Abstract
Staphylococcus aureus is a frequent cause of acute endophthalmitis, and infection with this virulent bacterium is often associated with a poor visual outcome. In this study, we investigated the bactericidal efficacy and the safety of intravitreal daptomycin (DAP), a lipopeptide antibiotic with broad-spectrum activity against Gram-positive bacteria, compared with those of intravitreal vancomycin (VAN) in a methicillin-resistant S. aureus endophthalmitis rabbit model. The pharmacokinetics and pharmacodynamics of daptomycin in the infected eyes were also studied. Rabbits were randomly divided into three treatment groups (n = 8) and one untreated group (n = 4), to compare the effect of single intravitreal injections of 0.2 mg and 1 mg of daptomycin (DAP 0.2 and DAP 1 groups, respectively) with that of 1 mg of intravitreal vancomycin (VAN 1 group). Vitreal aspirates were regularly collected and grading of ocular inflammation was regularly performed until euthanasia on day 7. In the DAP 0.2 group, 62.5% of the eyes were sterilized and the mean bacterial count presented a reduction of 1 log unit. In the DAP 1 and VAN 1 groups, the infection was eradicated (100% and 87.5% of eyes sterilized, respectively), with a 4-log-unit reduction of the mean bacterial count. The bactericidal efficacy in the DAP 1 group was not inferior to that in the VAN 1 group and was superior to that of the other regimens in limiting the ocular inflammation and preserving the architecture of the ocular structures (P < 0.05). The elimination half-life (t1/2β) of daptomycin was independent of the administered dose (38.8 ± 16.5 h and 40.9 ± 6.7 h, respectively, for the DAP 0.2 and DAP 1 groups) and was significantly longer than the t1/2β of vancomycin (20.5 ± 2.0 h for the VAN 1 group) (P < 0.05). This antibiotic could therefore be considered for the treatment of intraocular infections caused by Gram-positive bacteria.
INTRODUCTION
Acute bacterial endophthalmitis is a rare but vision-threatening eye disease. This infection and inflammation of the deep structures of the eye are mainly caused by colonization with exogenous bacteria following penetrating trauma (posttraumatic endophthalmitis) or intraocular surgery (postoperative endophthalmitis) and can result in blindness if not rapidly and properly managed. The leading causative organisms of posttraumatic and postoperative endophthalmitis are Gram-positive bacteria, preponderant on the normal ocular surface, with a significant percentage of the organisms being Staphylococcus aureus, an especially virulent pathogen causing ocular infections with poor visual outcomes (9, 12, 28). Current treatment regimens include direct intravitreal injection of 1 mg vancomycin (VAN), which shows broad-spectrum activity against Gram-positive bacteria (3, 16, 27). According to recent reports, even if the proportion of methicillin resistance among S. aureus isolates responsible for endophthalmitis is increasing (10), no vancomycin resistance has yet been declared. However, there is a need for a therapeutic alternative because of the trend for decreased susceptibility to vancomycin in nonocular S. aureus-related infections (7, 25).
Daptomycin (DAP) is a lipopeptide antibiotic approved for treatment of skin and soft tissue infections and bacteremia, including cases associated with right-sided endocarditis. Its spectrum of activity is similar to that of vancomycin, but daptomycin is also active against vancomycin-intermediate and vancomycin-resistant S. aureus strains and vancomycin-resistant enterococci (13). The use of daptomycin in an experimental endophthalmitis model has been reported only once: a single dose of 0.2 mg of intravitreal daptomycin is safe and effective in a rabbit model of coagulase-negative Staphylococcus epidermidis endophthalmitis (8).
The aim of this study was to compare the bactericidal activity and safety of different dosages of intravitreal daptomycin with those of 1 mg of intravitreal vancomycin, the reference treatment, in experimental endophthalmitis caused by S. aureus. In addition, we studied the pharmacokinetics and pharmacodynamics of daptomycin in the infected rabbit eyes.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
The S. aureus strain used in this study was isolated from a human corneal ulcer in the bacteriology laboratory of the University Hospital of Strasbourg (Strasbourg, France). Classification and identification used a matrix-assisted laser desorption ionization BioTyper system for mass spectrometry (Microflex; Bruker Daltonics, Bremen, Germany). The isolate was tested for susceptibility to oxacillin, vancomycin, and daptomycin using a Vitek 2 system (bioMérieux, Marcy l'Etoile, France). Susceptibility breakpoints and interpretive criteria from the 2011 recommendations and guidelines of the Antibiogram Committee of the French Microbiology Society were used (1). Etests were carried out to determine the vancomycin and daptomycin MICs (bioMérieux, Marcy l'Etoile, France). The presence of the Panton-Valentine leukocidin, A and B exfoliative toxins, and A, B, C, and D enterotoxins was studied by immunoprecipitation using the Ouchterlony method and reversed passive latex agglutination using a toxin detection kit (Oxoid, Dardilly, France) to determine the toxin profile of the S. aureus strain. S. aureus was cultivated on Columbia agar plates with 5% sheep blood (bioMérieux, Marcy l'Etoile, France) for 18 to 24 h in an aerobic atmosphere at 37°C.
Animal care.
Twenty-eight female New Zealand albino rabbits weighing between 3 and 4 kg were used in this study. They were obtained and cared for in the animal research facility of the Institute of Bacteriology of the University of Strasbourg in accordance with the recommendations of the European Community guidelines for the use of experimental animals (European Directive 2010/63/EU). Animals had ad libitum access to rabbit pellets and water throughout the study and were housed in individual cages with controlled temperature (21°C) and light cycle (12 h light/24 h). All in vivo testing conformed to the Association for Research in Vision and Ophthalmology (ARVO) statement on animal use, and the study protocol was approved by the regional veterinary department.
