3 months by measuring parasitemia in Giemsa-stained thin blood smears. To predict the presence of the Hobetsu parasites in the test samples, the remaining 100-μl aliquot of salivary gland homogenates was checked by PCR using a set of lineage-specific primers. DNA extraction and PCR testing were performed according to the methods described above.
Experimental transstadial transmission of the U.S. parasites by nymphal I. persulcatus.
To prepare naive larvae, we collected adult I. persulcatus females in Ebetsu, Hokkaido Island, where B. microti is not known to exist (31, 37). Female ticks were fed on hamsters free from parasite infection. The engorged ticks that dropped from the hamsters were placed at room temperature until eggs were laid in sealed glass tubes over a moistened filling of solidified plaster with activated charcoal. Eggs were aliquoted into small glass tubes and incubated until use. To ensure that the larvae were free of B. microti infection, the larvae hatched from the eggs were examined by nested PCRs, despite the reported lack of evidence for transovarial transmission of B. microti (33). Thereafter, the larvae at one or more months of age were fed on splenectomized hamsters that had been infected with B. microti NM69, a strain of the U.S. taxon originally isolated in Hokkaido, Japan (37). The infected hamsters were treated individually with 10 mg of prednisolone (Fujita Inc., Nagoya, Japan) intraperitoneally to keep the parasitemia at 10 to 20%. The engorged larvae dropped from the infected hamsters were collected and were sealed in glass tubes at room temperature as described above. After approximately 3 weeks of incubation, the larvae molted into nymphs. At 3 to 20 weeks after molting, the resulting nymphs at various ages (see Table 5) were fed on noninfected hamsters. All nymphs that engorged and dropped from the hamsters were subjected to lineage-specific PCR, to judge the existence of transstadially transmitted strain NM69. To monitor the parasite emergence in the hamsters, thin blood smears were prepared every 2 or 3 days for 3 months and at least 1,000 erythrocytes were observed under a light microscope.
Table 5.
Transstadial transmission of NM69 by I. persulcatus to hamstera
| Expt no. | Age of nymphs (wk)b | No. positive/no. examinedc | Transmission (prepatent period)d |
|---|---|---|---|
| 1 | 3 | 0/7 | — |
| 2 | 4 | 0/12 | — |
| 3 | 4 | 3/13 | — |
| 4 | 4 | 1/11 | — |
| 5 | 6 | 2/17 | 20 |
| 6 | 6 | 6/19 | 20 |
| 7 | 10 | 1/11 | 21 |
| 8 | 13 | 2/11 | 21 |
| 9 | 20 | 2/33 | 16 |
| 10 | 20 | 3/33 | 16 |
| Total | 20/167 |
I. persulcatus nymphs infected with strain NM69 of U.S. taxon at their larval stage were fed on Syrian hamsters.
Weeks after nymphs molted.
Number of engorged nymphs positive for U.S. lineage-specific DNA/number of engorged nymphs examined.
Days of emergence of parasites in erythrocytes after nymphs dropped from hamster (>0.05% presence in erythrocytes). —, absence of parasite infection was confirmed by PCR on hamster erythrocytes (see Materials and Methods).
RESULTS
Species, stages, and number of field-collected ticks.
