Abstract
We recently observed that the micafungin MICs for some Candida glabrata fks hot spot mutant isolates are less elevated than those for the other echinocandins, suggesting that the efficacy of micafungin may be differentially dependent on such mutations. Three clinical C. glabrata isolates with or without (S3) fks hot spot mutations R83 (Fks2p-S663F) and RR24 (Fks1p-S629P) and low, medium, and high echinocandin MICs, respectively, were evaluated to assess the in vivo efficacy in an immunocompetent mouse model using three doses of each echinocandin. Drug concentrations were determined in plasma and kidneys by high-performance liquid chromatography (HPLC). A pharmacokinetic-pharmacodynamic mathematical model was used to define the area under the concentration-time curve (AUC) that produced half- and near-maximal activity. Micafungin was equally efficacious against the S3 and R83 isolates. The estimates for the AUCs of each echinocandin that induced half-maximal effect (E50s) were 194.2 and 53.99 mg · h/liter, respectively. In contrast, the maximum effect (Emax) for caspofungin was higher against S3 than R83, but the estimates for E50 were similar (187.1 and 203.5 mg · h/liter, respectively). Anidulafungin failed to induce a ≥1-log reduction for any of the isolates (AUC range, 139 to 557 mg · h/liter). None of the echinocandins were efficacious in mice challenged with the RR24 isolate despite lower virulence (reduced maximal growth, prolonged lag phase, and lower kidney burden). The AUC associated with half-maximal effect was higher than the average human exposure for all drug-dose-bug combinations except micafungin and the R83 isolate. In conclusion, differences in micafungin MICs are associated with differential antifungal activities in the animal model. This study may have implications for clinical practice and echinocandin breakpoint determination, and further studies are warranted.
INTRODUCTION
The echinocandins are increasingly used as first-line therapy for the treatment of disseminated candidiasis. This class is characterized by favorable pharmacokinetics, few clinically relevant drug-drug interactions, safety in patients with end-organ dysfunction, and broad-spectrum anti-Candida activity (28, 31, 32, 39). Candida glabrata is increasingly seen and is consistently the second most common Candida species in the Northern Hemisphere (2, 37). The IDSA recommends the echinocandins as first-line agents for the treatment of C. glabrata infection, although there are no randomized clinical trial data that unequivocally support this position (31).
Following increased use, sporadic cases of failures associated with elevated MICs have been reported (3, 8, 12, 15, 16, 20, 27, 29, 30, 38). In the majority of cases, these failures have been associated with mutations in two hot spot regions of the FKS genes, which encode the target and major subunit of the 1,3-β-d-glucan synthase complex (3, 8, 26, 29, 30, 33, 34). The magnitude of the MIC increase depends on the location as well as the specific amino acid alteration. This has been demonstrated at the enzyme level and by broth microdilution and disk diffusion testing (5, 21, 22). We have recently observed that for some C. glabrata isolates with fks hot spot mutations, the MIC elevation for micafungin may be less pronounced than it is for caspofungin and anidulafungin (6, 23). This observation raises the question of whether the efficacy of micafungin may be less affected by certain mutations in the hot spot regions of C. glabrata than by those in the two other echinocandins. Thus, such isolates could be appropriate targets for micafungin and could be categorized as micafungin susceptible. In contrast, if this is rather an in vitro phenomenon and such isolates should be interpreted as inappropriate targets to all three echinocandins, separating these isolates from the wild-type population without misclassifying a considerable number of wild-type isolates as nonsusceptible will be challenging by in vitro susceptibility testing. In this study, we focused on this question by comparing the in vivo efficacies of the three echinocandins in an immunocompetent mouse model of hematogenous C. glabrata infection due to a wild-type isolate and two unique fks hot spot mutant isolates.
MATERIALS AND METHODS
Isolates.
From a previously reported strain collection of wild-type and fks mutant isolates (4–6), we selected one FKS wild-type and two fks hot spot mutant C. glabrata isolates to represent a low-micafungin-MIC fks mutant and a high-micafungin-MIC fks mutant. The susceptibility results by EUCAST and CLSI testing with and without bovine serum albumin (BSA) and by disk testing of this strain collection are shown in comparison with those for other wild-type and mutant isolates in Table 1, where the three isolates are indicated as S3 (susceptible FKS wild-type isolate), R83 (Fks2p-S663F), and RR24 (Fks1p-S629P) (6, 13, 14, 40).
Table 1.
Susceptibility data and Fks1p and Fks2p phenotypes for the three C. glabrata isolates used in animal experimentsa
| Strain | Fks alteration | Microdilution MIC (μg/ml)c |
CLSI disk diffusion zone diam (mm) for: |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EUCAST |
CLSI |
EUCAST-BSAb |
CLSI-BSA |
||||||||||||
| ANF | CSF | MCF | ANF | CSF | MCF | ANF | CSF | MCF | ANF | CSF | MCF | CSF | MCF | ||
| S3 | None | ≤0.03 | 0.25 | ≤0.03 | 0.031 | 0.031 | 0.125 | 1 | 0.25 | 1 | 1 | 0.25 | 1 | 31.8 | 31.0 |
| ≤0.03 | 0.25 | ≤0.03 | 0.25 | 0.125 | 0.064 | 1 | 0.25 | 2 | |||||||
| 0.06 | 0.25 | ≤0.03 | 0.25 | 0.125 | 0.064 | 1 | 0.5 | 1 | |||||||
| (≤0.042) | (0.25) | (≤0.03) | (0.177) | (0.094) | (0.083) | (1) | (0.333) | (1.333) | |||||||
| R83 | Fks2p-S663F | 0.5 | 1 | 0.06 | 0.25 | 0.125 | 0.25 | ≥32 | ≥32 | ≥32 | 4 | 4 | 8 | 19.5 | 22.1 |
| 0.5 | 1 | ≤0.03 | 1 | 1 | 1 | ≥32 | ≥32 | ≥32 | |||||||
| 0.25 | 1 | ≤0.03 | 2 | 1 | 0.5 | 8 | 4 | 16 | |||||||
| (1.083) | (1) | (≤0.042) | (1.083) | (0.708) | (0.583) | (≥24) | (≥22.67) | (≥26.67) | |||||||
| RR24 | Fks1p-S629P | 4 | 16 | 1 | 4 | 8 | 4 | ≥32 | ≥32 | ≥32 | ≥32 | ≥32 | ≥32 | 17.8 | 20.0 |
| 4 | 16 | 1 | 2 | 8 | 2 | ≥32 | ≥32 | ≥32 | |||||||
| 2 | ≥32 | 2 | 4 | 8 | 2 | ≥32 | ≥32 | ≥32 | |||||||
| (3.333) | (≥21.333) | (1.333) | (3.333) | (8) | (2.667) | (≥32) | (≥32) | (≥32) | |||||||
EUCAST, EUCAST-BSA, and CLSI tests were each done on three separate occasions, whereas a single run of CLSI-BSA and disk testing was performed.
