Abstract
Previous studies implicated loss of motility and mutations of the alsR and sigW regulatory genes in enhanced fitness of the Bacillus subtilis evolved strain WN716 over that of its ancestral strain WN624. The fitness of strains carrying knockout mutations alsR::spc, sigD::kan, and/or sigW::erm was measured and compared to that of the congenic ancestral strain by competition experiments.
TEXT
In order to understand the mechanisms leading to increased fitness during bacterial evolution under a particular set of environmental conditions, a long-term study was undertaken in which Bacillus subtilis was allowed to evolve for 6,000 generations in a rich medium that repressed selection for sporulation (9, 10). During this experiment, new strains which displayed numerous altered phenotypes, including significantly different colony and cell morphologies, loss of postexponential phenotypes, such as sporulation, competence, acetoin production, and motility, multiple auxotrophies, and increased competitive fitness, emerged (11). One of these evolved strains, B. subtilis WN716, was examined in greater detail using a combination of phenotypic characterizations, transcription microarray analyses, and whole-genome resequencing (3, 11). From these analyses, it appeared that loss of function of two different pleiotropic transcriptional regulators was implicated in the greater fitness of strain WN716: (i) SigW, an alternative extracytoplasmic function (ECF) sigma factor controlling resistance to bacteriocins and cell envelope-damaging compounds (5), and (ii) AlsR, a LysR family positive transcriptional regulator of the alsSD operon, encoding enzymes of the acetoin pathway (16). However, we were unable to assign loss of motility to the mutation in the sigD gene encoding the alternative sigma factor SigD, which controls expression of motility, chemotaxis, and cell wall proteins (1, 3, 11). In order to test the hypothesis that loss of motility and AlsR and SigW functions had led to an increase in strain WN716's fitness during its evolution, this communication describes (i) the construction of congenic knockout mutants in which SigW, SigD, and/or AlsR was disrupted, either singly or in all possible combinations, (ii) phenotypic characterization of the resulting mutant strains, and (iii) competition experiments to test the fitness of the mutants compared to that of the ancestral strain from which the evolution experiments were initiated.
The B. subtilis strains used are described in Table 1. Strains carrying knockout mutations inactivating alsR, sigD, or sigW were generous gifts from Elisabeth Härtig and John Helmann. The plasmid pCm::Tc for switching of the amyE::cat antibiotic resistance cassette to amyE::tet (17) was obtained from the Bacillus Genetic Stock Center, Columbus, OH. The media used were Luria-Bertani (LB) (12) and the sporulation-repressing (R) medium previously used for laboratory evolution experiments (9). For normal cultivation or motility assays, the medium was supplemented with agar at final concentrations of 1.5% or 0.3%, respectively. Where appropriate, the following antibiotics were added to the medium (final concentration): chloramphenicol (Chl; 5 μg/ml), erythromycin (Erm; 5 μg/ml), fosfomycin (Fos; 50 μg/ml), kanamycin (Kan; 10 μg/ml), spectinomycin (Spc; 100 μg/ml), or tetracycline (Tet; 10 μg/ml). Techniques used for B. subtilis transformation (2), enumeration of viable cells (15), assaying of phenotypic properties, and performance of competitions (14) have been described previously.
Table 1.
