Abstract
Effective sanitization is important in viral epizootic outbreaks to avoid further spread of the pathogen. This study examined thermal inactivation as a sanitizing treatment for manure inoculated with highly pathogenic avian influenza virus H7N1 and bacteriophages MS2 and ϕ6. Rapid inactivation of highly pathogenic avian influenza virus H7N1 was achieved at both mesophilic (35°C) and thermophilic (45 and 55°C) temperatures. Similar inactivation rates were observed for bacteriophage ϕ6, while bacteriophage MS2 proved too thermoresistant to be considered a valuable indicator organism for avian influenza virus during thermal treatments. Guidelines for treatment of litter in the event of emergency composting can be formulated based on the inactivation rates obtained in the study.
INTRODUCTION
In epizootic viral disease outbreaks, materials such as carcasses and litter, if not properly sanitized, can pose a health risk to humans and animals and can act as a source of further spread of the pathogen. Intensive poultry and egg production systems are rapidly expanding; for example, the global production of poultry meat increased by 75% between 1992 and 2002. In Asia, however, the industry expanded by 150% during that period (23). Thus, there is an increasing need for a safe sanitization method suitable for both small-scale and large-scale poultry production units. Methods available for the disposal of such materials include deposition in landfills, on-site burial, composting, treatment in rendering plants, and incineration.
Compared with other options, such as incineration, composting can be regarded as a relatively economical and environmentally friendly alternative. During the thermophilic phase of aerobic composting, the temperatures reached can be high enough to inactivate most pathogens, including avian influenza virus (AIV) (11, 20). In addition, composting can help prevent further transmission of AIV since the sanitization can be performed on-site, avoiding transport of contaminated materials.
In order to establish guidelines for sanitization treatments in the event of an epizootic outbreak, validation of treatment processes is required. Viral analysis can be expensive and time-consuming, and thus, the use of phages as a model organism is a good alternative. Bacteriophages have structures similar to those of viruses infecting humans and animals, but they are easier and less expensive to analyze. Various phages have been suggested as viral indicators (13) and can be used to monitor treatment efficiency in terms of viral inactivation. It is unclear whether there is a suitable phage that can be used as an indicator for AIV during thermal treatments. The MS2 phage has previously been suggested as an indicator for several different viruses (14). However, the characteristics of MS2 differ greatly from those of AIV. One of the more significant characteristic differences between the two viruses is that phage MS2 lacks an envelope. During thermal treatment, damage to the heat-sensitive envelope leads to rapid inactivation of enveloped viruses in comparison with the time for inactivation of nonenveloped viruses (27). In contrast to the MS2 phage, the size and overall characteristics of Pseudomonas phage ϕ6 are more similar to those of AIV. The ϕ6 phage is a enveloped, segmented double-stranded RNA phage (15) and has previously been shown to be a promising model for persistence and inactivation studies of an H5N1 AIV strain in water at 17 and 28°C (2).
The aim of the present study was to investigate the inactivation during mesophilic and thermophilic composting of an H7N1 highly pathogenic AIV (HPAIV) strain and the phages MS2 and ϕ6.
MATERIALS AND METHODS
Compost material and microorganisms.
The compost mixtures used in the study consisted of fresh poultry manure (free-range indoor hen flock) collected at Lövsta research station, SLU, Uppsala, Sweden, on two occasions and mixed with 3% straw by weight (mixture 1) and the same mixture with the addition of 25% unhatched eggs by weight (14-day-old embryos; Håtunaholm AB) (mixture 2). Sanitization of the compost mixture was monitored by analysis of the added infective microorganisms, HPAIV strain A/turkey/Italy/1387/00(H7N1) (Istituto Zooprofilattico Sperimentale delle Venezie, Legnaro, Italy), MS2 phage ATCC 15597-B1, and Pseudomonas phage ϕ6 ATCC 21781-B1.