A total of 4,010 ticks (3,514 adults and 496 nymphs) were collected by flagging vegetation in the nine forest areas on Hokkaido Island and one forest area on Awaji Island in Japan (Table 1). The collected ticks included six species, Ixodes ovatus, I. persulcatus, I. turdus, I. tanuki, Haemaphysalis flava, and H. douglasi. While the I. ovatus tick species was collected from all the areas surveyed, it was found in greatest abundance (64% of ticks) on Hokkaido Island (72%) and Awaji Island (62%). Since northern Japan has a subarctic climate while the southern part is subtropical, the vegetation and population of ticks collected varied between the islands. In Hokkaido, I. persulcatus ticks were collected in abundance (34%, 1,274/3,708) from all nine survey areas, whereas the collection frequencies varied considerably among the areas, from 8% to 99%. The third most frequently collected tick was H. douglasi, which was collected in three areas in Hokkaido with a 0.16% to 2.5% collection frequency. The minority of species collected were two I. turdus ticks at Horonobe, one I. tanuki tick at Ebetsu, and two H. flava ticks at Hobetsu. In Awaji Island, no I. persulcatus representatives were collected and H. flava was the predominant species (89/302, 29.5%). As the third most collected tick, I. turdus comprised 7.6% (23/302) of the total ticks collected in Awaji. Male and female ticks were collected at nearly the same ratio in all tick species (data not shown). Focusing on the areas of sympatry of the two parasite lineages, I. persulcatus (472/610, 77%), I. ovatus (137/610, 22%), and H. douglasi (1/610, 0.16%) in Nemuro and I. ovatus (190/302, 63%), H. flava (89/302, 29%), and I. turdus (23/302, 7.6%) in Sumoto were the most common species collected.
Detection of B. microti group parasites in field-collected ticks.
To assess the presence of B. microti group parasites in the field-collected ticks, nested PCR targeting the rRNA gene (38) was performed with DNA samples extracted from pooled or individual ticks (Table 2). Among 542 samples, positive signals were detected in 40, including 38 from I. ovatus (of 183 samples) and 2 from I. persulcatus (of 315 samples). No PCR product was amplified from 162 DNA samples from other tick species, I. turdus, I. tanuki, H. flava, and H. douglasi. I. ovatus ticks carrying B. microti group parasites were found in 6 of 9 areas examined. MIRs of these I. ovatus ticks varied among survey areas, from 12.3% at Kiyosato to 2.0% at Shimokawa. I. persulcatus ticks carrying B. microti group parasites were found only at Nemuro, where the MIRs were 1.4% in adults and 0% in nymphs. Focusing on areas of sympatry, I. ovatus and I. persulcatus in Nemuro were found to be infected, with MIRs of 8.3% (4/48) and 0.6% (2/335, adult and nymph), respectively. In Sumoto, only an I. ovatus tick was found to be infected with a B. microti group member, with an MIR of 6.1% (11/180).
Genotyping of B. microti group parasites found in I. ovatus and I. persulcatus.
To classify the B. microti group parasites detected, all of the rRNA-positive samples were further examined by lineage-specific PCR based on the β-tubulin gene (38) (Table 3). By the use of universal primers that amplify β-tubulin sequences of all lineages of B. microti group parasites, all of the samples positive for parasite rRNA were confirmed to be PCR positive (Table 3, B. microti group). Subsequent tests using lineage-specific primers revealed that only the Hobetsu-specific and not the U.S.- or Kobe-specific sequences were detected, regardless of the tick collection area, in the 38 I. ovatus samples examined (Table 3, parasite lineage). From the two I. persulcatus samples determined to be parasite positive by our screening tests, only the U.S.- and never the Hobetsu- or Kobe-specific sequences were amplified (Table 3, parasite lineage).
Table 3.
Genotyping of B. microti group found in field-collected ticks
| Tick species | No. of positive samples/no. of samples examined |
|||
|---|---|---|---|---|
| B. microti groupa | Parasite lineageb |
|||
| Hobetsu | U.S. | Kobe | ||
| I. ovatus | 38/38 | 38/38 | 0/38 | 0/38 |
| I. persulcatus | 2/2 | 0/2 | 2/2 | 0/2 |
Nested PCRs were performed using universal primers targeting B. microti group β-tubulin sequences.
Nested PCRs were performed using lineage-specific primers targeting β-tubulin sequences of each lineage.
Infectivity of salivary gland sporozoites of the Hobetsu lineage from I. ovatus ticks.