B. subtilis strains used in this studya
| Strain | Genotype and/or phenotype | Source, reference, and/or transformation processb |
|---|---|---|
| WN624 | trpC2, amyE::spc; Spcr ancestral strain | 9 |
| WN628 | trpC2, amyE::cat; Chlr ancestral strain | 9 |
| WN715 | trpC2, amyE::cat; Chlr strain isolated before WN716 population sweep | 11 |
| WN716 | trpC2, amyE::spc; Spcr strain evolved from WN624 to greater fitness | 11 |
| WN1148 | Strain AMBs2; trpC2, pheA1, alsR::spc; Spcr | E. Härtig (6) |
| WN1220 | B. subtilis subsp. spizizenii strain NRRL B-14821 | M. Roberts (13) |
| WN1237 | Strain HB4035; sigD::kan | J. Helmann (18) |
| WN1238 | Strain HB0042; sigW::kan | J. Helmann |
| WN1239 | Strain HB0020; sigW::erm | J. Helmann (4) |
| WN1241 | trpC2, amyE::cat, alsR::spc; Chlr, Spcr | WN1148→WN628; Spcr/Chlr |
| WN1242 | trpC2, amyE::cat, sigD::kan; Chlr, Kanr | WN1237→WN628; Kanr/Chlr |
| WN1243 | trpC2, amyE::cat, sigW::kan; Chlr, Kanr | WN1238→WN628; Kanr/Chlr |
| WN1244 | trpC2, amyE::cat, sigW::erm; Chlr, Ermr | WN1239→WN628; Ermr/Chlr |
| WN1262 | trpC2, amyE::tet, Tetr | pCm::Tc→WN628; Tetr/Chls |
| WN1265 | trpC2, amyE::cat, alsR::spc, sigD::kan; Chlr, Kanr, Spcr | WN1242→WN1241; Kanr/Chlr, Spcr |
| WN1266 | trpC2, amyE::cat, alsR::spc, sigW::erm; Chlr, Spcr, Ermr | WN1244→WN1241; Ermr/Chlr, Spcr |
| WN1267 | trpC2, amyE::cat, sigD::kan, sigW::erm; Chlr, Kanr, Ermr | WN1242→WN1244; Kanr/Chlr, Ermr |
| WN1268 | trpC2, amyE::cat, alsR::spc, sigD::kan, sigW::erm; Chlr, Spcr, Kanr, Ermr | WN1242→WN1266; Kanr/Chlr, Spcr, Ermr |
Spcr, spectinomycin resistant; Chls, chloramphenicol sensitive.
→, transformation from donor to recipient strain. The first marker after the semicolon denotes the selected marker. The marker(s) after the forward slash denotes the screened marker(s).
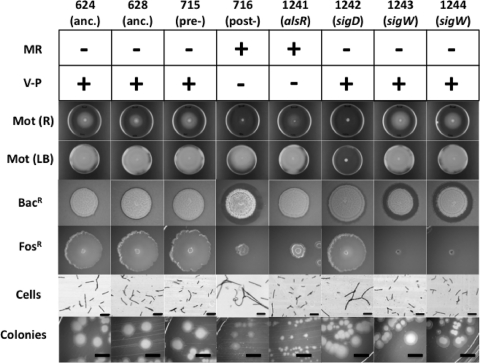
The evolved strain WN716 exhibited a number of altered phenotypes likely controlled by the alsR, sigD, or sigW gene products, including a small-colony phenotype and lack of motility on R medium, cell filamentation, sensitivity to bacteriocins and fosfomycin, loss of acetoin production, and acidification of liquid medium by overproduction of acetate (Fig. 1). Therefore, the phenotypes displayed by ancestral strains WN624, WN628, and WN715 (11), as well as by evolved strain WN716 (11), were compared to those of congenic strains carrying knockout mutations of alsR::spc (strain WN1241), sigD::kan (strain WN1242), sigW::kan (strain WN1243), and sigW::erm (strain WN1244) (Fig. 1). Strain WN716 was observed to resemble the alsR::spc knockout strain in its production of acid (methyl red [MR] positive) but not acetoin (Voges-Proskauer [V-P] negative), its reduced motility on R motility agar, and its small-colony phenotype on R agar (Fig. 1). Strain WN716 resembled the sigD::kan knockout strain in its cell filamentation in R liquid medium and resembled the sigW::kan and sigW::erm knockout strains in its sensitivity to fosfomycin and the bacteriocin produced by B. subtilis subsp. spizizenii (Fig. 1). Note, however, that strain WN716 had not completely lost motility, as would be predicted for a sigD mutant strain, but was more similar to the alsR::spc knockout strain in that motility was reduced on R motility agar but was normal on LB motility agar (Fig. 1).
Fig 1.
Phenotypic characterization of ancestral strains WN624 and WN628 (anc. 624 and 628, respectively), presweep strain WN715 (pre-), and postsweep evolved strain WN716 (post-). For comparative purposes, the phenotypes are compared with those of congenic strains carrying knockout mutations alsR::spc (strain WN1241), sigD::kan (strain WN1242), sigW::kan (strain WN1243), and sigW::erm (strain WN1244). The phenotypes tested were the acidification of medium using the methyl red (MR) test, acetoin production by the Voges-Proskauer (V-P) test, motility (Mot) on R or LB plates, and resistance to the bacteriocin produced by B. subtilis subsp. spizizenii (Bacr) or to fosfomycin (Fosr). All photos were of typical plates or microscopic fields; all photos in each row were taken of cultures at the same time of incubation. Size bars, 10 μm for cells and 5 mm for colonies.