The H7N1 HPAIV strain was cultured in 11-day embryonated specific-pathogen-free (SPF) eggs (Håtunaholm AB) by injecting 0.2 ml containing 4 log10 50% tissue culture infectious doses (TCID50) of virus in physiological saline into the allantoic cavity. The eggs were incubated for 2 days at 37°C and 40 to 60% humidity and refrigerated overnight, and the allantoic fluid was harvested. The virus batch was aliquoted and stored at −70°C before use. Propagation of the MS2 phage was performed in nutrient broth (NB; Oxoid AB, Sweden) using the host strain Salmonella enterica serotype Typhimurium WG 49 (ATCC 700730). The ϕ6 phage was propagated on lawns of its host strain, Pseudomonas phaseolicola HB10Y (ATCC), on Luria-Bertani (LB) agar plates using the double layer agar method (1). Phages were harvested by resuspending the top agar in 3 ml of LB broth. Cell-free lysate of the two phages was prepared by centrifugation at 3,000 × g for 10 min followed by filtration of the supernatant (Filtropur S, 0.45 μm; Sarstedt AB, Sweden). Stock solutions with bacteriophages were stored at 4 to 6°C before use.
Experimental design. (i) Thermal treatment.
The moisture content of the materials used during the thermal treatment experiments was 68% ± 0.6% (mean ± standard deviation) in mixture 1 and 67% ± 0.3% in mixture 2. Trials were performed with 3 replicates for material inoculated with H7N1 HPAIV and with 6 replicates for the two phages. Compost material was inoculated with the three viruses, not naturally occurring in the material, in separate tubes to an initial content of approximately 5.5 log10 TCID50 g−1 of the H7N1 HPAIV strain and 7.5 log10 PFU g−1 of phages MS2 and ϕ6. In the case of the MS2 phage, the material was mixed manually and 1 g of material was weighed into tubes before thermal treatment in a block thermostat (Grant digital QBD2) for 9 days. Sampling in triplicate was started when the material had reached the required temperature. In trials where the material was inoculated with the H7N1 HPAIV strain or the ϕ6 phage, the material was mixed manually and 1 g of material was weighed into tubes and heated to the required temperature before the addition of virus, whereupon the initial samples were taken. Thereafter, thermal treatment took place during 24 h in a water bath (Grant OLS200) or block thermostat (Grant digital QBD2) for the H7N1 HPAIV strain and the ϕ6 phage, respectively. The pH was measured in 1:10 compost mixture/deionized water after incubation for 1 h at room temperature (22°C), using a pH meter (inoLab pH 720) in the case of the phages and using pH sticks (Merck KGaA, Darmstadt, Germany) in samples inoculated with H7N1 HPAIV.
(ii) Composting in laboratory-scale reactors.
Trials with composting in laboratory-scale reactors were performed in 2 replicates for each mixture and organism. The compost materials were inoculated with one of the three viruses, H7N1 HPAIV strain, MS2 phage, or ϕ6 phage, to an initial content corresponding to the concentration in the thermal treatments. The first samples (day zero) were taken after the inoculated compost mixtures had been mixed manually, and then the material was divided among separate 1.5-liter compost reactors (Dewar flask; Fisher Scientific, United Kingdom) with Styrofoam lids loosely placed on top and kept at 20°C. The temperature within the compost reactors was monitored by using a Tinytag logger (Intab Interface-Teknik AB, Sweden). Sampling was performed on days 0, 1, 2, 4, and 7. On each sampling occasion, samples for enumeration of viruses were collected from the top, the middle, and the bottom of the reactor, comprising approximately 3 g of material from each location, and put on ice to stop the reaction. In addition, samples were collected at the same locations and pooled for pH measurement. Following sampling, the compost was thoroughly mixed. The pH was measured in 1:10 compost mixture/deionized water after incubation for 1 h at room temperature (22°C) using a pH meter (inoLab pH 720) for samples inoculated with the MS2 phage or the ϕ6 phage and using pH sticks (Merck KGaA, Darmstadt, Germany) for H7N1 HPAIV. The material was also analyzed for dry matter content, nonvolatile solids, and C/N ratio on day 0 and day 7 in reactors inoculated with phages, with similar values assumed for compost inoculated with H7N1 HPAIV (Table 1).
Table 1.