To confirm that Hobetsu lineage parasites occurring in I. ovatus ticks were infectious to mammals, we tested the sporozoite infectivity by inoculating the salivary gland homogenates of I. ovatus ticks into naive hamsters (Table 4). Four pools of activated salivary glands were prepared from 77 I. ovatus females, which were collected in an area where the Hobetsu lineage is endemic. A portion of each pool was examined for the existence of parasites by PCR, and the remainder was inoculated into. By lineage-specific PCR, two pools were proven to contain Hobetsu-lineage parasites, whereas others tested negative (Table 4). All four of the pools were individually inoculated into naive hamsters (Table 4). At 10 and 19 days after inoculation, two of these hamsters started to show parasitemia in which intraerythrocytic parasites exhibited multiple morphologies: dot, ring, ovoid, piriform, amoeboid, crescent-arch, and Maltese cross (Table 4). The parasites grown in two hamsters were both confirmed to be of Hobetsu lineage by the lineage-specific PCR, and thus this is the first direct evidence that the Hobetsu parasites in I. ovatus salivary glands were immediately infectious to the vertebrate hosts. The other two hamsters which were inoculated with PCR-negative samples did not develop parasitemia over a 3-month period and showed negative results on the nested PCR for parasite DNA in the blood.
Table 4.
Infection of hamsters by Hobetsu sporozoites in the salivary glands of field-collected I. ovatus ticks
| Expt no. | No. of ticks pooleda | Lineage-specific PCRb | Infection of hamstersc |
|---|---|---|---|
| 1 | 12 | — | − |
| 2 | 20 | Hobetsu | + |
| 3 | 20 | Hobetsu | + |
| 4 | 25 | — | − |
Salivary glands were dissected from the indicated number of field-collected ticks.
Results of lineage-specific PCR performed on the pooled salivary glands. —, absence of detectable PCR product in hamster erythrocytes.
Parasites were grown (or were unable to be grown) in the hamsters after intraperitoneal injection with the pooled tick salivary glands.
Transstadial transmission of U.S. parasites by nymphal I. persulcatus.
Since only a limited number of naturally infected I. persulcatus ticks could be collected (Table 2), we examined vector competence (vector ability to acquire, maintain, and transmit pathogens) by generating parasite-infected I. persulcatus ticks in the laboratory. Larvae hatched from eggs were fed on hamsters which had previously been infected with B. microti strain NM69, a U.S. lineage parasite isolated in Japan (37), and engorged larvae dropped from the infected hamsters were incubated to allow molting to the nymphal stage. To confirm that the larvae took up parasitized erythrocytes, the larvae dropped from the hamsters were individually examined by lineage-specific PCR. Parasitic load in the engorged larvae was 100% (40/40). However, a dramatic decrease in the positive rate was observed when the larvae were molted into nymphs: 10% (2/20) of the resulting nymphs were positive. Thereafter the rates reduced (Table 5) and remained nearly constant at 10 to 20% during the 16 weeks after molting (data not shown). To examine whether the resulting nymphs carrying NM69 were competent to transmit the parasites, the nymphs at various ages (3 to 20 weeks) were fed on naive hamsters (Table 5). A total of 167 nymphs were successfully fed, engorged, and dropped from the hamsters. Among the engorged nymphs, 20 were PCR positive and thus 12% (20/167) were believed to have become infected with NM69 (Table 5). Corresponding to the given PCR-positive rate, hamsters fed on by PCR-positive nymphs developed parasitemia (Table 5, experiments 5, 6, 7, 8, 9, and 10), except for the two hamsters which were fed on by the nymphs at the ages of 3 and 4 weeks (Table 5, experiments 3 and 4). Parasites were observed in erythrocytes as early as 16 to 20 days after the nymphs dropped from the hamsters (Table 5). Even in a hamster fed on by one infected nymph, the parasites grew successfully and an intense parasitemia developed (Table 5, experiment 7). The hamsters fed on by PCR-positive nymphs as early as 3 and 4 weeks after molting (Table 5, experiments 3 and 4), as well as those fed on by PCR-negative nymphs (Table 5, experiments 1 and 2), did not develop any detectable parasitemia during the 3-month follow-up period after challenge (Table 5, experiments 1 and 2). The absence of NM69 growth was further confirmed by lineage-specific PCR on the red blood cells taken from these hamsters. It was demonstrated conclusively that NM69, a Japanese strain of U.S. lineage, transmitted unambiguously from stage to stage in I. persulcatus ticks. It was also verified that the development of this parasite to a stage infective for mammalian hosts was completed within 6 weeks after molting in the I. persulcatus nymphs.
