Abstract
The antibacterial efficacies of tedizolid phosphate (TZD), linezolid, and vancomycin regimens simulating human exposures at the infection site against methicillin-resistant Staphylococcus aureus (MRSA) were compared in an in vivo mouse pneumonia model. Immunocompetent BALB/c mice were orally inoculated with one of three strains of MRSA and subsequently administered 20 mg/kg TZD every 24 hours (q24h), 120 mg/kg linezolid q12h, or 25 mg/kg vancomycin q12h over 24 h. These regimens produced epithelial lining fluid exposures comparable to human exposures observed following intravenous regimens of 200 mg TZD q24h, 600 mg linezolid q12h, and 1 g vancomycin q12h. The differences in CFU after 24 h of treatment were compared between control and treatment groups. Vehicle-dosed control groups increased in bacterial density an average of 1.1 logs. All treatments reduced the bacterial density at 24 h with an average of 1.2, 1.6, and 0.1 logs for TZD, linezolid, and vancomycin, respectively. The efficacy of TZD versus linezolid regimens against the three MRSA isolates was not statistically different (P > 0.05), although both treatments were significantly different from controls. In contrast, the vancomycin regimen was significantly different from TZD against one MRSA isolate and from linezolid against all isolates. The vancomycin regimen was less protective than either the TZD or linezolid regimens, with overall survival of 61.1% versus 94.7% or 89.5%, respectively. At human simulated exposures to epithelial lining fluid, vancomycin resulted in minimal reductions in bacterial counts and higher mortality compared to those of either TZD or linezolid. TZD and linezolid showed similar efficacies in this MRSA pneumonia model.
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA), including community-associated (CA) and health care-associated (HA) strains, continue to dominate the infectious disease landscape. MRSA strains USA100, USA300, and USA400 are increasingly more resistant to antimicrobials and are spread over the United States and globally (3, 10, 13, 23, 27). As this species continues to adapt, the treatment of both CA-MRSA and HA-MRSA pneumonia has become more difficult (27, 33). Tedizolid phosphate (TZD; formerly torezolid phosphate) is a novel oxazolidinone antibiotic that is under investigation in phase III trials for acute bacterial skin and skin structure infections (ABSSSI) and has proven to be a powerful new agent against Gram-positive pathogens, including MRSA, Streptococcus pneumonia, and enterococci (2, 29). TZD is a prodrug antibiotic that is rapidly converted in vivo to the microbiologically active moiety tedizolid, also known as TR-700. Human pharmacokinetic studies of tedizolid showed improved pharmacokinetic results compared to those of linezolid and support a once-daily dose of 200 mg (25, 29). Additionally, tedizolid was shown to have in vivo efficacy in a pneumonia model of infection (26).
In this current study, the epithelial lining fluid (ELF) profile observed after the administration of 200 mg tedizolid every 24 hours (q24h) was studied in a mouse pneumonia infection model against both CA-MRSA and HA-MRSA strains. The determination of concentrations at the site of infection is now recommended for investigational drugs, and the approach of simulating human dosing exposures may show that such endeavors have additional significance (15, 24, 35). We have examined the efficacy of tedizolid compared to that of the sole FDA-approved oxazolidinone, linezolid (LZD), and the therapeutic standard, vancomycin (VAN), using ELF drug exposures in mice, which simulated those observed in humans.
MATERIALS AND METHODS
Bacterial strains.
Three MRSA strains were used for these in vivo analyses. Two were CA-MRSA strains, 156 (USA300, Panton-Valentine leukocidin [PVL] negative, staphylococcal cassette chromosome mec type IV [SCCmec IV]) and 464 (PVL negative), and one was HA-MRSA, strain 56 (494 from Anti-Infective Research Laboratory, Detroit Receiving Hospital and University Health Center, Detroit, MI; USA100). Strains were maintained at −80°C and transferred onto agar medium for viability and to ensure uncontaminated growth before use in either in vitro or in vivo studies.
Antimicrobial agents.
The analytical-grade TZD for in vivo studies and tedizolid for in vitro testing were both supplied by Albany Molecular Research Inc., Albany, NY. Linezolid was provided from Pfizer, Inc., Groton, CT. Vancomycin was acquired from Sigma-Aldrich Chemicals, St. Louis, IL. The antimicrobials were weighed and reconstituted in appropriate diluents to achieve the desired concentration each day of in vivo experimentation immediately prior to use. TZD was diluted in 0.025 M phosphate buffer, LZD in sterile water for injection, and VAN in normal saline. Both LZD and VAN solutions were stored under refrigeration pending the subsequent 12-h dose.
