Abstract
Scientific and regulatory interest in assessing clinical endpoints after 48 to 72 h of treatment for acute bacterial skin and skin structure infections (ABSSSI) has increased. Historical, pre-antibiotic-era data suggest that a treatment effect relative to untreated controls can be discerned in this time interval. Ceftaroline fosamil, a broad-spectrum bactericidal cephalosporin with activity against Gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA), and Gram-negative organisms was efficacious in two phase 3 trials of complicated skin infections (CANVAS 1 and 2) using clinical cure rates at the test-of-cure visit. To assess an early clinical response in the CANVAS trials, a retrospective analysis using a day 3 clinical endpoint was conducted. Adults with ABSSSI received intravenous ceftaroline fosamil at 600 mg every 12 h (q12h) or vancomycin at 1 g plus aztreonam at 1 g (V/A) q12h for 5 to 14 days. Clinical response at day 3, defined as cessation of infection spread and absence of fever, was analyzed in patients with a lesion size of ≥75 cm2 and either deep and/or extensive cellulitis, major abscess, or an infected wound. Day 3 integrated CANVAS clinical response rates were 74.0% (296/400) for ceftaroline and 66.2% (263/397) for V/A (difference, 7.8%; 95% confidence interval [CI], 1.3% to 14.0%). In the individual studies, absolute treatment differences of 9.4% (CANVAS 1) and 5.9% (CANVAS 2) favoring ceftaroline were observed. For ABSSSI due to MRSA, response rates were 81.7% and 77.4% in the ceftaroline and V/A groups, respectively. In this retrospective analysis, ceftaroline fosamil monotherapy had a numerically higher clinical response than V/A at day 3 in the treatment of ABSSSI.
INTRODUCTION
Complicated skin and skin structure infections (cSSSI), such as wound infections, deep and/or extensive cellulitis, or major abscess, can be serious or life-threatening conditions requiring systemic antimicrobial therapy, surgical management, and hospitalization (3, 5, 6, 10).
Over the past few decades, efficacy endpoints for clinical registration trials to evaluate antibacterial agents in the treatment of cSSSI have undergone revision (17, 18). Until recently, noninferiority trials incorporating a test-of-cure (TOC) visit as the timing for the primary clinical efficacy assessment were used to evaluate clinical cure at a point in time after completion of therapy (11, 16, 18). Typically, clinical cure has been defined as total resolution of all signs and symptoms of the baseline infection or improvement to such an extent that no further antimicrobial therapy is necessary.
Per the 2010 U.S. Food and Drug Administration (FDA) draft guidance document Acute Bacterial Skin and Skin Structure Infections: Developing Drugs for Treatment (17), which includes consideration of available historical data, the types of skin infections that should be included in clinical trials to support an indication for treatment have been reevaluated. Previously referred to as uncomplicated and complicated skin and skin structure infections (uSSSI and cSSSI), these are now termed acute bacterial skin and skin structure infections (ABSSSI). These infections should have a minimum surface area of measurable erythema, edema, and/or induration (i.e., ≥75 cm2 of cellulitis). This definition also provides a measurable objective extent of disease with which to potentially monitor clinical improvement or worsening. Furthermore, in response to ongoing efforts in the scientific community regarding clinical trial design for the treatment of ABSSSI, the FDA recommended that trials include evaluation of clinical response at 48 to 72 h after initiation of therapy as the primary endpoint (17). This recommendation was based on historical data indicating that cessation of lesion spread plus the absence of fever in patients with serious skin infection reflected the greatest antimicrobial treatment effect after approximately 48 to 72 h of antibacterial therapy (13, 14). Evidence of an antimicrobial treatment effect was supported by reduced rates of recurrence and sepsis compared with control therapy. Of interest, others have recently attempted to define treatment effects for alternative endpoints and noninferiority margins for complicated skin and skin structure infections, without general acceptance (15).