DISCUSSION
In order to identify the principal vector for the three lineages of B. microti group parasites, U.S., Kobe, and Hobetsu, in Japan, we conducted a field study at two Japanese sites, Nemuro on Hokkaido Island and Sumoto on Awaji Island, where an anticipated high yield of up to two vectors could be expected in various combinations within the same or similar reservoir hosts (31, 34, 37). Geographical distributions and frequencies of tick species collected in the present study in the two study areas as well in the eight surrounding areas (Table 1) corresponded largely to previously reported data for Japan (5, 13, 27, 28, 35). I. ovatus and I. persulcatus on Hokkaido Island and I. ovatus and H. flava on Awaji Island were the two most abundant tick species. I. persulcatus, a species phylogenetically similar to I. scapularis and I. ricinus (I. ricinus species complex) (8) and reported to be distributed widely from the taiga forests of Eastern Europe to East Asia, including the northern half of Japan (3, 13, 27), was frequently collected throughout Hokkaido (9 of 9 areas) but was not found at Awaji Island (Table 1). I. ovatus, a species clustering unambiguously into a group distinct from the I. ricinus species complex (8) and known to be distributed widely throughout East Asia, including Japan, was detected in all surveyed areas at high frequencies: 74% (2738/3708) on Hokkaido Island and 63% (190/302) on Awaji Island (Table 1). Interestingly, adult ticks of these two species, I. persulcatus and I. ovatus, are known to be the most abundant species encountered on humans in Japan and are recognized as a Lyme disease vector (15) and tick-borne encephalitis vector (29), respectively.
Subsequently, we assessed the tick prevalence of B. microti infection at Nemuro, Hokkaido Island, where Hobetsu and U.S. lineages of B. microti parasites occur in the same or similar reservoir hosts (31, 37). I. ovatus and I. persulcatus were predominantly collected and were shown to be positive for the Babesia rRNA gene by nested PCR (Table 1 and 2). At the larval and nymphal stages they have overlapping sets of mammalian hosts (5, 7, 27, 28), such as Apodemus and Myodes rodents and Sorex shrews, which were identified to be infected with B. microti group parasites in Japan (31, 34, 37). Considering the known feeding habits of the larval and nymphal stages, the likelihoods of the two ticks to acquire their respective parasites are presumed to be equal, as evidenced by their geographical distributions and densities (Table 1) (5, 7, 27, 28) and by the similar frequencies of the two parasite lineages among the main reservoir mice, Myodes (both equal to 12% of lineage-specific infections and an additional case of mixed infection with both lineages), at Nemuro (38). However, the Hobetsu lineage was detected only in I. ovatus (4 of 19 samples, including 48 ticks) and not in any of the 335 I. persulcatus ticks (Tables 2 and 3). Likewise, the lineage was detected in I. ovatus but not I. persulcatus collected in the surrounding areas of Kiyosato, Shimokawa, Furano, and Hobetsu (Tables 2 and 3). These results indicate that I. ovatus but not I. persulcatus is a vector for Hobetsu lineage. Although it is not demonstrated by laboratory experiments (16), we speculate, based on the results of the epidemiological survey (Table 2) and transstadial transmission study using U.S.-lineage parasites, that Hobetsu parasites, once having acquired equal exposure to I. ovatus and I. persulcatus, might be cleared from I. persulcatus in the time period of development from nymph to adult. In contrast, the parasites acquired by I. ovatus might multiply and undergo further development. As for the U.S. lineage, 33 samples of I. persulcatus (including 139 adult ticks), but not 19 samples of I. ovatus (including 48 adult ticks), collected in Nemuro contained two positive samples (Table 3), indicating, possibly, an etiological vector in nature. Indeed, vector competence of the tick was successfully demonstrated in our laboratory experiments (Table 5). Because the field incidence was not sufficient, at this point, we could not eliminate the possibility of I. ovatus as a vector for U.S.-lineage parasites and arrive at a conclusion concerning the specific parasite-tick epidemiological relevance. Nevertheless, the entirely negative PCR results for Hobetsu and U.S. parasites (Table 2), respectively, with 677 I. persulcatus ticks from all areas in Hokkaido and with 48 I. ovatus ticks at Nemuro with a very low tick species richness (Table 1), were a meaningful finding. An increase in field collections and/or additional laboratory experiments with all possible parasite-vector combinations may define more clearly the parasite-vector relationships occurring naturally in Japan.
