Abstract
Bacteriophage endolysins have an interesting potential as antimicrobials. The endolysin LysH5, encoded by Staphylococcus aureus phage vB_SauS-phi-IPLA88, was expressed and secreted in Lactococcus lactis using the signal peptide of bacteriocin lactococcin 972 and lactococcal constitutive and inducible promoters. Up to 80 U/mg of extracellular active endolysin was detected in culture supernatants, but most of the protein (up to 323 U/mg) remained in the cell extracts.
TEXT
Staphylococcus aureus is a cause of serious concern in clinical settings especially due to the emergence of methicillin-resistant S. aureus (MRSA) and vancomycin-resistant S. aureus (VRSA) (13). Moreover, S. aureus is a threat to food safety (3). In the dairy environment, this pathogen is recognized as a frequent cause of subclinical intramammary infections in dairy cows (28). Bacteriophage endolysins mediate lysis of the host bacteria at the end of the lytic cycle to release phage progeny (39). They have a huge potential as novel therapeutic antibacterial agents (7), as biopreservatives in foods (27), in pathogen detection (38), and as disinfectants of industrial facilities (32).
Lactococcus lactis, a GRAS (generally regarded as safe) microorganism with a long history of safe use in food fermentations, has been proven as a suitable host for expression and purification of heterologous proteins with food safety applications such as bacteriocins (1, 23) and bacteriophage lytic enzymes against Listeria monocytogenes (8, 36) and Clostridium difficile (24). To facilitate secretion, proteins have been fused to bacteriocin signals (12) or to the signal peptide (SP) of Usp45 (SPusp45), the major Sec-dependent protein secreted by L. lactis (1). The capacity of this microorganism for protein secretion can be exploited to improve large-scale production processes and downstream purification steps (19).
LysH5 endolysin from S. aureus bacteriophage vB_SauS-phi-IPLA88 has been characterized. LysH5 is a 53.7-kDa protein, encoded by a 1,446-bp gene (10), which lysed a wide range of staphylococci and also inhibits S. aureus growth in milk (11, 27).
The aim of this work was cloning and expression of the LysH5-encoding gene in an L. lactis strain under the control of lactococcal inducible and constitutive promoters to facilitate the potential application of this endolysin as a food preservative. For secretion, the signal peptide (SPLcn972) of the bacteriocin lactococcin 972 (Lcn972), which shows a Sec-dependent processing signal (22), has been tagged with LysH5 endolysin.
Heterologous expression of the endolysin LysH5 in L. lactis.
Prior to the construction of an L. lactis secretion system for the endolysin LysH5, we assessed the ability of this bacterium to express active LysH5 protein. Phage phiIPLA88 DNA (9) was used to amplify the LysH5-encoding gene with primers AMI-1 and AMI-2 (see Table S1 in the supplemental material). PCR amplifications were carried out using the PureTaq Ready-To-Go PCR bead kit (GE Healthcare, United Kingdom), and PCR fragments were purified using the GenElute PCR cleanup kit (Sigma, St. Louis, MO). The resulting PCR product was cloned into the plasmid pNZ8020 (5) using the restriction sites PstI and BamHI. The recombinant plasmid pNZ8020-LysH5, bearing the LysH5-encoding gene under the control of the PnisA promoter, was transformed into L. lactis NZ9000 (15). This recombinant plasmid and those constructed thereof were confirmed by DNA sequencing. Aliquots of cultures L. lactis NZ9000(pNZ8020) and L. lactis NZ9000(pNZ8020-LysH5) at an optical density at 600 nm (OD600) of 0.4 were induced for 4 h at 30°C with nisin A (10 ng/ml). Cells from 10-ml cultures were resuspended in 50 mM sodium phosphate buffer, pH 7, and lysed using glass beads in a FastPrep system (ThermoSavant-Bio 101/Q-Biogen, Holbrook, NY). Lysates were centrifuged at 10,000 × g for 10 min at 4°C and tested for lytic activity by turbidity reduction assay against S. aureus Sa9 cell suspensions (27). A specific activity of 166.85 ± 34.8 U/mg (Table 1) was observed, indicating that LysH5 is synthesized in L. lactis as an active protein. No lysis of S. aureus Sa9 was detected in extracts of the control strain L. lactis NZ9000(pNZ8020).
Table 1.