Moisture content and C/N ratio in compost mixtures at the start of the trial and in compost reactors 1 and 2 on day 7a
| Organism | Day | Mixture 1 |
Mixture 2 |
||
|---|---|---|---|---|---|
| MCb (%) | C/N ratio | MC (%) | C/N ratio | ||
| HPAIV | 0 | 65 | 10.1 | 58 | 8.4 |
| 7 | NDc | ND | ND | ND | |
| ϕ6 | 0 | 64 | 8.8 | 65 | 8.7 |
| 7 | 56, 60 | 10.9, 11.5 | 54, 58 | 11.1, 10.3 | |
| MS2 | 0 | 63 | 12.2 | 64 | 12.6 |
| 7 | 59, 64 | 17.1, 17.4 | 68, 54 | 15.4, 14.1 | |
Samples were taken from each compost mixture prior to division between compost reactors on day 0 and from each compost reactor on day 7 (reactor 1, reactor 2).
MC, moisture content.
ND, not determined due to biosafety considerations.
Enumeration of microorganisms.
For the HPAIV analysis, the samples were extracted by diluting them 10-fold in prechilled Eagle's minimal essential medium with 120 mg liter−1 penicillin G sodium salt, 100 mg liter−1 streptomycin sulfate (produced at SVA), 0.75 μg ml−1 Fungizone (Bristol-Myers Squibb), and 10% fetal bovine serum (Invitrogen) and then mixing and freezing them at −70°C. After thawing for 1 h at room temperature, the tubes were vortexed for 1 min, followed by centrifugation at 3,000 × g for 10 min. The supernatant was gel filtered through Sephadex G-25 PD-10 columns (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) and sterile filtered through a 0.45-μm filter (order number 83.1826; Sarstedt). The filtrate was analyzed by an endpoint titration method through cell culture cytopathic effect in 96-well plates, using MDCK cells and 8 replicates per dilution, as previously described (9). Virus titers were calculated after 6 to 8 days according to the Spearmann Kärber formula (17) and expressed as log10 TCID50 values per gram of wet-weight compost, with a detection limit of 1.8 log TCID50 g−1. The detection limit was set through the viral interference test (described below) by extracting and treating a sample from each mixture as described above. The viral interference test showed that the samples had to be diluted 1:10 in order not to give any interference. Thus, by testing a sample volume of 15 ml (diluted 1:10) of the total volume of 30 ml (3-g sample diluted to 30 ml), the detection limit was set to 1.8 TCID50 g−1.
Samples containing the MS2 phage were extracted by diluting them 10-fold in prechilled phosphate M15 buffer, pH 7.2 (SVA, Uppsala, Sweden), mixing, and freezing at −70°C. Following thawing at room temperature for 1 h, the samples were vortexed for 1 min prior to sterile filtration through a 0.45-μm filter (Sarstedt AB, Helsingborg, Sweden). For the ϕ6 phage analysis, the samples were diluted 10-fold in prechilled phosphate buffer and then vortexed for 1 min, followed by sterile filtration. Enumeration of the phages was performed according to the double-layer agar method (1), using host strains S. Typhimurium WG49 (ATCC 700730) for the MS2 phage and Pseudomonas phaseolicola HB10Y for the ϕ6 phage. In brief, samples were 10-fold serially diluted in saline solution (0.86% to 0.9% NaCl). Suitable dilutions were mixed with the host strain and soft agar and poured onto blood agar base (BAB) plates (Oxoid) for the MS2 phage and LB agar plates for the ϕ6 phage. The BAB plates were incubated at 37°C and the LB agar plates at 23°C for 18 h ± 2 h. Thereafter, plaques were counted, using a detection limit of 10 PFU per gram of wet-weight compost material.
Cytotoxicity and viral interference test for the H7N1 HPAIV strain.
In order to assess the cytotoxicity and interference properties of the compost material regarding viral infection of cells and set the assay detection limits, cytotoxicity and viral interference assays were performed as previously described (9). In samples where no virus was detected, the detection limit of the titration assay was calculated according to the Poisson distribution by using the formula c = −ln p/V, where 1 − p is the 95% probability that the aliquot is free of infectious virus (P = 0.05), V is the volume, and c is the virus concentration (4).
Statistical analysis.