Anesthesia and euthanasia drug protocols.
The anesthesia protocol, used prior to each eye puncture, consisted of an intramuscular injection of a mixture of 30 mg/kg body weight of ketamine (Virbac, Carros, France) and 4 mg/kg body weight of xylazine (Rompun, 2%; Bayer Pharma, Puteaux, France). Additionally, a drop of oxybuprocaine (oxybuprocaine faure, 1.6 mg/0.4 ml; Novartis Pharma SAS, Rueil Malmaison, France) was instilled. At the end of the study, the animals were euthanatized with 5 ml of sodium pentobarbital (Dolethal; Vetoquinol SA, Lure, France) administered intravenously.
Experimental endophthalmitis model.
A 27-gauge needle assembled on a 1-ml tuberculin syringe was inserted 3 mm from the limbus and directed toward the center of the eyeball, avoiding the crystalline lens. The right eye of all animals received approximately 500 CFU of S. aureus in a volume of 0.1 ml. This bacterial suspension was prepared from S. aureus colonies harvested from an 18-h to 24-h culture and diluted in sterile 0.9% (wt/vol) NaCl. Purity and counts were verified by plating aliquots of the serially diluted samples on 5% sheep blood agar plates, which were incubated overnight at 37°C. Left eyes served as noninfected control eyes.
Preparation of the antibiotic intravitreal injection.
Daptomycin (Cubicin; Novartis France, Paris, France) and vancomycin (Vancomycine Sandoz; Sandoz SAS, Levallois-Perret, France) were obtained from commercial sources. Intravitreal solutions were freshly prepared from injectable powders, which were diluted in sterile ophthalmic balanced salt solution (BSS) to the desired concentrations.
Study design.
After the clinical onset of endophthalmitis, all the rabbits received 0.1 ml antibiotic or BSS intravitreal injection in the infected right eye. Rabbits were randomly divided into four groups: three treatment groups and one untreated group. One group of eight animals received 0.2 mg of daptomycin (DAP 0.2 group), and the second and the third groups, each composed of eight animals, received 1.0 mg of daptomycin (DAP 1 group) and 1 mg of vancomycin (VAN 1 group). In the untreated group, 0.1 ml of BSS was injected into four animals (BSS group). Left control eyes received no injection. Day 0 corresponded to the day of this antibiotic intravitreal injection. All the eyes were clinically examined on day 0 and 2, 4, and 7 days after. Serial 50- to 100-μl vitreous samples were withdrawn from the right eyes under sterile conditions and through 27-gauge needles on days 0, 1, 2, 4, and 7 for bacterial counts and/or dosage of antibiotic residual concentrations. Vitreous samples obtained from the left eyes (control group) served for use for bacterial cultures on day 7. All animals were eventually euthanatized on day 7, and four animals from each group were enucleated for histological assessment.
Bacterial count.
Fifty microliters of the vitreous samples was serially 10-fold diluted in 450 μl of sterile 0.9% (wt/vol) NaCl. A 100-μl volume of each dilution was spread on a 5% sheep blood agar plate and incubated overnight at 37°C, and counts were obtained after 24 h. The limit of detection was 1 log10 CFU/ml.
Clinical examination of the eyes.
The clinical investigation was conducted by a single ophthalmologist (M.S.) and according to the Nussenblatt criteria (17). Briefly, five increasing levels of severity of damage were scored for the anterior segment and annexes, and five other levels were scored for the posterior segment observed by direct ophthalmoscopy (Heine). The sum of these two scores constituted the ocular inflammation score (on a scale of from 0, indicating normal findings, to increasing values corresponding to increasing severity of abnormal findings, with 8 being the maximum score). Endophthalmitis corresponded to a Nussenblatt score higher than 2.
Histopathologic examination of the eyes.
Immediately after enucleation, the eyes were fixed in 4% (vol/vol) buffered formaldehyde. After being embedded in paraffin, they were sectioned in 5-μm-thick transverse sections and stained with hematoxylin-eosin. The slides were analyzed histopathologically by an ophthalmic pathologist (L.M.) and graded according to the scheme detailed in Table 1 (18, 20).
Table 1.
Grading of endophthalmitis severity
| Grade | Description |
|||
|---|---|---|---|---|
| Cornea | Anterior chamber | Vitreous humor | Retina | |
| 0 | No infiltration of inflammatory cells | Normal | No inflammation | Normal |
| 1 | Partial-thickness infiltration of inflammatory cells | Partially filled with fibrin, no inflammatory cells | Inflammatory cells visible, no focal abscesses | Partially infiltrated and necrotic, some normal retina seen |
| 2 | Segmental full-thickness infiltration of inflammatory cells | Partially filled with fibrin, inflammatory cells | Partially filled with abscesses of infiltrate | Totally infiltrated and partially necrotic, no normal retina, some retinal layers intact |
| 3 | Total full-thickness infiltration of inflammatory cells | Completely filled with fibrin, inflammatory cells | Completely filled with infiltrate | Totally necrotic, no retinal layer intact |
Assay of daptomycin and vancomycin in rabbit vitreous humor.