BSA, bovine serum albumin-supplemented susceptibility test growth medium.
ANF, anidulafungin; CSF, caspofungin; MCF, micafungin. Mean MICs from the three testing occasions, in cases where three tests were administered, are given in parentheses.
FKS gene sequence analysis.
C. glabrata genomic DNA was extracted from yeast cells grown overnight on CHROMagar (SSI Diagnostika, Hillerød, Denmark) using the patented 2-step buffer extraction method, as previously described by Brillowska-Dabrowska (10) (patent WO2006133701). Pre- and postinoculation isolates from kidneys of untreated control mice were sequenced to confirm genotypes. PCR and sequencing primers were based on C. glabrata FKS1 and FKS2 (GenBank accession nos. XM_446406 and XM_448401, respectively) (11). The amplicons containing hot spot regions of the FKS genes (35) of all the strains included in this study were submitted for sequencing at Macrogen Europe (The Netherlands) and analyzed using the bioinformatics software CLC DNA Workbench (CLC Bio, Denmark). The specific mutations are shown in Table 1.
In vitro growth rate determination.
The in vitro growth rates of the three C. glabrata isolates were determined (including preinoculation isolates as well as isolates harvested from the kidney of the first control mouse from each group in the first micafungin experiment; see below) by measuring the change in optical density at 490 nm with the use of a microtiter plate spectrophotometer over a 48-h period. Candida cells were harvested from 2-day-old colonies (CHROMagar plates) and adjusted to an inoculum of 1 × 106 to 5 × 106 cells/ml in sterile water for each isolate, and 100 μl, was used to inoculate 12 wells containing 100 μl of double-strength RPMI-1640 2% glucose medium buffered with MOPS (morpholinepropanesulfonic acid) (40). Plates were incubated with shaking at 35°C in an incubation enzyme-linked immunosorbent assay (ELISA) reader (Powerwave 340; BioTek Instruments, Inc.) and optical density was read every 15 min at 490 nm for 48 h.
Murine model of disseminated infection with Candida glabrata.
All in vivo experiments were performed using a nonneutropenic murine model of disseminated C. glabrata infection. Experiments were approved by the Danish Animal Experimentation Committee under the Ministry of Justice (number 2009/561-1637). NMRI mice weighing 26 to 30 g (Harlan Scandinavia, Allerød, Denmark) were housed with free access to food and water. On day 0, mice received 200 μl of a suspension of C. glabrata containing 1 × 108 to 5 × 108 CFU intravenously (i.v.) via the tail vein. Exposure-response relationships for the strains S3 (wild type), R83 (Fks2p-S663F, moderately resistant), and RR24 (Fks1p-S629P, highly resistant) were determined. Treatment with an echinocandin commenced 24 h postinoculation and was administered intraperitoneally (i.p.) in a 0.5-ml volume at 24, 48, and 72 h postinoculation. Doses were as follows: anidulafungin, 0, 3, 6, and 12 mg/kg of body weight; caspofungin, 0, 1.5, 3, and 6 mg/kg; and micafungin, 0, 3, 6, and 12 mg/kg. These dosages were chosen on the basis of previous pharmacodynamic studies of echinocandins against C. glabrata (1, 25) and a desire to achieve drug exposures in mice that were comparable to those in humans receiving currently licensed echinocandin regimens. The latter was achieved using allometric scaling with the following relationship: mouse dose = human dose/17,200 cm2 × 65 cm2, where 17,200 cm2 and 65 cm2 are estimates of surface areas for human and mouse, respectively (17, 19). Thus, the doses tested for anidulafungin and micafungin were 2-fold higher than those for caspofungin in agreement with the ratio between the recommended human maintenance doses (100 mg/day anidulafungin and micafungin and 50 mg/day caspofungin).
Pharmacokinetics.
The pharmacokinetics for each compound were estimated using groups of 3 mice challenged with R83 (Fks2p-S663F) per dose-time point combination (n = 81 per drug). A destructive experimental design was employed. Plasma samples were obtained 24.5 h, 26 h, 28 h, 31 h, 71 h, 73 h, 75 h, and 78 h postinoculation. A final sample was also taken for drug concentrations on day 4 at the time of the pharmacodynamic experiments (see below). Samples were stored at −80°C before analysis.
The pharmacokinetics in tissues were simultaneously estimated. At the time of blood sampling, kidneys were removed, weighed, and placed in sterile saline (500 μl per kidney) for CFU determinations and drug concentration determination. All organs were stored at −80°C before homogenization with a homogenizer (RW 16 Basic; IKA Labortechnik, Bie & Berntsen, Denmark).
Pharmacodynamics.
