Abstract
To evaluate the relationship between bacterial genotypes and stress resistance patterns, we exposed 57 strains of Shiga toxin-producing Escherichia coli (STEC) O157 to acid, freeze-thaw, heat, osmotic, oxidative, and starvation stresses. Inactivation rates were calculated in each assay and subjected to univariate and multivariate analyses, including principal component analysis (PCA) and cluster analysis. The stx genotype was determined for each strain as was the lineage-specific polymorphism assay (LSPA6) genotype. In univariate analyses, strains of the stx1 stx2 genotype showed greater resistance to heat than strains of the stx1 stx2c genotype; moreover, strains of the stx1 stx2 genotype showed greater resistance to starvation than strains of the stx2 or stx2c genotypes. LSPA6 lineage I (LI) strains showed greater resistance to heat and starvation than LSPA6 lineage II (LII) strains. PCA revealed a general trend that a strain with greater resistance to one type of stress tended to have greater resistance to other types of stresses. In cluster analysis, STEC O157 strains were grouped into stress-resistant, stress-sensitive, and intermediate clusters. In stx genotypes, all strains of the stx1 stx2 genotype were grouped with the stress-resistant cluster, whereas 72.7% (8/11) of strains of the stx1 stx2c genotype grouped with the stress-sensitive cluster. In LI strains, 77.8% (14/18) of the strains were grouped with the stress-resistant cluster, whereas 64.7% (11/17) of LII strains were grouped with the stress-sensitive cluster. These results indicate that the genotypes of STEC O157 that are frequently associated with human illness, i.e., LI or the stx1 stx2 genotype, have greater multiple stress resistance than do strains of other genotypes.
INTRODUCTION
Shiga toxin-producing Escherichia coli (STEC) strains are some of the most common food-borne pathogens worldwide. Among various STEC serotypes, O157 is the most prevalent serogroup in food-borne infections, and STEC O157 infections have often been associated with severe conditions, such as hemolytic uremic syndrome (HUS) (26, 38). The low infectious dose and serious nature of STEC O157 has stimulated interest in the determinants of the survival of the organism in food and the environment. STEC O157 may encounter various environmental stresses, including nutrient depletion in the environment and changes in temperature and osmotic pressure. In the human stomach, STEC O157 must survive the low pH of gastric fluid. In order to understand the ecology of STEC O157 and establish effective control measures against the bacterium, extensive research has been done on bacterial stress responses (5, 24). Although some of these studies have shown the importance of cross-protection in stress resistance (5, 20, 33), the association between more than three types of stress has seldom been investigated. In practice, multiple stress resistance of a pathogen is important, because control measures in the food supply industry are based on the concept of employing multiple hurdles to decrease pathogen survival (14).
Recently, several studies have suggested that the bacterial populations of STEC O157 isolated from humans and cattle are genetically distinct (4, 19, 21, 45). Zhang et al. (44) and Abu-Ali et al. (1) reported that Shiga toxin production and the ability to adhere to intestinal cells are related to the genetic lineages of STEC O157. Stress resistance is an additional phenotype that may be related to genotype. Microarray and quantitative reverse transcriptase PCR analyses of STEC O157 strains have shown that several genes involved in the stress response are differentially expressed between human- and bovine-biased genotypes (7, 39). However, the difference in stress resistance among genotypes has been shown with respect to acid stress (25, 28, 34, 39). In other bacterial species, multivariate analyses, including principal component analysis (PCA) and cluster analysis, have revealed correlations between stress resistance, metabolic patterns, and their environmental niches (16, 29, 35). These methods would be useful to elucidate associations between genotype and several types of stress resistance phenotypes in STEC O157.
In this study, patterns of stress response in STEC O157 isolates were investigated in six different assays: acid, heat, freeze-thaw, high osmotic pressure, oxidative stress, and starvation. These stresses were selected because STEC O157 often encounters these stresses in food and host environments (5, 36). The relationships between stress resistance patterns and genotypes were further analyzed by univariate and multivariate methods. Genotypic traits were characterized by stx typing and a lineage-specific polymorphism assay with 6 markers (LSPA6), because these genotypic traits are known to reflect the divergence of STEC O157 (18, 19, 42). In this study, our objective was to examine the differences in stress resistance patterns among these genotypes of STEC O157.
MATERIALS AND METHODS
Bacterial strains.
A total of 57 STEC O157 strains, including 27 human isolates and 30 cattle isolates, were used in this study (Table 1). The human isolates comprised 23 isolates from enteritis patients and enteritis-linked isolates and four reference strains obtained from the American Type Culture Collection. All of these strains, except the four reference strains, were isolated in Japan between 1995 and 2009. The presence of stx1, stx2, stx2c, and LSPA6 lineage has been determined previously (19). The LSPA genotype was determined using octamer-based genome scanning, which uses short sequences from the E. coli genome for genotyping (17). Therefore, polymorphisms in these six loci for LSPA6 are regarded as selectively neutral (42). LSPA6 lineages are associated with stx genotypes and the genetic clades defined by single nucleotide polymorphisms (SNP clades). In previous studies, LSPA6 lineage I (LI) strains were more likely to carry stx2 than LSPA lineage II (LII) strains (19, 43, 45). Only limited information is available about the association between LSPA6 lineage and SNP clade; however, the hypervirulent SNP clade, clade 8, belongs to LSPA6 lineage I/II (LI/II) (12, 18).
Table 1.
