Abstract
This study assessed the pulmonary disposition of tedizolid, an oxazolidinone, in adult volunteers receiving 200 mg of the prodrug tedizolid phosphate orally every 24 h for 3 days to steady state. Plasma samples were collected over the dosing interval, and participants were randomized to undergo bronchoalveolar lavage (BAL) at 2, 6, 12, or 24 h after the last dose. Drug concentrations in plasma, BAL fluid, and alveolar macrophages (AM) were determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS), and the urea correction method was used to calculate epithelial lining fluid (ELF) concentrations. Pharmacokinetic parameters were estimated by noncompartmental methods followed by compartmental population pharmacokinetics. Penetration was calculated as the area under the concentration-time curve during the dosing interval (AUC0–24) for ELF and AM relative to the free AUC0–24 (fAUC0–24) in plasma. The half-life and volume of distribution in plasma were 9.23 ± 2.04 h and 108.25 ± 20.53 liters (means ± standard deviations), respectively. Total AUC0–24 in plasma was 25.13 ± 5.78 μg · h/ml. Protein binding was 89.44% ± 1.58%, resulting in a mean fAUC0–24 of 2.65 ± 0.72 μg · h/ml in plasma. Mean concentrations (μg/ml) at 2, 6, 12, and 24 h were 9.05 ± 3.83, 4.45 ± 2.18, 5.62 ± 1.99, and 1.33 ± 0.59 in ELF and 3.67 ± 1.02, 4.38 ± 2.18, 1.42 ± 0.63, and 1.04 ± 0.52 in AM. ELF and AM penetration ratios were 41.2 and 20.0. The mean ELF penetration ratio after population analyses was 39.7. This study demonstrates that tedizolid penetrates into ELF and AM to levels approximately 40-fold and 20-fold, respectively, higher than free-drug exposures in plasma.
INTRODUCTION
Staphylococcus aureus and Streptococcus pneumoniae are the most common causes of hospital-acquired and community-acquired bacterial pneumonia, respectively (9, 10). Resistance to currently available antibiotics can be substantial, particularly for S. aureus, as methicillin resistance has been reported in upwards of 60% of S. aureus isolates (13). With respect to methicillin-resistant S. aureus (MRSA), few antibiotics are available to treat pneumonia caused by this organism. Vancomycin and linezolid are recommended as first-line therapy by recent guidelines (16). These agents are not without limitations, including nephrotoxicity and the requirement for therapeutic drug monitoring for vancomycin and myelosuppression for linezolid (29, 32). Newer agents, including telavancin and ceftaroline, are not yet approved for MRSA pneumonia.
Tedizolid phosphate free acid (TR-701 FA) is the prodrug of tedizolid (TR-700), an oxazolidinone active against many common respiratory Gram-positive bacteria, including methicillin-susceptible and -resistant S. aureus and S. pneumoniae (11, 17, 31). MICs for MRSA and pneumococcus are approximately 8-fold lower than those of linezolid (11). Because of this spectrum of activity and near-equivalent oral and intravenous (i.v.) bioavailability (2), tedizolid phosphate is being developed as i.v. and oral agents for the treatment of pneumonia. Murine pneumonia experiment results further support the development of this drug. Against S. pneumoniae, tedizolid achieved bacteriostatic and 1-log CFU killing at a dose 4.6- to 5.5-fold lower than the linezolid dose needed to achieve the same level of bacterial reductions (4). Another murine pharmacodynamic study demonstrated that ratios of the free area under the curve (fAUC) to MIC of 10 and 25 in plasma were required for bacteriostasis and a 1-log CFU killing against S. aureus isolates, respectively (22). These pharmacodynamic exposure targets were similar for linezolid and tedizolid.
While murine infection model data provide insight into the possibility of tedizolid phosphate as a potential agent for the treatment of pulmonary infections, there are currently no data describing the extent of penetration into the site of infection. For pneumonia, the epithelial lining fluid (ELF) is presumed to be the site of infection for extracellular organisms such as S. aureus and S. pneumoniae. In contrast, alveolar cellular space is thought to be the site for infections caused by intracellular pathogens and can be estimated by measuring penetration into the alveolar macrophages.