Endolysin LysH5 production in supernatants and cell extracts from recombinant strains
| Lactococcal strain | Lytic activity (sp act, U/mg) |
|
|---|---|---|
| Supernatant | Cell extract | |
| L. lactis NZ9000(pNZ8020) | 0 | 0 |
| L. lactis NZ9000(pIL252) | 0 | 0 |
| L. lactis NZ9000(pMG36c) | 0 | 0 |
| L. lactis NZ9000(pNZ8020-LysH5) | 0 | 166.85 ± 34.8 |
| L. lactis NZ9000(pNZ8020-SPLcn972-LysH5) | 40 ± 7 | 116.36 ± 9.6 |
| L. lactis NZ9000(pNZ8020-SPLcn972-LysH5opt) | 80 ± 3 | 323.63 ± 17.1 |
| L. lactis NZ9000(pIL252-Pllmg0169-SPLcn972-LysH5opt) | 0.01 ± 0.005 | 3.63 ± 0.5 |
| L. lactis NZ9000(pMG36c-P32-SPLcn972-LysH5opt) | 0.01 ± 0.003 | 3.34 ± 1.1 |
Lcn972 signal peptide (SPLcn972) leads to LysH5 secretion by L. lactis.
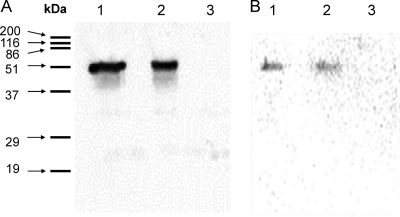
To obtain a recombinant strain able to release active LysH5 into the culture supernatants, a fusion between SPLcn972 and the complete LysH5 was designed. Plasmid pBL1 (31) was used as the template to amplify an 81-bp fragment containing SPLcn972 using the primers Lcn-AMI and Lcn-AMI-3 (see Table S1 in the supplemental material). A PCR fragment containing the LysH5-encoding gene was obtained after amplification of phage phiIPLA88 DNA with primers AMI-2 and AMI-7 (see Table S1). The two PCR fragments were merged by a PCR with primers AMI-2 and Lcn-AMI (see Table S1). The resulting PCR product (1,566 bp) was cleaved with BamHI and PstI and cloned into plasmid pNZ8020 to generate the recombinant plasmid pNZ8020-SPLcn972-LysH5. Transformants containing this plasmid were induced with nisin A as indicated above. Expression and secretion of LysH5 by L. lactis NZ9000 under the control of the nisin A promoter (PnisA) were tested in both cell extracts and culture supernatants by immunoblotting and lytic activity assays. Rabbit polyclonal antibodies were raised against purified LysH5 according to a previous protocol (30). Cell extracts and supernatants from induced cultures were subjected to Western blotting and immunological detection (chemiluminescent Western blotting kit; Roche) using anti-LysH5 (1:4,000 dilution). As shown in Fig. 1A (lane 2), a single protein band of about 53 kDa reacted with the antibody in the cell extract from L. lactis NZ9000(pNZ8020-SPLcn972-LysH5). A weak band was also detected in the corresponding supernatants. These results agree well with the predicted mass of LysH5. The functional expression of LysH5 was determined and quantified by using S. aureus Sa9 cell suspensions in a turbidity reduction assay (Table 1). The cell extract from L. lactis NZ9000(pNZ8020-SPLcn972-LysH5) showed a significantly stronger activity than did the supernatant. Although several examples have been published supporting the idea that the best production yields in L. lactis are obtained when proteins are secreted to the culture medium (19), LysH5 production did not follow this pattern. It is well known that protein size, the nature of the signal peptides (SPs), and the presence of propeptides are factors that may interfere with protein secretion (4). Moreover, secretion efficiency might be also influenced by the proper combination between the mature protein and the SP used to drive secretion (20).
Fig 1.
Detection of heterologously synthesized LysH5 by immunoblotting in cell extracts (A) and in supernatants of cultures (B) of L. lactis NZ9000(pNZ8020-SPLcn972-LysH5opt) (lanes 1), L. lactis NZ9000(pNZ8020-SPLcn972-LysH5) (lanes 2), and L. lactis NZ9000(pNZ8020) (lanes 3). Protein molecular mass markers with sizes in kilodaltons are indicated in the left margin.