Genotypic characteristic and IRI values of the strains used in this study
| Strain | Source | stx genotype | LSPA6 genotypea | LSPA6 lineagea | IRI valueb (stress) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Acid | Freeze-thaw | Heat | Osmotic | Oxidative | Starvation | |||||
| ESC226 | Cattle | stx1 | 111111 | I | −0.98 | −2.29 | 0.06 | −2.54 | 0.75 | −4.70 |
| ESC343 | Cattle | stx1 | 111111 | I | −0.91 | −3.07 | 0.37 | −3.94 | 0.09 | −3.12 |
| ATCC35150 | Human | stx1 | 111111 | I | −1.12 | −3.19 | −0.20 | −3.35 | −0.27 | −4.80 |
| ATCC43890 | Human | stx1 | 111111 | I | 0.56 | −3.15 | 0.58 | −2.48 | −0.06 | −3.09 |
| EC55 | Human | stx2 | 111111 | I | 0.52 | −3.03 | 0.49 | −3.22 | −0.33 | −3.13 |
| ESC361 | Human | stx2 | 111111 | I | −0.96 | −3.23 | 0.15 | −5.30 | −0.25 | −3.10 |
| EC10 | Human | stx1stx2 | 111111 | I | −0.57 | −3.30 | −0.03 | −3.65 | 0.11 | −4.68 |
| EC18 | Human | stx1stx2 | 111111 | I | −0.93 | −3.11 | 0.34 | −2.31 | 0.60 | −5.95 |
| EC28 | Human | stx1stx2 | 111111 | I | −0.97 | −3.05 | 0.80 | −2.87 | 0.98 | −4.13 |
| EC32 | Human | stx1stx2 | 111111 | I | −0.82 | −2.84 | 0.87 | −2.87 | 0.53 | −4.03 |
| EC33 | Human | stx1stx2 | 111111 | I | −0.83 | −2.42 | 0.20 | −2.97 | 0.92 | −4.91 |
| EC43 | Human | stx1stx2 | 111111 | I | −1.04 | −3.05 | −0.71 | −3.79 | −0.01 | −5.49 |
| EC52 | Human | stx1stx2 | 111111 | I | −0.89 | −3.11 | −0.33 | −3.35 | 0.13 | −4.92 |
| EC70 | Human | stx1stx2 | 111111 | I | −1.84 | −3.17 | 0.30 | −3.65 | −0.09 | −4.40 |
| EC170 | Cattle | stx1stx2 | 111111 | I | −1.08 | −3.24 | −0.43 | −3.46 | 0.12 | −4.57 |
| ESC228 | Cattle | stx1stx2 | 111111 | I | −0.82 | −3.17 | −0.12 | −2.40 | 0.88 | −4.22 |
| ESC342 | Cattle | stx1stx2 | 111111 | I | −1.11 | −3.06 | 0.29 | −3.51 | −0.05 | −4.61 |
| ATCC43895 | Human | stx1stx2 | 111111 | I | −1.22 | −3.41 | −1.20 | −3.71 | −0.21 | −5.01 |
| EC160 | Cattle | stx2 | 211111 | I/II | −0.51 | −2.83 | 0.13 | −3.64 | −0.40 | −3.11 |
| ESC225 | Cattle | stx2 | 211111 | I/II | −0.92 | −2.49 | −0.44 | −2.04 | 1.00 | −3.77 |
| ESC344 | Cattle | stx2 | 211111 | I/II | −1.01 | −3.18 | −0.36 | −3.89 | 0.32 | −5.34 |
| ESC349 | Human | stx2 | 211111 | I/II | −1.21 | −3.10 | −0.26 | −3.53 | 0.18 | −4.53 |
| ESC360 | Human | stx2 | 211111 | I/II | −0.83 | −2.47 | 0.89 | −2.51 | 0.74 | −3.03 |
| EC59 | Human | stx2c | 211111 | I/II | −0.93 | −3.00 | −0.01 | −2.32 | 0.80 | −4.13 |
| EC157 | Cattle | stx2c | 211111 | I/II | −1.09 | −3.17 | −0.89 | −3.61 | −0.23 | −5.09 |
| ESC206 | Cattle | stx2c | 211111 | I/II | −0.38 | −2.75 | −0.44 | −2.87 | 0.80 | −3.96 |
| ESC219 | Cattle | stx2c | 211111 | I/II | −0.10 | −3.28 | 0.88 | −1.96 | 0.79 | −3.84 |
| EC66 | Human | stx1stx2c | 211111 | I/II | −0.93 | −2.11 | 0.16 | −2.19 | 0.93 | −4.14 |
| ESC138 | Cattle | stx1stx2c | 211111 | I/II | −1.26 | −2.89 | 0.92 | −2.39 | 0.75 | −4.14 |
| EC1 | Human | stx1stx2c | 211111 | I/II | −1.01 | −2.95 | 0.49 | −2.32 | 0.71 | −4.18 |
| EC42 | Human | stx1stx2c | 211111 | I/II | −1.20 | −3.00 | 0.39 | −2.61 | 0.57 | −4.16 |
| ESC362 | Human | stx1stx2c | 211111 | I/II | −1.12 | −2.66 | 0.00 | −2.33 | 0.55 | −3.94 |
| ATCC43889 | Human | stx1stx2c | 211111 | I/II | −0.77 | −2.85 | 0.94 | −2.84 | 1.21 | −3.70 |
| ESC356 | Human | stx2c | 221212 | II | −1.05 | −2.92 | 0.11 | −3.03 | 0.31 | −4.35 |
| EC37 | Human | stx2c | 222212 | II | −0.23 | −2.97 | 0.68 | −1.94 | 0.38 | −3.53 |
| EC38 | Human | stx2c | 222212 | II | −0.60 | −2.70 | 0.99 | −2.63 | 1.00 | −3.16 |
| EC169 | Cattle | stx2c | 222212 | II | −0.83 | −2.80 | 0.37 | −3.87 | −0.49 | −3.06 |
| EC183 | Cattle | stx2c | 222212 | II | −0.91 | −2.99 | 0.61 | −4.24 | −0.43 | −3.03 |
| ESC209 | Cattle | stx2c | 222212 | II | −0.78 | −3.08 | 0.32 | −2.31 | 1.35 | −4.07 |
| ESC340 | Cattle | stx2c | 222212 | II | −0.84 | −2.87 | 0.70 | −2.83 | 0.81 | −3.04 |
| ESC211 | Cattle | stx2c | 222222 | II | −0.92 | −2.88 | 0.48 | −3.26 | 0.38 | −3.09 |
| EC181 | Cattle | stx1stx2c | 221222 | II | −1.11 | −3.09 | −0.33 | −3.71 | −0.35 | −4.72 |
| EC164 | Cattle | stx1stx2c | 222222 | II | −1.28 | −3.26 | −0.41 | −3.47 | −0.33 | −5.04 |
| ESC213 | Cattle | stx1stx2c | 222222 | II | −0.34 | −2.56 | 1.45 | −1.77 | 1.75 | −4.02 |
| ESC214 | Cattle | stx1stx2c | 222222 | II | −0.86 | −2.65 | 1.34 | −2.38 | 1.06 | −3.02 |