MATERIALS AND METHODS
Study design.
This was a prospective, open-label, multiple-dose pharmacokinetic study which took place at the Clinical Research Center and Same Day Surgi-Center at Hartford Hospital, Hartford CT. The protocol was approved by the Hartford Hospital Institutional Review Board, and all participants provided written informed consent prior to screening.
Participants.
Twenty healthy adult volunteer participants were included in the study. The inclusion criteria were as follows: males or nonpregnant, nonlactating females, 18 to 55 years of age, with a body mass index (BMI) between 20 to 34.9 kg/m2, who were considered healthy with no significant underlying medical or surgical history. A screening evaluation performed within 28 days of study drug administration consisted of a detailed medical and surgical history, physical examination, and clinical laboratory testing. Participants underwent clinical laboratory testing to confirm no changes from the screening visit on the day prior to study drug administration as well as at the end of the study period.
Participants were excluded from the study for any of the following reasons: evidence of clinically significant disease or illness, allergy to tedizolid phosphate, linezolid or their components, allergy to lidocaine, midazolam, or other anesthetics of similar classes, evidence or history of clinically significant medical abnormalities on physical examination, predefined abnormal laboratory values of chemistry, liver panel, or complete blood counts, history of regular alcohol consumption exceeding 7 drinks/week for females and 14 drinks/week for males, use of tobacco or nicotine-containing products within 6 months prior to admittance to the study center, use of prescription or nonprescription drugs, vitamins, and dietary supplements within 14 days of first dose of study drug with the exception of acetaminophen at doses less than or equal to 1 g/day, the use of any investigational drug within 30 days or 5 half-lives, whichever was longer, and previous enrollment in any tedizolid phosphate trial.
Study medication.
Tedizolid phosphate 200-mg tablets were supplied by Trius Therapeutics, Inc. (San Diego, CA). Medications were stored at room temperature according to the manufacturer's recommendations until administration.
Dosing and plasma sampling.
Participants received 200 mg tedizolid phosphate with 120 ml water every 24 h for 3 days to achieve steady state. Participants were required to fast for 4 h prior to and 2 h after study drug administration and 1 h prior to and after administration with regard to water. Blood samples were collected on day 3 from a peripheral i.v. catheter at 0 h (immediately before drug administration) and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, and 24 h after study drug administration for plasma drug concentration determination. Blood samples were collected in a 10 ml Monoject tube (Tyco Healthcare Group LP, Mansfield, MA) containing 15% K3 EDTA. Blood for urea analysis was also collected simultaneously with the bronchoscopy and bronchoalveolar lavage (BAL) procedure. Blood samples were centrifuged at 2,000 × g for 10 min at 4°C within 30 min of collection to obtain the separated plasma and stored at −80°C until further analysis.
Protein binding assessment.
Protein binding was assessed in triplicate at 1 h after dose administration on day 3. In previous studies, protein binding has been shown to be independent of concentration over the range of therapeutic exposures (unpublished data). Blood samples were collected in a 10-ml Monoject tube containing 15% K3 EDTA and centrifuged at 2,000 × g for 10 min at 4°C to obtain separated plasma. Aliquots of 0.9 ml of plasma were transferred into three ultrafiltration devices (Centrifree centrifugal filters; Millipore Corporation, Billerica, MA) with a molecular mass cutoff of 30 kDa and centrifuged at 2,000 × g using a fixed-angle rotor for 45 min at 10°C to obtain the ultrafiltrate and stored frozen at −80°C until analysis. Nonspecific protein binding was negligible when these ultrafiltration devices were used (K. Bartizal, Trius Therapeutics, Inc.], personal communication). Protein binding was calculated using the following equation: % protein binding = 100 − (100 × Cultrafiltrate/Cplasma), where Cultrafiltrate is the concentration of the ultrafiltrate and Cplasma is the total concentration of tedizolid at 1 h.