The CANVAS (ceftaroline versus vancomycin in skin and skin structure infections) 1 and 2 registration trials (ClinicalTrials.gov identifiers NCT00424190 and NCT00423657) were two identically designed, randomized, multinational, double-blind, phase 3, noninferiority trials involving a total of 1,378 adults with clinically documented cSSSI (2, 19). These trials were initiated in 2007, before the recent FDA recommendations were issued, and thus, the study designs included a traditional primary endpoint of noninferiority of the clinical cure rate for ceftaroline fosamil at TOC (8 to 15 days after the end of therapy) compared with vancomycin plus aztreonam (V/A). Study results demonstrated that ceftaroline was noninferior to V/A, with the lower limit of the 95% confidence interval (CI) (using a 10% margin) around the treatment difference (ceftaroline − V/A) being greater than −10% (−6.6% in CANVAS 1, −4.4% in CANVAS 2, and −4.2% in the integrated CANVAS trials) (1).
Although the phase 3 CANVAS trials used a traditional study design with a clinical cure evaluation at TOC, relevant data were collected during the study to allow analysis of clinical response rates (i.e., cessation of lesion spread and absence of fever) at day 3. A retrospective analysis of the individual and combined CANVAS trials was performed using a clinical response endpoint at day 3 in a subgroup of patients who met the FDA definition of ABSSSI. This is the first analysis conducted in this indication for a new drug application approval that is based on the recent FDA guidance. The results of the individual trials were instrumental in the FDA approval for marketing of ceftaroline fosamil.
MATERIALS AND METHODS
Study design and treatment.
CANVAS 1 and 2 were two identically designed, randomized, multinational, double-blind, phase 3, noninferiority trials that compared the efficacy and safety of intravenous (IV) ceftaroline (600 mg every 12 h [q12h]) versus IV V/A (both at 1 g q12h) for 5 to 14 days in adults with cSSSI (2, 18). The trials were designed to allow pooling of results for a larger database of pathogens and safety information (1). A total of 111 study centers in Europe, Latin America, and the United States participated in the trials. (See the work of Corey et al. [1] for details of the original integrated trials.) The original CANVAS trials were designed and powered to examine clinical cure rates at the TOC in cSSSI (1). ABSSSI was defined after the CANVAS trials were completed. The FDA definitions were applied to the CANVAS data set, resulting in a reduced sample size consisting of approximately 60% of the patients from the integrated trials that were included in this post hoc analysis.
Study population.
The exploratory modified intent-to-treat (E-MITT) population included all randomized patients who received any study drug, had a lesion size of ≥75 cm2, and had deep and/or extensive cellulitis (including extensive cellulitis due to infected arthropod bites), a major abscess with a component of cellulitis (defined as erythema ≥5 cm from each margin), an infected wound, or a lower-extremity abscess or cellulitis with diabetes mellitus or peripheral vascular disease (categories were condensed to cellulitis, abscess, infected wound, and infected arthropod bite for analysis). The size of the primary infection site was defined by the margin of erythema and/or induration. The length and width of the primary infection site were measured in centimeters, with the length being measured along the head-to-toe axis and the width being defined by the widest point on a perpendicular axis. Approximately 75% of infected wounds were the consequence of trauma. The remainder were surgical wound infections, evenly distributed between different types of surgery without any predominance of any specific type of surgery. Infected burns, infected ulcers, and other less frequent types of ABSSSI not already specified were excluded from the analysis. In addition, anyone who did not meet the FDA criteria for ABSSSI or did not receive study drug was excluded from the analysis. Of the total treated population (MITT) from the original integrated CANVAS trials, 42.2% (581/1,378) were excluded from the E-MITT population.
Efficacy assessments.
Clinical response at day 3 was defined as meeting both of the following criteria: cessation of infection spread (no increase in baseline lesion width or length measurement) and absence of fever (temperature ≤ 37.6°C). Patients who did not meet both of these criteria were considered nonresponders. In addition, patients who were considered by the investigator as clinical failures on day 3 or who had missing or incomplete information on day 3 were also considered nonresponders.
Microbiological assessments.