Antimicrobial susceptibility testing.
The MRSA isolates were tested in accordance with Clinical and Laboratory Standards Institute by broth microdilution in triplicate for each compound (7). The median MICs and MIC ranges were reported.
Mouse infection model.
Specific pathogen-free BALB/c female mice weighing approximately 20 g were obtained from Harlan Sprague Dawley, Inc. (Indianapolis, IN). Animals were maintained and used in accordance with National Research Council recommendations and allowed food and water ad libitum. This study was reviewed and approved by the Hartford Hospital Institutional Animal Care and Use Committee.
The immunocompetent S. aureus pneumonia model has been described elsewhere (9, 17, 20). In short, a volume of 0.05 ml of 109 CFU/ml MRSA bacterial suspension (in saline with 3% hog gastric mucin) was inoculated into each mouse. While the mice were anesthetized (2% isofluorane), the bacterial suspension was orally instilled and nares were blocked. The mouse aspirated suspension into the lungs while being held vertically for 60 s. Mice were randomized into vehicle or antimicrobial treatment groups consisting of six infected mice each and received the first administration of subcutaneous saline, intraperitoneal TZD, subcutaneous LZD, or subcutaneous VAN 3 h after inoculation. Mice were sacrificed and whole lung tissues were harvested from groups of animals prior to (0 h) and 24 h after commencement of dosing. The change in log10 CFU was calculated as the difference in the average number of CFU per group at 24 h minus the initial (0-h control) CFU level. Bacterial densities outside one standard deviation (SD) were excluded from the group average. The survival of the animals over the 24 h was monitored.
Determination of the ELF concentration profile.
Single doses of each antimicrobial were administered to separate cohorts of six infected mice. Bronchoalveolar lavage fluid (BAL fluid) and blood (for urea only) was collected as described previously (17, 21) from groups of mice over the 12- to 24-h dosing intervals. TZD was administered via intraperitoneal injection at 6, 8.4, 18, and 20 mg/kg; LZD subcutaneously at 60, 120, and 180 mg/kg; and VAN subcutaneously at 20, 25, and 110 mg/kg. Cells in the BAL fluid were removed by centrifugation, and supernatant was stored at −80°C until analysis for antimicrobial agents. Blood samples for urea determination were separated by centrifugation and stored at −80°C.
Concentrations of tedizolid in BAL fluid were analyzed by matrix-validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) by Midwest Bioresearch, Skokie, IL, as previously reported (29). The upper and lower limits of quantification were 1,000 ng/ml and 10 ng/ml, respectively.
LZD was assayed by validated high-performance liquid chromatography (HPLC) procedures (37). Briefly trichloroacetic acid and an internal standard (4-nitroaniline) were added to the BAL fluid samples. After centrifugation, the aqueous layer was injected onto a C18 column with a pH 5 sodium acetate buffer-acetonitrile (80:20, vol/vol) with peaks monitored at 251 nm. The ratio of peak height to internal standard relative to the linear regression equation from prepared stock solutions was used to determine the sample concentration. Assay limits over 0.2 to 30 μg/ml were linear (r2 ≥ 0.99), with percent coefficient of variation between 1.4 to 4.5%.
VAN concentrations were assayed by high-pressure liquid chromatography. BAL fluid samples underwent centrifugation after the addition of the internal standard (caffeine) and acetonitrile. The aqueous phase was evaporated to dryness under nitrogen. Reconstitution was made with 200 μl of mobile phase consisting of phosphate buffer-acetonitrile at 89:11 (vol/vol). Samples were injected onto a C18 column (Spherisorb, 5 μm; Phenominex, Inc., Torrance, CA) and monitored at 198 nm. Linearity of the assay was well defined (r2 = 1.0). The sample concentration was determined as described above for LZD; assay limits were 0.1 to 10 μg/ml with variability between 2.8 to 4.3%.