The pharmacodynamics of the three echinocandins against each of the strains were estimated over the course of four separate independent experiments using a total of 492 mice and dosing schemes as described above. Experiments 1 to 3 each included 144 mice inoculated with S3 (n = 24), R83 (n = 96, including 72 mice for drug concentration determinations), and RR24 (n = 24) and treated in groups of 6 with different doses of anidulafungin (experiment 1), caspofungin (experiment 2), and micafungin (experiment 3). Experiment 4 included 60 mice inoculated with S3 (total n = 30) and R83 (total n = 30 mice) and treated in groups of 3 with each of the three echinocandins in order to control for potential interexperimental variation. Isolates from the end of each experiment were retrieved and the FKS genes resequenced to ensure there had not been progressive mutational events nor inadvertent mixing of strains.
Measurement of echinocandin concentrations in plasma and kidneys.
Anidulafungin concentrations in plasma and kidney tissue were measured using high-performance liquid chromatography (HPLC) with a Shimadzu Prominence (Shimadzu, Milton Keynes, United Kingdom). The anidulafungin method used a Kinetex 2.6-μm C18 column of 75 by 4.6 mm (Phenomenex, Macclesfield, United Kingdom) and a 5-μl injection volume. A standard curve encompassing 0.05 to 100 mg/liter in plasma and kidney was constructed from stock solutions of anidulafungin at 1,000 mg/liter in dimethyl sulfoxide (DMSO) further diluted in methanol (Fisher Scientific, Loughborough, United Kingdom). The internal standard was micafungin. The mobile phase was 65% of 0.1% trifluoroacetic acid (TFA) in water and 35% of acetonitrile with 0.1% TFA (vol/vol), with a gradient profile changing to 30% and 70%, respectively, over 4 min with an overall run time of 6.25 min and a flow rate of 1 ml/min. Internal standard and anidulafungin were detected using fluorescence with excitation (Ex) of 273 nm and emission (Em) of 464 nm; they eluted after 3.4 and 4.5 min, respectively. For plasma, the percentage coefficient of variation (CV%) was <2.4% over a concentration range of 0.05 to 10 mg/liter. The limit of detection was 0.05 mg/liter. The intra- and interday variation was <2.4%.
Caspofungin concentrations were measured using HPLC and used a Kinetex 2.6-μm C18 column of 75 by 4.6 mm as detailed for anidulafungin with a 30-μl injection volume. A standard curve encompassing 0.125 to 100 mg/liter in plasma and kidney was constructed from stock solutions of caspofungin at 1,000 mg/liter in DMSO further diluted in methanol. The internal standard was 4-hexylresorcinol. The mobile phase was 75% of 0.1% TFA in water and 25% of acetonitrile with 0.1% TFA (vol/vol) with a gradient profile changing to 45% and 55%, respectively, over 5 min with an overall run time of 7 min and a flow rate of 1.4 ml/min. Caspofungin and internal standard were detected using fluorescence with Ex of 224 nm and Em of 304 nm; they eluted after 4.2 and 5.8 min, respectively. For plasma, the CV% was <6.1% over a concentration range of 0.125 to 100 mg/liter. The limit of detection was 0.125 mg/liter. The intra- and interday variation was <6.4%.
Micafungin concentrations in plasma and kidney tissue were measured using high-performance liquid chromatography (HPLC) using a Hypersil BDS C18 5-μm column of 250 by 4.6 mm (Thermo Fisher Scientific, Loughborough, United Kingdom) and a 10-μl injection volume. A standard curve encompassing 0.1 to 25 mg/liter in plasma and kidney was constructed from stock solutions of micafungin at 1,000 mg/liter in DMSO further diluted in methanol. A gradient method was used with initial concentrations of 70% 0.02 M potassium dihydrogen phosphate and 30% acetonitrile changing to 30% and 70%, respectively, over 12 min with an overall run time of 16 min. A flow rate of 1 ml/min was used. Micafungin and the internal standard were detected using fluorescence with excitation of 273 nm and emission of 464 nm; they eluted after 10 and 13.4 min, respectively. For plasma and kidney, the CV% was <10% over a concentration range of 0.1 to 25 mg/liter. The limit of detection was 0.1 mg/liter. The intra- and interday variation was <10%.
Pharmacokinetic and pharmacodynamic modeling.
The pharmacokinetic data from plasma and kidney for each echinocandin were modeled using a population methodology and using the Big version of the program nonparametric adaptive grid (BIG NPAG) as previously described (25).
The pharmacodynamic data were modeled using an inhibitory sigmoid maximum effect (Emax) model taking the following form: Effect = Econ − [(Emax × exposureH)/(E50 + exposureH)], where Econ is the fungal density in the kidney in the absence of therapy, Emax is the asymptotic reduction in fungal density induced by echinocandin therapy, E50 is the AUC of each echinocandin that induced half-maximal effect, exposure is the echinocandin AUC, and H is the slope (or Hill) function. The model was fitted to the data using the pharmacokinetic program ADAPT 5. The mean fungal density in the kidney was used, and the data were weighted by the observed variance.
Statistical analysis.
Treatment responses were analyzed comparing the three treatment and control groups using the Kruskal-Wallis test; a P value of <0.05 was regarded as significant. Individual groups were subsequently compared using Dunn's multiple comparison test, again with a significance level set at a P value of <0.05.
RESULTS
In vitro susceptibility and FKS phenotype.
The MICs for a panel of wild-type and fks1 mutant C. glabrata isolates determined by CLSI and EUCAST methodologies, with and without supplementation of bovine serum albumin, and by CLSI disk testing have been reported previously (4–6). For the present study, an Fks2p-S663F mutant isolate (R83) for which the micafungin MIC was close to the MIC of wild-type isolates for the CLSI and EUCAST reference methods was chosen and compared with a typical wild-type (S3) and a highly resistant (RR24, Fks1p-S629P) isolate. The results of repetitive MIC testing of these isolates are shown in Table 1.
In vitro growth rates.