All patients had a microbiological specimen collected from the infection site at baseline. For cellulitis, a specimen was obtained by leading-edge needle aspiration or punch biopsy. For other types of skin infections (e.g., surgical wound infections and abscesses), a deep-site specimen was obtained via biopsy or needle aspiration or from surgically obtained tissue or fluid or purulent matter that was physically contiguous with the lesion. Superficial swabs of infected areas were not acceptable. In addition, aerobic and anaerobic blood cultures (one aerobic bottle and one anaerobic bottle each from two separate sites) were obtained at baseline and as medically indicated throughout the study and were repeated upon observation of a positive result until resolution of bacteremia was confirmed. All isolates identified at the local laboratories were sent to a central laboratory for identification verification and susceptibility testing using broth microdilution and Kirby-Bauer disk diffusion tests, and final pathogen determination was based on the genus and species identification from the central laboratory.
Statistical methods.
This was a retrospective analysis to evaluate clinical response at day 3 (approximately 48 h) after initiation of antibacterial therapy as a primary endpoint based on the new FDA recommendations described earlier. The exploratory endpoint was the per-patient clinical response (cessation of infection spread and absence of fever) rate at day 3 in the E-MITT population. Other exploratory analyses included the per-patient clinical response in various subgroups of the E-MITT population as well as the per-pathogen clinical response at day 3 in the microbiological E-MITT population.
A 95% CI for the observed difference in the outcome measure between the ceftaroline and the V/A groups was calculated using the method of Miettinen and Nurminen (9) stratified by study.
RESULTS
Patient disposition and analysis populations.
The phase 3 CANVAS 1 and 2 trials enrolled 1,378 patients with cSSSI (ceftaroline, 693; V/A, 685). Of these, 797 (ceftaroline, 400; V/A, 397) met the FDA criteria for ABSSSI and were included in the E-MITT population.
Patient demographics and baseline medical characteristics.
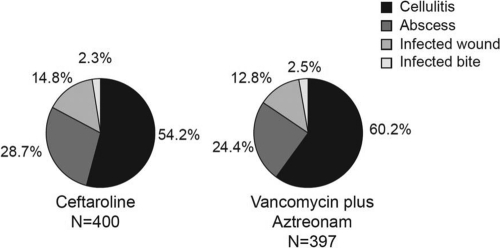
Patients in both treatment groups in the individual studies and in the integrated analysis had similar demographic characteristics, types and sites of ABSSSI, and relevant medical histories (Table 1). The integrated E-MITT population was predominantly male and well matched for age, with the majority being from the United States and Eastern Europe. Comorbid conditions included diabetes mellitus in 15.5% and 19.1% of patients in the ceftaroline and V/A groups, respectively, and peripheral vascular disease in 9.0% and 9.8% of patients, respectively (Table 1). Fever (body temperature ≥ 38°C) was present in 44% and elevated white blood cell count was present in 47% of the E-MITT population. Infection types occurred with similar frequency in the ceftaroline and V/A groups, with cellulitis accounting for the majority of infections (Table 1; Fig. 1). The median infection area was 240 cm2 for the ceftaroline group and 245 cm2 for the V/A group.
Table 1.