Bronchoscopy and BAL.
Participants were randomized to undergo a single bronchoscopy and BAL at 2, 6, 12, or 24 h (5 participants per time point) after the third dose of study drug. Participants fasted for 6 h prior to the procedure and were then administered 4% aerosolized lidocaine in the nares and oropharynx and 2% lidocaine jelly in the nasal passageway within 30 min of bronchoscopy. A fiber optic bronchoscope (BF-Q180; Olympus America Inc., Center Valley, PA) was inserted into the medial segment of the right middle lobe of the lung. Four aliquots of 50 ml 0.9% sodium chloride was instilled and immediately aspirated individually via the bronchoscope. The first sample of collected BAL fluid was discarded to prevent contamination with larger cell particles and lidocaine, and the remaining three aspirate volumes were recorded and pooled. Two 4-ml samples were taken from the pooled sample for complete cell count with differential, while the remaining fluid was placed on ice for processing. BAL fluid was centrifuged at 400 × g at 4°C for 10 min to separate the supernatant and the cell pellet. A sample of the supernatant was collected for urea concentration determination. The cell pellet was resuspended at a volume equal to 5% of the total pooled BAL volume. The supernatant and cell pellet were then stored at −80°C until further analysis.
Analytical concentration determination.
Plasma, BAL fluid supernatant, and BAL fluid cell pellet samples were assayed at Covance, Inc. (Madison, WI), for tedizolid concentrations by validated liquid chromatography with tandem mass spectrometric detection (LC-MS/MS). Tedizolid phosphate concentrations were not determined in this study.
Plasma.
The method for plasma tedizolid concentration determination was recently published (24). The standard curve range for tedizolid was 5 to 1,000 ng/ml. Quality control (QC) samples were prepared and analyzed at 15, 150, and 750 ng/ml.
BAL fluid supernatant and alveolar cells.
The methodology for tedizolid concentration determination in the BAL fluid supernatant and alveolar cells is described below. The standard curve range for tedizolid in BAL fluid supernatant and alveolar cells was 10 to 1,000 ng/ml, and the QC samples were prepared and analyzed at 30, 150, and 750 ng/ml. Human BAL fluid was obtained from commercial sources to obtain BAL supernatant. The BAL fluid was centrifuged at 400 × g for 10 min at room temperature to obtain the BAL fluid supernatant and alveolar cell pellet. BAL fluid supernatant was used for all calibration standards, QC validations, and analyses of BAL fluid supernatant samples. The BAL fluid supernatant was spiked with an appropriate concentration of tedizolid for preparation of calibration standards and QC samples. A 20-μl internal standard of stable labeled tedizolid obtained from Kalexysn, Inc. (50 ng/ml), was added to a 20-μl sample aliquot. The sample was mixed for 2 min and then centrifuged at 1,600 × g for 5 min. A 500-μl sample of acetonitrile-water (50:50, vol/vol) was added to a 96-well collection plate to which 50 μl of supernatant was added and mixed for 2 min. The extract was chromatographed on a Hypersil Gold aQ column (50 by 3 mm, 5-μm particle size; Thermo Scientific) coupled with a column heater set at 40°C. The mobile phases were 55:45 (vol/vol) 20 mM ammonium formate in water (pH 9.0 ± 0.2) (mobile phase A) and methanol (mobile phase B) and were run in an isocratic condition. The liquid chromatography system consisted of a solvent delivery system (LC-20AD; Shimadzu) and autoinjector (SIL-20AC, Prominence; Shimadzu) interfaced with a Sciex API 4000 triple-quadrupole mass spectrometer operated in the multiple-reaction-monitoring, positive-ion mode, and this system monitored the transition ions m/z 371 → 343 for tedizolid and 375 → 347 for stable labeled tedizolid, respectively. The range of the BAL supernatant standard curve was 10 to 1,000 ng/ml and was linear (r2 ≥ 0.9994) over the concentration range with QC sample concentrations of 30, 150, and 750 ng/ml. Intraday assay accuracy values for the QC samples ranged from 94.0 to 98.4%, with precision (coefficient of variation [CV]) values between 0.8 and 4.6%. Interday assay accuracy values for the QC samples ranged from 95.3 to 96.3% with precision values between 1.5 and 3.3%.