| ESC215 | Cattle | stx1stx2c | 222222 | II | −0.93 | −2.94 | 1.04 | −2.45 | 0.99 | −3.07 |
| ESC216 | Cattle | stx1stx2c | 222222 | II | −0.51 | −2.69 | 1.83 | −1.79 | 0.93 | −4.32 |
| ESC220 | Cattle | stx1stx2c | 222222 | II | −0.65 | −2.83 | 0.89 | −1.91 | 0.55 | −3.37 |
| ESC222 | Cattle | stx1stx2c | 222222 | II | −0.72 | −2.78 | 0.99 | −2.25 | 1.13 | −4.17 |
| ESC229 | Cattle | stx1stx2c | 222222 | II | −0.67 | −2.97 | 1.30 | −2.55 | 1.18 | −3.05 |
| EC44 | Human | stx2 | 231111 | Other | −0.68 | −2.65 | 1.14 | −2.49 | 0.85 | −3.07 |
| EC45 | Human | stx2 | 231111 | Other | −0.70 | −2.06 | 0.09 | −2.35 | 1.07 | −4.37 |
| EC175 | Human | stx2c | 212111 | Other | −1.06 | −3.08 | 0.41 | −4.36 | −0.57 | −3.35 |
| ESC223 | Cattle | stx2c | 212211 | Other | −0.91 | −1.38 | 0.86 | −3.07 | 0.58 | −3.07 |
| ESC231 | Cattle | stx2c | 221111 | Other | −0.97 | −2.90 | 0.08 | −2.58 | 0.94 | −4.83 |
| ESC367 | Cattle | stx2c | 221211 | Other | −0.91 | −3.23 | 0.35 | −2.57 | 0.77 | −4.91 |
| ESC339 | Cattle | stx2c | 252211 | Other | −1.09 | −3.12 | 1.09 | −2.85 | 1.09 | −4.36 |
The LSPA6 alleles were placed in the following order: folD sfmA, Z5935 gene, yhcG, rtcB, rbsB, and arp-iclR. LSPA6 genotypes of 222222, 221222, 222212, and 221212 were regarded as LII.
These values are calculated from inactivation rate. Because of log transformation, some values are positive, whereas the others are negative. Smaller IRI values correspond to greater stress resistance.
Stress resistance assays.
Strains were grown in tryptic soy broth (TSB; Becton, Dickinson and Company, NJ) at 37°C (Incubator MIR-262; Sanyo, Osaka, Japan) for 20 h. For acid stress, the culture of each strain was diluted 100-fold into 10 ml of minimal E glucose medium (EG medium) (41) acidified with hydrogen chloride (Kanto Chemical Co., Inc., Tokyo, Japan) to pH 2.5. The broth was incubated at 37°C, and viable cell counts were performed at 0, 2, 6, 8, and 12 h. To enumerate the viable cells, the inoculated broth was serially diluted with phosphate-buffered saline (PBS; Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) and pour-plated onto tryptone soya agar (TSA; Oxoid Ltd., Hampshire, United Kingdom). All plates were incubated at 37°C, and colonies were counted after 48 h. For heat stress, the culture of each strain was diluted 100-fold in 1.5 ml of TSB, and the broth was submerged in a water bath (Thermominder EX; Taitec Cooperation, Saitama, Japan) at 52°C. Viable cell counts were performed at 0, 2, 4, and 6 h as described above. For freeze-thaw stress, the culture of each strain was diluted 100-fold in 10 ml of TSB, and the broth was subsequently frozen at −20°C (Medicool MPR-411 FRS; Sanyo). One freeze-thaw cycle consisted of freezing at −20°C for 22.5 h followed by thawing at 37°C for 1.5 h. Freeze-thaw cycles were repeated seven times for each sample. Viable cell counts were performed at days 0, 1, 2, 4, and 7 as described above. For high osmotic pressure stress, the culture of each strain was diluted 100-fold in 10 ml of a solution of 20% (wt/vol) NaCl (Wako Pure Chemical Industries Ltd., Tokyo, Japan). The solution was incubated at 37°C, and viable cell counts were performed at days 0, 1, 2, 4, and 7 as described above. For oxidative stress, the culture of each strain was diluted 100-fold in 10 ml of PBS containing 1 mM H2O2 (Wako). The solution was incubated at 37°C, and viable cell counts were performed at 0, 2, 4, 6, 8, and 12 h as described above. For starvation stress, cells were harvested by centrifugation at 4,000 × g for 20 min and washed twice with 10 ml of distilled water (DW) (Nihon Millipore Ltd., Tokyo, Japan). Thereafter, the cells were resuspended in 10 ml of DW at 103 CFU/ml. The suspension was then incubated at 25°C. Viable cell counts were performed at days 0, 7, 14, 21, and 28 as described above. DW was used, because the population of STEC decreases very slowly in other nutrient-depleted media, such as phosphate-buffered saline (11), and it is not appropriate to measure an inactivation rate. Therefore, this assay evaluated the bacterial response against starvation in the environment as well as low osmotic pressure.
Calculation and validation of inactivation rate index.