Demographic and baseline characteristics (E-MITT population)
| Characteristic | No. (%) of subjects in: |
|||||
|---|---|---|---|---|---|---|
| CANVAS 1 |
CANVAS 2 |
Integrated CANVAS |
||||
| Ceftaroline (n = 200) | V/A (n = 209) | Ceftaroline (n = 200) | V/A (n = 188) | Ceftaroline (n = 400) | V/A (n = 397) | |
| Age (yr) | ||||||
| <65 | 168 (84.0) | 168 (80.4) | 170 (85.0) | 162 (86.2) | 338 (84.5) | 330 (83.1) |
| ≥65 | 32 (16.0) | 41 (19.6) | 30 (15.0) | 26 (13.8) | 62 (15.5) | 67 (16.9) |
| <75 | 186 (93.0) | 193 (92.3) | 185 (92.5) | 177 (94.1) | 371 (92.8) | 370 (93.2) |
| ≥75 | 14 (7.0) | 16 (7.7) | 15 (7.5) | 11 (5.9) | 29 (7.3) | 27 (6.8) |
| Gender, male | 125 (62.5) | 129 (61.7) | 143 (71.5) | 120 (63.8) | 268 (67.0) | 249 (62.7) |
| Region of enrollment | ||||||
| United States | 81 (40.5) | 85 (40.7) | 100 (50.0) | 85 (45.2) | 181 (45.3) | 170 (42.8) |
| Eastern Europe | 81 (40.5) | 83 (39.7) | 71 (35.5) | 74 (39.4) | 152 (38.0) | 157 (39.5) |
| Latin America | 21 (10.5) | 23 (11.0) | 20 (10.0) | 17 (9.0) | 41 (10.3) | 40 (10.1) |
| Western Europe | 17 (8.5) | 18 (8.6) | 9 (4.5) | 12 (6.4) | 26 (6.5) | 30 (7.6) |
| Comorbid condition | ||||||
| Diabetes mellitus | 29 (14.5) | 47 (22.5) | 33 (16.5) | 29 (15.4) | 62 (15.5) | 76 (19.1) |
| Peripheral vascular disease | 19 (9.5) | 25 (12.0) | 17 (8.5) | 14 (7.4) | 36 (9.0) | 39 (9.8) |
| CrCl (ml/min)a | ||||||
| >80 | 167 (83.5) | 172 (82.3) | 169 (84.5) | 149 (79.3) | 336 (84.0) | 321 (80.9) |
| >50 to ≤80 | 27 (13.5) | 32 (15.3) | 23 (11.5) | 34 (18.1) | 50 (12.5) | 66 (16.6) |
| >30 to ≤50 | 6 (3.0) | 5 (2.4) | 7 (3.5) | 5 (2.7) | 13 (3.3) | 10 (2.5) |
| Fever | 88 (44.0) | 91 (43.5) | 82 (41.0) | 88 (46.8) | 170 (42.5) | 179 (45.1) |
| Elevated white blood cell count (no. positive/total) | 76/181 (42.0) | 88/189 (46.6) | 87/175 (49.7) | 80/164 (48.8) | 163/356 (45.8) | 168/353 (47.6) |
| Bacteremia | 14 (7.0) | 5 (2.4) | 7(3.5) | 11 (5.9) | 21 (5.3) | 16 (4.0) |
| Median infection area, cm2 (range) | 246.9 (75, 3,150) | 255 (75, 2,451) | 224 (75.6, 2,860) | 237 (80, 4,950) | 240 (75, 3,150) | 245 (75, 4,950) |
CrCl, creatinine clearance.
Fig 1.
Infection type at baseline (exploratory modified intent-to-treat population).
The most common pathogen isolated was Staphylococcus aureus, with methicillin-resistant S. aureus (MRSA) accounting for 42.3% (104/246) of isolates in the ceftaroline group and 35.4% (84/237) in the V/A group. A majority of the MRSA isolates tested were positive for the Panton-Valentine leukocidin (PVL) gene (ceftaroline group, 82.8% [77/93]; V/A group, 87.1% [61/90]), while the majority of the methicillin-susceptible S. aureus (MSSA) isolates tested were PVL negative (ceftaroline group, 73.3% [99/135]; V/A group, 70.3% [104/148]). Bacteremia occurred in 5.3% and 4.0% of patients in the ceftaroline and V/A groups, respectively. Approximately half of all patients had received antimicrobial therapy within 96 h prior to the start of study drug administration.
Clinical outcomes.
The exploratory endpoint (the per-patient clinical response rates at day 3) was 74.0% (296/400) for the ceftaroline group and 66.2% (263/397) for the V/A group (treatment difference, 7.8%; 95% CI, 1.3 to 14.0) (Table 2). In the individual trials, absolute treatment differences of 9.4% (95% CI, 0.4 to 18.2; CANVAS 1) and 5.9% (95% CI, −3.1 to 14.9; CANVAS 2) in favor of ceftaroline were observed. The lower limit of the 95% CI was >0 in CANVAS 1 and the integrated trials and >−4% in CANVAS 2.
Table 2.
