Abstract
This work determined the impact of irrigation on the seasonal dynamics of populations of Pseudomonas spp. producing the antibiotics phenazine-1-carboxylic acid (Phz+) and 2,4-diacetylphloroglucinol (Phl+) in the rhizosphere of wheat grown in the low-precipitation zone (150 to 300 mm annually) of the Columbia Plateau of the Inland Pacific Northwest. Population sizes and plant colonization frequencies of Phz+ and Phl+ Pseudomonas spp. were determined in winter and spring wheat collected during the growing seasons from 2008 to 2009 from selected commercial dryland and irrigated fields in central Washington State. Only Phz+ bacteria were detected on dryland winter wheat, with populations ranging from 4.8 to 6.3 log CFU g−1 of root and rhizosphere colonization frequencies of 67 to 100%. The ranges of population densities of Phl+ and Phz+ Pseudomonas spp. recovered from wheat grown under irrigation were similar, but 58 to 100% of root systems were colonized by Phl+ bacteria whereas only 8 to 50% of plants harbored Phz+ bacteria. In addition, Phz+ Pseudomonas spp. were abundant in the rhizosphere of native plant species growing in nonirrigated areas adjacent to the sampled dryland wheat fields. This is the first report that documents the impact of irrigation on indigenous populations of two closely related groups of antibiotic-producing pseudomonads that coinhabit the rhizosphere of an economically important cereal crop. These results demonstrate how crop management practices can influence indigenous populations of antibiotic-producing pseudomonads with the capacity to suppress soilborne diseases of wheat.
INTRODUCTION
Wheat in the Pacific Northwest of the United States is grown throughout a wide range of agriclimatic zones. Foremost among these is the low-precipitation zone of the Columbia Plateau, which stretches from north-central Washington State into northeastern Oregon and is the largest contiguous cropping system in the western United States (31). The region is characterized by cool, moist winters and warm and dry summers, with annual precipitation ranging from only 150 to 300 mm. Wheat has been grown in this extreme environment for 125 years, typically under dryland conditions with an alternate winter wheat-summer fallow rotation (6, 31). The year of fallow allows sufficient water to accumulate in the soil profile to support a wheat crop the following year. Since the 1950s, however, wheat has increasingly been produced under irrigation, allowing annual cropping and raising yields 2- to 4-fold higher than dryland wheat yields.
Under both dryland and irrigated conditions, necrotrophic soilborne fungal pathogens are ubiquitous and pose major yield constraints to wheat production (31). In the traditional winter wheat-summer fallow rotation, Fusarium crown rot caused by Fusarium culmorum and Fusarium pseudograminearum historically has been the most important disease. More recently, the increasing use of reduced tillage to control soil erosion has resulted in a significant increase in Rhizoctonia root rot caused by Rhizoctonia solani AG-8 and Rhizoctonia oryzae (9). Take-all, caused by Gaeumannomyces graminis var. tritici, predominates in irrigated fields, where Fusarium crown rot and Rhizoctonia root rot are rarely seen (7, 9). Wheat and barley lack genetic resistance to all of these pathogens (7, 32) and instead have developed an alternative microbially based defense strategy that involves the stimulation, enrichment, and support of specific populations of antagonistic rhizosphere microorganisms (8). Among these, antibiotic-producing Pseudomonas spp. have especially important roles in the promotion of plant growth and the suppression of soilborne diseases of wheat and other crops (12, 13, 33). For example, isolates of Pseudomonas fluorescens that produce the broad-spectrum polyketide antibiotic 2,4-diacetylphloroglucinol (2,4-DAPG) are responsible for the natural suppression of take-all in the Pacific Northwest (35), and phenazine-producing pseudomonads contribute to the natural suppression of Fusarium wilt in soils of the Châteaurenard region of France (22).
Recently, Mavrodi et al. (20) reported the presence of abundant populations (>105 CFU g−1 [fresh weight] of root) of indigenous fluorescent Pseudomonas spp. producing the broad-spectrum antibiotic phenazine-1-carboxylic acid (PCA) and sizeable accumulations of PCA in the rhizosphere of cereals grown in commercial dryland fields in the low-precipitation zone of the Columbia Plateau (20, 21). These indigenous populations include at least 31 diverse genotypes of the P. fluorescens complex, all of which carry the conserved phenazine biosynthesis gene phzF and synthesize PCA in vitro (23). Surprisingly, Mavrodi et al. (20) also found that the frequency of wheat root systems colonized by these Phz+ pseudomonads was inversely related to annual precipitation, indicating that they thrive in dry soils. Many of these pseudomonads are strongly inhibitory to R. solani AG-8 in vitro and in situ, and our preliminary results suggest that they may contribute to the protection of wheat and barley against Rhizoctonia root rot in dryland cropping systems (20).
Our results to date, and those of previous studies (20, 29), led us to hypothesize that indigenous 2,4-DAPG- and PCA-producing Pseudomonas spp. are differentially enriched and supported in the rhizosphere of wheat grown in irrigated and dryland fields. The aim of the present study was to further explore how crop management, and in particular irrigation, modulates the population dynamics of these two groups of antibiotic-producing pseudomonads. To do this, we sampled dryland and irrigated commercial fields in the low-precipitation zone of the Columbia Plateau. This region provides an ideal natural laboratory in which to address this question on an ecosystem scale because irrigated fields are interspersed among traditional dryland fields, allowing side-by-side comparisons.