B. microti has been discovered in as many as 5% of nymphal I. dammini ticks collected on Nantucket Island, MA, where human babesiosis (Nantucket fever) was first identified (20). Babesiosis due to B. microti emerged as a recognized human disease only in the late 1960s, and I. dammini was first recognized at that time. By the mid-1970s, babesiosis in the United States had become more commonly associated with black-legged ticks (I. dammini). These ticks have expanded their geographic range along the borders of the northeastern and the upper midwestern United States (20). Interestingly, the Hobetsu taxon, first described by Tsuji et al. in 2001 (31), is known to be widely distributed throughout Japan (31, 38), and the geographic pattern of distribution in reservoir hosts was comparable to that seen for I. ovatus ticks (Table 2). On the other hand, the geographical distribution of I. persulcatus that occurred widely throughout Hokkaido was, interestingly, not correlated with the local prevalence of U.S. taxon but was confined narrowly to the Nemuro area in association with both vector ticks and reservoir hosts (38) (Tables 1 and 2). It is our speculation that the vector competence of ticks may vary greatly among the parasites of the B. microti group, and the difference in vector competency may be the primary factor in determining the emergence and geographical spread of the lineage of parasites. An explanation for the disparate distribution of Hobetsu and U.S. lineages may rightly be attributed to the different competencies of these vectors. In fact, the prevalence of the Hobetsu taxon in the population of host-seeking I. ovatus ticks appears to range from almost twice to almost 6 times (∼12.3% and 8.3% at Nemuro) (Table 2), respectively, those of I. dammini on Nantucket Island (5% for the U.S. lineage) and I. persulcatus at Nemuro (1.4% for the U.S. lineage) (Table 2).
Kobe lineage occurs in wild rodents in a few narrowly defined focal areas in Japan (26, 31, 34). Three tick species, I. ovatus, H. flava, and I. turdus, were collected (Table 1) at Sumoto on Awaji Island, where two lineages, Kobe and Hobetsu, are reported to coexist in Apodemus mice at an equal incidence (29%) (31, 34). A Kobe-specific β-tubulin sequence, however, was not detectable from any of the tick species examined (Tables 2 and 3). By broadly reactive PCR assay for the rRNA gene of Babesia, I. ovatus alone was positive (Table 2) and, from the rRNA gene-positive samples, only the Hobetsu-specific β-tubulin sequence was detectable (Tables 2 and 3). A negative PCR result with large-enough sample sizes of 190 I. ovatus ticks and 89 H. flava ticks (Tables 2 and 3) for Kobe parasites was a meaningful finding; therefore, at least two tick species are unlikely to be the principal vector for Kobe lineage. We also hypothesize that the tick candidate for Kobe lineage retains a high vector competency and feeds on two or three different hosts, as evidenced by the finding that Sumoto on Awaji Island is the place where the blood donors who were the Japanese index cases for babesiosis presumably were infected by tick bites containing the Kobe parasite (31, 34).
There have been two further reports that describe the presence of sporoblasts detected by electron microscopy in the salivary glands of I. ovatus ticks that are positive for the Hobetsu (referred to as Otsu)-specific rRNA gene sequence (23, 36). The results of our present study were subjected to vigorous analysis and are, to our knowledge, the first to prove that Hobetsu and perhaps U.S. and Kobe lineages in nature are vectored independently by the tick species I. ovatus, I. persulcatus, and possibly another ixodid species.