Concentrations of daptomycin were determined using a high-performance liquid chromatography (HPLC) assay developed and validated for the quantification of the drug in vitreous samples at the bacteriology laboratory of the University Hospital of Strasbourg (S.L.). Vitreous samples collected from rabbits were stored at −80°C until analysis. Frozen vitreous samples were thawed at room temperature and subjected to protein precipitation, as follows. Vitreous aliquots of 50 μl were prepared by addition of an equal volume of a 1:2 (vol/vol) methanol-acetonitrile mixture and vortexed for 5 s. After a slow rotation at 30 rpm for 10 min, this mixture was centrifuged at 10,000 × g for 5 min. Finally, 20 μl of the supernatant was injected into a Prominence HPLC system (Shimadzu USA Manufacturing Inc., Canby, OR) comprising an LP-20AT pump and a SPD-20A UV-visible detector. A Rheodyne model 7725i manual sample injector (Rheodyne, Rohnert Park, CA) was used with a 20-μl loop. Chromatographic separation was achieved on a reversed-phase C18, 150- by 4.6-mm, 3-μm Uptisphère column (Interchim, Montluçon, France) with a mobile phase consisting of an isocratic mixture of 20 mM phosphate buffer and 40% acetonitrile, and the pH was adjusted to 3.5 with phosphoric acid. A 1.0-ml/min flow rate was used, and the detection wavelength was set at 224 nm. Under these conditions, the retention time for daptomycin was 7.5 min. The LC solution Shimadzu software (Shimadzu France, Champs sur Marne, France) was used for acquiring and processing the data. The quantification limit of the daptomycin assay was 0.5 μg/ml in vitreous humor. The method was linear over the concentration range of 0.5 to 500 μg/ml, with a mean correlation coefficient of 0.9988. Quality control standards were prepared at final concentrations of 1.0, 50.0, and 250 μg/ml. Interday and intraday accuracies of the method ranged from 92.0 to 103% and 93.8 to 101%, respectively. The precision values (coefficients of variation) ranged from 0.98 to 3.58% for the intraday precision and 2.66 to 3.03% for the interday precision. Vitreous levels of vancomycin were determined by enzyme multiplied immunoassay (Viva-E drug testing system; Siemens). Hemorrhagic vitreous samples were excluded from the study.
PK/PD analysis.
To address the problem of intersubject variation, we calculated individual pharmacokinetic (PK) parameters for each rabbit of the three different treatment groups (DAP 0.2, DAP 1, and VAN 1 groups). The measured vitreous concentration-time data were graphically displayed with a semilogarithmic scale. On the basis of the correlation coefficient (R2) and least-squares regression analysis, with each animal providing three or four concentration values at different time points, vitreous antibiotic concentration-time data best fit a monoexponential equation. Exponential least-squares regression analysis was realized for all the animals of the three groups. Antibiotic maximum concentrations in vitreous humor (Cmaxs), corresponding to initial concentrations immediately after the antibiotic intravitreal injection, were estimated by extrapolation of the regression curves to time zero. The respective equations of the regression curves were used to calculate the following pharmacokinetic parameters for each animal: the terminal elimination rate constant (kβ) was the slope of the regression line, the terminal elimination half-life (t1/2β) was calculated as ln 2/kβ, and areas under the concentration-time curves (AUCs) were measured using the log trapezoidal rule, as follows: AUC1–2 = (C1 − C2)/kβ, where AUC1–2 is the AUC from 1 to 2 days after the intravitreal antibiotic injection and C1 and C2 are the concentrations at 1 and 2 days after the intravitreal antibiotic injection, respectively. We calculated the AUC between 0 and 1 day (AUC0-1) and the AUC between 1 and 7 days (AUC1-7). Finally, the Cmax/MIC and AUC0-1/MIC ratios were calculated for the pharmacodynamic (PD) analysis.
Statistical analysis.
All data were expressed as mean ± standard deviation. Differences among the groups were tested for significance using a nonparametric Kruskal-Wallis test (Prism software, version 5.04; GraphPad Software Inc., San Diego, CA). Then, if a significant difference occurred, Dunn's multiple-comparison posttest was used to compare each pair of groups. Dose proportionality assessment of daptomycin was realized using the approach proposed by Smith et al. (26). Log-transformed, dose-normalized AUC1–7 values for the two daptomycin groups (DAP 0.2 and DAP 1) were evaluated by one-way analysis of variance, and ratios of geometric means and their corresponding 90% confidence intervals (CIs) were reported and compared with the accepted interval defined by FDA guidance (26). We used the R software (R Development Core Team, 2011; Foundation for Statistical Computing, Vienna, Austria) to realize this statistical analysis.
RESULTS
The mass spectrum of the S. aureus strain was similar to that of the S. aureus ATCC 29737 reference strain, with a log score of 2.332. This strain was methicillin resistant and susceptible to vancomycin and daptomycin. Oxacillin, vancomycin, and daptomycin MICs for the S. aureus strain were >2 (resistant), 0.5 (susceptible), and 0.064 (susceptible) mg/liter, respectively. Study of the toxin profiling (Table 2) revealed the presence of type A enterotoxin and the absence of Panton-Valentine leukocidin.
Table 2.
Toxin profiling of S. aureus ATCC 29737
| Profiling technique and toxina | Presence (+) or absence (−) |
|---|---|
| Immunoprecipitation | |
| LukFPV, LukSPV | − |
| EtA | − |
| EtB | − |
| Reversed passive latex agglutination | |
| SEA | + |
| SEB | − |
| SEC | − |
| SED | − |
Immunoprecipitation was done using the Ouchterlony method, and reversed passive latex agglutination was done using a toxin detection kit (Oxoid, Dardilly, France). LukFPV and LukSPV, proteins F and P of the Panton-Valentine leukocidin, respectively; EtA and EtB, A and B exfoliative toxins, respectively; SEA, SEB, SEC, and SED, A, B, C, and D enterotoxins, respectively.
Bacterial count.