Regarding the low specific activity of LysH5 in the supernatants of L. lactis cultures, this could be due, at least partially, to the activation of housekeeping proteases by the stress response to overexpression of secreted proteins (2). In fact, the inactivation of HtrA, the unique housekeeping protease in the L. lactis cell surface, results in higher heterologous protein yields (26). The low specific activity of the extracellular LysH5 may also be ascribed to inactivation by low pH as previously observed (27).
In order to improve these results, we proceeded to the codon optimization of the SPLcn972-LysH5-encoding gene based on the Lactococcus lactis subsp. cremoris MG1363 codon usage (Genescript) (17). The resulting DNA fragment was cloned into BamHI/PstI restriction sites from pNZ8020 to generate pNZ8020-SPLcn972-LysH5opt. Expression of the optimized version of the LysH5 gene under the control of the PnisA promoter was evaluated (Table 1; Fig. 1, lanes 1). The codon optimization of the LysH5-encoding gene resulted in higher levels of endolysin activity in both cell extracts and supernatants from L. lactis NZ9000(pNZ8020-SPLcn972-LysH5opt). Increases of 2.7- and 2-fold in the cell extract and supernatant activities, respectively, were obtained over the wild-type version of the LysH5 gene (Table 1; Fig. 1, lanes 2).
Induction of LysH5 production by coculturing with the nisin Z producer strain L. lactis IPLA 729.
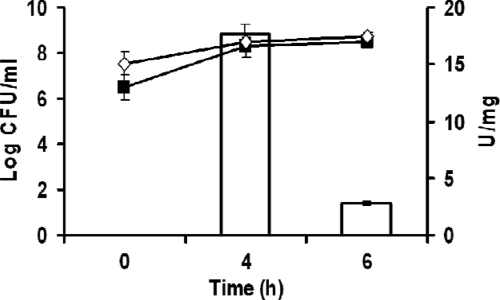
In regard to the industrial production of LysH5, nisin addition remains costly. Therefore, the activity of LysH5 produced by cultures of L. lactis NZ9000 carrying pNZ8020 derivatives in the presence of the nisin Z producer strain L. lactis IPLA 729 (29) was assessed as an alternative approach. Initially, we determined the survival of the L. lactis NZ9000 strains in the presence of L. lactis IPLA 729. We defined the optimal coculture conditions as an inoculum of 0.01% (vol/vol) L. lactis IPLA 729 (3 × 106 CFU/ml) and 3% (vol/vol) L. lactis NZ9000(pNZ8020-LysH5) or L. lactis NZ9000(pNZ8020-SPLcn972-LysH5opt) (3 × 107 CFU/ml) (data not shown). Then, L. lactis IPLA 729 and L. lactis NZ9000(pNZ8020) derivatives were grown to stationary phase, centrifuged, and resuspended at the same cell density. Cocultures (20 ml) of L. lactis NZ9000(pNZ8020) derivatives (3%, vol/vol) and L. lactis IPLA 729 (nisZ+) (0.01%, vol/vol) were incubated for 4 and 6 h at 30°C. To determine the viable counts of each strain, culture dilutions were plated onto GM17 and GM17 containing chloramphenicol (10 μg/ml). LysH5 activity was tested in both cell extracts and supernatants of cocultures. LysH5 activity was detected in the cell extract of L. lactis NZ9000(pNZ8020-LysH5), the highest specific activity being 17.74 ± 0.85 U/mg after 4 h of incubation (Fig. 2) and matching the highest nisin Z concentration in the supernatants (400 AU/ml). This result suggests that the bacteriocin secreted by the nisin Z-producing strain induced production of the endolysin. A similar result was obtained when cocultures of L. lactis NZ9000(pNZ8020-SPLcn972-LysH5opt) and L. lactis IPLA 729 were used (data not shown). However, no LysH5 activity was detected in the supernatant from these cocultures, although the presence of LysH5 was detected by Western blot analysis (data not shown). This could indicate that secreted nisin Z was not enough to induce LysH5 in L. lactis(pNZ8020-Lcn-LysH5opt). Therefore, further coculturing conditions and alternative nisin Z producers should be tested.
Fig 2.
Coculture of L. lactis NZ9000(pNZ8020) derivative with L. lactis IPLA 729 (nisin Z producer). Survival of both strains, L. lactis NZ9000(pNZ8020-LysH5) (♢) and L. lactis IPLA 729 (■), was monitored as viable counts at different incubation times. Bars indicate the endolysin LysH5 activity expressed by L. lactis NZ9000(pNZ8020-LysH5) in coculture with L. lactis IPLA 729.