Each blood and BAL fluid sample was tested for urea concentration using a commercially available urea assay (TecoDiagnostics, Anaheim, CA). Linearity was highly consistent with correlation of ≥0.99 over the assay range of 0.1 to 2.0 mg/dl; variability was 0.2% to 2.9%. Accuracies of quality-control samples were between 93.1 and 98.3% recovery and 1.3% to 3.3% variance. The drug concentrations in ELF were calculated from the following formula: ELF concentration = BAL fluid concentration × (blood urea concentration/BAL fluid urea concentration) (21, 42). The area under the drug concentration-time curve (AUC) in ELF for all three regimens was calculated using the trapezoidal rule. The target AUC from 0 to 24 h (AUC0–24) ELF exposures after administration of 200 mg TZD q24h, 600 mg LZD q12h, and 1 g VAN q12h were 109, 960, and 92 mg · h/liter, respectively (8, 16, 30, 38), in healthy adults.
Statistical comparison of antimicrobial efficacies.
The differences between the average change in log10 CFU in lung from control and treatment groups at 24 h was evaluated using a one-way analysis of variance (ANOVA; SigmaStat version 2.03; SPSS, Inc., San Rafael, CA). A P value of <0.05 was considered significant.
RESULTS
MICs.
The tedizolid, LZD, and VAN MIC values for each MRSA isolate are listed in Table 1. All isolates were susceptible to both LZD and VAN. Likewise, these isolates would be interpreted as susceptible with the proposed ≤2 μg/ml breakpoint for tedizolid and represent the MIC90 (2, 5). Tedizolid had 2- to 8-fold greater potency than VAN and LZD against these MRSA isolates.
Table 1.
MICs and characteristics of MRSA strains used during in vivo testing
| Isolatea | Characteristics | Median MIC (range) (μg/ml) |
||
|---|---|---|---|---|
| Tedizolid | LZD | VAN | ||
| 56 | HA-MRSA, USA100 | 0.5 | 4 (4–8) | 1 |
| 156 | CA-MRSA, USA300 | 0.5 | 4 (2–4) | 1 |
| 464 | CA-MRSA, USA100 | 0.5 | 4 (2–4) | 1 (0.5–1) |
Internal designation.
Analysis of pharmacokinetic data to produce human simulated regimens of tedizolid, LZD, and VAN in ELF.
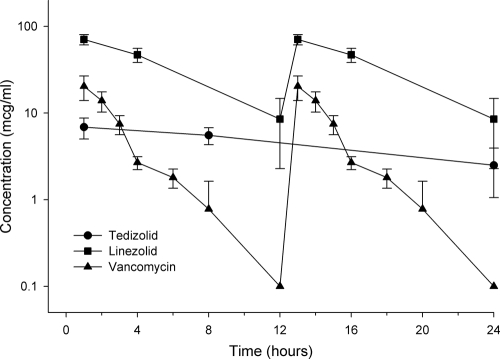
The pharmacokinetic profile for each antimicrobial in mouse ELF is displayed in Fig. 1. The mouse concentration profiles for tedizolid, LZD, and VAN replicated the AUC0–24 exposures in humans and also demonstrated peak concentrations comparable to that observed in humans (8, 16, 30). The relevant ELF exposures (AUC0–24) for tedizolid, LZD, and VAN are shown in Table 2.
Fig 1.
ELF concentration time course of 20 mg/kg tedizolid q24h (circle), 120 mg/kg LZD q12h (square), and 25 mg/kg VAN q12h (triangle) over 24 h in mice.
Table 2.
Comparative ELF exposures of tedizolid, linezolid, and vancomycin in human and mouse pneumonia models
| Compound | Human ELF AUC0-24 | Mouse ELF AUC0-24 | Mouse regimen |
|---|---|---|---|
| Tedizolid | 109 | 111 | 20 mg/kg q24h |
| LZD | 960 | 910 | 120 mg/kg q12h |
| VAN | 92 | 104 | 25 mg/kg q12h |
Comparative efficacy studies in the in vivo pneumonia model.