MATERIALS AND METHODS
Field sampling.
Commercial wheat fields located in the low-precipitation zone of the Columbia Plateau in Washington State were sampled from March through June of 2008 and 2009. Wheat was produced in these fields using the traditional winter wheat-summer fallow rotation or under central pivot irrigation. The location of each sampling site was recorded with latitudinal and longitudinal coordinates by using a global positioning system (GPS) device. At each site, plants were selected along four transects running in different directions across the field. Along each transect, clumps of plants were chosen randomly every few meters, dug with a shovel to a depth of about 18 cm, and placed in a large plastic bag. Each bag was treated as a replicate. Plants were brought to the laboratory and stored at 4°C for no more than 24 h before being processed. From each of the four replicate bags, 4 plants were assayed separately to determine both the bacterial population size and the frequency at which the individual root systems were colonized by pseudomonads containing either PCA (Phz+) or 2,4-DAPG (Phl+) biosynthesis genes. Strains carrying these genetic markers routinely produce the respective antibiotics in culture (23, 35). In addition, populations of total aerobic culturable rhizobacteria were determined. Population sizes of Phz+ and Phl+ bacteria were also determined on roots of native plant species collected from a previously noncropped (virgin) site located 8.9 km west of Ritzville, WA (47°8′26″N, 118°29′39″W). For each native plant species, four plants were processed essentially as described below.
Quantification of Phz+, Phl+, and total culturable bacteria.
The root system with adhering rhizosphere soil of a single plant was placed in a tube with sterile distilled water, and the tube was vortexed (1 min) and then sonicated in an ultrasonic cleaner (1 min). Bacterial population sizes were determined using the dilution endpoint assay (11, 20). An aliquot (100 μl) of each root wash was serially diluted in water (200 μl per well) in a 96-well microtiter plate, and the resulting dilutions were then used to inoculate two other microtiter plates prefilled with (i) a semiselective growth medium for fluorescent Pseudomonas spp. comprised of one-third-strength King's medium B (1/3 KMB) (16) supplemented with cycloheximide (100 μg/ml), chloramphenicol (13 μg/ml), and ampicillin (40 μg/ml) and (ii) one-tenth-strength tryptic soy broth (1/10 TSB) (BD Biosciences, Franklin Lakes, NJ) supplemented with cycloheximide (100 μg/ml). These microplates were incubated for 72 h at room temperature, after which the turbidity in each well was measured with a Bio-Rad model 680 microplate reader (Bio-Rad, Hercules, CA). An optical density at 600 nm of 0.1 was considered positive for bacterial growth (11, 20). For each sample, dilutions in 1/3 KMB plus antibiotics containing bacterial growth were screened for the presence of Phz+ and Phl+ pseudomonads by PCR, with primers targeting the antibiotic biosynthesis genes phzF (primers Ps_up1 and Ps_low1 [21]) and phlD (primers B2BF and BPR4 [11]). Population densities of Phz+ and Phl+ pseudomonads were calculated from the final dilution at which a phzF or phlD signal appeared (11, 20). With this assay, the detection limit of the Phz+ and Phl+ isolates was 3.2 log CFU g−1 (fresh weight) of root. The final dilution at which bacterial growth occurred in 1/10 TSB with cycloheximide was used to calculate populations of total aerobic culturable rhizosphere bacteria.
Determination of the persistence of Phz+ and Phl+ pseudomonads in bulk soil.
Bulk soil samples (Ritzville silt loam) were obtained from the Kagele (47°7′56″N, 118°34′26″W) and Jirava (47°8′34″N, 118°28′22″W) farms (7 km apart) located near Ritzville, WA. The Kagele soil was collected from an irrigated field that had been cropped to winter wheat for three consecutive years and in which take-all occurred. The Jirava farm soil was collected from a dryland field in a winter wheat-summer fallow rotation. The soils were collected from the upper 25 cm of the soil profile using a shovel in September to October of 2007 and transported to Pullman, WA, in plastic buckets, air dried, and sieved through a 0.5-cm mesh screen before being planted. Six pregerminated seeds of spring wheat (Triticum aestivum cv. Penawawa) were sown in square pots (6.5 cm high by 7 cm wide) containing 200 g of raw Kagele or Jirava farm soil and watered with 50 ml of metalaxyl solution (2.5 mg of active ingredient ml−1; Syngenta, Wilmington, DE). Metalaxyl is specific for Pythium spp., which are ubiquitous in wheat fields and can cause seed and seedling damping-off of wheat grown under controlled conditions. Plants were grown in each soil for 3 weeks in a controlled-environment chamber (15°C, 12-h photoperiod). Treatments were arranged in the chamber in a completely randomized design, and each pot served as a replicate. For each soil, population densities of indigenous Phz+ and Phl+ pseudomonads and total aerobic culturable rhizosphere bacteria from six randomly chosen plants were determined by the dilution endpoint assay as described above. We used this “baiting” approach to determine the presence of Phz+ and Phl+ pseudomonads because population densities of these bacteria typically drop below the detection limit of our assay in bulk soil.
Data analysis.