LysH5 expression under different L. lactis promoters.
For a constitutive secretion of LysH5, the plasmid pGM36c (37) containing the P32 promoter was used. Primers Lcn-Lys-1 and Lcn-Lys-2 (see Table S1 in the supplemental material) were used to amplify a 1,573-bp fragment from plasmid pNZ8020-SPLcn972-LysH5opt. The PCR-generated fragment was cloned into SacI/PstI restriction sites of plasmid pMG36c to generate the recombinant plasmid pMG36c-P32-SPLcn972-LysH5opt. The heterologous production of LysH5 was determined in both supernatants and cell extracts. The results showed a very low production of LysH5 in both the cell extracts and the supernatants (Table 1). To test a presumably more efficient promoter, the inducible Pllmg0169 promoter of the L. lactis MG1363 llmg0169 gene was amplified from plasmid pAB0169 (21) using primers pAB0169BamHI and pAB2164SmaI (see Table S1). The PCR-generated fragment (241 bp) was cloned into the BamHI/SmaI restriction sites of plasmid pIL252 (33). Then, the gene encoding SPLcn972-LysH5opt fusion protein was cloned into BamHI/PstI restriction sites from plasmid pIL252-Pllmg0169, resulting in the plasmid pIL252-Pllmg0169-SPLcn972-LysH5opt. With the purpose of increasing the transcription under the Pllmg0169 promoter, transformed cultures (OD600, 0.4) were induced with bacitracin (1 μg/ml) for 4 h at 30°C. As occurred when the P32 promoter was used, L. lactis(pIL252-Pllmg0169-SPLcn972-LysH5opt) extracts also showed low endolysin activity, whereas only scant lytic activity was observed in the supernatants (Table 1). The lower production of LysH5 by these derivatives than by the pNZ8020-based plasmids may be due to copy number differences but, more likely, is caused by the intrinsic differences among promoters used to drive gene expression (6, 14). Thus, the highest endolysin activity levels were obtained under the transcriptional control of PnisA, the promoter of the NICE system, which has been extensively used to produce proteins in L. lactis (16, 19, 25). In contrast, LysH5 activity was dramatically reduced in the constructs carrying the constitutive P32 promoter, which was previously used to successfully drive the cytoplasmic production of the Listeria endolysins Ply118 and Ply511 in L. lactis (8).
Production of heterologous proteins is determined by a number of host genes. Different L. lactis mutants with higher lysostaphin and endolysin Ply511 activities were obtained by insertions in four different genes related to cell wall biosynthesis (35). In addition, other tools to enhance protein secretion have been developed, such as the fusion of a short synthetic propeptide between the SP and the mature moiety (18). Recently, the overexpression of the lactococcal CsiA protein, which inhibits the last stages of cell wall biosynthesis, promoted the efficient release of the heterologous intracellular Listeria endolysin LM4 in its active form without affecting cell viability (34). In conclusion, the synthesis of active LysH5 in L. lactis has been achieved, supporting the use of this GRAS host for its production as a food additive or for therapeutic purposes. However, further optimization to enhance secretion that facilitates downstream purification is needed for cost-effective LysH5 large-scale production.
Supplementary Material
ACKNOWLEDGMENTS
This research study was supported by grants AGL2009-13144-C02-01 (Ministry of Science and Innovation, Spain), IB08-052 (Science, Technology and Innovation Programme, Principado de Asturias, Spain), and PIE200970I090 (CSIC, Spain). L.R.-R. is a fellow of the Science, Technology and Innovation Programme (Principado de Asturias, Spain).