Linear regression based on the data from the thermal treatments was performed using Minitab (Minitab 15.1.20.0; Minitab, Inc.). The results obtained from the linear regression analysis were used to calculate the inactivation rate, expressed as the D-value (time required to reach a 1-log10 reduction) for the inoculated microorganisms. The D-values obtained in the study were used to extrapolate the time needed for a 12-log10 reduction in the organisms. The 99% confidence intervals (99% CI) were calculated only for material and temperature combinations with data available from at least three sampling occasions, so in this case, the time for a 12-log10 reduction in the organisms was based on the upper 99% CI.
RESULTS
Inactivation of virus during thermal treatment at 35, 45, and 55°C.
Linear regression analysis of the log10-transformed data was used to determine D-values for inactivation of virus during thermal treatment at 35, 45, and 55°C, and the time needed to achieve a 12-log10 reduction in virus was extrapolated based on the upper 99% CI (Tables 2 and 3).
Table 2.
D-values with 99% CI and times needed for a 12-log10 reduction in H7N1 HPAIV, ϕ6 phage, and MS2 phage in compost material consisting of manure and straw (mixture 1) treated at various temperaturesa
| Temp (°C) | Virus or phage | D-value (99% CI) | Time to 12-log10 reduction |
|---|---|---|---|
| 35 | AIV | 0.36 h (0.27–0.53) | 6.4 h |
| ϕ6 | 0.23 h (0.16–0.45) | 5.4 h | |
| MS2 | NIa | ||
| 45 | AIV | 4.2 min (3.0–8.4) | 1.7 h |
| ϕ6 | 9 min (6.6–15.0) | 3.0 h | |
| MS2 | 3.3 days (2.8–4.0) | 48 days | |
| 55 | AIV | 2.4 minb | 29 min |
| ϕ6 | 0.6 minb | 7.2 min | |
| MS2 | 1.0 days (0.86–1.2) | 14.4 days |
NI, not included due to the estimated D-value exceeding the study period.
99% CI not calculated due to insufficient data.
Table 3.
D-values with 99% CI and times needed for a 12-log10 reduction in H7N1 HPAIV, based on the upper 99% CI, and in ϕ6 phage and MS2 phage in compost material consisting of manure, straw, and embryonated eggs (mixture 2) treated at various temperatures
| Temp (°C) | Virus or phage | D-value (99% CI) | Time to 12-log10 reduction |
|---|---|---|---|
| 35 | HPAIV | 0.39 h (0.28–0.63) | 7.6 h |
| ϕ6 | 0.17 h (0.10–0.44) | 5.3 h | |
| MS2 | NIa | ||
| 45 | HPAIV | 7.2 min (4.2–49.2) | 9.8 h |
| ϕ6 | 6.6 min (5.4–10.2) | 2.0 h | |
| MS2 | 2.6 days (2.3–2.9) | 34.8 days | |
| 55 | HPAIV | 2.5 minb | 30 min |
| ϕ6 | 1.2 minb | 14.4 min | |
| MS2 | 1.2 days (1.0–1.4) | 16.8 days |
NI, not included due to the estimated D-value exceeding the study period.
99% CI was not calculated due to insufficient data.
No clear pattern of differences in the persistence of the ϕ6 phage and the H7N1 HPAIV strain could be found at 35 or 45°C. At 55°C, the speed of inactivation was too fast for regression analysis to be performed and the 99% CI could not be calculated. The MS2 phage was between 501 and 3,488 times more persistent than the H7N1 HPAIV strain and the ϕ6 phage in all three thermal treatments. At 35°C, the reduction of the MS2 phage was less than 1 log10 during the study period. The inactivation rate constants were −0.001 and −0.002 log day−1 in mixture 1 and mixture 2, respectively (P < 0.001; standard error, 0.0003 and 0.0005; R2, 37.5% and 31.8%; n = 38). No consistent differences in inactivation rates could be seen between compost mixture 1, consisting of manure and straw, and mixture 2, consisting of manure, straw, and unhatched embryonated eggs.
Inactivation of virus and phages during laboratory-scale composting.