Statistical analyses were performed by using appropriate parametric and nonparametric procedures with STATISTIX 8.0 software (Analytical Software, St. Paul, MN). All population data were converted to log CFU per gram (fresh weight) of root. Maps were constructed with the global positioning system (GPS) coordinates of sampled fields using ArcGIS 9.3.1 software (ESRI, Redlands, CA).
RESULTS
Populations of Phz+ and Phl+ Pseudomonas spp. and plant colonization frequencies in wheat sampled from irrigated and dryland commercial farm fields.
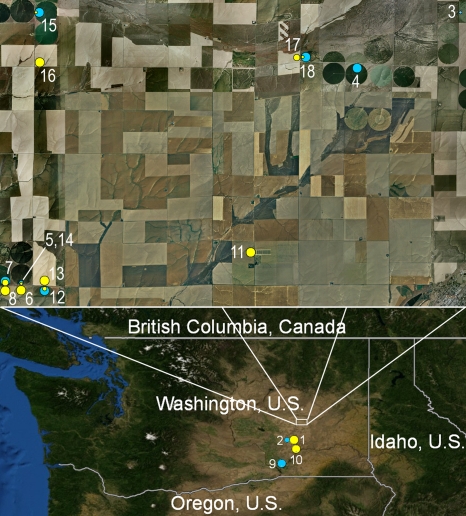
In 2008 and 2009, we sampled winter wheat from 8 dryland (150 to 300 mm precipitation annually) and 10 irrigated fields located in the heart of the low-precipitation zone. The plants were used to determine population sizes of Phz+ and Phl+ pseudomonads in the rhizosphere and the frequency at which root systems were colonized by these bacteria. Plants from all dryland fields were colonized by Phz+ bacteria, with mean population sizes of 5.5 ± 0.5 log CFU g−1 (fresh weight) of root (Table 1; Fig. 1); 67 to 100% of the root systems sampled were colonized by Phz+ pseudomonads, with those from only two fields (locations 11 and 17) having colonization frequencies below 83% (Table 1). In contrast, Phl+ pseudomonads were detected in only one dryland field (location 10), where 8% of the sampled plants were colonized with a mean population size of 3.9 ± 0.1 log CFU g−1 (fresh weight) of root. The distribution of Phz+ and Phl+ pseudomonads in wheat samples collected from irrigated fields displayed a reverse trend compared to that in plants collected from dryland fields. Plants from all irrigated fields (sites 2, 3, 4, 5, 7, 9, 12, 14, 15, and 18) harbored Phl+ pseudomonads, and Phz+ pseudomonads were below the detection limit in 3 of these 10 sites (Table 1). The ranges of population densities of Phl+ and Phz+ bacteria in irrigated fields were similar (4.1 to 6.8 and 4.1 to 7.1 log CFU g−1 [fresh weight] of root, respectively), but the frequencies of root systems colonized differed substantially. Colonization frequencies for Phl+ bacteria (58 to 100%) were always higher than those for Phz+ bacteria (8 to 50%). Samples that did not follow the trend were defined as atypical samples and were collected from (i) irrigated sites 2 and 3, where only 25% and 13% of plants, respectively, carried Phl+ bacteria on roots and Phz+ bacteria were not detectable, and (ii) site 7, with high colonization frequencies for both Phl+ and Phz+ bacteria (100% and 63%, respectively). In addition, population densities of total culturable aerobic bacteria varied from 7.7 to 8.1 and 7.8 to 9.1 log CFU g−1 (fresh weight) of root (data not shown) in dryland and irrigated fields, respectively.
Table 1.
Population sizes of Phz+ and Phl+ rhizobacteria and plant colonization frequencies in wheat sampled from irrigated and dryland farm fields of east-central Washington State
| Irrigation | Locationa | Cropb | Geographic coordinates (degrees/minutes/seconds) | Sampling date (mo/day/yr) | Phz+ bacteria |
Phl+ bacteria |
||
|---|---|---|---|---|---|---|---|---|
| Populationc (mean log CFU g−1 [fresh wt] of root ± SD) | Frequencyd | Populationc (mean log CFU g−1 [fresh wt] of root ± SD) | Frequencyd | |||||
| No | 1 | WW | 46°47′40″N | 05/16/08 | 6.3 ± 0.6 | 0.88 | NDe | ND |
| 118°39′42″W | ||||||||
| Yes | 2 | WW | 46°47′36″N | 05/16/08 | ND | ND | 4.8 ± 0.2 | 0.25 |
| 118°48′3″W | ||||||||
| Yes | 3 | SW | 46°13′1″N | 06/09/08 | ND | ND | 5.3 ± 0 | 0.13 |
| 118°23′8″W | ||||||||
| Yes | 4 | SW | 47°12′24″N | 06/09/08 | ND | ND | 6.8 ± 1.1 | 0.88 |
| 118°25′8″W | ||||||||
| Yes | 5 | WW | 47°7′57″N | 06/09/08 | 7.1 | 0.13 | 5.7 ± 1.1 | 0.75 |
| 118°35′7″W | ||||||||
| No | 6 | WW | 47°7′57″N | 06/09/08 | 5.9 ± 1.1 | 1.00 | ND | ND |
| 118°35′7″W | ||||||||
| Yes | 7 | WW | 47°7′56″N | 06/09/08 | 4.8 ± 0.2 | 0.63 | 5.7 ± 0.3 | 1.0 |
| 118°35′44″W | ||||||||
| No | 8 | WW | 47°7′56″N | 06/09/08 | 5.7 ± 0.9 | 0.83 | ND | ND |
| 118°35′44″W | ||||||||