Clinical response at different time points (E-MITT population)
| Time and study | No. (%) of patients with status/total |
Difference (95% CI)b | Pc | |||
|---|---|---|---|---|---|---|
| Respondera |
Nonresponder |
|||||
| Ceftaroline | V/A | Ceftaroline | V/A | |||
| Day 3 | ||||||
| CANVAS 1 | 148/200 (74.0) | 135/209 (64.6) | 52/200 (26.0) | 74/209 (35.4) | 9.4 (0.4, 18.2) | 0.04 |
| CANVAS 2 | 148/200 (74.0) | 128/188 (68.1) | 52/200 (26.0) | 60/188 (31.9) | 5.9 (−3.1, 14.9) | 0.2 |
| Integrated CANVAS | 296/400 (74.0) | 263/397 (66.2) | 104/400 (26.0) | 134/397 (33.8) | 7.7 (1.3, 14.0) | 0.018 |
| Cure |
Failure |
||||
|---|---|---|---|---|---|
| Ceftaroline | V/A | Ceftaroline | V/A | ||
| TOC | |||||
| CANVAS 1 | 177/200 (88.5) | 178/209 (85.2) | 23/200 (11.5) | 31/209 (14.8) | 3.3 (−3.3, 10.0) |
| CANVAS 2 | 172/200 (86.0) | 161/188 (85.6) | 28/200 (14.0) | 27/188 (14.4) | 0.4 (−6.7, 7.5) |
| Integrated CANVAS | 349/400 (87.3) | 339/397 (85.4) | 51/400 (12.8) | 58/397 (14.6) | 1.9 (−2.9, 6.7) |
Defined as a patient who exhibits cessation of lesion spread, is afebrile (temperature ≤ 37.6°C), and is not considered a clinical failure by the investigator on day 3.
Difference in clinical response rates, i.e., ceftaroline group − comparator group. Differences for CANVAS 1 and 2 are crude differences; those for integrated CANVAS are weighted differences (stratified by study). Confidence intervals were calculated using the method of Miettinen and Nurminen (9) method without adjustments except for integrated trials (stratified by study).
Calculated by a two-sided test of ceftaroline versus comparator using the Miettinen and Nurminen method, with a delta value of 0. A P value of <0.05 is suggestive of superiority of ceftaroline in day 3 response rate. Integrated analysis was stratified by study. Analyses were exploratory and conducted retrospectively.
In contrast to the clinical response rates at day 3 seen in the current analysis, the response rates reported in the integrated CANVAS trials in the clinically evaluable population at the TOC were higher and were similar (ceftaroline, 91.6%; V/A, 92.7%; difference, −1.1 [95% CI, −4.2 to 2.0]) (1). The clinical response rates in the E-MITT population at the TOC were also similar between treatment groups (Table 2) (1, 2, 19). This is what would be expected in a traditional controlled trial designed to show noninferiority.
In other exploratory analyses, the per-pathogen clinical response rates at day 3 associated with MRSA were similar in the ceftaroline group (81.7%, 85/104) and in the V/A group (77.4%, 65/84). The difference in per-pathogen clinical response rates with MSSA was higher in the ceftaroline group (71.8%, 102/142) than the V/A group (60.1%, 92/153) (Table 3). For Streptococcus pyogenes, the response rates were also similar (53.2% versus 57.1%). The numbers of other baseline pathogens were too small to draw meaningful conclusions.
Table 3.
Clinical response rates in integrated CANVAS of patients positive for selected baseline isolates at day 3 (E-MITT population)
| Organisma | No. of responders/total (%) |
|
|---|---|---|
| Ceftaroline (n = 400) | V/A (n = 397) | |
| Staphylococcus aureus | 188/246 (76.4) | 156/236a (66.1) |
| MRSA | 85/104 (81.7) | 65/84 (77.4) |
| MSSA | 102/142 (71.8) | 92/153 (60.1) |
| Streptococcus pyogenes | 25/47 (53.2) | 28/49 (57.1) |
| Streptococcus agalactiae | 9/13 (69.2) | 6/7 (85.7) |
| Enterococcus faecalis | 8/13 (61.5) | 6/10 (60.0) |
| Streptococcus anginosus group | 8/9 (88.9) | 6/10 (60.0) |
| Streptococcus dysgalactiae | 6/8 (75.0) | 4/8 (50.0) |
| Escherichia coli | 5/8 (62.5) | 7/13 (53.8) |
| Proteus mirabilis | 7/10 (70.0) | 7/12 (58.3) |
| Klebsiella pneumoniae | 5/9 (55.6) | 1/7 (14.3) |
| Klebsiella oxytoca | 6/8 (75.0) | 3/6 (50.0) |
The table lists all Enterobacteriaceae isolates, including those producing extended-spectrum β-lactamases. One patient had both MSSA and MRSA and was counted once in the S. aureus total.