We used the raw count data to construct an inactivation rate index (IRI) representing, for each strain, the rate of reduction in the bacterial count caused by each stress assay. Given an initial concentration (C0) of bacteria in an aliquot, we assumed the subsequent concentration undergoes a constant rate of log reduction, so that at sampling time ti the log concentration is log Ci = log C0 − αti, where α represents the rate of inactivation or hazard rate and i is 1, 2, 3, etc. Even if the inactivation curve is not exponential, α is still a meaningful phenotypic measure, because it represents the average rate of inactivation. We are not attempting here to produce an accurate inactivation model so have avoided multiparameter models such as the Weibull (30). The observed count at sampling time ti is obtained from a plated volume Di (the volume of dilution), and thus the expected count is μi = DiCi. We assume then that the observed count has a Poisson distribution with mean μi, for which log μi = log DiCi = log Di + log Ci = log Di + log C0 − αti.
Thus, α may be estimated as the negative slope in a Poisson regression model with log Di as an offset. This was fitted using the glm function of R 2.11.2 (32) to obtain α values for each stress-strain combination, with smaller IRI values corresponding to greater stress resistance. Graphical inspection of α values showed that they were highly skewed, and thus they were log transformed before they were submitted for further analysis to give a reasonably symmetrical, approximately normal, distribution. This transformation can be further justified, in a wider context, by noting that modeling of the log hazard is a common approach in survival analysis (6), corresponding to an assumption of proportional hazards. Thus, the IRI used in this study was log α, the log of the average inactivation rate.
Univariate analyses of different STEC O157 genotypes and their resistance to stress.
The IRI values of stx genotypes or LSPA6 lineages were compared using multiple Student's t tests with Tukey's adjusted P value.
Multivariate analyses of IRI values in all stress resistance assays.
To gain insight into patterns of stress resistance, IRI values from all of the six stress resistance assays were analyzed by PCA and cluster analysis. PCA is useful for identifying a trend in a multivariate data set or a correlation between variables (40). The transformation of PCA consolidates the information of a data set into a few new variables, principal components (PCs). PCAs were computed from the following equation: Zi = aiacidxacid + aifreezexfreeze + aiheatxheat + aiosmoticxosmotic + aioxidativexoxidative + aistarvationxstarvation, where Zi refers to a PC score in one strain in the ith PC, a refers to a factor loading, and x refers to the IRI value of the strain (Table 1) in a stress resistance assay. The a values are calculated from the correlation matrix of the observed data. First, a1 values are computed to maximize the variance of Z1, constrained so that the sum of the squares of factor loadings is one. This is termed the first PC, and the maximized variance the first eigenvalue. Then, a2 is calculated to maximize the variance of Z2 subject to orthogonality to Z1. The computation can be continued until the sixth (the number of the variables) PC is calculated. However, PCA is usually stopped when the cumulative variance reaches 70% or when the next eigenvalue becomes less than 1.0 (13, 27). Factor loadings for each variable and PC scores for each PC have a specific interpretation. In our data set, PCs described stress resistance patterns of STEC O157. For each PC, factor loadings of each variable represent the contribution of the variable to that particular pattern. On the other hand, PC scores represent relative values of each strain among all of the strains. Therefore, the interpretations for PC scores are made according to the loadings in each PC. PCA is also useful for graphic representation. In a PCA biplot, each strain is plotted according to the first and second PC scores to show the largest variation in the data set. In the biplot, strains that have similar stress resistance patterns will be closer to each other. PCA was performed using the princomp function of R with default settings.
To see a general trend of multiple stress resistance, cluster analysis was performed using the partitioning-around-medoids (PAM) clustering method (15) in the library “cluster” in R with default settings. This method was used to inform grouping of STEC O157 strains on the basis of similarities in their IRI values for the six stress resistance assays. The number of clusters was evaluated from 2 to 10 by the silhouette width (15) and visual inspection of PCA biplots. The stress resistance pattern of each cluster was characterized by mean IRI values, and mean IRI values were compared using multiple Student's t tests with adjusted P values. From these results, the number of clusters for subsequent analysis was decided, where the majority of strains can be defined by the mean IRI values (e.g., stress resistant, susceptible, etc.). Associations between resistance patterns and stx genotypes or LSPA6 lineages were explored by the distribution of the IRI-based clusters among genotypes.
RESULTS
Calculation and validation of IRI values.
IRI values were obtained from the results of the six stress resistance assays (Table 1). Although IRI values were calculated from the inactivation rate, positive and negative values resulted from subsequent log transformation. These transformed IRI values gave an approximately normal distribution and thus were appropriate for multivariate analyses. The range of IRI values varied among the assays; therefore, to exclude the effect of the ranges of IRI values among the assays, IRI values were standardized before multivariate analyses. For standardization, within each assay, the mean was subtracted from each IRI value and then divided by the standard deviation (31).
Univariate analyses of STEC O157 genotypes and their stress resistance.
Associations between the source or genotype of STEC O157 and the IRI values from each stress resistance assay were evaluated. No significant differences in IRI values between human and cattle isolates of STEC O157 were observed. However, in comparisons between IRI values and genotypes, significant associations were observed in the heat and starvation assays. Strains of the stx1 stx2 genotype (strains with both stx1 and stx2 genes) showed significantly greater resistance to heat than strains of the stx1 stx2c genotype (P = 0.011). Strains of the stx1 stx2 genotype also showed greater resistance to the starvation assay than strains of the stx2 or stx2c genotypes (stx1 stx2 strains versus stx2 strains, P = 0.019; stx1 stx2 strains versus stx2c strains, P = 0.011). LI and LI/II strains showed significantly greater resistance to heat than LII strains (LI strains versus LII strains, P = 0.005; LI/II strains versus LII strains, P = 0.015). In the starvation assay, LI strains showed greater resistance than LII strains (P = 0.024).
Multivariate analyses of IRI values in all stress resistance assays.