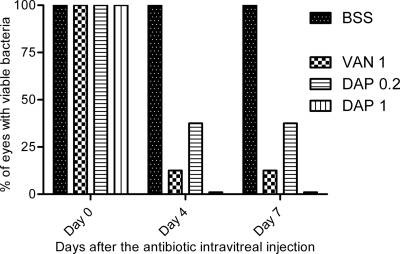
The sizes of the injected bacterial inocula did not differ significantly among the different treatment groups (P > 0.05, Kruskal-Wallis test). Just before the antibiotic intravitreal injection (day 0), all infected right eyes displayed bacterial growth with comparable bacterial counts (P > 0.05, Kruskal-Wallis test). On day 4 and day 7, 62.5% of the eyes treated with a 0.2-mg intravitreal injection of daptomycin (DAP 0.2 group), 87.5% of the eyes treated with a 1-mg intravitreal injection of vancomycin (VAN 1 group), and 100% of the eyes that received a 1-mg intravitreal injection of daptomycin (DAP 1 group) were sterilized (Fig. 1). Eyes from the BSS group (100%) showed increasing bacterial growth from day 0 to day 7, while cultures of vitreous humor from the control left eyes did not demonstrate any bacterial growth. Bacterial counts are presented in Table 3. Mass spectrometry confirmed the sole presence of S. aureus in all the colonies which were isolated from positive vitreous samples.
Fig 1.
Percentage of eyes with viable bacteria at different time points after a single intravitreal injection of 1 mg of vancomycin (VAN 1) and 0.2 mg (DAP 0.2) or 1 mg (DAP 1) of daptomycin in rabbit eyes infected by methicillin-resistant S. aureus. BSS corresponds to the infected but untreated eyes group.
Table 3.
Bacterial counts of the vitreous samples at different time points after a single intravitreal injection of 1 mg of vancomycin or 0.2 mg or 1 mg of daptomycin in rabbit eyes infected by methicillin-resistant S. aureus
| Rabbit group | Bacterial count (log10 CFU/ml)a |
||
|---|---|---|---|
| Day 0 | Day 4 | Day 7 | |
| BSS (n = 4) | 4.95 ± 0.94 | 6.82 ± 1.06 | 6.18 ± 2.42 |
| VAN 1 (n = 8) | 5.65 ± 1.53 | 1.59 ± 1.66** | 1.54 ± 1.52** |
| DAP 0.2 (n = 8) | 4.14 ± 1.48 | 2.16 ± 1.74* | 2.86 ± 2.58 |
| DAP 1 (n = 8) | 4.65 ± 1.92 | 1.0 ± 0.00*** | 1.0 ± 0.00** |
| P (Kruskal-Wallis test) | >0.05 | 0.0006 | 0.003 |
The bacterial counts are expressed as mean ± standard deviation. BSS corresponds to the infected but untreated eyes group. The limit of detection is 1 log10 CFU/ml. Asterisks represent a statistically significant difference in comparison with the BSS group (Dunn's multiple-comparison posttest): *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
Clinical examination.
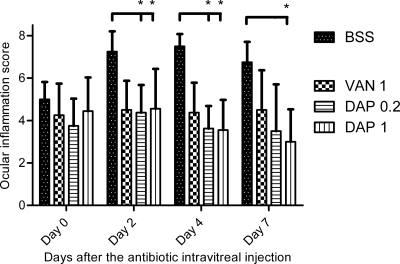
The baseline clinical examination revealed normal ocular structures, and both eyes of all rabbits scored zero on the ocular inflammation grading scale, using Nussenblatt criteria. Throughout the study, no inflammation was observed in the left eyes. The clinical scores of the inoculated eyes at the different time points are presented in Fig. 2. On day 0, endophthalmitis was clinically diagnosed for all inoculated eyes (scores higher than 2) and the ocular inflammatory scores were not significantly different between the different groups (P > 0.05, Kruskal-Wallis test). For all time points, the clinical scores were similar among the different antibiotic-treated groups (VAN 1, DAP 0.2, and DAP 1 groups) (P > 0.05, Kruskal-Wallis test). On days 2 and 4, while clinical scores in the DAP groups (DAP 0.2 and DAP 1) were significantly lower than those in the BSS group, this difference was not found with the VAN 1 group. On day 7, only the DAP 1 group differed from the untreated BSS group (P < 0.05, Dunn's multiple-comparison test).
Fig 2.
Effects of a single intravitreal injection of 1 mg of vancomycin (VAN 1) and 0.2 mg (DAP 0.2) or 1 mg (DAP 1) of daptomycin on the ocular inflammation score (expressed in cumulative average points) in infected rabbit eyes. Error bars represent the standard deviation. BSS corresponds to the infected but untreated eyes group. Asterisks represent a statistically significant difference in comparison with the BSS group (Dunn's multiple-comparison posttest): *, P < 0.05.
Histopathologic analysis.
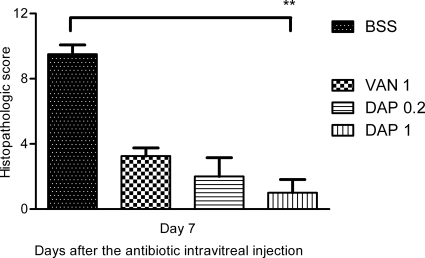
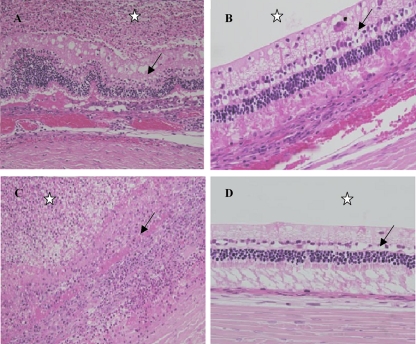
The left control rabbit eyes displayed normal and well-distinguished layers. The histopathologic scores of the inoculated rabbit eyes are presented in Fig. 3, and when the scores are compared with the score for the untreated group (BSS group; score, 9.5 ± 0.58), a significant difference appeared only with the DAP 1 group (score, 1 ± 0.82) (P < 0.01, Dunn's posttest). Eyes from the DAP 0.2 and DAP 1 groups presented a normal or partially inflamed vitreous humor and always had a normal retinal architecture (Fig. 4A). The anterior chamber was free of inflammatory cells but was sometimes partially filled with fibrin. These eyes were less inflamed than those in the VAN 1 group. Indeed, the retinas of the VAN 1 group eyes were partially infiltrated and necrotic, even if some well-organized retinal layers were seen. Inflammatory cells were observed migrating from the vitreous humor into the retina (Fig. 4B). The eyes from the BSS group exhibited the most severe inflammation in all ocular structures. Inflammatory cells migrated in greater numbers into the corneal periphery, forming corneal ring abscesses. Inflammatory cells, fibrin, and sometimes erythrocytes filled the anterior segment and the vitreous humor. The retinal structures were indistinguishable because of the necrosis (Fig. 4C).