Footnotes
Published ahead of print 17 February 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Borrero J, et al. 2011. Use of the usp45 lactococcal secretion signal sequence to drive the secretion and functional expression of enterococcal bacteriocins in Lactococcus lactis. Appl. Microbiol. Biotechnol. 89:131–143 [DOI] [PubMed] [Google Scholar]
- 2. Darmon E, et al. 2002. A novel class of heat and secretion stress responsive genes is controlled by the autoregulated CssRS two-component system of Bacillus subtilis. J. Bacteriol. 184:5661–5671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Buyser ML, Dufour B, Maire M, Lafarge V. 2001. Implication of milk and milk products in food-borne diseases in France and in different industrialised countries. Int. J. Food Microbiol. 67:1–17 [DOI] [PubMed] [Google Scholar]
- 4. Degering C, et al. 2010. Optimization of protease secretion in Bacillus subtilis and Bacillus licheniformis by screening of homologous and heterologous signal peptides. Appl. Environ. Microbiol. 76:6370–6376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Ruyter PG, Kuipers OP, de Vos WM. 1996. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl. Environ. Microbiol. 62:3662–3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Vos WM. 1987. Gene cloning and expression in lactic streptococci. FEMS Microbiol. Rev. 46:281–295 [Google Scholar]
- 7. Fischetti VA. 2010. Bacteriophage endolysins: a novel anti-infective to control Gram-positive pathogens. Int. J. Med. Microbiol. 300:357–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gaeng S, Scherer S, Neve H, Loessner MJ. 2000. Gene cloning and expression and secretion of Listeria monocytogenes bacteriophage-lytic enzymes in Lactococcus lactis. Appl. Environ. Microbiol. 66:2951–2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. García P, Madera C, Martínez B, Rodríguez A. 2007. Biocontrol of Staphylococcus aureus in curd manufacturing processes using bacteriophages. Int. Dairy J. 17:1232–1239 [Google Scholar]
- 10. García P, et al. 2009. Functional genomic analysis of two Staphylococcus aureus phages isolated from the dairy environment. Appl. Environ. Microbiol. 75:7663–7673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. García P, Martínez B, Rodríguez L, Rodríguez A. 2010. Synergy between the phage endolysin LysH5 and nisin to kill Staphylococcus aureus in pasteurized milk. Int. J. Food Microbiol. 141:151–155 [DOI] [PubMed] [Google Scholar]
- 12. Gutiérrez J, Larsen R, Cintas LM, Kok J, Hernández PE. 2006. High-level heterologous production and functional expression of the Sec-dependent bacteriocin from Enterococcus faecium P13, in Lactococcus lactis. Appl. Microbiol. Biotechnol. 72:41–51 [DOI] [PubMed] [Google Scholar]
- 13. Köck R, et al. 2010. Methicillin-resistant Staphylococcus aureus (MRSA): burden of disease and control challenges in Europe. Euro Surveill. 15:19688.(Erratum, 15:19694.) [DOI] [PubMed] [Google Scholar]
- 14. Kok J, Jos MB, van der Vossen M, Venema G. 1984. Construction of plasmid cloning vectors for lactic streptococci which also replicate in Bacillus subtilis and Escherichia coli. Appl. Environ. Microbiol. 48:726–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuipers OP, de Ruyter P, Kleerebezem M, de Vos WM. 1998. Quorum sensing-controlled gene expression in lactic acid bacteria. J. Biotechnol. 64:15–21 [Google Scholar]
- 16. Kunji ER, Slotboom DJ, Poolman B. 2003. Lactococcus lactis as host for overproduction of functional membrane proteins. Biochim. Biophys. Acta 1610:97–108 [DOI] [PubMed] [Google Scholar]
- 17. Laird MW, et al. 2005. Optimization of BLyS production and purification from Escherichia coli. Protein Expr. Purif. 39:237–246 [DOI] [PubMed] [Google Scholar]
- 18. Le Loir Y, et al. 2001. Signal peptide and propeptide optimization for heterologous protein secretion in Lactococcus lactis. Appl. Environ. Microbiol. 67:4119–4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Le Loir Y, et al. 2005. Protein secretion in Lactococcus lactis: an efficient way to increase the overall heterologous protein production. Microb. Cell Fact. 4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lindholm A, Smeds A, Palva A. 2004. Receptor binding domain of Escherichia coli F18 fimbrial adhesin FedF can be both efficiently secreted and surface displayed in a functional form in Lactococcus lactis. Appl. Environ. Microbiol. 70:2061–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martínez B, Zomer AL, Rodríguez A, Kok J, Kuipers OP. 2007. Cell envelope stress induced by the bacteriocin Lcn972 is sensed by the lactococcal two-component system CesSR. Mol. Microbiol. 64:473–486 [DOI] [PubMed] [Google Scholar]