A surrogate matrix of 0.9% sodium chloride was used for the alveolar cells. The procedure for analytical concentration determination in the alveolar cells was the same as mentioned above. The tested range was 10 to 1,000 ng/ml. The assay was linear (r2 ≥ 0.9994) over the concentration range with QC sample concentrations of 30, 150, and 750 ng/ml. Intraday accuracy values for the QC samples ranged from 102.7 to 105.5%, with precision values between 0.8 to 2.8%. Interassay accuracy ranged from 103.3 to 104.9%, with precision values ranging from 1.2 to 1.9%.
Urea concentration determination.
The urea concentrations in serum and BAL fluid collected simultaneously at the time of bronchoscopy were analyzed by a colorimetric enzymatic assay (Teco Diagnostics, Anaheim, CA) by a spectrophotometer detection method (Cary 50 series; Varian, Walnut Creek, CA). The assay was linear (r2 ≥ 0.9999) for the urea concentrations in both BAL fluid and serum over the range of 0.1 to 2 mg/dl. The intraday and interday variability of the QC samples (0.15 and 1.5 mg/dl in both matrixes) was <5%.
Calculation of drug concentrations in ELF and AM.
The volume of ELF within the BAL fluid was calculated by the urea dilution method (25) as previously described by our group (3, 21). The number of alveolar macrophages (AM) within the BAL fluid was determined from the mean proportion present in the two manual cell counts, and the total volume of these cells in the cell pellet was calculated by using a mean AM volume of 2.42 μl/106 cells.
Pharmacokinetic analyses.
The plasma pharmacokinetics of tedizolid were estimated for each participant by noncompartmental methods (WinNonlin 5.3; Pharsight Corporation, Mountain View, CA) and compartmental population methods (see below). For noncompartmental analyses, the area under the concentration-time curve from time zero to the end of the dosing interval (AUC0–24) was calculated by use of the linear-log trapezoidal rule. The elimination rate constant (λz) was estimated as the slope of the best-fit linear regression line for the concentrations collected after the maximum tedizolid concentration during the dosing interval (Cmax). The time to maximum drug concentrations (Tmax) and the lowest concentration after the Cmax (Cmin) were both observed from the plotted concentration-time profile for each participant. The half-life for each participant was estimated as ln2/λz. Clearance (CL/F) was estimated as the dose/AUC0–24. Volume of distribution at steady state (VSS/F) was estimated using the mean residence time extrapolated to infinity multiplied by the clearance. ELF and AM AUC0–24 as well as the half-life of each pulmonary compartment were calculated using the mean concentrations for the five participants at each time point. Drug penetration was estimated by the ratio of the AUC0–24 for ELF or AM to the mean free AUC0–24 (fAUC0–24) in plasma for the population.
Population pharmacokinetic modeling.
All plasma and ELF samples were comodeled in a population sense using the nonparametric grid (NPAG) program with adaptive γ of Leary et al. (14). Multiple models were evaluated and were discriminated employing the Akaike information criterion (33) and the likelihood ratio test. Weighting started with identifying the estimate of variance for an observation using the intraday coefficient of variation of the assay for both plasma and BAL fluid supernatant. A run was performed first with adaptive γ activated. This value of γ was then used to multiply the original estimates from the assay to provide an approximation to a homoscedastic weighting scheme. These weights were then employed without further use of adaptive γ. The final model was a three-compartment model (absorption, central, and ELF compartments) with a lag time. The mean weighted error was the estimate of bias. The bias-adjusted mean weighted squared error was employed as the estimate of precision.
