Abstract
Twenty-five serial passages of Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus and 50 passages of methicillin-resistant Staphylococcus aureus resulted in no significant increase in NVC-422 MICs, while ciprofloxacin MICs increased 256-fold for E. coli and 32-fold for P. aeruginosa and S. aureus. Mupirocin, fusidic acid, and retapamulin MICs for MRSA increased 64-, 256-, and 16-fold, respectively. No cross-resistance to NVC-422 was observed with mupirocin-, fusidic acid-, and retapamulin-resistant strains.
TEXT
Multiple-passage studies determine the effect of selective pressure of antibiotics on microorganisms (15), resulting in cumulative acquisition of mutations at the genetic level (3, 5, 6, 13). The development of resistance is a result of the frequency of mutation, the number and type of mutations required to express resistance, the potency and concentration of the treating drug, and other factors (9, 10, 14). Currently, there is an unmet medical need for novel antimicrobial agents that are effective against resistant pathogens (14, 18).
NVC-422 (N,N-dichloro-2,2-dimethyltaurine) (20) is active against Gram-positive and Gram-negative bacteria (including drug-resistant pathogens), fungi, and viruses (21). NVC-422 mechanism-of-action studies (22) indicate that it kills microorganisms by inactivating proteins via oxidative modification of Met and Cys. This mechanism differentiates NVC-422 from antibiotics by attacking multiple targets, thereby making it highly unlikely for microorganisms to develop resistance.
NVC-422 is currently in clinical development for impetigo, urinary catheter blockage and encrustation, and adenoviral conjunctivitis.
In this study, we investigated the propensity of NVC-422 to select for resistance by passaging bacteria at subinhibitory concentrations. Representative organisms Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and methicillin-resistant S. aureus (MRSA) were selected (1, 3, 12, 17). Ciprofloxacin was selected as a broad-spectrum comparator. Mupirocin, fusidic acid, and retapamulin were selected as comparators against MRSA, as these antibiotics are used for the treatment of topical infections (6, 18, 19).
The synthesis of NVC-422 has been described previously (20). The purity of NVC-422 was 99.83% by high-performance liquid chromatography (HPLC). The stabilities of NVC-422 (100 μg/ml) in cation-adjusted Mueller-Hinton broth (CAMHB) and M9 minimal medium were tested at 37°C using the Agilent HPLC-1200 system (Agilent Technologies, Palo Alto, CA) with a diode array UV detector.
E. coli ATCC 25922, S. aureus ATCC 29213, and MRSA ATCC 33591 were purchased from the American Type Culture Collection (ATCC, Manassas, VA); P. aeruginosa PAO1 was obtained from Queen's University (Kingston, Ontario, Canada). All strains were grown on tryptic soy agar (Difco Laboratories, Detroit, MI) at 37°C. Antibiotics were obtained from MP Biomedical (Santa Ana, CA), Sigma-Aldrich (St. Louis, MO), and APAC Pharmaceuticals, LLC (Columbia, MD).
Determination of MICs was in accordance with the Clinical and Laboratory Standards Institute (CLSI) methodology (4) for using CAMHB or M9 medium (Teknova, Hollister, CA). In order to detect incremental changes in drug susceptibility, an extended gradient was created by combining two sets of 2-fold serial dilutions from two different starting concentrations (16.38 mg/ml and 12.28 mg/ml) for NVC-422, which extended over two rows of the 96-well plate. Serial passage experiments were performed using a published method (7).
Due to the reactive nature of NVC-422, there was the possibility that its activity would be quenched with media. To evaluate this possibility, we tested the stabilities of NVC-422 in CAMHB and M9 medium using HPLC. At 37°C, the half-life (t1/2) of NVC-422 was less than 10 min in CAMHB. NVC-422, however, showed a much longer t1/2 (10 h) in M9 medium. The rate of kill for NVC-422 is rapid, with complete kill of S. aureus and E. coli in <5 min (21), making testing in the media relevant despite the short t1/2. The MICs of NVC-422 in M9 minimal medium were 4 μg/ml and 64 μg/ml for E. coli and P. aeruginosa, respectively. The MICs of NVC-422 in CAMHB were 256 μg/ml for E. coli and 384 μg/ml for S. aureus and MRSA. The higher MIC of NVC-422 obtained with testing in CAMHB than with testing in M9 medium is the result of the shorter t1/2 of NVC-422 in CAMHB. To maximize bacterial exposure time and minimize the quenching of NVC-422 activity, we conducted resistance studies with Gram-negative bacteria in M9 minimal medium.