- 22. Martínez B, Fernández M, Suárez JE, Rodríguez A. 1999. Synthesis of lactococcin 972, a bacteriocin produced by Lactococcus lactis IPLA 972, depends on the expression of a plasmid-encoded bicistronic operon. Microbiology 145:3155–3161 [DOI] [PubMed] [Google Scholar]
- 23. Martínez JM, Kok J, Sanders JW, Hernandez PE. 2000. Heterologous coproduction of enterocin A and pediocin PA-1 by Lactococcus lactis: detection by specific peptide-directed antibodies. Appl. Environ. Microbiol. 66:3543–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mayer MJ, Narbad A, Gasson JM. 2008. Molecular characterization of a Clostridium difficile bacteriophage and its cloned biologically active endolysin. J. Bacteriol. 190:6734–6740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mierau I, Kleerebezem M. 2005. 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl. Microbiol. Biotechnol. 68:705–717 [DOI] [PubMed] [Google Scholar]
- 26. Morello E, et al. 2008. Lactococcus lactis, an efficient cell factory for recombinant protein production and secretion. J. Mol. Microbiol. Biotechnol. 14:48–58 [DOI] [PubMed] [Google Scholar]
- 27. Obeso JM, Martínez B, Rodríguez A, García P. 2008. Lytic activity of the recombinant staphylococcal bacteriophage phiH5 endolysin active against Staphylococcus aureus in milk. Int. J. Food Microbiol. 128:212–218 [DOI] [PubMed] [Google Scholar]
- 28. Oliveira M, Bexiga R, Nunes SF, Vilela CL. 2011. Invasive potential of biofilm-forming staphylococci bovine subclinical mastitis isolates. J. Vet. Sci. 12:95–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rilla N, Martínez B, Delgado T, Rodríguez A. 2003. Inhibition of Clostridium tyrobutyricum in Vidiago cheese by Lactococcus lactis ssp. lactis IPLA 729, a nisin Z producer. Int. J. Food Microbiol. 85:23–33 [DOI] [PubMed] [Google Scholar]
- 30. Rodríguez I, García P, Suárez JE. 2005. A second case of −1 ribosomal frameshifting affecting a major virion protein of the Lactobacillus bacteriophage A2. J. Bacteriol. 187:8201–8204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sánchez C, et al. 2000. Nucleotide sequence and analysis of pBL1, a bacteriocin-producing plasmid from Lactococcus lactis IPLA 972. Plasmid 44:239–249 [DOI] [PubMed] [Google Scholar]
- 32. Sass P, Bierbaum G. 2007. Lytic activity of recombinant bacteriophage Φ11 and Φ12 endolysins on whole cells and biofilms of Staphylococcus aureus. Appl. Environ. Microbiol. 73:347–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Simon D, Chopin A. 1988. Construction of a vector plasmid family and its use for molecular cloning in Streptococcus lactis. Biochemie 70:559–566 [DOI] [PubMed] [Google Scholar]
- 34. Stentz R, Bongaerts RJ, Gunning AP, Gasson M, Shearman C. 2010. Controlled release of protein from viable Lactococcus lactis cells. Appl. Environ. Microbiol. 76:3026–3031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tan YP, Giffard PM, Barry DG, Huston WM, Turner MS. 2008. Random mutagenesis identifies novel genes involved in the secretion of antimicrobial, cell wall-lytic enzymes by Lactococcus lactis. Appl. Environ. Microbiol. 74:7490–7496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Turner MS, Waldherr F, Loessner MJ, Giffard PM. 2007. Antimicrobial activity of lysostaphin and a Listeria monocytogenes bacteriophage endolysin produced and secreted by lactic acid bacteria. Syst. Appl. Microbiol. 30:58–67 [DOI] [PubMed] [Google Scholar]
- 37. van de Guchte M, van der Vossen JM, Kok J, Venema G. 1989. Construction of a lactococcal expression vector: expression of hen egg white lysozyme in Lactococcus lactis subsp. lactis. Appl. Environ. Microbiol. 55:224–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Walcher G, et al. 2010. Evaluation of paramagnetic beads coated with recombinant Listeria phage endolysin-derived cell-wall-binding domain proteins for separation of Listeria monocytogenes from raw milk in combination with culture-based and real-time polymerase chain reaction-based quantification. Foodborne Pathog. Dis. 7:1019–1024 [DOI] [PubMed] [Google Scholar]
- 39. Young R. 1992. Bacteriophage lysis: mechanism and regulation. Microbiol. Rev. 56:430–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.