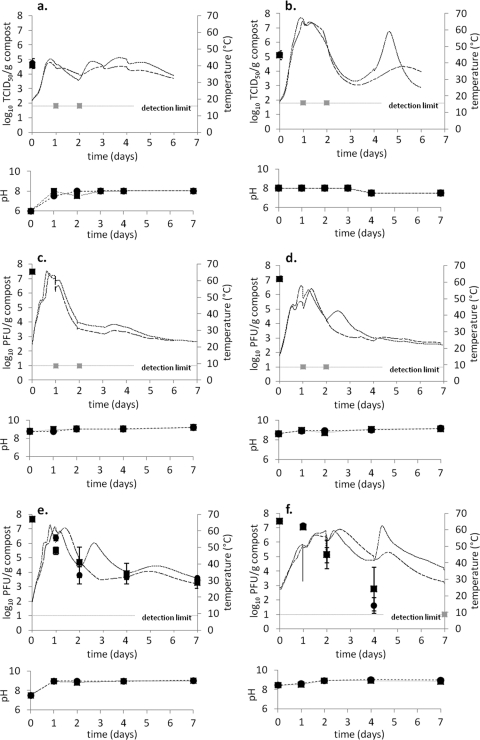
Temperature profiles, pH changes, and viral inactivation for the laboratory-scale compost reactors are presented in Fig. 1. The thermophilic phase with temperatures above 50°C was quickly reached and maintained for 1 to 2 days with the exception of mixture 1 inoculated with the H7N1 HPAIV strain, where the highest temperature achieved was 43°C (Fig. 1a). Following the thermophilic phase, a period with mesophilic temperatures could be seen. Throughout the trial, a decrease in moisture content and an increase in C/N ratio was observed in reactors inoculated with the ϕ6 and MS2 phages (Table 1).
Fig 1.
Changes in temperature (°C) and survival (log10 TCID50/PFU g−1 compost) of H7N1 HPAIV (a, b), ϕ6 phage (c, d), and MS2 phage (e, f) during composting in laboratory-scale compost reactor 1 (■, solid line), reactor 2 (●, dashed line), mixture 1 (left), and mixture 2 (right). Gray markers (square, circle) represent samplings where the number of viruses was found to be below the detection limit.
Both the H7N1 HPAIV strain and the ϕ6 phage were inactivated to levels below the detection limit within 24 h from the start of the compost trial, with peak temperatures between 42 and 67°C, and no infective particles of either virus could be found throughout the trial. During the 7-day trial, an inactivation of the MS2 phage corresponding to more than 7 log10 PFU g−1 compost was achieved in mixture 2, while in mixture 1, an inactivation of approximately 4 log10 PFU g−1 compost was achieved. The inactivation rate for the MS2 phage was at its highest between days 1 and 4, which corresponds well to the high temperatures achieved in the compost reactors in that period.
DISCUSSION
In the past decade, there have been increasing numbers of avian influenza (AI) outbreaks in poultry, and these have not only had a negative effect on the industry but are also a cause of public health concerns and an economic burden in those countries affected by the virus (7). In the case of an outbreak of an epizootic disease such as AI, sanitization treatment of all materials originating from the infected farm is important, since the virus might otherwise end up in the environment, causing further transmission of the disease. The strain used in the present study, H7N1 HPAIV, has been shown to survive for more than a year in manure-amended soil at 5°C and for 7 weeks in laboratory trials at 22°C (E. Emmoth, J. Elving, A. Albihn, K. Nyberg, B. Vinnerås, and J. R. Ottoson, unpublished data). AIV can also survive for long periods in water, depending on parameters such as temperature, salinity, and pH (6). Due to the risk of spread and long-term survival in the environment, runoff to water bodies, and leakage into groundwater, disposal options such as deposition in a landfill or burial are not viable alternatives for handling these materials. Composting, on the other hand, is a well-established technology, and if properly managed, it is known to reduce most pathogenic viruses, bacteria, fungi, protozoa, and helminth ova, with the exception of endospore-forming bacteria and prions (5, 12, 16, 28), thus resulting in a safe end product. Furthermore, in the case of AI outbreaks, on-site sanitization treatments such as composting are preferable to treatment at, e.g., rendering plants, since transport of the material has been shown to be a risk factor for spread of AIV between poultry farms (18). Thus, reactor or in-house composting of carcasses and litter is one of the preferred disposal options from a risk perspective during an AI outbreak (3, 19).