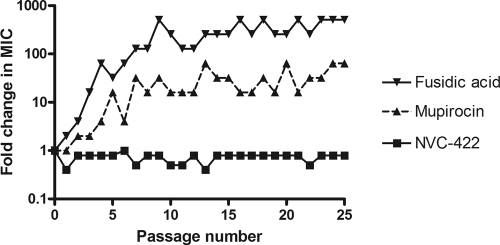
Multiple independent cultures were tested for each organism to enhance the probability of resistance development. A control organism previously unexposed to the antimicrobials was run at every passage. Strains with decreased susceptibility to ciprofloxacin were generated by 25 serial passages of E. coli, P. aeruginosa, and S. aureus and were 256-fold less susceptible for E. coli and 32-fold less susceptible for P. aeruginosa and S. aureus. Strains with decreased susceptibility to mupirocin, fusidic acid (Fig. 1), and retapamulin were generated by serial passages of MRSA (4- to 32-, 128- to 512-, and 4- to 16-fold decreases, respectively, over 25 passages for mupirocin and fusidic acid and over 50 passages for retapamulin) (Table 1). This decrease in susceptibility corresponds to low-level resistance (MICs of 8 to 64 μg/ml) (16) to mupirocin and high-level resistance (MICs of >128 μg/ml) (2, 16) to fusidic acid as defined by CLSI. While there are no CLSI breakpoints for retapamulin, the MICs of generated mutants (2 to 8 μg/ml) were above the EUCAST epidemiological cutoff value for retapamulin of <0.5 μg/ml. Mutants with retapamulin MICs of 4 to 16 μg/ml for S. aureus isolates, including MRSA, were generated in previously reported passage studies (6, 13). The decrease in susceptibility was most rapid for fusidic acid, where the MIC was already increased 16-fold on passage 3. The development of resistance to retapamulin was slower, consistent with reported results (6, 8, 13, 18). It took 34 passages for the MIC of retapamulin against MRSA to increase 16-fold. Equal numbers of independent cultures were passaged with NVC-422 for 25 passages in E. coli, P. aeruginosa, and S. aureus and for 50 passages in MRSA (Table 1). No significant (>2-fold) increase of the NVC-422 MIC was observed for any of these organisms (Table 1 and Fig. 1). The baseline MICs of NVC-422 were 4 μg/ml and 64 μg/ml for E. coli and P. aeruginosa, respectively, with testing in M9 minimal medium and 384 μg/ml for S. aureus and MRSA with testing in CAMHB. The maximal increase in NVC-422 MIC observed in these studies was the 2-fold increase to 768 μg/ml for MRSA (Table 1). These concentrations are within the therapeutic range, considering that 1.5% NVC-422 gel (15,000 μg/ml) was determined to be safe and efficacious in the impetigo clinical trial (11).
Fig 1.
Changes in MICs of NVC-422, mupirocin, and fusidic acid for MRSA ATCC 33591 during 25 passages at 0.5 MIC (determined by the previous passage). Each line shows the result for one representative culture out of seven independent cultures passaged with each antibacterial compound.
Table 1.
Summarized tabular representation of serial passage data
| Microorganism | Antimicrobial | No. of passages | No. of independent cultures passaged | MIC range (μg/ml) |
Passage at which resistance emerges in at least one culturea | |
|---|---|---|---|---|---|---|
| Initial | Final | |||||
| E. coli ATCC 25922 | Ciprofloxacin | 25 | 2 | 0.0078 | 0.0039–1 | 11 |
| NVC-422 | 25 | 2 | 1.5–4 | 1.5–4 | No resistance | |
| P. aeruginosa PAO1 | Ciprofloxacin | 25 | 5 | 0.0625 | 1–2 | 3 |
| NVC-422 | 25 | 5 | 6–12 | 6–12 | No resistance | |
| S. aureus ATCC 29213 | Ciprofloxacin | 25 | 5 | 0.25–0.5 | 8 | 15 |
| NVC-422 | 25 | 5 | 384–512 | 256–512 | No resistance | |
| MRSA ATCC 33591 | Mupirocin | 50 | 7 | 0.25–0.5 | 4–16 | 9 |
| Fusidic acid | 50 | 7 | 0.5 | 8–256 | 2 | |
| Retapamulin | 50 | 7 | 0.0625–0.25 | 0.25–8 | 35 | |
| NVC-422 | 50 | 7 | 96–768 | 96–512 | No resistance | |
Resistance is defined as a sustained 4-fold increase in original MIC (4).
No cross-resistance to NVC-422 was observed between fusidic acid-resistant strains, mupirocin-resistant strains, or retapamulin-resistant strains generated by serial passages. Additionally, no cross-resistance was observed between the three control antibiotics (data not shown).
In summary, our study confirms that development of resistance to NVC-422 is highly unlikely. This observation, combined with broad-spectrum, rapid microbiocidal activity, makes NVC-422 a desirable agent for topical antimicrobial therapy.