Fig 3.
Effects of a single intravitreal injection of 1 mg of vancomycin (VAN 1) and 0.2 mg (DAP 0.2) or 1 mg (DAP 1) of daptomycin on the histopathologic score (expressed in cumulative average points) in infected rabbit eyes. Error bars represent the standard deviation. BSS corresponds to the infected but untreated eyes group. Asterisks represent a statistically significant difference in comparison with the BSS group (Dunn's multiple-comparison posttest): **, P < 0.01.
Fig 4.
Histopathologic sections from enucleation specimen stained with hematoxylin-eosin, showing vitreous humor (star) and retina (arrow) in the different rabbit groups. (A) Right eye after a 1-mg intravitreal injection of vancomycin (VAN 1 group) showing an infiltrated vitreous humor and a partially infiltrated and necrotic retina with some normal retina. Magnification, ×200. (B) Right eye after a 1-mg intravitreal injection of daptomycin (DAP 1 group) showing a noninfiltrated vitreous humor and a normal retina. Magnification, ×400. (C) Right eye after an intravitreal injection of a balanced salt solution (BSS group) showing a completely filled vitreous humor with infiltrate and a totally necrotic and disorganized retina with no retinal layer intact. Magnification, ×200. (D) Left control eye with no inflammation of vitreous humor and a normal retina. Magnification, ×400.
Antibiotic assay.
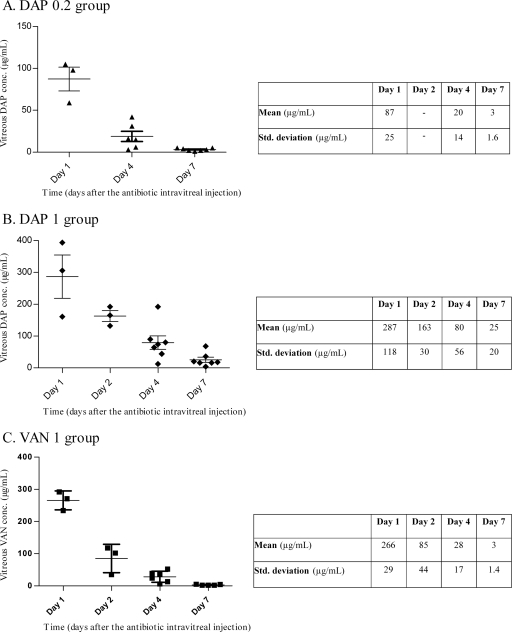
The time course of the measured antibiotic vitreous humor concentrations and corresponding statistical data (mean ± standard deviation) are presented in Fig. 5. Twenty-four hours (day 1) after the intravitreal injection of 0.2 mg (DAP 0.2 group) and 1 mg (DAP 1 group) of daptomycin, antibiotic vitreous humor concentrations were, respectively, 87 ± 25.0 and 287 ± 118.0 μg/ml, while the residual concentrations reached, respectively, 3 ± 1.6 and 25 ± 20.0 μg/ml at day 7. Vancomycin vitreous humor concentrations were 266 ± 29.0 μg/ml on day 1 and 3 ± 1.4 μg/ml on day 7 in the VAN 1 group.
Fig 5.
Daptomycin and vancomycin vitreous humor concentrations versus time after administration of a single intravitreal injection of 0.2 mg (A) or 1 mg (B) of daptomycin and 1 mg of vancomycin (C).
PK/PD analysis.
In the DAP 0.2, DAP 1, and VAN 1 groups, the plot of the log of measured vitreous humor antibiotic concentrations versus time gave nearly straight lines with correlation coefficients close to 1 (R2 for DAP 0.2 = 0.974 ± 0.035, R2 for DAP 1 = 0.979 ± 0.018, and R2 for VAN 1 = 0.943 ± 0.085). t1/2βs were 38.8 ± 16.5 h, 40.9 ± 6.7 h, and 20.5 ± 2.0 h, respectively, for the DAP 0.2, DAP 1, and VAN 1 groups. The t1/2βs of the DAP 0.2 and DAP 1 groups were similar, while a significant difference was observed between the VAN 1 group and both daptomycin groups (P < 0.05, Dunn's posttest). The AUC1-7s were 3,629 ± 2,705 mg · h/liter, 15,417 ± 9,734 mg · h/liter, and 11,052 ± 4,935 mg · h/liter, respectively, for the DAP 0.2, DAP 1, and VAN 1 groups. The estimated ratio of AUC1-7 for DAP 1/AUC1-7 for DAP 0.2 was equal to 4.62, with a 90% CI of 1.91 to 11.2. Concerning the pharmacodynamic parameters of the different treatment groups, Cmax/MIC ratios ranged from 1,817 to 7,157 mg/liter, while AUC0-1/MIC ratios ranged from 29,359 to 138,934 mg · h/liter. The results are presented in Table 4.