Monte Carlo simulation.
The ADAPT II package of D'Argenio and Schumitzky (7) was employed to estimate the mean penetration into the ELF and variability of the penetration estimates. Both normal and log-normal distributions were evaluated and discriminated by the fidelity with which the original point estimates of the mean and variance were recapitulated. In all instances, concentration profiles in plasma and ELF are for 9,999 subjects. The AUC0–24 was estimated in each compartment after simulation. Plasma AUC was corrected by the mean free fraction determined for these 20 participants prior to calculation of penetration ratios. Statistical analysis was performed with the SYSTAT for Windows package, v11.
Safety assessment.
The safety and tolerability of tedizolid phosphate were monitored by recording adverse events that occurred during the study. Each study participant went through an exit evaluation which involved a physical examination and clinical laboratory testing upon exiting the Clinical Research Center.
RESULTS
Participants.
Twenty participants were enrolled and completed the study. Of these participants, 17 were male; 15 were Caucasian and 5 were African American. Four of the 20 participants were of Hispanic ethnicity. Their ages ranged from 20 to 50 years, with a mean of 28 ± 9 years. The weight and BMI (mean ± standard deviation [SD]) were 82.4 ± 12.5 kg and 26.7 ± 3.3 kg/m2, respectively.
Plasma pharmacokinetics and protein binding.
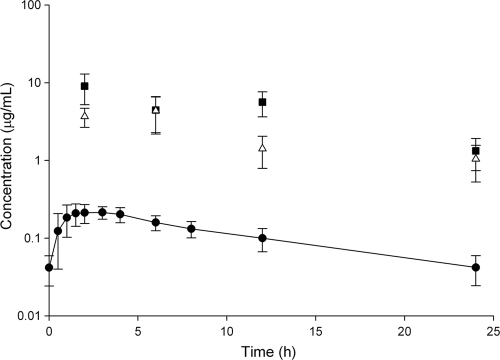
The plasma pharmacokinetics of tedizolid are listed in Table 1 and are compared with noncompartmental estimates reported elsewhere. Drug concentrations taken immediately prior to the third dose and at 24 h after the third dose were not statistically different (P = 0.947), indicating that concentrations were at steady state. Protein binding ranged from 86.1% to 91.9%, with a mean of 89.44 ± 1.58%. During the dosing interval, this resulted in a steady state fAUC0–24 of 2.65 ± 0.72 μg · h/ml. The mean concentration-time profile of free drug is depicted in Fig. 1.
Table 1.
Plasma pharmacokinetics of oral tedizolid phosphatea
| Data sourceb | Cmax (μg/ml) | Tmax (h) | Total drug AUC (μg · h/ml) | VSS/F (liters) | CL/F (liters/h) | t1/2 (h) |
|---|---|---|---|---|---|---|
| BAL study (day 3) | 2.4 (0.4) | 2 (0.5–4) | 25.1 (5.8) | 108 (21) | 8.4 (2.2) | 9.2 (2.0) |
| Bien (single dose) (1) | 2.0 (0.4) | 3 (1–4) | 25.4 (4.6) | 128 (31)c | 8.1 (1.5) | 11.2 (3.6) |
| Prokocimer (multiple dose, day 1) (23) | 1.8 (1.2) | 3 (1.5–4) | 21.6 (6.5) | 155 (29)c | 10.0 (2.8) | 11.1 (1.2) |
| Prokocimer (multiple dose, steady state) (23) | 1.8 (0.4) | 3 (2–4) | 22.5 (6.5) | 143 (51)c | 9.5 (2.7) | 10.2 (2.0) |
Data are means (SD) except Tmax, which are means (range). Cmax, maximum drug concentration; Tmax, time to Cmax; AUC, area under the curve from time zero to infinity (for single dose) or for the 0- to 24-h dosing interval (for multiple doses); VSS/F, volume of distribution at steady-state; CL/F, total body clearance; t1/2, elimination half-life.