| Yes | 9 | WW | 46°17′1″N | 03/17/09 | 5.7 | 0.08 | 4.9 ± 0.9 | 0.83 |
| 118°55′18″W | ||||||||
| No | 10 | WW | 46°36′3″N | 03/17/09 | 5.1 ± 1.3 | 0.83 | 3.9 ± 0.1 | 0.08 |
| 118°36′21″W | ||||||||
| No | 11 | WW | 47°8′34″N | 06/15/09 | 5.7 ± 1.2 | 0.75 | ND | ND |
| 118°28′22″W | ||||||||
| Yes | 12 | SW | 47°7′56″N | 06/15/09 | 4.2 ± 0.8 | 0.5 | 6.7 ± 0.5 | 1.0 |
| 118°34′26″W | ||||||||
| No | 13 | WW | 47°7′56″N | 06/15/09 | 4.8 ± 1.0 | 0.92 | ND | ND |
| 118°34′26″W | ||||||||
| Yes | 14 | WW | 47°7′57″N | 06/15/09 | 4.1 ± 1.2 | 0.33 | 4.1 ± 0.9 | 0.58 |
| 118°35′7″W | ||||||||
| Yes | 15 | WW | 47°13′35″N | 06/15/09 | 6.1 | 0.08 | 4.4 ± 0.7 | 0.83 |
| 118°34′43″W | ||||||||
| No | 16 | WW | 47°12′20″N | 06/15/09 | 5.0 ± 1.3 | 0.83 | ND | ND |
| 118°34′41″W | ||||||||
| No | 17 | WW | 47°12′28″N | 06/15/09 | 5.4 ± 1.1 | 0.67 | ND | ND |
| 118°27′4″W | ||||||||
| Yes | 18 | WW | 47°12′28″N | 06/15/09 | 4.4 ± 0.3 | 0.25 | 4.2 ± 0.9 | 0.92 |
| 118°27′4″W | ||||||||
The numbering of sampled locations corresponds to that in Fig. 1.
WW, winter wheat; SW, spring wheat.
As determined by the PCR-based endpoint dilution assay with phzF- and phlD-specific primers.
Frequency of individual rhizospheres colonized by Phz+ or Phl+ bacteria.
ND, none detected.
Fig 1.
Distribution of indigenous Phz+ and Phl+ bacteria in the rhizospheres of wheat plants sampled in 2008 and 2009 from dryland and irrigated commercial fields in the low-precipitation zone of the Columbia Plateau. The numbering of sampled sites corresponds to that listed in Table 1. The top panel depicts a satellite image of the surveyed area northwest of Ritzville, WA, which covers approximately 320 km2. Wheat fields watered with center pivot irrigation systems appear on the satellite image as large circles. The frequencies of wheat rhizospheres colonized with Phz+ and Phl+ pseudomonads are represented by the inserted smaller yellow and blue circles, respectively, with the circle size being proportional to the frequency of plants colonized by Phz+ or Phl+ bacteria. Satellite images and global positioning system (GPS) coordinates of sampled fields were managed with ArcGIS 9.3.1 software. The map was created using ArcGIS and ArcMap software (ESRI).
Populations of Phz+ and Phl+ bacteria on roots in noncropped sites.
We also assessed population densities of Phz+ and Phl+ bacteria in the rhizosphere of plants collected from a noncropped (virgin) area located near Ritzille, WA. Phz+ pseudomonads were abundant in the rhizosphere of diverse plant hosts, including native and introduced grass species [Elymus glaucus Buckley, Agropyron cristatum (L.) Gaertn., Poa sp., and Bromus tectorum L.], yarrow (Achillea millefolium L.), tarweed fiddleneck (Amsinckia lycopsoide Lehm.), and common sagebrush (Artemisia tridentata Nutt.). A total of 83% of the sampled plants were colonized by Phz+ pseudomonads, with a mean population size of 4.7 ± 0.9 log CFU g−1 (fresh weight) of root. In contrast, Phl+ pseudomonads were detected only in the rhizosphere of members of the Poaceae, with 33% of sampled plants colonized with a mean population size of 4.9 ± 0.1 log CFU g−1 (fresh weight) of root.
Seasonal dynamics of Phz+ and Phl+ rhizobacteria.
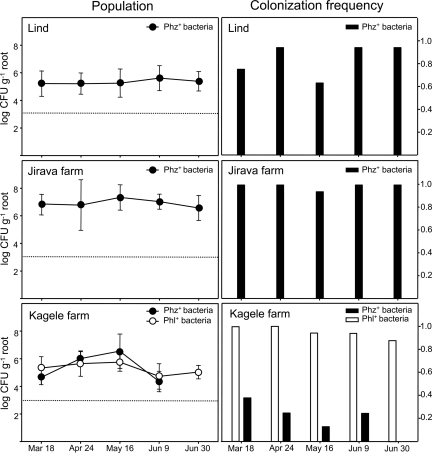
In 2008, we monitored the seasonal population dynamics of indigenous Phz+ and Phl+ pseudomonads on winter wheat in one irrigated and two dryland commercial fields. The dryland sites were located 16.9 km apart and included (i) a field at the Jirava farm near Ritzville (site 11 in Table 1 and Fig. 1) and (ii) a commercial field adjacent to the WSU Dryland Research Station near Lind, WA (47°0′1″N, 118°33′45″W). The irrigated site was located at the Kagele farm near Ritzville, WA (site 12 in Table 1 and Fig. 1). Wheat rhizospheres from both dryland fields were colonized only by Phz+ pseudomonads, whose populations remained almost constant throughout the period from March through June 2008 (Fig. 2). The frequencies of root systems colonized by Phz+ bacteria were 94 to 100% and 63 to 94% for the Jirava and Lind dryland fields, respectively.