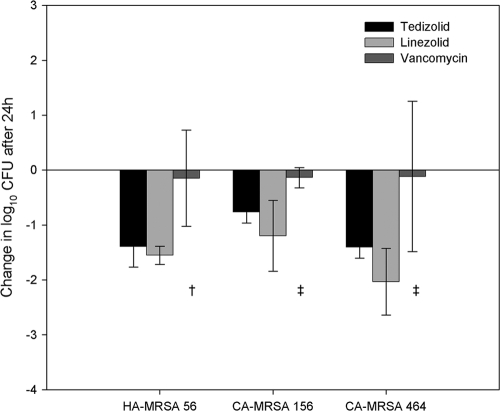
Studies to evaluate the efficacies of the above-described dosages of TZD, LZD, and VAN as ELF exposures against the three MRSA strains were conducted in immunocompetent mice. The change in log10 CFU/ml for each treatment against each of the MRSA isolates is graphically displayed in Fig. 2. At the start of therapy, mice had between 7.28 to 7.54 log10 CFU in lung tissues for all MRSA isolates. After 24 h, bacterial densities in lung tissues from control groups contained 8.41 to 8.59 log10 CFU, an increase of approximately 1 log. Tedizolid diminished CFU loads by 0.76 to 1.4 logs at 24 h. Likewise, the LZD regimen lowered bacterial density ranging between 1.2 and 2.0 logs. No statistical differences were found between the tedizolid and LZD against any of the three MRSA isolates (P > 0.05); however, both regimens resulted in a statistically significant reduction in bacterial density compared with that of the vehicle-dose controls (P ≤ 0.005). The VAN treatment was not nearly as efficacious; the bacterial density change averaged only slightly better than stasis, with an average decrease of 0.13 logs against the three MRSA isolates after 24 h. A statistical difference was found between the VAN regimens and control against two MRSA isolates (56 and 156; P = 0.012) but not the third, MRSA isolate 464 (P = 0.142). The variability around the average VAN CFU counts against S. aureus isolate 464 was large, possibly inhibiting detection of any divergence. Both tedizolid and LZD were more efficacious against MRSA isolate 56 versus VAN (P = 0.016 and 0.013, respectively). Additionally, the VAN regimen was statistically less efficacious than LZD against MRSA 156 (P = 0.026) and MRSA 464 (P = 0.023).
Fig 2.
Changes in bacterial density after 24 h for tedizolid (black bar)-, LZD (light-gray bar)-, and VAN (dark-gray bar)-treated groups (bar level represents average change in log10 CFU of group from initial density; error bars, ±1 SD). †, significantly different from tedizolid and LZD, P ≤ 0.016; ‡, significantly different from LZD, P ≤ 0.026.
Average survival following each treatment regimen and in control groups was monitored. With the VAN treatment, an average of 39% of the mice perished before the end of the 24-h treatment interval for the three S. aureus isolates, a value similar to the 32% mortality noted in untreated controls. The TZD and LZD treatments were entirely protective against the MRSA isolates 56 and 464 and only one and two animals infected with S. aureus isolate 156 expired during TZD and LZD treatment, respectively.
DISCUSSION
Staphylococcus aureus is a frequently identified etiologic agent in bacterial pneumonia (13, 39, 40). As the incidence of methicillin-resistant S. aureus strains is increasing, the severity of infection and health care costs have increased (13, 23, 27, 33, 34). While vancomycin remains a frequently used treatment for pneumonia caused by MRSA, clinical failures in this setting have been increasingly recognized (12, 31, 32). As such, additional treatment options appear necessary as MRSA isolates with reduced VAN susceptibilities (MIC, 2 μg/ml) are becoming more prevalent and may contribute to poorer clinical outcomes (22, 32, 41). In addition to reductions in potency, the relatively low penetration (∼50%) of vancomycin into ELF has also been suggested as a reason for the compound's diminished clinical efficacy (6, 22, 30, 35).
In a novel class of antimicrobial with which to treat MRSA pneumonia, LZD, an oxazolidinone, offers better human pharmacokinetics than VAN, and penetration of LZD into ELF is approximately 400% compared to that of blood (4, 8, 28). Moreover, unlike vancomycin, LZD does not require therapeutic monitoring to optimize drug exposures while minimizing its toxic potential. LZD is a commonly used alternative to VAN for the treatment of pneumonia due to S. aureus, including MRSA (19, 28, 39, 40). TZD, a next-generation oxazolidinone, has recently undergone clinical efficacy assessments for treatment of skin and soft-tissue infections, including MRSA (1, 11). Owing to its long half-life, excellent ELF penetration ratios, and potent in vitro activity, TZD is being considered a potential therapeutic agent for pneumonia caused by susceptible microorganisms, including MRSA (1, 16, 25, 26, 29).