All IRI values in the six stress resistance assays were included in the multivariate analyses. First, in PCA, the first and second PCs accounted for 45.8% and 22.1%, respectively, of the variance within the multivariate data set. Because most of the variation (67.9%) was contained in the first and second PC and no other PCs possessed eigenvalues of >1.0, we focused on these PCs. All of the factor loadings on the first PC were negative values, indicating that the first PC described multiple stress resistance, with higher scores associated with greater resistance to the six stresses, and lower scores associated with susceptibility to them (Table 2). On the other hand, higher scores in the second PC were associated with resistance to the acid, heat, and starvation stresses and susceptibility to the osmotic, oxidative, and freeze-thaw stresses. Pairwise comparisons of PC scores in the first PC and stx genotypes or LSPA6 lineages showed higher scores in the strains of the stx1 stx2 genotype (P = 0.0099) and LI strains (P = 0.0088) than in the strains of the stx1 stx2c genotype and LII strains, respectively (Table 3). This result indicates the general trend of greater multiple stress resistance of the strains of the stx1 stx2 genotype and LI strains than those of the stx1 stx2c genotype and LII strains, respectively. In PC scores in the second PC, there was no significant difference among genotypes.
Table 2.
Principal component analysis of inactivation rate index values in six stress resistance assays
| Variable | Factor loadinga in: |
|
|---|---|---|
| 1st PC | 2nd PC | |
| Acid | −0.30 | −0.43 |
| Freeze-thaw | −0.36 | 0.12 |
| Heat | −0.48 | −0.21 |
| Osmotic | −0.48 | 0.37 |
| Oxidative | −0.47 | 0.44 |
| Starvation | −0.31 | −0.65 |
| Proportion of variance | 0.46 | 0.22 |
| Cumulative proportion | 0.46 | 0.68 |
Factor loading indicates the relative weight of the individual stress resistance assay on each principal component (PC).
Table 3.
Differences in PC scores between stx genotypes and LSPA6 lineage
| Genotype | PC score (mean ± SD) in: |
|
|---|---|---|
| 1st PCa | 2nd PCb | |
| stx genotype | ||
| stx1 | 0.33 ± 1.51 | −0.67 ± 1.87 |
| stx2 | 0.07 ± 1.78 | −0.59 ± 1.67 |
| stx2c | −0.14 ± 1.38 | −0.25 ± 1.16 |
| stx1stx2 | 1.21 ± 1.37c | 0.59 ± 0.66 |
| stx1stx2c | −1.11 ± 1.98c | 0.26 ± 0.62 |
| stx2stx2c | −0.49 ± 0.64 | 0.59 ± 0.34 |
| LSPA6 lineage | ||
| I | 0.99 ± 1.41d | −0.07 ± 1.49 |
| I/II | −0.06 ± 1.44 | 0.34 ± 0.87 |
| II | −0.75 ± 1.79d | −0.32 ± 0.92 |
| Other | −0.60 ± 1.46 | 0.23 ± 1.28 |
Higher PC scores indicate greater resistance to the six stresses.
Higher PC scores indicate greater resistance to acid, heat, and starvation stresses and lesser resistance to freeze-thaw, high osmotic pressure, and oxidataive stresses.
Significant (P = 0.0099) difference in mean PC scores in a pairwise t test with adjusted P values.
Significant (P = 0.0088) difference in mean PC scores in a pairwise t test with adjusted P values.
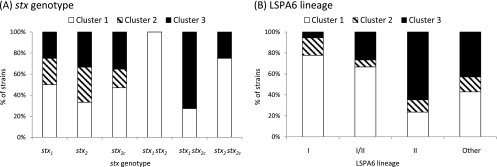
In cluster analysis, the average silhouette width was the largest when the number of clusters was six (see Fig. S1 in the supplemental material); however, the number was not decisive, because its standard deviation was large. According to the comparison of IRI values among clusters, most of the strains (>85%) were grouped to stress-resistant or -sensitive clusters when the numbers of clusters were two and three (see Table S1 in the supplemental material). When the number of clusters was two, each strain was grouped essentially according to its PC score in the first PC (see Fig. S2 in the supplemental material). Therefore, in order to describe the diversity of stress resistance pattern, three clusters were considered appropriate for the analysis. When the number of clusters was predefined as three, 31, 7, and 19 strains were grouped into clusters 1, 2, and 3, respectively (Fig. 1). To characterize stress resistance patterns, mean IRI values were compared among these clusters (Table 4). The mean IRI value of cluster 1 was significantly lower than that of cluster 3 in all of the six assays. It suggested that cluster 1 is a group relatively resistant to the stresses in this study and that cluster 3 is a stress-susceptible group. Cluster 2 can be characterized as a group of intermediate or variable stress resistance. The mean IRI values for cluster 2 in the acid, freeze-thaw, and heat stress assays were between those for clusters 1 and 3. In the osmotic and oxidative stress assays, strains in cluster 2 showed the greatest stress resistance; however, they showed the most susceptibility to starvation stress (Table 4). Among these clusters, genotypes of STEC O157 were not distributed evenly (Fig. 2). All strains of the stx1 stx2 genotype were grouped to cluster 1, whereas most of the strains (72.7%) of the stx1 stx2c genotype were grouped to cluster 3. Three of four strains (75.0%) of the stx2 stx2c genotype were grouped to cluster 1. The difference in distribution of clusters was more apparent for the LSPA6 lineage. The most common cluster for LI strains was cluster 1; on the other hand, LII was the most common in cluster 3. LI/II strains showed an intermediate pattern between LI and LII strains. In the strains carrying only one type of stx and strains of atypical LSPA6 lineage, none of the clusters held the majority.
Fig 1.
The results of cluster analysis in biplots by principal component analysis (PCA). Distances between plots represent the similarities between strains. Each stx genotype (A) and LSPA6 lineage (B) of STEC O157 corresponds to a color, as shown. Plots located inside the circle belong to each cluster: red, cluster 1; green, cluster 2; blue, cluster 3. The distance between plots shows the similarity of their stress resistance patterns. PCA shows that higher values on the x axis indicate greater multiple stress resistance to six stresses and higher values on the y axis indicate greater resistance to acid, heat, and starvation stresses and lower resistance to freeze-thaw, osmotic pressure, and oxidative stresses.
Table 4.