The per-patient clinical response rates at day 3 in various patient subgroups by baseline characteristic are outlined in Table 4. The day 3 response rates for all baseline characteristics were numerically higher for ceftaroline, excluding the rates seen in patients with diabetes as comorbidity. The use of prior antimicrobial therapy did not alter the day 3 response rate in either treatment group, and the numerically higher clinical response rates with ceftaroline were maintained in patients with or without prior antimicrobial therapy (Table 4).
Table 4.
Clinical response rates in integrated CANVAS by patient demographics and baseline characteristics at day 3 (E-MITT population)
| Characteristic | No. of responders/total (%) |
|
|---|---|---|
| Ceftaroline (n = 400) | V/A (n = 397) | |
| Age (yr) | ||
| <65 | 246/338 (72.8) | 218/330 (66.1) |
| ≥65 | 50/62 (80.6) | 45/67 (67.2) |
| <75 | 271/371 (73.0) | 247/370 (66.8) |
| ≥75 | 25/29 (86.2) | 16/27 (59.3) |
| Region of enrollment | ||
| United States | 150/181 (82.9) | 127/170 (74.7) |
| Eastern Europe | 95/152 (62.5) | 85/157 (54.1) |
| Latin America | 33/41 (80.6) | 31/40 (77.5) |
| Western Europe | 18/26 (69.2) | 20/30 (66.7) |
| Diabetes mellitus | ||
| Yes | 40/62 (64.5) | 56/76 (73.7) |
| No | 256/338 (75.7) | 207/321 (64.5) |
| CrCl, ml/mina | ||
| >80 | 246/336 (73.2) | 214/321 (66.7) |
| >50 to ≤80 | 39/50 (78.0) | 41/66 (62.1) |
| >30 to ≤50 | 11/13 (84.6) | 8/10 (80.0) |
| Fever | ||
| Yes | 97/170 (57.1) | 90/179 (50.3) |
| No | 199/230 (86.5) | 173/218 (79.4) |
| Bacteremia | ||
| Yes | 15/21 (71.4) | 8/16 (50.0) |
| No | 281/379 (74.1) | 255/381 (66.9) |
| Infection type | ||
| Cellulitis | 152/217 (70.0) | 151/239 (63.2) |
| Abscess | 95/115 (82.6) | 76/97 (78.4) |
| Infected wound | 41/59 (69.5) | 30/51 (58.8) |
| Infected bite | 8/9 (88.9) | 6/10 (60.0) |
| Prior antimicrobial therapy | ||
| Yes | 143/198 (72.2) | 128/190 (67.4) |
| No | 153/202 (75.7) | 135/207 (65.2) |
CrCl, creatinine clearance.
DISCUSSION
Until very recently, the primary efficacy endpoint in noninferiority studies for cSSSI has been resolution of signs and symptoms of infection at a time point several days to weeks after completion of therapy (e.g., at the TOC visit) (17, 18). Although a known treatment effect size is essential for a noninferiority trial design, historical data for the estimation of treatment effects on resolution of signs and symptoms several days to weeks after completion of therapy are generally not available. However, data from the pre-antibiotic era show antibacterial drug treatment effects at day 3 in the course of treatment of cSSSI (13, 14). In medical practice, day 3 clinical endpoints can be very useful and have strong therapeutic relevance. Early indication of treatment failure can guide reselection of antimicrobial treatment within 72 h, thus avoiding prolonged use of inappropriate antimicrobial agents, which has been reported to negatively impact overall morbidity and mortality (4). In addition, evaluation at day 3 with subsequent cultures can aid in the decision to de-escalate antibiotic treatment to a narrower-spectrum agent as well as the decision to switch from IV to oral therapy and subsequently discharge the patient, based on clinical improvement (7, 8, 12).