Table 4.
Ocular pharmacokinetic/pharmacodynamic parametersa
| Treatment group | kβ | t1/2β (h) | AUC1-7 (mg · h/liter) | MIC (mg/liter) | Cmax (mg/liter) | AUC0-24 | Cmax/MIC (mg/liter) | AUC0-1/MIC (mg · h/liter) |
|---|---|---|---|---|---|---|---|---|
| DAP 0.2 | 0.48 ± 0.16 | 38.8 ± 16.5 | 3,629 ± 2,705 | 0.064 | 160 ± 159 | 2,896 ± 2,755 | 2,499 ± 2,487 | 45,257 ± 43,043 |
| DAP 1 | 0.42 ± 0.08 | 40.9 ± 6.7 | 15,417 ± 9,734 | 0.064 | 458 ± 296 | 8,892 ± 5,589 | 7,157 ± 4,627 | 138,934 ± 87,332 |
| VAN 1 | 0.82 ± 0.09 | 20.5 ± 2.0 | 11,052 ± 4,935 | 0.5 | 908 ± 533 | 14,679 ± 8,100 | 1,817 ± 1,066 | 29,359 ± 16,199 |
AUC1-7 and AUC0-1, area under the curve between 1 and 7 days and between 0 and 1 day after the intravitreal antibiotic injection, respectively; kβ, terminal elimination rate constant; t1/2β, terminal elimination half-life; Cmax, antibiotic maximum concentration in vitreous humor. All parameters are expressed as mean ± standard deviation.
DISCUSSION
The rabbit model is considered suitable for evaluation of the activity, toxicity, and pharmacokinetics of antimicrobials in experimental endophthalmitis (globe size, aqueous humor turnover rate, and blood-ocular barriers are comparable to those of human eyes) (2, 4). In this study, we used a strain of methicillin-resistant S. aureus, a very virulent pathogen often responsible for poor visual outcomes. The antibacterial activity of daptomycin was assessed here in vivo because of its known rapid and concentration-dependent bactericidal in vitro efficacy against S. aureus in comparison with the slow and time-dependent bactericidal in vitro efficacy of vancomycin (29).
Bactericidal efficacy.
In this study, increasing doses of daptomycin improved the bactericidal efficacy in the eye. A single 0.2-mg intravitreal injection of daptomycin was not effective in sterilizing all infected eyes. It resulted in an approximately 1-log-unit reduction in the mean bacterial count 7 days after the antibiotic intravitreal injection. This bacterial count reduction failed to reach a level of statistical significance compared with the result for the untreated group (BSS group). In contrast, single 1.0-mg intravitreal injections of daptomycin or vancomycin reduced bacterial counts in the eyes by approximately 4 log units and eradicated the ocular infection. However, the failure of the 0.2-mg daptomycin intravitreal treatment is surprising since the daptomycin vitreous humor concentration in the DAP 0.2 group was approximately 2,000 times higher than the daptomycin MIC90 of the inoculated S. aureus strain on day 1 and 47 times higher on day 7. In pharmacodynamic studies of antibiotics with concentration-dependent efficacies, when antibiotic concentrations are eight times higher than the MIC, bactericidal activity was predominantly and strongly associated with a clinical response (6, 11).
PK/PD analysis.
After a 0.2- or 1.0-mg intravitreal injection (DAP 0.2 and DAP 1 groups), daptomycin vitreous humor concentration-time curves showed a single exponential decline during the period from 1 to 7 days after administration. With a first sampling time at 24 h, a distribution phase could have been missed to predict the model followed by the ocular daptomycin distribution. In this study, we did not take a sample of the vitreous humor immediately after intravitreal injection to limit the number of the eye punctures and to favor the collection of samples during an extended period (7 days). We decided to use this attitude to analyze the process of ocular elimination of daptomycin. The results showed that the terminal elimination half-life of daptomycin was constant and independent of the administered dose (38.8 h and 40.9 h, respectively, for the DAP 0.2 and DAP 1 groups) and that the t1/2βs of both daptomycin groups were significantly longer than the t1/2β of vancomycin (20.5 h in the VAN 1 group). The 90% CI (1.91 to 11.2) of the ratio of the AUC1–7 for DAP 1/AUC1–7 for DAP 0.2 was not contained completely within the acceptance interval (4 to 6.25). Consequently, it is not statistically possible to conclude that the AUC is proportional to both doses studied. Further studies are needed to draw conclusions about the distribution model and the dose proportionality of daptomycin after administration by intravitreal injection in this endophthalmitis model.
According to the literature, daptomycin displays concentration-dependent killing against S. aureus, and efficacy is well-correlated with the Cmax/MIC and AUC0-1/MIC ratios, using data based on serum concentrations (14, 22). However, the ocular pharmacokinetic/pharmacodynamic relationship determining the efficacy of daptomycin against S. aureus in the eye, especially after a direct intravitreal injection, is not known. In this experimental model of endophthalmitis, very high Cmax/MIC and AUC0-1/MIC ratios did not appear to be predictive of antibacterial efficacy. Indeed, in the DAP 0.2 group, a Cmax/MIC of 2,499 and an AUC0-1/MIC of 45,257 mg · h/liter were not sufficient to provide satisfactory bactericidal activity, but 1 mg of daptomycin, with a Cmax/MIC of 7,157 and an AUC0-1/MIC of 138,934 mg · h/liter, was a bactericidal treatment presenting no failure in curing the endophthalmitis. The pharmacodynamic profile should be accurately described in future studies. The composition of vitreous humor with severe inflammation in infected eyes may impact the bactericidal activity of daptomycin. Marked binding to vitreal proteins and/or conformational modifications could affect the percentage of free, stable, and active drug. The importance of high protein binding (90 to 93% of plasma protein binding, according to Benvenuto et al. [5]) has already been underlined by Safdar et al. (22). In a neutropenic murine model of S. aureus thigh infection, the bactericidal activity and in vivo efficacy of daptomycin were analyzed by using free drug concentrations. In our study, daptomycin vitreous humor concentrations were very high and protein binding could have been saturated in the DAP 1 group but not in the DAP 0.2 group. This could be one explanation for the failure of the low dosage of daptomycin in the present study, in spite of the very high pharmacodynamics ratios.