Within the B. microti group, the four intragroup taxa—Hobetsu, U.S., Kobe, and Munich—are each shown to demonstrate differences in the presentation of several genetic traits. (i) There are substantial levels of sequence divergence that are closely comparable to those found between pairs of the β-tubulin and CCT7 genes found in such well-recognized species as B. odocoilei and B. divergens (14, 32, 38). (ii) CCT7 introns, regardless of a great degree of size diversity among taxa (19 to 254 nucleotides) (6, 14), exhibit uniformity in size within each given taxon (less than one base difference) at their respective positions, thus distinguishing them one from the other (6, 14). (iii) The estimated patterns of genetic population structures based on the sequences of CCT7 introns are different for each taxon. Hobetsu and Munich taxa, common to Japan and Europe, respectively, exhibit little or no pairwise sequence divergence among geographically diverse samples; this suggests the occurrence of an extreme population bottleneck during recent history. Conversely, U.S. and Kobe taxa, widely distributed over the Northern Hemisphere and within several narrowly defined areas in Japan, show extreme genetic divergence among geographical samples (6). In addition to the genetic disparities within the lineages described above and slight cross-reactivities of antigens (31), it was suggested for the first time that the Hobetsu taxon differs biologically in vector specificity from the U.S. taxon (B. microti sensu stricto). Judging from the biologic and genetic characteristics, it may be necessary to designate a new and separate species for the Hobetsu lineage.
ACKNOWLEDGMENTS
We are grateful to Sam R. Telford III (Tufts University) for the excellent advice on transmission experiments. We thank Chiharu Morita (Rakuno-Gakuen University) for his help in the epidemiological survey and Takako K. Kurata (Rakuno-Gakuen University) for her excellent technical assistance. With heartfelt appreciation, we also thank Danny H.-Kauffmann Jokl (Columbia University Medical Center) for critical reading of the manuscript and helpful suggestions.
This work was supported in part by Grants-in-Aid from the Ministry of Education, Science and Culture of Japan, by Health Science Grants for Research in Emerging and Re-emerging Infectious Diseases from the Ministry of Health, Labor and Welfare of Japan, by a Grant-in-Aid to Cooperative Research from Rakuno-Gakuen University, and by Gakujutsu Frontier Cooperative Research in Rakuno-Gakuen University.
Footnotes
Published ahead of print 2 March 2012
REFERENCES
- 1. Arai S, et al. 2003. Retrospective seroepidemiological survey for human babesiosis in an area in Japan where a tick-borne disease is endemic. J. Vet. Med. Sci. 65:335–340 [DOI] [PubMed] [Google Scholar]
- 2. Beck R, et al. 2011. Molecular survey of Babesia microti in wild rodents in central Croatia. Vector Borne Zoonotic Dis. 11:81–83 [DOI] [PubMed] [Google Scholar]
- 3. Brown RN, Lane RS, Dennis DT. 2005. Geographic distributions of tick-borne diseases and their vectors, p 363–391 In Goodman JL, Dennis DT, Sonenshine DE. (ed), Tick-borne diseases of humans. ASM Press, Washington, DC [Google Scholar]
- 4. Duh D, Petrovec M, Trilar T, Avsic-Zupanc T. 2003. The molecular evidence of Babesia microti infection in small mammals collected in Slovenia. Parasitology 126:113–117 [DOI] [PubMed] [Google Scholar]
- 5. Ehara S. 1980. Family Ixodidae. In Ehara S. (ed), Illustrations of the mites and ticks in Japan. Zenkoku Noson Kyoiku Kyokai, Tokyo, Japan: (In Japanese) [Google Scholar]
- 6. Fujisawa K, et al. 2011. Intron sequences in the CCT7 gene show diverse evolutionary histories of the four lineages within the “Babesia microti group,” a genetically related species-complex including pathogens of humans. Jpn. J. Infect. Dis. 64:403–410 [PubMed] [Google Scholar]