Difference in IRI values between clusters in each stress resistance assay
| Cluster | Mean ± SD of IRI values (stress)a |
|||||
|---|---|---|---|---|---|---|
| Acid | Freeze-thaw | Heat | Osmotic | Oxidative | Starvation | |
| 1 | −1.02 ± 0.24 AB | −3.00 ± 0.26 B | 0.02 ± 0.53 B | −3.03 ± 0.56 B | 0.41 ± 0.45 B | −4.55 ± 0.57 BC |
| 2 | −0.67 ± 0.55 A | −3.00 ± 0.15 | 0.36 ± 0.17 A | −4.08 ± 0.66 B | −0.34 ± 0.22 B | −3.13 ± 0.10 B |
| 3 | −0.61 ± 0.37 B | −2.68 ± 0.44 B | 0.90 ± 0.43 AB | −2.35 ± 0.36 B | 0.91 ± 0.39 B | −3.53 ± 0.51 C |
A, values with the same letter designation in each assay have a statistically significant difference (P < 0.05) in mean IRI values in a pairwise t test with adjusted P values; B and C, values with the same letter designation in each assay have a statistically significant difference (P < 0.01) in mean IRI values in a pairwise t test with adjusted P values.
Fig 2.
Associations between clusters and stx genotypes (A) and LSPA6 lineages (B) of STEC O157. Cluster 1 and cluster 3 were characterized as stress-resistant and stress-susceptible groups, respectively. Stress resistances of strains in cluster 2 varied depending on the stress.
DISCUSSION
In this study, we showed an association between genotypes of STEC O157 and stress resistance in six different assays by using univariate and multivariate analyses. Because only a subset of the genotypes is likely to be involved in human infection (9, 19), our results provide an insight into how selective pressures may affect the transmission of STEC O157 from cattle to humans via food and the environment.
Significant associations were found between genotypic traits of STEC O157 and their stress responses, although no significant associations were observed between the strain sources and their stress responses. Because human and cattle isolates of STEC O157 have been reported to share a subpopulation (9, 18, 19), it is potentially more revealing to examine the distribution of phenotypes of the bacterium among the genotypes rather than the sources (19). When IRI values were compared among stx genotypes or LSPA6 lineages, significant differences were observed in the heat and starvation stresses. Interestingly, the stx1 stx2 genotype and LI and LI/II strains, which showed greater resistance to these stresses, have been shown to be more frequently associated with human infection (19, 43, 45). This suggests that responses of STEC O157 to these two stresses play more important roles in their ecology than other stresses do. In E. coli, heat and starvation stresses induce general stress responses and affect various components of the bacterial cell via heat shock protein and rpoS regulons, respectively (5, 8, 37). The broad spectrum of effects on bacterial metabolism and cell components might be reflected by these results; however, rpoS sequences of the strains used in this study were highly homogeneous, and there was no apparent correlation when this was investigated by uni- and multivariate analyses (see Fig. S3 in the supplemental material). It is likely that variations in other stress-related genes are responsible for the diversity in the stress response among strains.
To characterize stress resistance patterns of the strains, all IRI values were subjected to multivariate analyses, including PCA and cluster analysis. These analyses revealed the influence of all six stresses, including four stresses that did not yield significant results in the univariate analyses. In PCA, the factor loading values for all of the six assays in the first PC were in the same direction (negative values). This finding implies that the largest contribution of variation to the stress resistance pattern is in multiple stress resistance, that is, a general trend that an STEC O157 strain that is resistant to one stress is also resistant to other stresses. This finding is in agreement with the results of Benito et al. (2), who showed a similar trend in resistance to hydrostatic pressure, mild-heat, acid, oxidative, and osmotic stresses for E. coli O157 strains.
In cluster analysis, STEC O157 strains were grouped into three clusters according to their stress resistance patterns. These clusters were characterized by mean IRI values (Table 4). Multiple comparisons elucidated that strains in cluster 1 showed greater resistance in all of the six assays than strains in cluster 3 did. These results corroborate the first PC in PCA, which showed that the most discriminative factor is multiple stress resistance. Interestingly, the clusters were not distributed evenly among genotypes of STEC O157. This suggests that the stress resistance patterns are associated with the phylogeny of STEC O157 to some extent. In contrast to the predominance of cluster 1 in LI strains, LII strains were predominantly comprised of cluster 3. LI/II strains showed an intermediate distribution among the clusters (Fig. 2), consistent with the intermediate genetic properties in this lineage (18, 45). Similar trends were observed in strains carrying two different types of stx, i.e., stx1 stx2, stx1 stx2c, and stx2 stx2c genotypes. This could be attributable to the strong association between the stx genotype and the LSPA6 lineage (12, 19, 43, 45). In a previous study, most of the strains (>85%) of the stx1 stx2, stx2 stx2c, and stx1 stx2c genotypes were shown to belong to LI, LI/II, and LII, respectively (19). On the other hand, strains carrying only one type of stx showed a more diverse stress resistance pattern. This result is in concordance with the result of a study that showed that these strains are genetically more diverse than strains carrying two types of stx (19). The second PC showed that the stress responses to acid, heat, and starvation stress tend to be similar to each other and likewise osmotic, oxidative, and freeze-thaw stresses. Although we cannot infer whether or not this is the result of overlapping genetic responses, these stress responses may have a similar role in the ecology of STEC O157.
An important implication of the clustering result is that human-biased genotypes, such as LI strains and strains of the stx1 stx2 or stx2 stx2c genotype were more likely to be stress resistant than the other genotypes. In food and the environment, pathogens encounter various types of stress. Those stressors could explain a pressure for a subset of genotypes to survive and consequently cause human infections. In Listeria monocytogenes and lactic acid bacteria, relationships between stress resistance patterns and their environmental niches have been shown (3, 29, 46). It is plausible that a similar selective pressure exists for STEC O157. Previously, differences in acid resistance among STEC O157 strains from different sources and of different genotypes have been reported (25, 28, 39). In our study, however, no significant difference of IRI values in acid resistance assays was observed among the genotypes. Further studies are required to draw any conclusion regarding the acid resistance in STEC O157. In other studies on stress resistance of STEC O157, the results of Benito et al. (2) are concordant with our results, while the results of Malone et al. (22) are not. In order to compare the results across studies, additional work is needed to confirm the genotypes under investigation. In further studies on the stress resistance of STEC O157, the stx genotype and LSPA6 lineage would be useful, because differences in distribution of these genotypes among sources have been identified in several population genetics analyses (18, 19).