Clinical examination and histopathologic analysis.
Direct intravitreal injection is the best route to treat bacterial endophthalmitis because it shunts the blood-ocular barrier, which limits the ocular penetration of antibiotics from the bloodstream, providing intravitreal antibiotic concentrations higher than those obtained after systemic administration. However, ocular toxicity is a real problem correlated with high antibiotic concentrations in the eye. Indeed, aminoglycosides may induce retinal toxicity and macular ischemia with permanent damage after a single intravitreal injection (21, 23). In this study, the 1-mg daptomycin dosage directly injected into the eye was superior to other regimens in limiting the ocular inflammation and preserving the architecture of the ocular structures. It also appeared that the retina, including the photoreceptor layers, was preserved histologically. However, the toxicity of such doses of intravitreal daptomycin on retinal function must be assessed in further studies.
One of the limitations of this study is that repeated punctures of the infected eyeball of each animal may reduce the antibiotic concentration after dilution in the newly secreted aqueous humor. There is also a risk of breaching the blood-ocular barrier and of damage to the ocular tissues. However, the same procedure was performed in the vancomycin group, and it is likely that modifications occurred in the same proportion in all groups. Additionally, hemorrhagic samples were excluded from the analysis because of the possible impact on antibiotic elimination and the clinical and histopathologic examinations. The findings in this study show differences with those of a previously published study. Comer et al. (8) show the safety and efficacy of 0.2 mg intravitreal daptomycin in an adult pigmented rabbit eye model of S. epidermidis endophthalmitis. No growth was observed in any infected eyes (seven eyes) 48 h after the antibiotic injection. No changes were found on the electroretinogram pattern. The photoreceptor layer was preserved. Therefore, the 0.2-mg dose appeared to be safe for the retinal functions and retinal toxicity was observed only for higher doses (i.e., moderate and severe scotopic and photopic waveforms and a missing photoreceptor layer). This type of photoreceptor destruction was not found in DAP 1 group samples. The difference in the strain (respectively, Dutch belted versus New Zealand) and weight (respectively, 2.2 to 2.5 kg versus 3.0 to 4.0 kg) of the rabbits used could partially explain the difference observed, because the vitreous volume of the tested rabbits could vary significantly. Ocular pigmentation could also play a role, since the affinity of daptomycin to melatonin could affect the intraocular pharmacokinetics of this antibiotic, as it has already been implicated in some studies comparing intraocular penetration of systemic antibiotics in albino and pigmented rabbits (15, 19). The difference in the tested bacterial species (S. aureus versus S. epidermidis) could modify the bacterial toxin profiling and could explain the differences in strain virulence and histopathologic analysis. Finally, the absence of a vitreous humor daptomycin assay in the study of Comer et al. (8) makes it impossible to compare these studies.
To our knowledge, there is only one study dealing with ocular penetration of daptomycin in human (24). In this study, daptomycin was administered intravenously as a single 10-mg/kg dose to a patient presenting with methicillin-resistant S. aureus endogenous endophthalmitis. The vitreous humor concentration 42 h after administration of the dose was 12.43 mg/liter, and therapeutic efficacy was not assessed in the absence of cultures of intraocular samples. No data for samples obtained after direct intravitreal administration in human are available. Even if numerous ocular PK/PD studies are conducted on rabbits, because of the anatomic characteristics of the rabbit eye (i.e., globe size and aqueous humor turnover rate are comparable to those of human eyes), caution must be taken in extrapolating these results to humans.
Conclusion.
In conclusion, this study demonstrates evidence of the effectiveness of daptomycin for the treatment of experimental staphylococcal endophthalmitis. A 1-mg dose of daptomycin ensures noninferiority from the bactericidal efficacy of a 1-mg dose of vancomycin. This is the first report to study the ocular pharmacokinetics and pharmacodynamics of daptomycin in the eye. Daptomycin presented a long terminal elimination half-life significantly longer than that of vancomycin for the doses studied (P < 0.05). Clinical and histopathologic examinations showed limited ocular inflammation and preservation of the architecture of the ocular structures after sterilization of the treated eye. Daptomycin should therefore be considered for the treatment of intraocular infections caused by Gram-positive bacteria. Further studies are needed to determine the toxicity of daptomycin on visual function and the PK/PD profile.
ACKNOWLEDGMENTS
We thank Julien Godet for conducting the statistical analysis of the dose proportionality of daptomycin.
We have no conflict of interest to declare, and none of the authors has financial interest in this work.