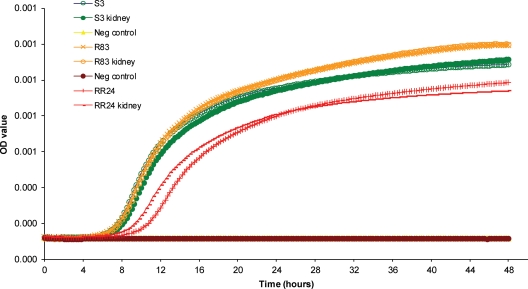
The in vitro growth rates of the three isolates are compared in Fig. 1. The growth curves for isolates S3 and R83 were superimposable over the first 12 to 16 h, after which the growth of the R83 isolate was slightly high compared to that of the wild-type isolate (S3). In comparison, the lag phase of the RR24 isolate was notably longer and the subsequent growth weaker than for the two other isolates.
Fig 1.
In vitro growth kinetics in liquid medium for each of the three isolates used for the animal experiments (S3, R83, and RR24) and the same isolates recovered from the kidneys of inoculated control mice (designated kidney).
Pharmacokinetics.
The estimates for the mean, median, and standard deviation for each parameter are summarized in Table 2. The estimates for the steady-state AUC in plasma and kidney for the three agents are shown in Table 3. The pharmacokinetics for each echinocandin were linear. The AUCs in the plasma and kidney were high for anidulafungin (1.5 and 1.8 times, respectively) and for caspofungin (2.1 and 2.5, times, respectively) compared to those for micafungin (Table 3). The mean AUCs of anidulafungin, caspofungin, and micafungin achieved in humans receiving currently licensed echinocandin regimens are 110, 101, and 115 mg · h/liter, respectively (18, 24, 42, 43).
Table 2.
Means, medians, and standard deviations for pharmacokinetic parameters
| Parametera | Anidulafungin |
Caspofungin |
Micafungin |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | SD | Mean | Median | SD | Mean | Median | SD | |
| ka (h−1) | 4.73 | 3.14 | 4.4 | 10.56 | 9.15 | 8.47 | 11.3 | 7.04 | 9.31 |
| SCL (liter/h) | 0.0005 | 0.0005 | 0.00009 | 0.0002 | 0.0001 | 0.0001 | 0.0008 | 0.0008 | 0.0001 |
| Vc (liter) | 0.004 | 0.003 | 0.0016 | 0.002 | 0.003 | 0.001 | 0.0065 | 0.0036 | 0.01 |
| kck (h−1) | 12.62 | 12.04 | 5.23 | 24.93 | 26.32 | 9.6 | 33.07 | 31.72 | 6.77 |
| kkc (h−1) | 8.2 | 8.55 | 2.37 | 23.91 | 32.82 | 12.55 | 25.9 | 24.66 | 3.99 |
| kcp (h−1) | 20.59 | 22.32 | 7.46 | 26.63 | 33 | 13.98 | 8.12 | 5.78 | 6.38 |
| kpc (h−1) | 17.76 | 15.58 | 6.49 | 28.44 | 29.41 | 7.53 | 18.43 | 11.9 | 9.82 |
| Vkidney (liter) | 0.004 | 0.004 | 0.0007 | 0.003 | 0.002 | 0.002 | 0.004 | 0.004 | 0.001 |
SCL is the clearance (liter/h); Vc and Vkidney are the volumes of central compartment and kidney, respectively (liters); ka, kcp, kpc, kck, and kkc are the first-order rate constants that connect the respective compartments.
Table 3.
Drug exposure at steady state in serum and kidneys and log reduction of median kidney burden of mice receiving echinocandinsc
| Compound and dosages | AUC (mg · h/liter) |
Log reduction in kidneys |
||||||
|---|---|---|---|---|---|---|---|---|
| Serum | Kidney | Experiments 1 to 3a |
Experiment 4b |
|||||
| S3 | R83 | RR24 | S3 | R83 | RR24 | |||
| Anidulafungin | ||||||||
| High dose (12 mg/kg) | 556.5 | 812.0 | 0.794 | 0.805 | −0.982 | 0.901 | 1.053 | ND |
| Medium dose (6 mg/kg) | 278.1 | 406.0 | 0.924 | 0.831 | −0.097 | 0.717 | 0.673 | ND |
| Low dose (3 mg/kg) | 139.0 | 203.0 | −0.515 | 0.147 | −0.130 | 0.340 | 0.522 | ND |
| Caspofungin | ||||||||
| High dose (6 mg/kg) | 786.2 | 1091.0 | 2.728* | 1.850* | −1.139 | 3.942 | 2.655 | ND |
| Medium dose (3 mg/kg) | 393.1 | 545.4 | 2.517* | 1.445 | −0.176 | 3.067 | 2.053 | ND |
| Low dose (1.5 mg/kg) | 196.6 | 272.7 | 1.878 | 0.367 | −1.181 | 1.845 | 1.372 | ND |
| Micafungin | ||||||||
| High dose (12 mg/kg) | 373.7 | 443.6 | 1.431* | 1.558* | 0.021 | 1.747 | 1.528 | ND |
| Medium dose (6 mg/kg) | 186.9 | 221.8 | 0.618 | 1.170 | −0.854 | 1.243 | 1.535 | ND |
| Low dose (3 mg/kg) | 93.4 | 110.9 | 0.437 | 0.768 | 0.021 | 0.757 | 1.462 | ND |
Six animals per group.
Three animals per group. ND, not done.
The echinocandins studied were anidulafungin (3, 6, and 12 mg/kg), caspofungin (1.5, 3, and 6 mg/kg), and micafungin (3, 6, and 12 mg/kg). Negative values indicate higher CFU counts than in untreated control kidneys. S3 indicates C. glabrata wild-type-challenged mice, R83 indicates Fks2p-S663F mutant-challenged mice, and RR24 indicates Fks1p S629P mutant-challenged mice. Significant log reduction is indicated by an asterisk.
Pharmacodynamics.