Fig 2.
Seasonal population dynamics and frequency of root systems of individual plants colonized by Phz+ and Phl+ Pseudomonas spp. in winter wheat sampled in 2008 at dryland fields near Lind, WA (47°0′1″N, 118°33′45″W), at the Jirava farm near Ritzville, WA (47°8′34″N, 118°28′22″W), and at the irrigated field on the Kagele farm near Ritzville, WA (47°7′56″N, 118°34′26″W). Wheat was collected from 4 transects in different directions in the field and placed in separate plastic bags. Each bag was treated as a replicate, and 4 plants from each bag were used to determine both bacterial population sizes and the frequency of root systems colonized by pseudomonads containing the phenazine biosynthesis gene phzF or the 2,4-DAPG biosynthesis gene phlD. With this assay, the detection limit of the Phz+ and Phl+ bacteria was 3.2 log CFU g−1 (fresh weight) of root. At the Lind and Jirava farms, population densities of Phl+ pseudomonads were below the detection level. At the Kagele field, populations of Phz+ pseudomonads dropped below the detection level on 30 June.
Both Phl+ and Phz+ pseudomonads were detected on roots from the irrigated Kagele field (Fig. 2). Their population sizes were above 5 log CFU g−1 (fresh weight) of root and remained constant throughout most of the monitored period (18 March to 9 June). However, on 30 June, the population of Phz+ pseudomonads dropped below the detection limit of 3.2 log CFU g−1 (fresh weight) of root. In contrast to the population densities, the colonization frequencies of Phl+ and Phz+ pseudomonads differed substantially on roots from the Kagele irrigated field. Only 13 to 38% of the root systems harbored Phz+ bacteria, whereas the colonization frequency by Phl+ pseudomonads was 88 to 100% (Fig. 2). On 30 June, the colonization frequency of Phz+ bacteria was 0%.
Indigenous Phz+ and Phl+ bacteria persist in bulk field soil between crops.
Soil from a dryland field at the Jirava farm (Table 1, site 11), which was in fallow, and from the irrigated field at the Kagele farm (Table 1, site 12) was collected in the late fall of 2007, transported to Pullman, and sown to spring wheat (Triticum aestivum cv. Penawawa). Plants were grown for 3 weeks, harvested, and used to determine population sizes of indigenous Phz+, Phl+, and total aerobic culturable rhizosphere bacteria. Only Phz+ pseudomonads were detected on roots of wheat grown in the Jirava bulk soil, and their population size 3 weeks after being planted was 4.7 ± 0.9 log CFU g−1 (fresh weight) of root. Wheat grown in the soil from the irrigated Kagele field was colonized only by Phl+ bacteria, with a population size of 5.6 ± 0.4 log CFU g−1 (fresh weight) of root. Population densities of total culturable aerobic rhizosphere bacteria from wheat grown in both soils ranged between 8.7 and 9.0 log CFU g−1 (fresh weight) of root (data not shown). The experiment was performed twice with similar results.
DISCUSSION
In this study, we focused on the impact of irrigation on the ecology and population dynamics of Phz+ and Phl+ pseudomonads in the rhizosphere of wheat grown in commercial fields in the low-precipitation zone of the Columbia Plateau. These two groups of Pseudomonas spp. are of special interest because they are well-studied, highly effective biological control agents (19, 35) and contribute to the natural suppressiveness of soils to certain soilborne diseases (22, 35, 36). This study was prompted by the recent report of Mavrodi et al. (20), who found that Phz+ Pseudomonas spp. are abundant on the roots of dryland cereal crops in the low-precipitation zone. They hypothesized that Phz+ Pseudomonas spp. are “uniquely adapted to growing and surviving in the rhizosphere under conditions of water stress and that soil moisture represents a major abiotic factor that drives the development of the indigenous phenazine-producing microbial community” (20). The occurrence of a highly significant inverse correlation between the proportion of wheat rhizospheres colonized by Phz+ Pseudomonas spp. and local annual precipitation values supported this hypothesis (20).