The measurement of drug in the blood, while well established in the literature, may not always be indicative of concentrations at the site of infection, such as in central nervous system or respiratory infections. The concentration of drug at the site of infection must be assessed in order to better evaluate the antibacterial activity of a given antimicrobial and is a current requirement of the FDA (15, 24, 35). Many mouse studies have described the efficacy of antimicrobials based on the human simulated blood concentrations; however, few have simulated the in vivo exposure of humans at the site of infection (9, 18, 36).
In this study, the activities of TZD, LZD, and VAN were compared at the infection site at exposures in mice reproducing those found in human ELF. Our study also incorporated the effect of an intact immune system in mice, thus incorporating the proposed theory of increased granulocyte impact on bacterial kill as proposed for tedizolid (14).
The efficacy data from this study is consistent with that from another pneumonia study with tedizolid. In the dose-ranging study by Pichereau et al., tedizolid demonstrated greater efficacy than LZD in a neutropenic murine pneumonia model (26). The TZD and LZD doses which produced a 1-log net decrease against MRSA were approximately 10 and 60 mg/kg, respectively, against MRSA. In our current study, the LZD regimen needed to simulate the human ELF exposure was 12-fold higher than that of the TZD; however, the in vivo efficacies of the two compounds were comparable. Although the doses needed to produce approximately a 1-log decrease in bacterial density differed between the studies, this was likely due to the varied host conditions (i.e., neutropenic versus immunocompetent) and the resultant differences in antimicrobial exposures among these models (17).
Our study revealed a previously unidentified aspect of treatment outcomes. While drug ELF exposures were consistent with that in humans, this short-term efficacy trial revealed not only minimal reductions in bacterial load with VAN but also that a larger percentage of the mice perished before the 24-h treatment was completed; only the vehicle-dosed controls fared worse over this period. Conversely, 95% and 89% survived during TZD and LZD treatment, respectively.
In summary, the efficacy of TZD, a novel oxazolidinone antibiotic, was compared with linezolid and vancomycin against strains of MRSA in an in vivo pneumonia model using dosing regimens that simulated human exposure at the site of infection. Both TZD and LZD were effective in this rigorous pneumonia model, while VAN showed minimal efficacy and higher mortality compared to those of either TZD or LZD. These data support the use of LZD and TZD for the treatment of pneumonia due to MRSA and support the continued development of TZD for this indication.
ACKNOWLEDGMENTS
This study was sponsored by a grant from Trius Therapeutics, San Diego, CA.
Thanks are extended to Michael J. Rybak for providing MRSA 56 (no. 494). We acknowledge the superior assistance of Deborah Santini, Lindsay Tuttle, Jennifer Hull, Henry Christensen, Mary Banevicius, Christina Sutherland, Mao Hagihara, and Seth Housman in the performance of this study.
Footnotes
Published ahead of print 21 February 2012
REFERENCES
- 1. Bien P, De Anda C, Prokocimer P. 2010. Microbial efficacy of torezolid phosphate in patients with complicated skin and skin structure infections, abstr F-1592. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 2. Betriu C, et al. 2010. Comparative activities of TR-700 (torezolid) against staphylococcal blood isolates collected in Spain. Antimicrob. Agents Chemother. 54:2212–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boucher HW, Corey GR. 2008. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 46(Suppl. 5):S344–S349 [DOI] [PubMed] [Google Scholar]
- 4. Bozdogan B, Appelbaum PC. 2004. Oxazolidinones: activity, mode of action, and mechanism of resistance. Int. J. Antimicrob. Agents. 23:113–119 [DOI] [PubMed] [Google Scholar]