The exposure-response relationships obtained in each of the initial three experiments for anidulafungin, caspofungin, and micafungin were reproducible (Table 3). The pharmacodynamic data were well described by the sigmoid Emax model. In general, the exposure-response relationships were relatively shallow except for that for caspofungin and the wild-type isolate (Fig. 2 to 4).
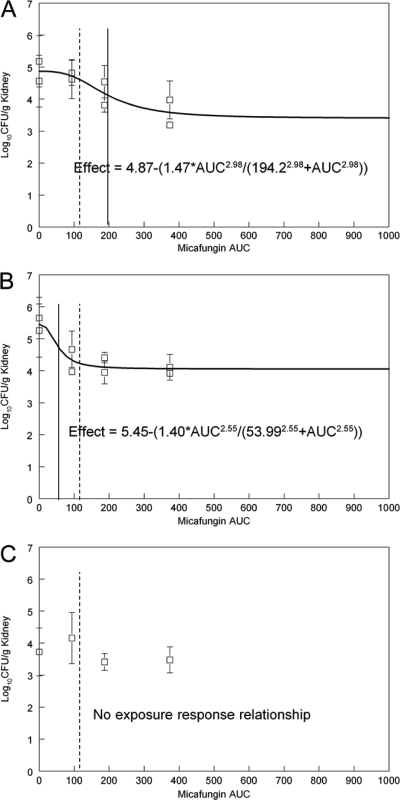
Fig 2.
Exposure-response relationships for micafungin for the various strains. (A) Wild type (S3); (B) R83, Fks2p-S663F; (C) RR24, Fks1p-S629P. The E50 is indicated by a solid vertical line, and the average human exposure is indicated by a dotted line for comparison.
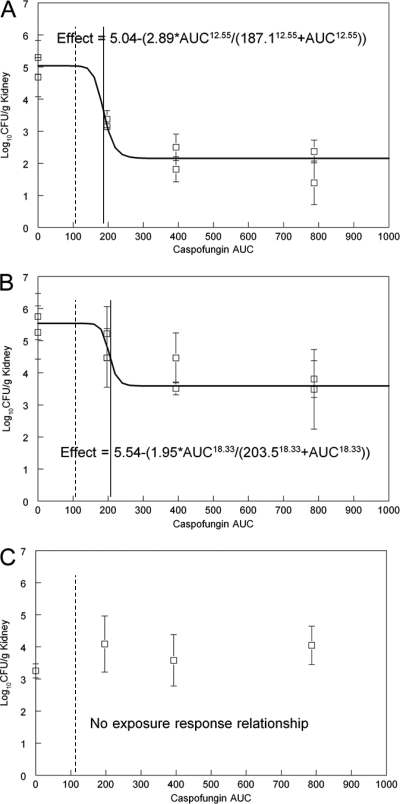
Fig 4.
Exposure-response relationships for caspofungin for the various strains. (A) Wild type (S3); (B) R83, Fks2p-S663F; (C) RR24, Fks1p-S629P. The E50 is indicated by a solid vertical line, and the average human exposure is indicated by a dotted line for comparison.
The exposure-response relationships for micafungin are shown in Fig. 2. A circa-1.5-log drop in fungal density in the kidney was induced for the S3 wild-type and the R83 Fks2p-S663F mutant isolates and was numerically greater for the latter (Fig. 2 and Table 3). The estimates for E50 for the wild type and the Fks2p-S663F mutant were 194.2 and 53.99 mg · h/liter, respectively. In contrast, there was no demonstrable exposure-response relationship for the RR24 Fks1p-S629P isolate (Fig. 2C). This strain appeared less fit, with the achievement of only ∼4 logs in the kidney after 96 h of growth in untreated animals (Fig. 2C) in contrast to ∼5 and 5.5 logs for the S3 wild-type and the R83 Fks2p-S663F mutant isolates, respectively. The average AUC for patients receiving 100 mg of micafungin/day, which is considerably lower than the E50 for the wild type but not that for the Fks2p-S663F mutant, is shown in Fig. 2.
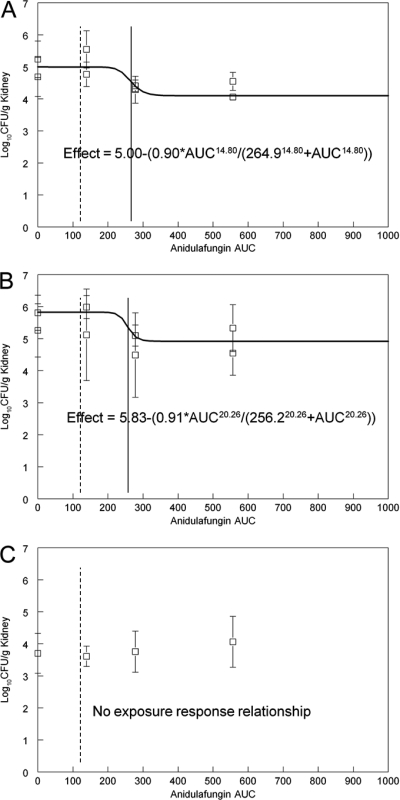
The administration of anidulafungin induced an almost 1-log drop in the fungal density in the kidney both for the S3 wild type and for the R83 Fks2p-S663F mutant. However, it did not reach statistical significance compared to what was seen for untreated control mice (Table 3). The estimates for E50 for these two strains were comparable (264.9 and 256.2 mg · h/liter, respectively) (Fig. 3A and B). Similar to what was seen for micafungin, there was no appreciable exposure-response relationship for the RR24 Fks1p-S629P mutant (Fig. 3). The average AUC for patients receiving 100 mg anidulafungin/day is shown in Fig. 3 and is lower than the E50 for both the wild type and the Fks2p-S663F mutant.
Fig 3.