ACKNOWLEDGMENTS
We thank Keith Poole, Queen's University, for providing the P. aeruginosa PAO1 strain, John A. Soderquist, University of Puerto Rico, for the critical review of the manuscript, and Louella Landicho for excellent graphics services.
Footnotes
Published ahead of print 21 February 2012
REFERENCES
- 1. Archer G. 1998. Staphylococcus aureus: a well-armed pathogen. Clin. Infect. Dis. 26:1179–1181 [DOI] [PubMed] [Google Scholar]
- 2. Chen HJ, et al. 2010. Fusidic acid resistance determinants in Staphylococcus aureus clinical isolates. Antimicrob. Agents Chemother. 54:4985–4991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clark C, McGhee P, Appelbaum PC, Kosowska-Shick K. 2011. Multistep resistance development studies of ceftaroline in gram-positive and -negative bacteria. Antimicrob. Agents Chemother. 55:2344–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clinical and Laboratory Standards Institute 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A6. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 5. Davies TA, Pankuch GA, Dewasse BE, Jacobs MR, Appelbaum PC. 1999. In vitro development of resistance to five quinolones and amoxicillin-claulanate in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:1177–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Farrell DJ, Robbins M, Rhys-Williams W, Love WG. 2011. Investigation of the potential for mutational resistance to XF-73, retapamulin, mupirocin, fusidic Acid, daptomycin, and vancomycin in methicillin-resistant Staphylococcus aureus isolates during a 55-passage study. Antimicrob. Agents Chemother. 55:1177–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Friedman L, Alder JD, Silverman JA. 2006. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob. Agents Chemother. 50:2137–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gentry DR, et al. 2008. Genetic characterization of Vga ABC proteins conferring reduced susceptibility to pleuromutilins in Staphylococcus aureus. Antimicrob. Agents Chemother. 52:4507–4509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gilbert DN, et al. 2001. Phenotypic resistance of Staphylococcus aureus, selected Enterobacteriaceae, and Pseudomonas aeruginosa after single and multiple in vitro exposures to ciprofloxacin, levofloxacin, and trovafloxacin. Antimicrob. Agents Chemother. 45:883–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goossens H, Ferech M, Vander Stichele R, Elseviers M. 2005. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 365:579–587 [DOI] [PubMed] [Google Scholar]
- 11. Iovino SM, et al. 2011. NVC-422 topical gel for the treatment of impetigo. Int. J. Clin. Exp. Pathol. 4:587–595 [PMC free article] [PubMed] [Google Scholar]
- 12. Kaper JB, Nataro JP, Mobley HL. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123–140 [DOI] [PubMed] [Google Scholar]
- 13. Kosowska-Shick K, et al. 2006. Single- and multistep resistance selection studies on the activity of retapamulin compared to other agents against Staphylococcus aureus and Streptococcus pyogenes. Antimicrob. Agents Chemother. 50:765–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levy SB. 2000. Antibiotic and antiseptic resistance: impact on public health. Pediatr. Infect. Dis. J. 19:S120–S122 [DOI] [PubMed] [Google Scholar]
- 15. Martinez JL, Baquero F, Andersson DI. 2011. Beyond serial passages: new methods for predicting the emergence of resistance to novel antibiotics. Curr. Opin. Pharmacol. 11:439–445 [DOI] [PubMed] [Google Scholar]
- 16. Patel JB, Gorwitz RJ, Jernigan JA. 2009. Mupirocin resistance. Clin. Infect. Dis. 49:935–941 [DOI] [PubMed] [Google Scholar]
- 17. Poole K. 2011. Pseudomonas aeruginosa: resistance to the max. Front. Microbiol. 2:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shawar R, et al. 2009. Topical retapamulin in the management of infected traumatic skin lesions. Ther. Clin. Risk Manag. 5:41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Traczewski MM, Brown SD. 2008. Proposed MIC and disk diffusion microbiological cutoffs and spectrum of activity of retapamulin, a novel topical antimicrobial agent. Antimicrob. Agents Chemother. 51:3880–3886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang L, Khosrovi B, Najafi R. 2008. N-Chloro-2,2-dimethyltaurines: stable homologues of N-chlorotaurines. Tetrahedron Lett. 49:2193–2195 [Google Scholar]
- 21. Wang L, et al. 2011. Chemical characterization and biological properties of NVC-422, a novel, stable N-chlorotaurine analog. Antimicrob. Agents Chemother. 55:2688–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoon J, et al. 2011. Virucidal mechanism of action of NVC-422, a novel antimicrobial drug for the treatment of adenoviral conjunctivitis. Antiviral Res. 92:470–478 [DOI] [PubMed] [Google Scholar]