- 7. Fujita H, Takahashi M, Yamamoto S, Saito T, Machida K. 1981. Ixodid ticks (Acari: Ixodidae) parasitic on mammals and birds in Saitama and Gumma Prefectures, Central Japan. 1. Host-tick relationships, geographical and vertical distributions, and medical problems. Annu. Rep. Ohara Hosp. 24:13–27 (In Japanese) [Google Scholar]
- 8. Fukunaga M, Yabuki M, Hamase A, Oliver JH, Jr, Nakao M. 2000. Molecular phylogenetic analysis of ixodid ticks based on the ribosomal DNA spacer, internal transcribed spacer 2, sequences. J. Parasitol. 86:38–43 [DOI] [PubMed] [Google Scholar]
- 9. Goethert HK, Telford SR., III 2003. What is Babesia microti? Parasitology 127:301–309 [DOI] [PubMed] [Google Scholar]
- 10. Hildebrandt A, et al. 2007. First confirmed autochthonous case of human Babesia microti infection in Europe. Eur. J. Clin. Microbiol. Infect. Dis. 26:595–601 [DOI] [PubMed] [Google Scholar]
- 11. Homer MJ, Aguilar-Delfin I, Telford SR, III, Krause PJ, Persing DH. 2000. Babesiosis. Clin. Microbiol. Rev. 13:451–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kjemtrup AM, Conrad PA. 2000. Human babesiosis: an emerging tick-borne disease. Int. J. Parasitol. 30:1323–1337 [DOI] [PubMed] [Google Scholar]
- 13. Kolonin GV. 2009. Fauna of ixodid ticks of the world (Acari, Ixodidae). http://www.kolonin.org/1.html
- 14. Nakajima R, et al. 2009. Babesia microti-group parasites compared phylogenetically by complete sequencing of the CCTeta gene in 36 isolates. J. Vet. Med. Sci. 71:55–68 [DOI] [PubMed] [Google Scholar]
- 15. Nakao M, Miyamoto K, Fukunaga M, Hashimoto Y, Takahashi H. 1994. Comparative studies on Borrelia afzelii isolated from a patient of Lyme disease, Ixodes persulcatus ticks, and Apodemus speciosus rodents in Japan. Microbiol. Immunol. 38:413–420 [DOI] [PubMed] [Google Scholar]
- 16. Oliveira MR, Kreier JP. 1979. Transmission of Babesia microti using various species of ticks as vectors. J. Parasitol. 65:816–817 [PubMed] [Google Scholar]
- 17. Piesman J, Karakashian SJ, Lewengrub S, Rudzinska MA, Spielmank A. 1986. Development of Babesia microti sporozoites in adult Ixodes dammini. Int. J. Parasitol. 16:381–385 [DOI] [PubMed] [Google Scholar]
- 18. Piesman J, et al. 1987. Seasonal variation of transmission risk of Lyme disease and human babesiosis. Am. J. Epidemiol. 126:1187–1189 [DOI] [PubMed] [Google Scholar]
- 19. Piesman J, Spielman A. 1979. Host-associations and seasonal abandance of immature Ixodes dammini in southeastern Massachusetts. Ann. Entomol. Soc. Am. 72:829–832 [Google Scholar]
- 20. Piesman J, Spielman A. 1980. Human babesiosis on Nantucket Island: prevalence of Babesia microti in ticks. Am. J. Trop. Med. Hyg. 29:742–746 [DOI] [PubMed] [Google Scholar]
- 21. Rar VA, Epikhina TI, Livanova NN, Panov VV. 2011. Genetic diversity of Babesia in Ixodes persulcatus and small mammals from North Ural and West Siberia, Russia. Parasitology 138:175–182 [DOI] [PubMed] [Google Scholar]
- 22. Saito-Ito A, et al. 2000. Transfusion-acquired, autochthonous human babesiosis in Japan: isolation of Babesia microti-like parasites with hu-RBC-SCID mice. J. Clin. Microbiol. 38:4511–4516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saito-Ito A, Yano Y, Dantrakool A, Hashimoto T, Takada N. 2004. Survey of rodents and ticks in human babesiosis emergence area in Japan: first detection of Babesia microti-like parasites in Ixodes ovatus. J. Clin. Microbiol. 42:2268–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spielman A. 1976. Human babesiosis on Nantucket Island: transmission by nymphal Ixodes ticks. Am. J. Trop. Med. Hyg. 25:784–787 [DOI] [PubMed] [Google Scholar]
- 25. Reference deleted.