Drug for the BAL was 200 mg tedizolid phosphate (containing 164 mg of the active compound, tedizolid); drug for the other studies was 200 mg tedizolid phosphate disodium (containing 150 mg of tedizolid).
VZ/F (volume of distribution).
Fig 1.
Concentration-time profiles (mean ± SD) for tedizolid in free plasma (circles), ELF (squares), and AM (triangles) from 20 healthy adult participants. Each ELF and AM point represents the mean of 5 individual participants randomly assigned to sampling at that time.
Pulmonary pharmacokinetics.
Each bronchoscopy and bronchoalveolar lavage procedure was started within a 3-min window of the scheduled time, and the time required for the collection of all four samples of BAL fluid was 4 ± 1 min (mean ± SD). The mean cell counts, % of AM, volume of ELF, and volume of aspirated BAL fluid were not statistically different between BAL time points (Table 2). The mean ELF, AM, and plasma drug concentrations at each BAL time point are listed in Table 3. Of note, some AM concentrations toward the end of the dosing period were below the lower limit of quantification (LLQ). For AM concentrations in which an identifiable peak was present on the assay chromatogram, 1/2 the LLQ (5 ng/ml) was used in all calculated concentrations. The calculated AUC0-24 values for ELF and AM were 109.3 and 52.95 μg · h/ml, respectively. The estimated half-lives of tedizolid in the ELF and AM based on mean concentrations at each time point were 8.8 and 10.5 h, respectively. The ratio of penetration into the ELF and AM using the AUC0-24 in the respective compartment compared with the fAUC0-24 in plasma was approximately 40 and 20, respectively.
Table 2.
Characteristics of BAL fluid, ELF, and AM cell recoverya
| Time (h) after BAL | Cell count (cells/ml) | AM (%) | ELF vol (ml) | BAL vol (ml) |
|---|---|---|---|---|
| 2 | 3.22E + 05 (1.68E + 05) | 83.5 (13.3) | 1.65 (0.87) | 81.8 (12.6) |
| 6 | 2.29E + 05 (8.70E + 04) | 81.5 (18.9) | 2.46 (0.86) | 82.4 (18.3) |
| 12 | 1.26E + 05 (4.23E + 04) | 83.4 (12.4) | 1.30 (0.34) | 101.1 (19.9) |
| 24 | 2.02E + 05 (8.32E + 04) | 89.0 (6.1) | 2.53 (1.11) | 91.4 (17.6) |
Data are means (SD).
Table 3.
ELF, AM, and plasma concentrations at each BAL time point
| Time (h) after BAL | Concn (μg/ml)a in: |
|||
|---|---|---|---|---|
| ELF | AM | Total plasma | Free plasmab | |
| 2 | 9.05 (3.83) | 3.67 (1.02) | 2.01 (0.55) | 0.213 (0.058) |
| 6 | 4.45 (2.18) | 4.38 (2.18) | 1.51 (0.33) | 0.159 (0.035) |
| 12 | 5.62 (1.99) | 1.42 (0.63) | 0.946 (0.31) | 0.100 (0.033) |
| 24 | 1.33 (0.59) | 1.04 (0.52) | 0.398 (0.17) | 0.042 (0.018) |
Data are means (SD).
Calculated using each participant's derived free fraction.
Population modeling of ELF penetration.