Exposure-response relationships for anidulafungin for the various strains. (A) Wild type (S3); (B) R83, Fks2p-S663F; (C) RR24, Fks1p-S629P. The E50 is indicated by a solid vertical line, and the average human exposure is indicated by a dotted line for comparison.
The estimates for Emax (the maximum antifungal effect induced by antifungal therapy) for caspofungin against the S3 wild type were 2 to 3 times larger than that observed with either micafungin or anidulafungin (Table 3 and Fig. 2A, 3A, and 4A). The estimates for E50 for the S3 wild type and the R83 Fks2p-S663F mutant were similar (187.1 and 203.5 mg · h/liter, respectively), although the Emax was 1 log lower for the mutant (Table 3). In keeping with what was seen for both micafungin and anidulafungin, there was no appreciable antifungal effect for caspofungin versus the RR24 Fks1p-S629P mutant (Fig. 4C). The average AUC for patients receiving 50 mg of caspofungin/day is shown in Fig. 4 and is lower than the E50 for both the S3 wild type and the R83 Fks2p-S663F mutant.
DISCUSSION
There are increasing reports detailing the emergence of Candida isolates with reduced echinocandin susceptibility (3, 8, 12, 15, 16, 20, 27, 29, 30, 38). In the majority of these strains, the FKS molecular mechanism of resistance is apparent (3, 8, 26, 29, 30, 33, 34). It has previously been shown in animal models that the S645 mutation in the target protein Fks1p in C. albicans cannot be treated with conventional dosages of caspofungin or micafungin and that the Fks1p-F641S mutation failed to respond to doses of caspofungin as high as 10 mg/kg (41, 44). A clinical question arises as to whether all substitutions in Fks proteins are biologically similar. A further understanding of this question would facilitate more appropriate antifungal therapy. Our previous in vitro investigations suggest that this is not the case (4–6). Thus, for C. glabrata, MIC elevations were most profound for isolates with Fks1p-S629P and Fks2p-S663P alterations followed by Fks1p-F625S and Fks2p-F659S, whereas more-discrete MIC elevations are found for isolates with alterations at Fks1p-D632, Fks2p-D666, and Fks2p-P667. Furthermore, the specific alteration may not affect the efficacy of the three echinocandins to the same extent, as the MIC elevation was notably greater for anidulafungin and caspofungin than for micafungin for some mutants compared to wild-type isolates (4, 5). Our present in vivo comparison showed that the Emax values for micafungin and the R83 Fks2p-S663F mutant were equivalent to that for the wild-type isolate and moreover that the pharmacodynamic target was lower for this mutant and below the human AUC during standard dosing of micafungin. This suggests that this mutant may be an appropriate target for micafungin and that the MIC provides clinically meaningful information predicting the likelihood of in vivo response.
The fitness of homozygous Fks1p-S645P and -F641S mutants of C. albicans has recently been shown to be reduced, with reduced growth rates, filamentation, and virulence in fly and mouse models of candidiasis (9). In agreement with this, we observed a prolonged lag phase and a lower growth rate of the equivalent RR24 Fks1p-S629P C. glabrata mutant, and the kidney burden for untreated control mice was lower than that for the other two isolates in our mouse model. For the other mutant (the R83 Fks2p-S663F mutant), no trend toward loss of fitness was observed, either in vitro or in the mouse model. Hence, loss of virulence does not appear to be the reason for the low pharmacodynamic target for micafungin and this isolate compared to that seen for the wild type.
One of the advantages of a pharmacokinetic-pharmacodynamic approach is the ability to place the experimental findings in a clinical context. The mean predicted AUCs in humans resulting from current echinocandin regimens were all lower than the E50 for the wild-type isolate in mice and (at least in this model) were associated with a submaximal antifungal response for all three echinocandins. In this regard, our results are similar to previous studies (1, 25) that used profoundly immunosuppressed models of disseminated C. glabrata infection. Our results suggest that patients infected with at least some C. glabrata strains may benefit from dosage escalation. Micafungin doses of 100 and 150 mg/day have been compared head to head in one clinical trial (32). Overall, the response was numerically but not statistically poorer for the higher dose for each individual species except for C. glabrata (88.2 for the 150-mg dose versus 85.7% for 100 mg), at doses that according to our study would still be expected to be associated with submaximal effect. Further clinical trials are required to address this question. Our results also suggest that for other strains, significantly larger drug exposures do not result in any response to antifungal therapy and that an alternative antifungal agent should be used. This was the case for the high-MIC RR24 Fks1p-S629P mutant and thus is in agreement with observations for such mutants in C. albicans (41, 44). The MIC and genotype can be used to make this distinction and inform appropriate clinical decision making.
Interestingly, the estimates for Emax for caspofungin were larger both for the wild type and for the R83 Fks2p-S663F mutant than those for the other two agents (Fig. 3). The precise reason for this is unclear. High AUCs compared with those of the other two agents were achieved in the kidney, despite using doses translated from the human dose by surface area. However, comparing kidney reduction for equivalent exposures, e.g., low caspofungin and the medium and high doses of anidulafungin and micafungin, suggests that difference in exposure cannot explain the observed difference in Emax (Table 3). A previous direct comparison in a neutropenic hematogenous mouse model showed that less drug in terms of mg/kg dosage and a lower AUC/MIC target was required for caspofungin for efficacy compared to the other echinocandin drugs (1), and this observation appears robust across different mouse models. Currently, the only clinical trial with a head-to-head comparison of two echinocandins, caspofungin and micafungin, failed to show any indication of superiority of caspofungin in terms of the response rate of C. glabrata (32).