The other important explanation for genetic divergence is the presence of virulence factors. Higher levels of stx expression and adherence to colonic cells from cattle were observed in LI strains (44). Interestingly, simultaneous upregulation of genes relating to a DNA damage repair system, SOS response, and stx in LI strains was observed in a microarray study (7). These associations between regulation of stress response and virulence properties may enhance bacterial survival in the environment, as well as pathogenesis. On the other hand, Dowd and Ishizaki (7) showed that genes encoding heat and cold shock proteins were differentially expressed between LI and LII strains of STEC O157. Heat and cold shock proteins influence various cell components and regulatory genes (8, 10) and may therefore be candidates for mediators of multiple stress resistance and, possibly, virulence properties. In this study, the association between stress resistance and a highly virulent genotype, clade 8, defined by single nucleotide polymorphisms (23), remains unclear. Several studies showed that strains of clade 8 belong to LI/II (12, 18). In this study, LI/II strains showed intermediate stress resistance in cluster analysis (Fig. 2).
In conclusion, our study revealed that (i) in our collection of STEC O157 isolates, heat and starvation stresses appeared to be more important for characterizing the bacterial population than were other stresses; (ii) there was a positive correlation among the six types of stress resistance; and (iii) some genotypes showed an association with multiple stress resistance. It is of interest that human-biased genotypes, such as LI strains or strains of the stx1 stx2 or stx2 stx2c genotype, showed greater stress resistance than strains of other genotypes did. However, the practical importance and mechanisms of stress resistance need further investigation. Our results also showed the importance of using STEC O157 strains from different genotypes in survival studies.
Supplementary Material
ACKNOWLEDGMENTS
This research was partially supported by the International Training Program of the Japan Society for the Promotion of Science and a Health Sciences Research Grant from the Ministry of Health, Labor, and Welfare, Japan.
Footnotes
Published ahead of print 24 February 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Abu-Ali GS, et al. 2010. Increased adherence and expression of virulence genes in a lineage of Escherichia coli O157:H7 commonly associated with human infections. PLoS One 5:e10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benito A, Ventoura G, Casadei M, Robinson T, Mackey B. 1999. Variation in resistance of natural isolates of Escherichia coli O157 to high hydrostatic pressure, mild heat, and other stresses. Appl. Environ. Microbiol. 65:1564–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bergholz TM, den Bakker HC, Fortes ED, Boor KJ, Wiedmann M. 2010. Salt stress phenotypes in Listeria monocytogenes vary by genetic lineage and temperature. Foodborne Pathog. Dis. 7:1537–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Besser TE, et al. 2007. Greater diversity of Shiga toxin-encoding bacteriophage insertion sites among Escherichia coli O157:H7 isolates from cattle than in those from humans. Appl. Environ. Microbiol. 73:671–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chung HJ, Bang W, Drake MA. 2006. Stress response of Escherichia coli. Compr. Rev. Food. Sci. Food Saf. 5:52–64 [Google Scholar]
- 6. Collet D. 2003. Modelling survival data in medical research. CRC Press, Boca Raton, FL [Google Scholar]
- 7. Dowd SE, Ishizaki H. 2006. Microarray based comparison of two Escherichia coli O157:H7 lineages. BMC Microbiol. 6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guisbert E, Yura T, Rhodius VA, Gross CA. 2008. Convergence of molecular, modeling, and systems approaches for an understanding of the Escherichia coli heat shock response. Microbiol. Mol. Biol. Rev. 72:545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gyles CL. 2007. Shiga toxin-producing Escherichia coli: an overview. J. Anim. Sci. 85:E45–E62 [DOI] [PubMed] [Google Scholar]
- 10. Han MJ, Lee SY. 2006. The Escherichia coli proteome: Past, present, and future prospects. Microbiol. Mol. Biol. Rev. 70:362–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hara-Kudo Y, Miyahara M, Kumagai S. 2000. Loss of O157 O antigenicity of verotoxin-producing Escherichia coli O157:H7 surviving under starvation conditions. Appl. Environ. Microbiol. 66:5540–5543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hartzell A, et al. 2011. Escherichia coli O157:H7 of genotype lineage-specific polymorphism assay 211111 and clade 8 are common clinical isolates within Pennsylvania. Foodborne Pathog. Dis. 8:763–768 [DOI] [PubMed] [Google Scholar]
- 13. Jacobs J, Rhodes M, Sturgis B, Wood B. 2009. Influence of environmental gradients on the abundance and distribution of Mycobacterium spp. in a coastal lagoon estuary. Appl. Environ. Microbiol. 75:7378–7384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jay JM, Loessner MJ, Golden DA. 2005. Modern food microbiology, 7th ed Springer, New York, NY [Google Scholar]
- 15. Kaufman L, Rousseeuw PJ. 1990. Finding groups in data: an introduction to cluster analysis. Wiley, New York, NY [Google Scholar]