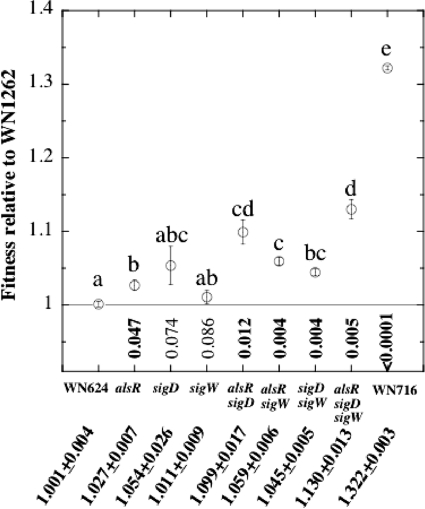
To test the hypothesis that inactivation of alsR, sigD, and/or sigW could confer greater competitive fitness, the appropriate strains were constructed and competed against the congenic ancestral strain WN1262 (with amyE::tet as its selective marker) in pairwise combinations in liquid R medium as described in detail previously (14). The results of these competition experiments showed that the alsR::spc single mutant was significantly more fit than the ancestor, whereas the sigD::kan and sigW::erm knockout strains were not significantly more fit at the P level of 0.05 (Fig. 2). All three strains carrying double mutations (alsR::spc sigD::kan, alsR::spc sigW::erm, and sigD::kan sigW::erm) were significantly more fit than the ancestor and, for the most part, significantly more fit than the single mutants (Fig. 2). The triple-knockout strain WN1268 (alsR::spc sigD::kan sigW::erm) was significantly more fit than all the single mutants and two of the double mutant strains (Fig. 2). However, the triple-knockout strain WN1268 (relative fitness of 1.130 ± 0.013) fell short of the high level of competitive fitness exhibited by evolved strain WN716 (relative fitness of 1.322 ± 0.0003) (Fig. 2).
Fig 2.
Results from pairwise competition experiments. Data points are presented as the averages ± standard deviations of duplicate experiments, with each consisting of 7 daily fitness determinations and performed as described previously (14). Below the strain designations are the actual numerical relative fitness values. Relative fitness is defined as 1 + s, where s is the selection coefficient, calculated as described previously (7). Lowercase letters directly above the data points denote groups of strains whose relative fitnesses were not significantly different from one another at the P level of ≤0.05 (by an analysis of variance [ANOVA]). Numbers below the data points on the x axis denote P values (ANOVA) of pairwise comparisons of the relative fitness of the indicated strain and that of the differentially marked ancestral strain WN1262; boldface numbers denote significant differences (P ≤ 0.05).
In summary, because evolved strain WN716 (i) exhibited higher fitness in R medium than its ancestral strain, (ii) carried mutations in alsR and sigW, as revealed by whole-genome sequencing, and (iii) demonstrated phenotypes consistent with mutations in alsR, sigD, and sigW (Fig. 1), I hypothesized that inactivation of the alsR, sigD, and/or sigW gene would lead to increased fitness of the resulting mutant strains over the ancestral strain in R medium. The data from pairwise competition experiments using engineered congenic strains carrying knockout mutations in alsR, sigD, and/or sigW supported this hypothesis; the single alsR knockout mutant, all three double-knockout mutants, and the alsR::spc sigD::kan sigW::erm triple-knockout mutant all exhibited significantly increased fitnesses relative to that of the ancestral strain (Fig. 2). These observations support and extend observations from large-scale flux analyses demonstrating that metabolic efficiency of B. subtilis cells was improved in sigD or sigW knockout mutants (8). However, no combination of knockout mutations was able to increase competitive fitness to the level seen in evolved strain WN716. I thus conclude that while inactivation of the pleiotropic regulators AlsR, SigD, and/or SigW does improve B. subtilis fitness in R medium, additional genetic factors are likely involved in the dramatic fitness increase of evolved strain WN716 over that of its ancestral strain. In the search for possible additional candidate genes, it should be noted that a whole-genome sequence analysis of WN716 uncovered, in addition to the mutations in alsR and sigW, a total of 43 other mutations within coding sequences (33 amino acid-changing single nucleotide polymorphisms [SNPs] and 10 +1 frameshift mutations) as well as 11 SNPs located within intergenic regions (3). These additional mutations will be targets for further exploration.