- 26. Tabara K, et al. 2007. Molecular survey of Babesia microti, Ehrlichia species and Candidatus neoehrlichia mikurensis in wild rodents from Shimane Prefecture, Japan. Microbiol. Immunol. 51:359–367 [DOI] [PubMed] [Google Scholar]
- 27. Takada N. 1990. A pictorial review of medical acarology in Japan. Kinpodo, Kyoto, Japan: (In Japanese.) [Google Scholar]
- 28. Takada N, Yamaguchi T. 1974. Studies on ixoidid fauna in the northern part of Honshu, Japan. 1. Ixoidid ticks (Ixodidae) parasitic on wild mammals and some cases of human infection. Jpn. J. Sanit. Zool. 25:35–40 (In Japanese) [Google Scholar]
- 29. Takeda T, et al. 1998. Isolation of tick-borne encephalitis virus from Ixodes ovatus (Acari: Ixodidae) in Japan. J. Med. Entomol. 35:227–231 [DOI] [PubMed] [Google Scholar]
- 30. Telford SR, III, Gorenflot A, Brasseur P, Spielman A. 1993. Babesial infections in humans and wildlife, p 1–47 In Kreier J. (ed), Parasitic protozoa, vol 5 Academic Press Inc, New York, NY [Google Scholar]
- 31. Tsuji M, et al. 2001. Human babesiosis in Japan: epizootiologic survey of rodent reservoir and isolation of new type of Babesia microti-like parasite. J. Clin. Microbiol. 39:4316–4322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsuji M, et al. 2006. Babesia microti-like parasites detected in Eurasian red squirrels (Sciurus vulgaris orientis) in Hokkaido, Japan. J. Vet. Med. Sci. 68:643–646 [DOI] [PubMed] [Google Scholar]
- 33. Walter G, Weber G. 1981. A study on the transmission (transstadial, transovarial) of Babesia microti, strain “Hannover i,” in its tick vector, Ixodes ricinus. Tropenmed. Parasitol. 32:228–230 [PubMed] [Google Scholar]
- 34. Wei Q, et al. 2001. Human babesiosis in Japan: isolation of Babesia microti-like parasites from an asymptomatic transfusion donor and from a rodent from an area where babesiosis is endemic. J. Clin. Microbiol. 39:2178–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yamaguti N, Tipton VJ, Keegan HL, Toshioka S. 1971. Ticks of Japan, Korea, and the Ryukyu Islands. Brigham Young Univ. Sci. Bull. Biol. Ser. 15:1–226 [Google Scholar]
- 36. Yano Y, Saito-Ito A, Anchalee D, Takada N. 2005. Japanese Babesia microti cytologically detected in salivary glands of naturally infected tick Ixodes ovatus. Microbiol. Immunol. 49:891–897 [DOI] [PubMed] [Google Scholar]
- 37. Zamoto A, et al. 2004. U.S.-type Babesia microti isolated from small wild mammals in Eastern Hokkaido, Japan. J. Vet. Med. Sci. 66:919–926 [DOI] [PubMed] [Google Scholar]
- 38. Zamoto A, et al. 2004. Epizootiologic survey for Babesia microti among small wild mammals in northeastern Eurasia and a geographic diversity in the beta-tubulin gene sequences. J. Vet. Med. Sci. 66:785–792 [DOI] [PubMed] [Google Scholar]