Our study provides further direct evidence that soil moisture (or the absence thereof) is a major factor driving the development of Phz+ and Phl+ Pseudomonas populations. As in the study by Mavrodi et al. (20), the roots of wheat grown in dryland fields were colonized almost exclusively by Phz+ pseudomonads. In contrast, the rhizospheres of plants grown in irrigated fields harbored bacteria that were either Phl+ or a mixture of Phl+ and Phz+ isolates. The impact of management practice and irrigation on these two groups of Pseudomonas spp. is especially notable in samples collected from paired sites 5 and 6, 7 and 8, 12 and 13, and 17 and 18 (Fig. 1; Table 1) because these adjacent irrigated and dryland wheat fields have identical soil types. With each pair of fields, plants sampled from the irrigated field harbored both Phl+ and Phz+ bacteria, with the Phl+ pseudomonads always being more abundant (Table 1), whereas in the corresponding dryland fields, all of the sampled plants were colonized exclusively by Phz+ bacteria. The variability in the relative abundance of Phl+ and Phz+ isolates in irrigated fields may reflect differences in their recent management history or their individual irrigation regimes. Irrigated wheat fields in the low-precipitation zone commonly receive about 23 cm of water from overhead irrigation between April and June, but the exact amounts of water from irrigation in the surveyed sites can differ markedly because growers have access to various amounts of water. To our knowledge, this is the first field study that documents the impact of irrigation on indigenous populations of two closely related groups of antibiotic-producing pseudomonads that coinhabit the rhizosphere of an economically important cereal crop.
The role of Phl+ Pseudomonas spp. in the natural biological control of take-all, an important soilborne root disease of wheat and barley, was first described by Raaijmakers et al. (29), who used dilution plating and colony hybridization to assess the frequency of Phz+ and Phl+ pseudomonads in the rhizospheres of wheat grown in soils from three different irrigated fields located in the low-precipitation zone of the Columbia Plateau. Each field had a history of more than 20 years of continuous wheat under irrigation, and all were highly suppressive to take-all. Raaijmakers et al. (29) showed that rhizospheres of wheat grown in these soils harbored high population densities of Phl+ Pseudomonas spp. (5 × 105 to 2 × 106 CFU g−1 [fresh weight] of root), but surprisingly, Phz+ pseudomonads were never detected. Our current findings provide an explanation for these earlier results (29) and indicate that with irrigation, a shift occurs in the composition of populations of pseudomonads away from Phz+ isolates and more toward Phl+ isolates. A snapshot of this transition was evident in the Kagele irrigated field (Fig. 1), which was in its 3rd year of continuous winter wheat after irrigation had been installed. By the end of the growing season in 2008, Phz+ pseudomonads were below the limit of detection. We think Raaijmakers et al. (29) failed to find Phz+ pseudomonads in the fields they sampled because Phz+ isolates had dropped below the limit of detection many growing seasons earlier due to irrigation.
Fluorescent Pseudomonas spp. thrive on roots, and it traditionally has been thought that they require water potentials near field capacity (15) and percolating water for rapid growth and metabolite production in the rhizosphere. An unexpected finding of our study was the consistently large population sizes of Phz+ pseudomonads (5 to 7 log CFU g−1 of root) detected throughout the growing season at Lind and Ritzville (Jirava farm) (Fig. 2) even as soils became progressively drier. The monthly precipitation levels at those locations in March, April, May, and June, when our sampling was done, were 2.59, 2.10, 2.20, and 1.49 cm (Lind) and 2.74, 2.13, 2.48, and 2.08 cm (Ritzville), respectively, and the water potentials in the top 10 cm and 20 cm of the soil steadily decreased, such that by 1 July the water potential values typically reach −750 kPa and −378 KPa, respectively (data not shown). These water potential values are far below that required for bacterial motility (10). Phz+ Pseudomonas population sizes remained above the threshold of 105 CFU g−1 of root, a density generally considered to be required for biologically significant activity in the rhizosphere (for example, induction of systemic resistance [27] or production of antibiotics [20, 26]). One important question that we are currently addressing is how far into the growing season and at what water potentials Phz+ populations remain sufficiently active to produce PCA.
The mechanism(s) underlying the differential effect of soil moisture on population levels of Phl+ and Phz+ Pseudomonas spp. on wheat remains unclear. One possible explanation is that the soil moisture level significantly changes the amount and/or composition of root exudates, which in turn differentially affects the growth rate and competitiveness of these pseudomonads in the rhizosphere. Another factor might be the displacement of Phz+ isolates by Phl+ isolates on irrigated wheat in root lesions caused by the take-all pathogen Gaeumannomyces graminis var. tritici (35). Phl+ P. fluorescens strains are highly aggressive colonists of wheat roots infected by this pathogen (28, 35), which typically causes only limited damage under dryland conditions because of its requirement for sustained high soil moisture but proliferates rapidly when a field is converted to irrigation. Finally, we speculate that phenazine producers are much better adapted for survival under conditions of moisture deficit or stress than Phl+ pseudomonads because of the production of PCA. Bacteria on root surfaces or in soil form structurally complex communities enmeshed in exopolymers and described as biofilms (1, 5), and biofilm formation is recognized as one of the physiological and molecular mechanisms that underlie the protection of bacteria against physical and chemical stresses and adaptation to survival in low-moisture habitats (2, 14), such as those that occur in the low-precipitation zone. Under water-limiting conditions, soil bacteria in biofilms increase the production of total exopolysaccharide and alginate, creating a hydrated microenvironment that helps to protect cells from dehydration-mediated membrane stress, accumulation of reactive oxygen species, and cell death (3, 4). Phenazines have been directly linked to biofilm formation (19). For example, cultures of Pseudomonas aureofaciens did not establish biofilms in the absence of phenazines, and biofilm architecture and bacterial dispersal rates were dependent on the identities and ratios of the phenazines produced (17, 18). Similarly, although Phz− mutants of Pseudomonas aeruginosa formed biofilms, the identities and amounts of amended phenazines influenced biofilm architecture and cell swarming activity (30). Price-Whelan et al. (25) suggested that because the diffusion rate of oxygen through biofilms is thought to be low, phenazines could help maintain the redox homeostasis of cells embedded in the film by acting as electron acceptors for the reoxidation of accumulating NADH. Indeed, in oxygen-limited stationary-phase cultures of P. aeruginosa, a decrease in intracellular NADH/NAD+ was correlated with the presence of pyocyanin in the culture (24), and phenazine-facilitated electron transfer promoted anaerobic survival but not growth under conditions of oxidant limitation (34). Interestingly, other electron shuttles that were reduced but not made by P. aeruginosa did not facilitate survival, suggesting that sophisticated systems are needed to control the reactivity of these molecules within the cell and that mechanisms have evolved in pseudomonads to be specific for the phenazines they produce (34).