ACKNOWLEDGMENTS
I thank Elisabeth Härtig, Mike Roberts, and John Helmann for generous donation of strains and Andrea Rivas-Castillo and Krystal Kerney for valuable technical assistance.
This work was supported in part by a grant from the NASA Astrobiology, Exobiology, and Evolutionary Biology program (NNX08AO15G).
Footnotes
Published ahead of print 17 February 2012
REFERENCES
- 1. Aizawa S-I, Zhulin IB, Márquez-Magaña L, Ordal G. 2003. Chemotaxis and motility, p 437–452 In Sonenshein AL, Hoch JA, Losick R. (ed), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC [Google Scholar]
- 2. Boylan RJ, Brooks D, Young FE, Mendelson NH. 1972. Regulation of the bacterial cell wall: analysis of a mutant of Bacillus subtilis defective in biosynthesis of teichoic acid. J. Bacteriol. 110:281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown CT, et al. 2011. Whole-genome sequencing and phenotypic analysis of Bacillus subtilis mutants following evolution under conditions of relaxed selection for sporulation. Appl. Environ. Microbiol. 77:6867–6877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Butcher BG, Helmann JD. 2006. Identification of Bacillus subtilis σW-dependent genes that provide intrinsic resistance to antimicrobial compounds produced by Bacilli. Mol. Microbiol. 60:765–782 [DOI] [PubMed] [Google Scholar]
- 5. Cao M, et al. 2002. Defining the Bacillus subtilis sigma(W) regulon: a comparative analysis of promoter consensus search, run-off transcription/macroarray analysis (ROMA), and transcriptional profiling approaches. J. Mol. Biol. 316:443–457 [DOI] [PubMed] [Google Scholar]
- 6. Cruz Ramos H, et al. 2000. Fermentative metabolism of Bacillus subtilis: physiology and regulation of gene expression. J. Bacteriol. 182:3072–3080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dykhuizen D. 1990. Experimental studies of natural selection in bacteria. Annu. Rev. Ecol. Syst. 21:373–398 [Google Scholar]
- 8. Fischer E, Sauer U. 2005. Large-scale in vivo flux analysis shows rigidity and suboptimal performance of Bacillus subtilis metabolism. Nat. Genet. 37:636–640 [DOI] [PubMed] [Google Scholar]
- 9. Maughan H, et al. 2006. The population genetics of phenotypic deterioration in experimental populations of Bacillus subtilis. Evolution 60:686–695 [PubMed] [Google Scholar]
- 10. Maughan H, Masel J, Birky C, Nicholson W. 2007. The roles of mutation accumulation and selection in loss of sporulation in experimental populations of Bacillus subtilis. Genetics 177:937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maughan H, Nicholson WL. 2011. Increased fitness and alteration of metabolic pathways during Bacillus subtilis evolution in the laboratory. Appl. Environ. Microbiol. 77:4105–4118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 13. Nakamura LK, Roberts MS, Cohan FM. 1999. Relationship of Bacillus subtilis clades associated with strains 168 and W23: a proposal for Bacillus subtilis subsp. subtilis subsp. nov. and Bacillus subtilis subsp. spizizenii subsp. nov. Int. J. Syst. Bacteriol. 49(Pt 3):1211–1215 [DOI] [PubMed] [Google Scholar]
- 14. Nicholson WL, et al. 2010. Exploring the low-pressure growth limit: evolution of Bacillus subtilis in the laboratory to enhanced growth at 5 kilopascals. Appl. Environ. Microbiol. 76:7559–7565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nicholson WL, Setlow P. 1990. Sporulation, germination, and outgrowth, p 391–450 In Harwood CR, Cutting SM. (ed), Molecular biological methods for Bacillus. J. Wiley & Sons, New York, NY [Google Scholar]
- 16. Renna MC, Najimudin N, Winik LR, Zahler SA. 1993. Regulation of the Bacillus subtilis alsS, alsD, and alsR genes involved in post-exponential-phase production of acetoin. J. Bacteriol. 175:3863–3875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Steinmetz M, Richter R. 1994. Plasmids designed to alter the antibiotic resistance expressed by insertion mutations in Bacillus subtilis, through in vivo recombination. Gene 142:79–83 [DOI] [PubMed] [Google Scholar]
- 18. Stöver AG, Driks A. 1999. Regulation of synthesis of the Bacillus subtilis transition-phase, spore-associated antibacterial protein TasA. J. Bacteriol. 181:5476–5481 [DOI] [PMC free article] [PubMed] [Google Scholar]