This analysis was conducted to support ongoing efforts within the scientific community to evaluate clinical response rates 48 to 72 h after initiation of therapy in clinical trials assessing treatment of ABSSSI. This analysis of the integrated CANVAS trials shows that among patients with lesion sizes of ≥75 cm2, the incidence of cessation of spread and absence of fever at day 3 was higher for patients in the ceftaroline group than for those in the V/A group, with a lower limit of the 95% CI around the treatment difference (ceftaroline − V/A) being >0, indicating superiority. However, superiority cannot be concluded based on this retrospective integrated analysis because this was not a preplanned analysis, nor was superiority seen in each individual study.
Greater improvement at day 3 was seen regardless of age, renal function status, presence of fever, bacteremia, prior antibiotic use, or infection type (Table 4). This trend was also generally preserved in the per-pathogen response rate (Table 3).
Potential limitations of this analysis include evaluation of an endpoint (i.e., cessation of lesion spread and absence of fever at day 3) that was not prespecified in the original CANVAS 1 and 2 study designs, data collection that was not optimized for this outcome measure, and lack of a prespecified hypothesis with the corresponding power calculations for this endpoint. Despite these limitations, the day 3 results of the individual trials were instrumental in the FDA approval for marketing of ceftaroline fosamil.
Conclusions.
In this analysis of an early treatment effect, the treatment difference in the individual CANVAS trials favored ceftaroline over V/A, suggesting that ceftaroline monotherapy may provide greater benefit than the V/A combination at day 3 in the treatment course of ABSSSI in terms of cessation of lesion spread plus the absence of fever.
ACKNOWLEDGMENTS
This work was supported by Cerexa, Inc., a wholly owned subsidiary of Forest Laboratories, Inc. Funding for editorial assistance was provided by Forest Laboratories, Inc.
We thank George H. Talbot (Talbot Advisors LLC, Anna Maria, FL) for contributions to the design of the CANVAS trials and especially for building in the collection of the data necessary for the day 3 analysis. We thank Mark Wilcox (Department of Microbiology, Leeds Teaching Hospitals and University of Leeds, Leeds, United Kingdom) and G. Ralph Corey (Division of Infectious Diseases, Duke Clinical Research Institute, Duke University Medical Center, Durham, NC) for overall contributions to the CANVAS trials. Stephanie A. Moore (Cerexa, Inc.) provided medical writing and editorial assistance on the manuscript. Scientific Therapeutics Information, Inc. (Springfield, NJ), provided editorial assistance on the manuscript.
H.D.F., T.O., D.B., D.R.R., L.L., and A.S. are employees of Cerexa, Inc. P.B.E., D.T., and G.W.W. were employees of Cerexa, Inc., and J.B.L. was an employee of Forest Research Institute, Inc., at the time the work and analysis were performed. H.D.F., D.B., P.B.E., D.R.R., L.L., G.W.W., A.S., and D.T. hold stock/stock options in Forest Laboratories, Inc.
Cerexa, Inc. and Forest Laboratories, Inc., were involved in the design of the study, the collection, analysis, and interpretation of data, and the decision to present these results. Cerexa, Inc. conducted the study, prepared the statistical analysis plan, and performed the analyses. The authors retained full control of the manuscript content and its conclusions. T.O. was the medical monitor and was involved in study design and data interpretation, H.D.F., P.B.E., D.B., and D.R.R. contributed to the statistical analysis plan, analysis of study data, and writing, editing, and approval of internal study reports. G.W.W. and J.B.L. outlined the content of the manuscript and wrote the first draft. L.L. and A.S. were involved in design of the statistical analysis plan, interpretation of the study data, and verification of study information. D.T. played a primary role in study design, design of statistical analysis plan, supervision of study conduct, training and oversight of clinical operations, analysis of data, and writing, editing, and approval of internal study reports. All authors contributed to the preparation and approval of the manuscript.