In conclusion, our results show for the first time under field conditions that indigenous populations of two major groups of biocontrol bacteria, Phz+ and Phl+ pseudomonads, are strongly influenced by the manner in which wheat is produced. Wheat grown in dryland fields was colonized predominantly by Phz+ bacteria, whereas rhizosphere populations of plants grown in irrigated fields were dominated by Phl+ bacteria. These results contribute to a better understanding of how management practices influence indigenous populations of antibiotic-producing pseudomonads that contribute to the natural control of soilborne diseases of wheat and other crops. Just as crop management practices modulate the incidence and severity of soilborne wheat root pathogens, there is a concomitant shift in antagonists that can provide a microbe-based defense against those pathogens. Consequently, Phl+ pseudomonads are enriched in irrigated fields to protect wheat against take-all (35), and Phz+ isolates are now hypothesized to protect wheat against Rhizoctonia root rot (20).
ACKNOWLEDGMENTS
We are grateful to Karen Adams, Renee Espinosa, and Karen Hansen for excellent technical assistance in processing of field samples.
Footnotes
Published ahead of print 2 March 2012
REFERENCES
- 1. Bloemberg GV, Wijfjes AH, Lamers GE, Stuurman N, Lugtenberg BJ. 2000. Simultaneous imaging of Pseudomonas fluorescens WCS365 populations expressing three different autofluorescent proteins in the rhizosphere: new perspectives for studying microbial communities. Mol. Plant Microbe Interact. 13:1170–1176 [DOI] [PubMed] [Google Scholar]
- 2. Chang WS, Halverson LJ. 2003. Reduced water availability influences the dynamics, development, and ultrastructural properties of Pseudomonas putida biofilms. J. Bacteriol. 185:6199–6204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chang WS, Li XH, Halverson LJ. 2009. Influence of water limitation on endogenous oxidative stress and cell death within unsaturated Pseudomonas putida biofilms. Environ. Microbiol. 11:1482–1492 [DOI] [PubMed] [Google Scholar]
- 4. Chang WS, et al. 2007. Alginate production by Pseudomonas putida creates a hydrated microenvironment and contributes to biofilm architecture and stress tolerance under water-limiting conditions. J. Bacteriol. 189:8290–8299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chin-A-Woeng TFC, et al. 1998. Biocontrol by phenazine-1-carboxamide-producing Pseudomonas chlororaphis PCL1391 of tomato root rot caused by Fusarium oxysporum f. sp. radicis-lycopersici. Mol. Plant Microbe Interact. 11:1069–1077 [Google Scholar]
- 6. Cook RJ. 1986. Wheat management systems in the Pacific Northwest. Plant Dis. 70:894–898 [Google Scholar]
- 7. Cook RJ, Schillinger WF, Christensen NW. 2002. Rhizoctonia root rot and take-all of wheat in diverse direct-seed spring cropping systems. Can. J. Plant Pathol. 24:349–358 [Google Scholar]
- 8. Cook RJ, et al. 1995. Molecular mechanisms of defense by rhizobacteria against root disease. Proc. Natl. Acad. Sci. U. S. A. 92:4197–4201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cook RJ, Veseth RJ. 1991. Wheat health management. APS Press, St. Paul, MN [Google Scholar]
- 10. Dechesne A, Wang G, Gulez G, Or D, Smets BF. 2010. Hydration-controlled bacterial motility and dispersal on surfaces. Proc. Natl. Acad. Sci. U. S. A. 107:14369–14372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gardener BBM, Mavrodi DV, Thomashow LS, Weller DM. 2001. A rapid polymerase chain reaction-based assay characterizing rhizosphere populations of 2,4-diacetylphloroglucinol-producing bacteria. Phytopathology 91:44–54 [DOI] [PubMed] [Google Scholar]
- 12. Glick BR. 1995. The enhancement of plant growth by free-living bacteria. Can. J. Microbiol. 41:109–117 [Google Scholar]
- 13. Haas D, Defago G. 2005. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 3:307–319 [DOI] [PubMed] [Google Scholar]
- 14. Harris RF. 1980. Effect of water potential on microbial growth and activity, p 23–95 In Parr JF, Gardner WR, Elliott LF. (ed), Water potential relations in soil microbiology. Soil Science Society of America, Madison, WI [Google Scholar]
- 15. Howie WJ, Cook RJ, Weller DM. 1987. Effects of soil matric potential and cell motility on wheat root colonization by fluorescent pseudomonads suppressive to take-all. Phytopathology 77:286–292 [Google Scholar]
- 16. King EO, Ward MK, Raney DE. 1954. Two simple media for the demonstration of pyocyanin and fluorescein. J. Lab. Clin. Med. 44:301–307 [PubMed] [Google Scholar]