The dose-response relationship for anidulafungin and C. glabrata was particularly shallow, and even our highest dose associated with an AUC five times higher than the one achieved in humans failed to reduce the kidney burden by 1 log. This is in agreement with previous studies showing that anidulafungin appears less potent on a mg/kg basis (1, 25). In a clinical trial comparing outcomes for patients with invasive candidiasis receiving anidulafungin or fluconazole, anidulafungin was superior to fluconazole due to superiority for patients with C. albicans and C. tropicalis but not for those with C. glabrata (36, 39). On the contrary, the outcome for candidemic patients with C. glabrata was significantly better if these patients were treated initially with caspofungin rather than fluconazole (7). Further clinical trials designed and powered to specifically address this question will be necessary to understand whether this differential maximal efficacy observed in the animal models may have any clinical implication.
In conclusion, we demonstrate here that differences in echinocandin MIC changes associated with the individual fks mutations appear to be associated with differential antifungal activity in an animal model. The mutant for which the MICs were clearly elevated above the MIC range for wild-type isolates was not treatable with any of the echinocandins, whereas the isolate for which the micafungin MIC was only marginally elevated, and which may be difficult to discriminate from wild-type isolates in routine testing, responded equally as well to micafungin as did the wild type. The study underscores the value of interpreting the MIC in the context of the species and underlying FKS genotype and thus has potential implications for clinical practice and echinocandin breakpoint determination.
ACKNOWLEDGMENTS
We thank Birgit Brandt for excellent technical assistance. We thank Astellas for providing micafungin pure substance and Mycamine, Merck for providing caspofungin pure substance, and Cancidas and Pfizer for providing anidulafungin pure substance and Ecalta.
The study was financially supported in part by an unrestricted research grant from the investigator-initiated study programs of Astellas Pharma. The opinions expressed in this paper are those of the authors and do not necessarily represent those of the pharmaceutical companies. D.S.P. is a shareholder in Merck, has acted as a consultant for Merck, Pfizer, and Astellas, is an advisory board member for Merck, Pfizer, Astellas, and Myconostica, has received research funding, although not for this particular study, from Merck, Pfizer, Astellas, and Myconostica, has been invited as a speaker at Merck, Pfizer, Astellas, and Myconostica, and has the following pending patent application: assays for resistance to echinocandin-class drugs (application 07763-O69WO1). M.C.A. has been a consultant for Astellas, Merck, Pfizer, and SpePharm, has been an invited speaker for Astellas, Cephalon, Merck Sharp & Dohme, Pfizer, Schering-Plough, and Swedish Orphan, and has received research funding for this particular study from Astellas and for other studies from Merck and Pfizer. J.G. has no conflicts. R.H.J. has received travel grants from Astellas and MSD Denmark. S.J.H. has received support grants from Gilead, Pfizer, and the Fungal Research Trust, has received travel grants from Astellas and Schering-Plough, has received equipment grants from the Fungal Research Trust, and has been paid for talks on behalf of Pfizer and Astellas. W.H. is supported by a National Institutes of Health Clinician Scientist Award. W.H. has been a consultant, given talks, and received research support from Astellas, Merck, and Pfizer.
Footnotes
Published ahead of print 21 February 2012
REFERENCES
- 1. Andes D, et al. 2010. In vivo comparison of the pharmacodynamic targets for echinocandin drugs against Candida species. Antimicrob. Agents Chemother. 54:2497–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arendrup MC. 2010. Epidemiology of invasive candidiasis. Curr. Opin. Crit. Care 16:445–452 [DOI] [PubMed] [Google Scholar]
- 3. Arendrup MC, et al. 2009. Breakthrough Aspergillus fumigatus and Candida albicans double infection during caspofungin treatment: laboratory characteristics and implication for susceptibility testing. Antimicrob. Agents Chemother. 53:1185–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arendrup MC, et al. 2010. Echinocandin susceptibility testing of Candida species: comparison of EUCAST EDef 7.1, CLSI M27-A3, Etest, disk diffusion, and agar dilution methods with RPMI and Isosensitest media. Antimicrob. Agents Chemother. 54:426–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arendrup MC, Park S, Brown S, Pfaller M, Perlin DS. 2011. Evaluation of caspofungin and micafungin CLSI M44-A2 disk diffusion and associated breakpoints testing using a well characterized panel of wild type and fks hot spot mutant Candida isolates. Antimicrob. Agents Chemother. 55:1891–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arendrup MC, et al. 2011. Echinocandin susceptibility testing of Candida spp. using the EUCAST EDef 7.1 and CLSI M27-A3 standard procedures: analysis of the influence of bovine serum albumin supplementation, storage time and drug lots. Antimicrob. Agents Chemother. 55:1580–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arendrup MC, et al. 2011. Diagnostic issues, clinical characteristics, and outcome for patients with fungemia. J. Clin. Microbiol. 49:3300–3308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baixench MT, et al. 2007. Acquired resistance to echinocandins in Candida albicans: case report and review. J. Antimicrob. Chemother. 59:1076–1083 [DOI] [PubMed] [Google Scholar]
- 9. Ben-Ami R, et al. 2011. Fitness and virulence costs of Candida albicans FKS1 hot spot mutations associated with echinocandin resistance. J. Infect. Dis. 204:626–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brillowska-Dabrowska A, Saunte DM, Arendrup MC. 2007. Five-hour diagnosis of dermatophyte nail infections with specific detection of Trichophyton rubrum. J. Clin. Microbiol. 45:1200–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Castanheira M, et al. 2010. Low prevalence of fks1 hot spot 1 mutations in a worldwide collection of Candida strains. Antimicrob. Agents Chemother. 54:2655–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cleary JD, Garcia-Effron G, Chapman SW, Perlin DS. 2008. Reduced Candida glabrata susceptibility secondary to an FKS1 mutation developed during candidemia treatment. Antimicrob. Agents Chemother. 52:2263–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard—third edition. CLSI document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 14. Clinical and Laboratory Standards Institute 2009. Method for antifungal disk diffusion susceptibility testing of yeasts; approved guideline—second edition. CLSI document M44-A2. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 15. Dannaoui E, et al. 2010. Infections due to Candida spp. with reduced susceptibility to caspofungin in France, abstr. O346. Abstr. 20th Eur. Congr. Clin. Microbiol. Infect. Dis [Google Scholar]