Footnotes
Published ahead of print 6 February 2012
REFERENCES
- 1. Corey GR, et al. 2010. Integrated analysis of CANVAS 1 and 2: phase 3, multicenter, randomized, double-blind studies to evaluate the safety and efficacy of ceftaroline versus vancomycin plus aztreonam in complicated skin and skin-structure infection. Clin. Infect. Dis. 51:641–650 [DOI] [PubMed] [Google Scholar]
- 2. Corey GR, et al. 2010. CANVAS 1: the first phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J. Antimicrob. Chemother. 65(Suppl.):iv41–iv51 [DOI] [PubMed] [Google Scholar]
- 3. DiNubile MJ, Lipsky BA. 2004. Complicated infections of skin and skin structures: when the infection is more than skin deep. J. Antimicrob. Chemother. 53(Suppl.):ii37–ii50 [DOI] [PubMed] [Google Scholar]
- 4. Edelsberg J, et al. 2008. Clinical and economic consequences of failure of initial antibiotic therapy for hospitalized patients with complicated skin and skin-structure infections. Infect. Control Hosp. Epidemiol. 29:160–169 [DOI] [PubMed] [Google Scholar]
- 5. Elston DM. 2005. Optimal antibacterial treatment of uncomplicated skin and skin structure infections: applying a novel treatment algorithm. J. Drugs Dermatol. 4(Suppl.):s15–s19 [PubMed] [Google Scholar]
- 6. Eron LJ, et al. 2003. Managing skin and soft tissue infections: expert panel recommendations on key decision points. J. Antimicrob. Chemother. 52(Suppl.):i3–i17 [DOI] [PubMed] [Google Scholar]
- 7. Jawesson P. 1994. Cost-effectiveness and value of an IV switch. Pharmacoeconomics 5(Suppl.):20–26 [DOI] [PubMed] [Google Scholar]
- 8. Mertz D, et al. 2009. Outcomes of early switching from intravenous to oral antibiotics on medical wards. J. Antimicrob. Chemother. 64:188–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miettinen O, Nurminen M. 1985. Comparative analysis of two rates. Stat. Med. 4:213–226 [DOI] [PubMed] [Google Scholar]
- 10. Nichols RL, Florman S. 2001. Clinical presentations of soft-tissue infections and surgical site infections. Clin. Infect. Dis. 33(Suppl.):S84–S93 [DOI] [PubMed] [Google Scholar]
- 11. Noel GJ, et al. 2008. Results of a double-blind, randomized trial of ceftobiprole treatment of complicated skin and skin structure infections caused by gram-positive bacteria. Antimicrob. Agents Chemother. 52:37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sevinç F, et al. 1999. Early switch from intravenous to oral antibiotics: guidelines and implementation in a large teaching hospital. J. Antimicrob. Chemother. 43:601–606 [DOI] [PubMed] [Google Scholar]
- 13. Snodgrass WR, Anderson T. 1937. Prontosil in the treatment of erysipelas. Br. Med. J. 2:101–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Snodgrass WR, Anderson T. 1937. Sulphanilamide in the treatment of erysipelas. Br. Med. J. 2:1156–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spellberg B, et al. 2009. Antimicrobial agents for complicated skin and skin structure infections: justification of noninferiority margins in the absence of placebo-controlled trials. Clin. Infect. Dis. 49:383–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Strijewski ME, et al. 2008. Televancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by gram-positive organisms. Clin. Infect. Dis. 46:1683–1693 [DOI] [PubMed] [Google Scholar]
- 17. U. S. Food and Drug Administration 2010. Guidance for industry. Acute bacterial skin and skin structure infections: developing drugs for treatment. U.S. Food and Drug Administration, Rockville, MD [Google Scholar]
- 18. U. S. Food and Drug Administration 1998. Guidance for industry. Uncomplicated and complicated skin and skin structure infections—developing antimicrobial drugs for treatment. U.S. Food and Drug Administration, Rockville, MD [Google Scholar]
- 19. Wilcox MH, et al. 2010. CANVAS 2: the second phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J. Antimicrob. Chemother. 65(Suppl.):iv53–iv65 [DOI] [PubMed] [Google Scholar]