- 17. Maddula VS, Pierson EA, Pierson LS., III 2008. Altering the ratio of phenazines in Pseudomonas chlororaphis (aureofaciens) strain 30-84: effects on biofilm formation and pathogen inhibition. J. Bacteriol. 190:2759–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maddula VS, Zhang Z, Pierson EA, Pierson LS., III 2006. Quorum sensing and phenazines are involved in biofilm formation by Pseudomonas chlororaphis (aureofaciens) strain 30-84. Microb. Ecol. 52:289–301 [DOI] [PubMed] [Google Scholar]
- 19. Mavrodi DV, Blankenfeldt W, Thomashow LS. 2006. Phenazine compounds in fluorescent Pseudomonas spp.: biosynthesis and regulation. Annu. Rev. Phytopathol. 44:417–445 [DOI] [PubMed] [Google Scholar]
- 20. Mavrodi DV, et al. 2012. Accumulation of the antibiotic phenazine-1-carboxylic acid in the rhizosphere of dryland cereals. Appl. Environ. Microbiol. 78:804–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mavrodi DV, et al. 2010. Diversity and evolution of the phenazine biosynthesis pathway. Appl. Environ. Microbiol. 76:866–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mazurier S, Corberand T, Lemanceau P, Raaijmakers JM. 2009. Phenazine antibiotics produced by fluorescent pseudomonads contribute to natural soil suppressiveness to Fusarium wilt. ISME J. 3:977–991 [DOI] [PubMed] [Google Scholar]
- 23. Parejko JA, Mavrodi DV, Mavrodi OV, Weller DM, Thomashow LS. Population structure and diversity of phenazine-1-carboxylic acid producing fluorescent Pseudomonas spp. from dryland cereal fields of central Washington State (U.S.A.). Microb. Ecol., in press [DOI] [PubMed] [Google Scholar]
- 24. Price-Whelan A, Dietrich LE, Newman DK. 2007. Pyocyanin alters redox homeostasis and carbon flux through central metabolic pathways in Pseudomonas aeruginosa PA14. J. Bacteriol. 189:6372–6381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Price-Whelan A, Dietrich LE, Newman DK. 2006. Rethinking ‘secondary’ metabolism: physiological roles for phenazine antibiotics. Nat. Chem. Biol. 2:71–78 [DOI] [PubMed] [Google Scholar]
- 26. Raaijmakers JM, Bonsall RE, Weller DM. 1999. Effect of population density of Pseudomonas fluorescens on production of 2,4-diacetylphloroglucinol in the rhizosphere of wheat. Phytopathology 89:470–475 [DOI] [PubMed] [Google Scholar]
- 27. Raaijmakers JM, et al. 1995. Dose-response relationships in biological control of Fusarium wilt of radish by Pseudomonas spp. Phytopathology 85:1075–1081 [Google Scholar]
- 28. Raaijmakers JM, Weller DM. 2001. Exploiting genotypic diversity of 2,4-diacetylphloroglucinol-producing Pseudomonas spp.: characterization of superior root-colonizing P. fluorescens strain Q8r1-96. Appl. Environ. Microbiol. 67:2545–2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Raaijmakers JM, Weller DM, Thomashow LS. 1997. Frequency of antibiotic-producing Pseudomonas spp. in natural environments. Appl. Environ. Microbiol. 63:881–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ramos I, Dietrich LE, Price-Whelan A, Newman DK. 2010. Phenazines affect biofilm formation by Pseudomonas aeruginosa in similar ways at various scales. Res. Microbiol. 161:187–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schillinger WF, Papendick RI. 2008. Then and now: 125 years of dryland wheat farming in the Inland Pacific Northwest. Agron. J. 100:S166–S182 [Google Scholar]
- 32. Schroeder KL. 2004. The dynamics of root diseases of wheat and barley in the transition from conventional tillage to direct seeding. Ph.D. thesis Washington State University, Pullman, WA: [DOI] [PubMed] [Google Scholar]
- 33. Thomashow LS, Weller DM. 1995. Current concepts in the use of introduced bacteria for biological disease control: mechanisms and antifungal metabolites, p 187–235 In Stacey G, Keen N. (ed), Plant-microbe interactions, vol 1 Chapman & Hall, New York, NY [Google Scholar]
- 34. Wang Y, Kern SE, Newman DK. 2010. Endogenous phenazine antibiotics promote anaerobic survival of Pseudomonas aeruginosa via extracellular electron transfer. J. Bacteriol. 192:365–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weller DM, et al. 2007. Role of 2,4-diacetylphloroglucinol-producing fluorescent Pseudomonas spp. in plant defense. Plant Biol. 9:4–20 [DOI] [PubMed] [Google Scholar]
- 36. Weller DM, Raaijmakers JM, Gardener BBM, Thomashow LS. 2002. Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu. Rev. Phytopathol. 40:309–348 [DOI] [PubMed] [Google Scholar]