Due to the biosafety risk while composting materials following an AI outbreak, it is preferable if the first stage of the composting process is performed in-house or in a reactor to avoid heat loss and further spread of the pathogen via vector animals, such as birds and rats. Compost material is often nonhomogeneous, and this can result in an uneven temperature distribution throughout the compost pile, with parts, such as the surface, of the compost not maintaining thermophilic temperature (>50°C). If these parts of the compost are allowed to remain at a lower temperature than the rest of the compost pile, there is a risk of pathogen survival there during treatment (8). However, as demonstrated here, acceptable inactivation of H7N1 HPAIV can be achieved rapidly within the compost material even at mesophilic temperature (35°C) during the first stage. In composting materials originating from an epizootic outbreak, special precautions are necessary and turning of the compost during the first stage of composting may not be desirable, since it can result in the formation of airborne particulates, spreading pathogens (10). Following completion of the first stage, turning can be performed if desired without extensive biosafety risks regarding AIV.
Our laboratory-scale compost reactors showed good repeatability between reactors, and temperatures above 35°C were reached within 1 day in all reactors. AIV has been shown to be sensitive to thermal treatment (21, 24, 26). However, considerable variation between different AIV strains has been demonstrated (24). The H7N1 HPAIV strain used in the present study has been shown to be relatively heat stable in comparison with other AIV strains (25). Rapid inactivation of the H7N1 HPAIV strain took place in the reactors during bench-scale composting, and no infective virus could be detected after 24 h of composting, corresponding to a reduction of more than 3 log10 TCID50 g−1. Considering the rapid inactivation during thermal treatment, with a time of less than 7.6 h required for a 12-log10 reduction based on the results obtained, this was not surprising. The fact that the laboratory-scale compost reactors maintained a temperature of more than 35°C for more than 36 h proves that composting can fulfill the sanitization requirements (overkill sterilization with a 12-log10 reduction) in outbreaks of AI. This is in agreement with studies on sanitization of materials from poultry farms in terms of HPAIV inactivation (11) and reported successful use of composting during AI outbreaks (22).
Properties such as being cheaper and easier to analyze make phages an attractive alternative to HPAIV for analyses such as those performed in the present study. One important trait of a good indicator organism is that it should behave similarly to the pathogenic virus but preferably be slightly more resistant to the treatments tested. Studies examining the persistence of the ϕ6 phage and two H5N1 HPAIV strains in fresh water at 17 and 28°C showed the ϕ6 phage to be more persistent than the HPAIV strains and, thus, a possible indicator for AIV (2). In the present study, no clear pattern in difference between the H7N1 HPAIV strain and the ϕ6 phage could be detected in terms of D-values at 35 or 45°C. At 55°C, inactivation of more than 2 log10 PFU occurred within 5 min and inactivation to below the detection limit within 15 min. There is no consistent evidence that the ϕ6 phage is more thermoresistant than the H7N1 HPAIV strain. During laboratory-scale composting in our reactors, rapid inactivation of the ϕ6 phage took place and no infective virus could be detected after 24 h of composting, i.e., similar results to those obtained for the H7N1 HPAIV strain. This corresponds to an inactivation of more than 6 log10 PFU g−1 for the ϕ6 phage. The data presented here show similar inactivation of H7N1 HPAIV and Pseudomonas phage ϕ6 at both mesophilic and thermophilic temperatures, making it a promising surrogate for H7N1 HPAIV during thermal inactivation studies.