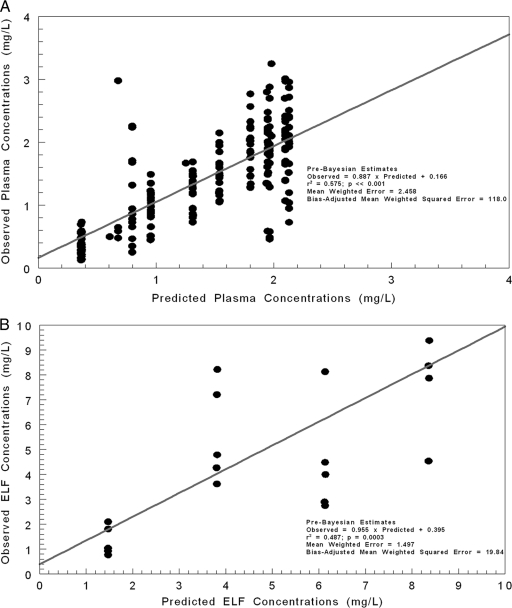
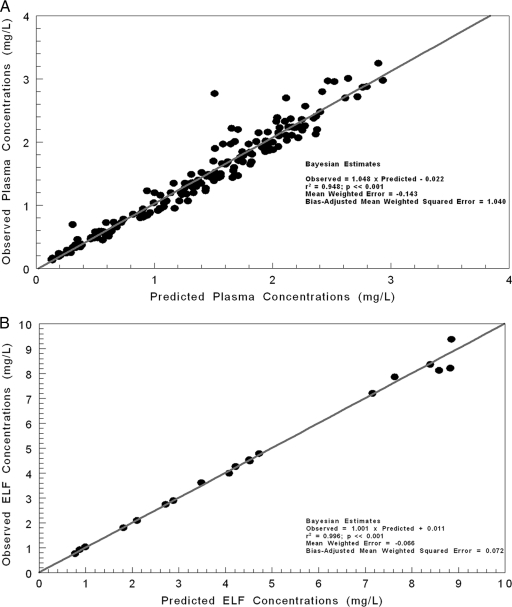
In Fig. 2, the predicted-observed plots are shown with regression for the pre-Bayesian analysis (using median parameter vector) for plasma (Fig. 2A) and ELF (Fig. 2B). The overall amount of the variance explained by the regression (r2) was 0.575 for plasma and 0.487 for ELF. Measures of bias and precision were acceptable for a pre-Bayesian analysis. In Fig. 3A and B, the Bayesian regressions are presented for the plasma and ELF output. The Bayesian analysis demonstrated slopes near 1.0 with small y intercepts, a large r2, and excellent bias and precision.
Fig 2.
Predicted-observed plots using the mean parameter vector (pre-Bayesian step) for plasma concentrations (A) and ELF concentrations (B).
Fig 3.
Predicted-observed plots using Bayesian parameter estimates for plasma concentrations (A) and ELF concentrations (B).
Table 4 provides the means, medians, and standard deviations for the model parameters. It is important to note that the estimate of drug clearance was highly concordant between the model-based analysis and the noncompartmental analysis, as it is with the population analysis in the work by Prokocimer et al. (23). Incorporation of a lag time provided a substantial difference in the log likelihood and was therefore retained in the model.
Table 4.
Parameter values from the population analysisa
| Calculation | Vc (liters) | CL (liters/h) | KC-ELF (h−1) | KELF-C (h−1) | VELF (liters) | Ka (h−1) | Tlag (h) |
|---|---|---|---|---|---|---|---|
| Mean | 51.65 | 8.46 | 15.67 | 16.08 | 15.06 | 4.86 | 0.52 |
| Median | 56.80 | 7.96 | 13.70 | 17.94 | 10.93 | 3.15 | 0.44 |
| SD | 13.45 | 2.15 | 4.80 | 5.40 | 8.90 | 4.28 | 0.35 |
Vc, volume of central compartment; CL, total clearance; KC-ELF, transfer rate constant between central and ELF compartments; KELF-C, transfer rate constant between ELF and central compartmenta; VELF, volume of ELF compartment (an estimate used as a scaler to correct the observed concentration to an amount during the population modeling); Ka, absorption rate constant; Tlag, lag time.
Monte Carlo simulation.
The Monte Carlo simulation was performed with a log-normal distribution (Table 5). The mean penetration ratio into ELF after Monte Carlo simulation was 39.7 and was almost identical to the estimate derived from the noncompartmental analysis and the composite ELF concentration profile. The median penetration ratio was 36.3, suggesting little variability in penetration into the ELF compartment. The lower 5% of the distribution still had an ELF penetration of 23.9-fold and an ELF AUC0–24 of 40.9 μg · h/ml.