Footnotes
Published ahead of print 27 February 2012
REFERENCES
- 1. Antibiogram Committee of the French Microbiology Society 2010. recommendations Antibiogram Committee of the French Microbiology Society, Paris, France: http://www.sfm-microbiologie.org/UserFiles/file/CASFM/casfm_2010.pdf [Google Scholar]
- 2. Barza M. 1989. Antibacterial agents in the treatment of ocular infections. Infect. Dis. Clin. North Am. 3:533–551 [PubMed] [Google Scholar]
- 3. Baum J, Peyman GA, Barza M. 1982. Intravitreal administration of antibiotic in the treatment of bacterial endophthalmitis. III. Consensus. Surv. Ophthalmol. 26:204–206 [DOI] [PubMed] [Google Scholar]
- 4. Belmatoug N, Fantin B. 1997. Contribution of animal models of infection for the evaluation of the activity of antimicrobial agents. Int. J. Antimicrob. Agents 9:73–82 [DOI] [PubMed] [Google Scholar]
- 5. Benvenuto M, Benziger DP, Yankelev S, Vigliani G. 2006. Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteers. Antimicrob. Agents Chemother. 50:3245–3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blaser J, Stone BB, Groner MC, Zinner SH. 1987. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob. Agents Chemother. 31:1054–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clark NC, Weigel LM, Patel JB, Tenover FC. 2005. Comparison of Tn1546-like elements in vancomycin-resistant Staphylococcus aureus isolates from Michigan and Pennsylvania. Antimicrob. Agents Chemother. 49:470–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Comer GM, et al. 2011. Intravitreal daptomycin: a safety and efficacy study. Retina 31:1199–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fisch A, et al. 1991. Epidemiology of infective endophthalmitis in France. The French Collaborative Study Group on Endophthalmitis. Lancet 338:1373–1376 [PubMed] [Google Scholar]
- 10. Freidlin J, et al. 2007. Spectrum of eye disease caused by methicillin-resistant Staphylococcus aureus. Am. J. Ophthalmol. 144:313–315 [DOI] [PubMed] [Google Scholar]
- 11. Gunderson SM, Hayes RA, Quinn JP, Danziger LH. 2004. In vitro pharmacodynamic activities of ABT-492, a novel quinolone, compared to those of levofloxacin against Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Antimicrob. Agents Chemother. 48:203–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Han DP, et al. 1996. Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis Vitrectomy Study. Am. J. Ophthalmol. 122:1–17 [DOI] [PubMed] [Google Scholar]
- 13. Kosmidis C, Levine DP. Daptomycin: pharmacology and clinical use. Expert Opin. Pharmacother. 11:615–625 [DOI] [PubMed] [Google Scholar]
- 14. Louie A, et al. 2001. Pharmacodynamics of daptomycin in a murine thigh model of Staphylococcus aureus infection. Antimicrob. Agents Chemother. 45:845–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mochizuki K, et al. 1994. Intraocular penetration of oral levofloxacin in rabbits. Nihon Ganka Gakkai Zasshi 98:1085–1090 (In Japanese.) [PubMed] [Google Scholar]
- 16. Novosad BD, Callegan MC. Severe bacterial endophthalmitis: towards improving clinical outcomes. Expert Rev. Ophthalmol. 5:689–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nussenblatt RB, Palestine AG, Chan CC, Roberge F. 1985. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology 92:467–471 [DOI] [PubMed] [Google Scholar]
- 18. Park SS, Samiy N, Ruoff K, D'Amico DJ, Baker AS. 1995. Effect of intravitreal dexamethasone in treatment of pneumococcal endophthalmitis in rabbits. Arch. Ophthalmol. 113:1324–1329 [DOI] [PubMed] [Google Scholar]
- 19. Perez S, et al. 2002. Pharmacokinetics and ocular penetration of grepafloxacin in albino and pigmented rabbits. J. Antimicrob. Chemother. 50:541–545 [DOI] [PubMed] [Google Scholar]
- 20. Peyman GA, Paque JT, Meisels HI, Bennett TO. 1975. Postoperative endophthalmitis: a comparison of methods for treatment and prophylaxis with gentamicin. Ophthalmic Surg. 6:45–55 [PubMed] [Google Scholar]
- 21. Piguet B, Chobaz C, Grounauer PA. 1996. Toxic retinopathy caused by intravitreal injection of amikacin and vancomycin. Klin. Monbl. Augenheilkd. 208:358–359 (In French.) [DOI] [PubMed] [Google Scholar]
- 22. Safdar N, Andes D, Craig WA. 2004. In vivo pharmacodynamic activity of daptomycin. Antimicrob. Agents Chemother. 48:63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seawright AA, Bourke RD, Cooling RJ. 1996. Macula toxicity after intravitreal amikacin. Aust. N. Z. J. Ophthalmol. 24:143–146 [DOI] [PubMed] [Google Scholar]
- 24. Sheridan KR, Potoski BA, Shields RK, Nau GJ. Presence of adequate intravitreal concentrations of daptomycin after systemic intravenous administration in a patient with endogenous endophthalmitis. Pharmacotherapy 30:1247–1251 [DOI] [PubMed] [Google Scholar]
- 25. Sievert DM, et al. 2008. Vancomycin-resistant Staphylococcus aureus in the United States, 2002-2006. Clin. Infect. Dis. 46:668–674 [DOI] [PubMed] [Google Scholar]
- 26. Smith BP, et al. 2000. Confidence interval criteria for assessment of dose proportionality. Pharm. Res. 17:1278–1283 [DOI] [PubMed] [Google Scholar]
- 27. Smith MA, et al. 1986. Treatment of experimental methicillin-resistant Staphylococcus epidermidis endophthalmitis with intravitreal vancomycin. Ophthalmology 93:1328–1335 [DOI] [PubMed] [Google Scholar]
- 28. Somani S, Grinbaum A, Slomovic AR. 1997. Postoperative endophthalmitis: incidence, predisposing surgery, clinical course and outcome. Can. J. Ophthalmol. 32:303–310 [PubMed] [Google Scholar]
- 29. Stratton CW, Weeks LS. 1990. Effect of human serum on the bactericidal activity of daptomycin and vancomycin against staphylococcal and enterococcal isolates as determined by time-kill kinetic studies. Diagn. Microbiol. Infect. Dis. 13:245–252 [DOI] [PubMed] [Google Scholar]