The inactivation rates of the MS2 phage were lower than those of H7N1 HPAIV at all three thermal treatment temperatures tested. In fact at 35°C, barely any inactivation of the MS2 phage occurred during the 9 days of the trial, compared with a D-value of less than 0.39 h for H7N1 HPAIV. In terms of physical characteristics, both H7N1 HPAIV and the MS2 phage are single-stranded RNA viruses, but H7N1 HPAIV is enveloped. Since enveloped viruses are known to be more sensitive to thermal treatments (27), the high inactivation rate of H7N1 HPAIV compared with that of the MS2 phage is not surprising. Due to the more thermoresistant nature of the MS2 phage, inactivation of the organism in the laboratory-scale compost reactors could be followed over a longer period, with the highest inactivation rate occurring between days 2 and 4. The comparatively low inactivation rate seen for the MS2 phage shows that it is far too heat resistant to be a valuable indicator for H7N1 HPAIV inactivation during composting. The use of such a thermoresistant indicator organism would lead to an underestimation of the sanitization efficiency of the process in terms of AIV inactivation. The MS2 phage could still be a relevant indicator for more thermoresistant viruses during composting, e.g., the nonenveloped avian hepatitis E virus.
In conclusion, to ensure sufficient inactivation of HPAIV during the composting process, recommended time and temperature combinations must be followed and handling, e.g., turning, of the material during the first stage of composting should be avoided. Due to loss of heat to the surrounding environment, the surface of the compost can have a lower temperature than the core. An insulating top layer could have a significant effect on sanitization performance by reducing the heat loss. Assuming that the surface temperature reflects the worst-case scenario (with the lowest temperature and, thus, the lowest inactivation), monitoring of the surface temperature can be used as a control parameter for the reduction of AIV during composting. In that case, the surface temperature should be kept above 35°C for at least 7.6 h.
ACKNOWLEDGMENTS
This study was funded by the European Union project Fluresist (SSPE-CT-2006-44311).
We thank Sarah Butcher and colleagues for kindly providing us with the ϕ6 phage and its host strain and Calogero Terrigino for providing us with the HPAIV strain A/turkey/Italy/1387/00 (H7N1).
Footnotes
Published ahead of print 2 March 2012
REFERENCES
- 1. Adams MH. 1959. Bacteriophages. Interscience Publishers, Inc, New York, NY [Google Scholar]
- 2. Adcock NJ, et al. 2009. The use of bacteriophages of the family Cystoviridae as surrogates for H5N1 highly pathogenic avian influenza viruses in persistence and inactivation studies. J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng. 44:1362–1366 [DOI] [PubMed] [Google Scholar]
- 3. Bendfeldt ES, Peer RW, Flory GA. 2006. In-house composting as a rapid response to avian influenza. BioCycle 47:38 [Google Scholar]
- 4. Blumel J, Schmidt I, Willkommen H, Lower J. 2002. Inactivation of parvovirus B19 during pasteurization of human serum albumin. Transfusion 42:1011–1018 [DOI] [PubMed] [Google Scholar]
- 5. Böhm R, Hunsinger B, Mardare C, Nagler C. 2008. Behaviour of prion-proteins in anaerobic and aerobic treatment of animal by-products, p 379–381 In Koutev V. (ed), 13th RAMIRAN International Conference—potential for simple technology solutions in organic manure management Network on Recycling of Agricultural Municipal and Industrial Residues in Agriculture (RAMIRAN), Albena, Bulgaria [Google Scholar]
- 6. Brown JD, Goekjian G, Poulson R, Valeika S, Stallknecht DE. 2009. Avian influenza virus in water: infectivity is dependent on pH, salinity and temperature. Vet. Microbiol. 136:20–26 [DOI] [PubMed] [Google Scholar]
- 7. Capua I, Alexander DJ. 2007. Animal and human health implications of avian influenza infections. Biosci. Rep. 27:359–372 [DOI] [PubMed] [Google Scholar]