- 5. Brown SD, Traczewski MM. 2010. Comparative in vitro antimicrobial activities of torezolid (TR-700), the active moiety of a new oxazolidinone, torezolid phosphate (TR-701), determination of tentative disk diffusion interpretive criteria, and quality control ranges. Antimicrob. Agents Chemother. 54:2063–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Butterfield JM, et al. 2011. Refining vancomycin protein binding estimates: identification of clinical factors that influence protein binding. Antimicrob. Agents Chemother. 55:4277–4282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clinical and Laboratory Standards Institute 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, approved standards, eighth ed. CLSI document M07-A8. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 8. Conte JE, Jr, Golden JA, Kipps J, Zurlinden E. 2002. Intrapulmonary pharmacokinetics of linezolid. Antimicrob. Agents Chemother. 46:1475–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crandon JL, Kuti JL, Nicolau DP. 2010. Comparative efficacies of human simulated exposures of telavancin and vancomycin against methicillin-resistant Staphylococcus aureus with a range of vancomycin MICs in a murine pneumonia model. Antimicrob. Agents Chemother. 54:5115–5119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. da Silva Coimbra MV, et al. 2003. Clonal spread of methicillin-resistant Staphylococcus aureus in a large geographic area of the United States. J. Hosp. Infect. 53:103–110 [DOI] [PubMed] [Google Scholar]
- 11. De Anda C, Das A, Fang E, Prokocimer P. 2011. Acute bacterial skin and skin structure infection (ABSSI) dose-ranging phase 2 tedizolid phosphate (TR-701) study: assessment of efficacy with new FDA guidance, abstr L1-1496. Abstr. 51st Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 12. Deleo FR, Otto M, Kreiswirth BN, Chambers HF. 2010. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 375:1557–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diekema DJ, et al. 2001. Survey of infections due to Staphylococcus species: Frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin. Infect. Dis. 32(Suppl 2):S114–S132 [DOI] [PubMed] [Google Scholar]
- 14. Drusano GL, Liu W, Kulawy R, Louie A. 2011. Impact of granulocytes on the antimicrobial effect of tedizolid in a mouse thigh infection model. Antimicrob. Agents Chemother. 51:5300–5305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Food and Drug Administration U. S. Department of Health and Human Services 1998. Guidance for industry. Developing antimicrobial drugs—general considerations for clinical trials. Drug Information Branch, Division of Communications Management, Rockville, MD: http://www.fda.gov/cder/guidance/dft.pdf [Google Scholar]
- 16. Housman ST, et al. 2011. Pulmonary disposition of tedizolid following once-daily oral 200 mg tedizolid phosphate in healthy adult volunteers. abstr A1-1747. Abstr. 51st Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Keel RA, Crandon JL, Nicolau DP. 2011. Pharmacokinetics and pulmonary disposition of tedizolid and linezolid in three murine models, abstr A1-1746. Abstr. 51st Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 18. Kim A, Banevicius MA, Nicolau DP. 2008. In vivo pharmacodynamic profiling of doripenem against Pseudomonas aeruginosa by simulating human exposures. Antimicrob. Agents Chemother. 52:2497–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kollef MH, Rello J, Cammarata SK, Croos-Dabrera RV, Wunderink RG. 2004. Clinical cure and survival in Gram-positive ventilator-associated pneumonia: retrospective analysis of two double-blind studies comparing linezolid with vancomycin. Int. Care Med. 30:388–394 [DOI] [PubMed] [Google Scholar]
- 20. Koomanachai P, Crandon JL, Banevicius MA, Peng L, Nicolau DP. 2009. Pharmacodynamic profile of tigecycline against methicillin-resistant Staphylococcus aureus in an experimental pneumonia model. Antimicrob. Agents Chemother. 53:5060–5063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laohavaleeson S, Tessier PR, Nicolau DP. 2008. Pharmacodynamic characterization of ceftobiprole in experimental pneumonia caused by phenotypically diverse Staphylococcus aureus strains. Antimicrob. Agents Chemother. 52:2389–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lodise TP, et al. 2011. Penetration of vancomycin into epithelial lining fluid in healthy volunteers. Antimicrob. Agents Chemother. 55:5507–5511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moran GJ, et al. 2006. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355:666–674 [DOI] [PubMed] [Google Scholar]
- 24. Müller M, de la Peña A, Derendorf H. 2004. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: distribution in tissue. Antimicrob. Agents Chemother. 48:1441–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Muñoz KA, et al. 2010. Improved pharmacokinetics of the novel oxazolidinone antibiotic torezolid phosphate compared to linezolid in healthy subjects, abstr P-1594. Abstr. 50th Annu. Intersci. Conf. Antimicrob. Agents Chemother., Boston, MA, 12 to 15 September, 2010 [Google Scholar]