- 16. Keymer DP, Miller MC, Schoolnik GK, Boehm AB. 2007. Genomic and phenotypic diversity of coastal Vibrio cholerae strains is linked to environmental factors. Appl. Environ. Microbiol. 73:3705–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim J, Nietfeldt J, Benson AK. 1999. Octamer-based genome scanning distinguishes a unique subpopulation of Escherichia coli O157:H7 strains in cattle. Proc. Natl. Acad. Sci. U. S. A. 96:13288–13293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laing CR, et al. 2009. In silico genomic analyses reveal three distinct lineages of Escherichia coli O157:H7, one of which is associated with hyper-virulence. BMC Genomics 10:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee K, et al. 2011. Multivariate analyses revealed distinctive features differentiating human and cattle isolates of Shiga toxin-producing Escherichia coli O157 in Japan. J. Clin. Microbiol. 49:1495–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leenanon B, Drake MA. 2001. Acid stress, starvation, and cold stress affect poststress behavior of Escherichia coli O157:H7 and nonpathogenic Escherichia coli. J. Food Prot. 64:970–974 [DOI] [PubMed] [Google Scholar]
- 21. Leopold SR, et al. 2009. A precise reconstruction of the emergence and constrained radiations of Escherichia coli O157 portrayed by backbone concatenomic analysis. Proc. Natl. Acad. Sci. U. S. A. 106:8713–8718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Malone AS, Yousef AE, LeJeune JT. 2007. Association of prophage antiterminator Q alleles and susceptibility to food-processing treatments applied to Escherichia coli O157 in laboratory media. J. Food Prot. 70:2617–2619 [DOI] [PubMed] [Google Scholar]
- 23. Manning SD, et al. 2008. Variation in virulence among clades of Escherichia coli O157:H7 associated with disease outbreaks. Proc. Natl. Acad. Sci. U. S. A. 105:4868–4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McClure PJ, Hall S. 2000. Survival of Escherichia coli in foods. Symp. Ser. Soc. Appl. Microbiol. 2000:61S–70S [DOI] [PubMed] [Google Scholar]
- 25. McKellar RC, Knight KP. 1999. Growth and survival of various strains of enterohemorrhagic Escherichia coli in hydrochloric and acetic acid. J. Food Prot. 62:1466–1469 [DOI] [PubMed] [Google Scholar]
- 26. Meng J, Doyle MP, Zhao T, Zhao S. 2007. Enterohemorrhagic Escherichia coli, p 249–269 In Doyle MP, Beuchat LR. (ed), Food microbiology: fundamentals and frontiers, 3rd ed ASM Press, Washington DC [Google Scholar]
- 27. Oda Y, Ouchi K. 1989. Principal component analysis of the characteristics desirable in bakers yeasts. Appl. Environ. Microbiol. 55:1495–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oh DH, et al. 2009. Escherichia coli O157:H7 strains isolated from environmental sources differ significantly in acetic acid resistance compared with human outbreak strains. J. Food Prot. 72:503–509 [DOI] [PubMed] [Google Scholar]
- 29. Parente E, et al. 2010. Diversity of stress tolerance in Lactobacillus plantarum, Lactobacillus pentosus and Lactobacillus paraplantarum: a multivariate screening study. Int. J. Food Microbiol. 144:270–279 [DOI] [PubMed] [Google Scholar]
- 30. Peleg M, Cole MB. 1998. Reinterpretation of microbial survival curves. Crit. Rev. Food Sci. Nutr. 38:353–380 [DOI] [PubMed] [Google Scholar]
- 31. Petrie A, Watson P. 2006. Statistics for veterinary and animal science, 2nd ed Blackwell Publishing Ltd., Oxford, United Kingdom [Google Scholar]
- 32. R Development Core Team 2010. R: a language and environment for statistical computing. R Foundation for Statistical Computing; http://www.R-project.org [Google Scholar]
- 33. Rowe MT, Kirk RB. 2000. Effect of nutrient starvation on the resistance of Escherichia coli O157:H7 to subsequent heat stress. J. Food Prot. 63:1745–1748 [DOI] [PubMed] [Google Scholar]
- 34. Saridakis CE, Johnson RP, Benson A, Ziebell K, Gyles CL. 2004. Influence of animal origin and lineage on survival of Escherichia coli O157:H7 strains in strong and weak acid challenges. J. Food Prot. 67:1591–1596 [DOI] [PubMed] [Google Scholar]
- 35. Siezen RJ, et al. 2010. Phenotypic and genomic diversity of Lactobacillus plantarum strains isolated from various environmental niches. Environ. Microbiol. 12:758–773 [DOI] [PubMed] [Google Scholar]
- 36. Stevens MP, van Diemen PM, Dziva F, Jones PW, Wallis TS. 2002. Options for the control of enterohaemorrhagic Escherichia coli in ruminants. Microbiology 148:3767–3778 [DOI] [PubMed] [Google Scholar]
- 37. Stortz G, Hengge-Aronis R. 2000. Bacterial stress responses. ASM Press, Washington, DC [Google Scholar]
- 38. Su CY, Brandt LJ. 1995. Escherichia coli O157:H7 infection in humans. Ann. Intern. Med. 123:698–714 [DOI] [PubMed] [Google Scholar]
- 39. Vanaja SK, Springman AC, Besser TE, Whittam TS, Manning SD. 2010. Differential expression of virulence and stress fitness genes between Escherichia coli O157:H7 strains with clinical or bovine-biased genotypes. Appl. Environ. Microbiol. 76:60–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Venables WN, Ripley BD. 2002. Modern applied statistics with S, 4th ed Springer-Verlag, Berlin, Germany [Google Scholar]
- 41. Vogel HJ, Bonner DM. 1956. Acetylornithinase of Escherichia coli: partial purification and some properties. J. Biol. Chem. 218:97–106 [PubMed] [Google Scholar]
- 42. Yang Z, et al. 2004. Identification of common subpopulations of non-sorbitol-fermenting, β-glucuronidase-negative Escherichia coli O157:H7 from bovine production environments and human clinical samples. Appl. Environ. Microbiol. 70:6846–6854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yokoyama E, et al. 2011. Biased distribution of IS629 among strains in different lineages of enterohemorrhagic Escherichia coli serovar O157. Infect. Genet. Evol. 11:78–82 [DOI] [PubMed] [Google Scholar]
- 44. Zhang YX, et al. 2010. Lineage and host source are both correlated with levels of Shiga toxin 2 production by Escherichia coli O157:H7 strains. Appl. Environ. Microbiol. 76:474–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ziebell K, et al. 2008. Genotypic characterization and prevalence of virulence factors among Canadian Escherichia coli O157:H7 strains. Appl. Environ. Microbiol. 74:4314–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zotta T, Ricciardi A, Ciocia F, Rossano R, Parente E. 2008. Diversity of stress responses in dairy thermophilic streptococci. Int. J. Food Microbiol. 124:34–42 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.