- 8. Elving J, Ottoson JR, Vinneras B, Albihn A. 2010. Growth potential of faecal bacteria in simulated psychrophilic/mesophilic zones during composting of organic waste. J. Appl. Microbiol. 108:1974–1981 [DOI] [PubMed] [Google Scholar]
- 9. Emmoth E, Ottoson J, Albihn A, Belak S, Vinneras B. 2011. Ammonia disinfection of hatchery waste for elimination of single-stranded RNA viruses. Appl. Environ. Microbiol. 77:3960–3966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Glanville TD, et al. 2006. Environmental impacts and biosecurity of composting for emergency disposal of livestock mortalities, p 5 Iowa Department of Natural Resources, Des Moines, IA [Google Scholar]
- 11. Guan J, et al. 2009. Survival of avian influenza and Newcastle disease viruses in compost and at ambient temperatures based on virus isolation and real-time reverse transcriptase PCR. Avian Dis. 53:26–33 [DOI] [PubMed] [Google Scholar]
- 12. Haug RT. 1993. The practical handbook of compost engineering. Lewis Publishers, Boca Raton, FL [Google Scholar]
- 13. Havelaar AH, et al. 1991. Bacteriophages as model viruses in water-quality control. Water Res. 25:529–545 [Google Scholar]
- 14. Havelaar AH, van Olphen M, Drost YC. 1993. F-specific RNA bacteriophages are adequate model organisms for enteric viruses in fresh water. Appl. Environ. Microbiol. 59:2956–2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huiskonen J, et al. 2006. Structure of the bacteriophage phi6 nucleocapsid suggests a mechanism for sequential RNA packaging. Structure 14:1039–1048 [DOI] [PubMed] [Google Scholar]
- 16. Kalbasi A, Mukhtar S, Hawkins SE, Auvermann BW. 2005. Carcass composting for managing farm mortalities: a review. Compost Sci. Util. 13:180–193 [Google Scholar]
- 17. Kärber G. 1931. Beitrag zur kollektiven bahandlung pharmakologischer reihenversuche. Arch. Exp. Pathol. Pharmakol. 162:480–483 [Google Scholar]
- 18. McQuiston JH, et al. 2005. Evaluation of risk factors for the spread of low pathogenicity H7N2 avian influenza virus among commercial poultry farms. J. Am. Vet. Med. Assoc. 226:767–772 [DOI] [PubMed] [Google Scholar]
- 19. Pollard SJT, et al. 2008. Exposure assessment of carcass disposal options in the event of a notifiable exotic animal disease: application to avian influenza virus. Environ. Sci. Technol. 42:3145–3154 [DOI] [PubMed] [Google Scholar]
- 20. Senne DA, Brundaban P, Morgan R. 1994. Effect of composting on survival of exotic avian viruses: highly pathogenic avian influenza (HPAI) virus and adenovirus of egg drop syndrome-76. Avian Dis. 38:733–737 [PubMed] [Google Scholar]
- 21. Shahid MA, Abubakar M, Hameed S, Hassan S. 2009. Avian influenza virus (H5N1): effects of physico-chemical factors on its survival. Virology J. 6:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spencer JL, Rennie B, Guan J. 2004. Emphasis on biosecurity for composting poultry and manure during an outbreak of highly pathogenic avian influenza in British Columbia. Can. Anim. Health Net Bull. 9:21–23 [Google Scholar]
- 23. Steinfeld H, Mooney HA, Schneider F, Neville LE. (ed). 2010. Livestock in a changing landscape—drivers, consequences, and responses, vol 1 Island Press, Washington, DC [Google Scholar]
- 24. Swayne DE, Beck JR. 2004. Heat inactivation of avian influenza and Newcastle disease viruses in egg products. Avian Pathol. 33:512–518 [DOI] [PubMed] [Google Scholar]
- 25. Terregino C, Beato MS, Bertoli E, Mancin M, Capua I. 2009. Unexpected heat resistance of Italian low-pathogenicity and high-pathogenicity avian influenza A viruses of H7 subtype to prolonged exposure at 37 degrees C. Avian Pathol. 38:519–522 [DOI] [PubMed] [Google Scholar]
- 26. Thomas C, King DJ, Swayne DE. 2008. Thermal inactivation of avian influenza and Newcastle disease viruses in chicken meat. J. Food Prot. 71:1214–1222 [DOI] [PubMed] [Google Scholar]
- 27. Wichuk KM, McCartney D. 2007. A review of the effectiveness of current time-temperature regulations on pathogen inactivation during composting. J. Environ. Eng. Sci. 6:573–586 [Google Scholar]
- 28. Wilkinson KG. 2007. The biosecurity of on-farm mortality composting. J. Appl. Microbiol. 102:609–618 [DOI] [PubMed] [Google Scholar]