- 26. Pichereau S, et al. 2009. Comparative pharmacodynamics of novel oxazolidinone, torezolid phosphate (TR-701), against S. aureus in the neutropenic murine pneumonia model, abstr A1-1939. Abstr. 49th Instersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA, 12 to 15 September 2009 [Google Scholar]
- 27. Pichereau S, Rose WE. 2010. Invasive community-associated MRSA infections: epidemiology and antimicrobial management. Expert Opin. Pharmacother. 11:3009–3025 [DOI] [PubMed] [Google Scholar]
- 28. Pletz MW, Burkhardt O, Welte T. 2010. Nosocomial methicillin-resistant Staphylococcus aureus (MRSA) pneumonia: linezolid or vancomycin? Comparison of pharmacology and clinical efficacy. Eur. J. Med. Res. 15:507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prokocimer P, et al. 2011. Phase 2, randomized, double-blind, dose-ranging study evaluating the safety, tolerability, population pharmacokinetics, and efficacy of oral torezolid phosphate in patients with complicated skin and skin structure infections. Antimicrob. Agents Chemother. 55:583–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rodvold KA, Gotfrield MH, Loutit JS, Porter SB. 2004. Plasma and intrapulmonary concentrations of oritavancin and vancomycin in normal healthy adults, abstr O254. Clin. Microbiol. Infect. 10(Suppl 3):44 [Google Scholar]
- 31. Sakoulas G, Moellering RC., Jr 2008. Increasing antibiotic resistance among methicillin-resistant Staphylococcus aureus strains. Clin. Infect. Dis. 46:S360–S367 [DOI] [PubMed] [Google Scholar]
- 32. Salerno D, Vahid B, Marik RE. 2007. Methicillin-resistant Staphylococcus aureus pneumonia after thoracic surgery: successful treatment with linezolid after failed vancomycin therapy. Ann. Thorac. Surg. 83:1888–1891 [DOI] [PubMed] [Google Scholar]
- 33. Shorr AF, et al. 2010. Clinical and economic outcomes for patients with health care-associated Staphylococcus aureus pneumonia. J. Clin. Microbiol. 48:3258–3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shorr AF, Combes A, Kollef MH, Chastre J. 2006. Methicillin-resistant Staphylococcus aureus prolongs intensive care unit stay in ventilator-associated pneumonia, despite initially appropriate antibiotic therapy. Crit. Care Med. 34:700–706 [DOI] [PubMed] [Google Scholar]
- 35. Stein GE, Wells EM. 2010. The importance of tissue penetration in achieving successful antimicrobial treatment of nosocomial pneumonia and complicated skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus: vancomycin and linezolid. Curr. Med. Res. Opin. 26:571–588 [DOI] [PubMed] [Google Scholar]
- 36. Sugihara K, et al. 2010. In Vivo pharmacodynamic activity of tomepenem (formerly CS-023) against Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus in a murine thigh infection model. Antimicrob. Agents and Chemother. 54:5298–5302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tobin CM, Sunderland J, White LO, MacGowan AP. 2001. A simple, isocratic high-performance liquid chromatography assay for linezolid in human serum. J. Antimicrob. Chemother. 48:605–608 [DOI] [PubMed] [Google Scholar]
- 38. Trius 15 March 2010. Investigator's brochure. TR-701 FA for oral and IV administration, 4th ed Trius Therapeutics, Inc., San Diego, CA [Google Scholar]
- 39. Wunderink RG, Mendelson MH, Fabian TC, May AK, Bhattacharyya Leeper HKV, Solomkin JS. 2008. Early microbiological response to linezolid vs vancomycin in ventilator-associated pneumonia due to methicillin-resistant Staphylococcus aureus. Chest 134:1200–1207 [DOI] [PubMed] [Google Scholar]
- 40. Wunderink RG, Rello J, Cammarator SK, Croos-Dabrera RV, Kollef MH. 2003. Linezolid vs. vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest 124:1789–1797 [PubMed] [Google Scholar]
- 41. Yoon YK, Kim JY, Park DW, Sohn JW, Kim MJ. 2010. Predictors of persistent methicillin-resistant Staphylococcus aureus bacteraemia in patients treated with vancomycin. J. Antimicrob. Chemother. 65:1015–1018 [DOI] [PubMed] [Google Scholar]
- 42. Ziglam HM, Baldwin DR, Daniels I, Andrew JM, Finch RG. 2002. Rifampicin concentrations in bronchial mucosa, epithelial lining fluid, alveolar macrophages and serum following a single 600 mg oral dose in patients undergoing fibre-optic bronchoscopy. J. Antimicrob. Chemother. 50:1011–1015 [DOI] [PubMed] [Google Scholar]