- 16. Desnos-Ollivier M, et al. 2008. Mutations in the fks1 gene in Candida albicans, C. tropicalis, and C. krusei correlate with elevated caspofungin MICs uncovered in AM3 medium using the EUCAST method. Antimicrob. Agents Chemother. 52:3092–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Diack SL. 1930. The determination of the surface area of the white rat. J. Nutr. 3:289–296 [Google Scholar]
- 18. Ecalta 2011. Ecalta: EPAR product information. European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000788/WC500020673.pdf [Google Scholar]
- 19. Frimodt-Moller N, Sebbesen O, Frolund TV. 1983. The pneumococcus and the mouse protection test: importance of the lag phase in vivo. Chemotherapy 29:128–134 [DOI] [PubMed] [Google Scholar]
- 20. Garcia-Effron G, Kontoyiannis DP, Lewis RE, Perlin DS. 2008. Caspofungin-resistant Candida tropicalis strains causing breakthrough fungemia in patients at high risk for hematologic malignancies. Antimicrob. Agents Chemother. 52:4181–4183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garcia-Effron G, Lee S, Park S, Cleary JD, Perlin DS. 2009. Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3-β-d-glucan synthase: implication for the existing susceptibility breakpoint. Antimicrob. Agents Chemother. 53:3690–3699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garcia-Effron G, Park S, Perlin DS. 2009. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: implications for interpretive breakpoints. Antimicrob. Agents Chemother. 53:112–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garcia-Effron G, Park S, Perlin DS. 2009. Improved Candida spp. echinocandin susceptibility determination by the addition of bovine serum albumin (BSA). Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-352 [Google Scholar]
- 24. Hiemenz J, et al. 2005. Pharmacokinetic and maximum tolerated dose study of micafungin in combination with fluconazole versus fluconazole alone for prophylaxis of fungal infections in adult patients undergoing a bone marrow or peripheral stem cell transplant. Antimicrob. Agents Chemother. 49:1331–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Howard SJ, et al. 2011. Pharmacodynamics of echinocandins against Candida glabrata: requirement for dosage escalation to achieve maximal antifungal activity in neutropenic hosts. Antimicrob. Agents Chemother. 55:4880–4887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Katiyar S, Pfaller M, Edlind T. 2006. Candida albicans and Candida glabrata clinical isolates exhibiting reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 50:2892–2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krogh-Madsen M, Arendrup MC, Heslet L, Knudsen JD. 2006. Amphotericin B and caspofungin resistance in Candida glabrata isolates recovered from a critically ill patient. Clin. Infect. Dis. 42:938–944 [DOI] [PubMed] [Google Scholar]
- 28. Kuse ER, et al. 2007. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 369:1519–1527 [DOI] [PubMed] [Google Scholar]
- 29. Laverdiere M, et al. 2006. Progressive loss of echinocandin activity following prolonged use for treatment of Candida albicans oesophagitis. J. Antimicrob. Chemother. 57:705–708 [DOI] [PubMed] [Google Scholar]
- 30. Miller CD, Lomaestro BW, Park S, Perlin DS. 2006. Progressive esophagitis caused by Candida albicans with reduced susceptibility to caspofungin. Pharmacotherapy 26:877–880 [DOI] [PubMed] [Google Scholar]
- 31. Pappas PG, et al. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48:503–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pappas PG, et al. 2007. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin. Infect. Dis. 45:883–893 [DOI] [PubMed] [Google Scholar]
- 33. Park S, et al. 2005. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 49:3264–3273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pasquale T, Tomada JR, Ghannoun M, Dipersio J, Bonilla H. 2008. Emergence of Candida tropicalis resistant to caspofungin. J. Antimicrob. Chemother. 61:219. [DOI] [PubMed] [Google Scholar]
- 35. Perlin DS. 2007. Resistance to echinocandin-class antifungal drugs. Drug Resist. Updat. 10:121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pfaller MA, Andes D, Diekema DJ, Espinel-Ingroff A, Sheehan D. 2010. Wild-type MIC distributions, epidemiological cutoff values and species-specific clinical breakpoints for fluconazole and Candida: time for harmonization of CLSI and EUCAST broth microdilution methods. Drug Resist. Updat. 13:180–195 [DOI] [PubMed] [Google Scholar]
- 37. Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20:133–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pfeiffer CD, et al. 2010. Breakthrough invasive candidiasis in patients on micafungin. J. Clin. Microbiol. 48:2373–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reboli AC, et al. 2007. Anidulafungin versus fluconazole for invasive candidiasis. N. Engl. J. Med. 356:2472–2482 [DOI] [PubMed] [Google Scholar]
- 40. Rodriguez-Tudela JL, et al. 2008. EUCAST definitive document EDef 7.1: method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin. Microbiol. Infect. 14:398–405 [DOI] [PubMed] [Google Scholar]
- 41. Slater JL, et al. 2011. Disseminated candidiasis caused by Candida albicans with amino acid substitutions in Fks1 at position Ser645 cannot be successfully treated with micafungin. Antimicrob. Agents Chemother. 55:3075–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stone JA, et al. 2002. Single- and multiple-dose pharmacokinetics of caspofungin in healthy men. Antimicrob. Agents Chemother. 46:739–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wiederhold NP, Lewis JS. 2007. The echinocandin micafungin: a review of the pharmacology, spectrum of activity, clinical efficacy and safety. Expert Opin. Pharmacother. 8:1155–1166 [DOI] [PubMed] [Google Scholar]
- 44. Wiederhold NP, Najvar LK, Bocanegra RA, Kirkpatrick WR, Patterson TF. 2011. Caspofungin dose escalation for invasive candidiasis due to resistant Candida albicans. Antimicrob. Agents Chemother. 55:3254–3260 [DOI] [PMC free article] [PubMed] [Google Scholar]