Table 5.
ELF penetration and AUC0-24 values in plasma and ELF after 9,999-subject Monte Carlo simulation
| Calculation | Penetration ratio | AUC0-24 |
|
|---|---|---|---|
| Free plasmaa | ELF | ||
| Mean | 39.7 | 2.67 | 106.0 |
| Median | 36.3 | 2.59 | 93.9 |
| SD | 82.3 | 0.68 | 55.9 |
| Percentile of the distribution | |||
| 5th | 23.9 | 1.71 | 40.9 |
| 10th | 26.3 | 1.87 | 49.3 |
| 25th | 30.6 | 2.19 | 66.9 |
| 50th | 36.3 | 2.59 | 93.9 |
| 75th | 42.8 | 3.07 | 131.4 |
| 90th | 49.7 | 3.57 | 177.7 |
| 95th | 54.6 | 3.90 | 213.0 |
The plasma AUC is corrected for mean protein binding (89.44%).
Safety and tolerability.
Overall, tedizolid phosphate was well tolerated by all participants, with no serious adverse events reported. The most commonly reported adverse events possibly related to tedizolid phosphate were mild and included bradycardia (n = 2), headache (n = 1), and nausea (n = 1). The two volunteers with bradycardia experienced asymptomatic resting heart rates of 46 to 59 beats per minute 30 to 60 min after study drug administration on day 1. Resting heart rate for the two participants returned to baseline. Adverse events unrelated to the study medication included inflammation at the i.v. blood collection site (n = 1), a syncopal episode during blood collection (n = 1), and sore throat (n = 1). Upon discharge from the Clinical Research Center, two participants experienced elevations in white blood cell count that returned to baseline upon repeating the laboratory testing.
DISCUSSION
In this study, we analyzed the pulmonary disposition and pharmacokinetics of tedizolid after administration of oral tedizolid phosphate in healthy adults. Steady-state plasma pharmacokinetics in this study were similar to that found in previously reported data from healthy participants. We observed ELF and AM concentrations that were higher than free plasma concentrations over the entire dosing interval, suggesting extensive penetration into both the extracellular (i.e., ELF) and intracellular (i.e., AM) pulmonary compartments. Population pharmacokinetic estimations yielded similar estimates for clearance as that for the noncompartmental analyses, thus suggesting the robustness of the model and internal consistency. Relative to free drug exposures in plasma, tedizolid penetration ratios were approximately 40-fold in the ELF and 20-fold in alveolar macrophages. Mean penetration into the ELF was consistent between the noncompartmental and population model methods.
The observed high penetration of tedizolid into the ELF may be facilitated by passive diffusion across the alveolar capillary wall, active transport by macrophages or other cells, or additional undefined mechanisms (12), as the exact mechanism of penetration is unknown. Relative to linezolid, the only other oxazolidinone with comparable data (6), tedizolid penetration ratios were higher in both ELF and AM. After correction for protein binding (∼30%), linezolid penetration ratios into ELF and AM were 5.2 and 0.2, respectively (6). The mean ELF AUC0–24 for linezolid was 960 μg · h/ml, compared with 109.3 μg · h/ml for tedizolid in this study. Given that the reported MIC90s against methicillin-susceptible S. aureus (MSSA), MRSA, and community-acquired MRSA are 0.5 μg/ml for tedizolid and 4 μg/ml for linezolid, the AUC0-24/MIC90 achieved in ELF would be 219 for tedizolid and 240 for linezolid (6, 30).
In contrast, the AM AUC0-24 for linezolid was 40.8, compared with 52.95 for tedizolid, resulting in AUC0-24/MIC90 ratios of 10.2 and 106 for linezolid and tedizolid, respectively. Tedizolid's increased penetration into AM compared with linezolid has been observed previously with human macrophages and human endothelial cells, in which tedizolid was 5- to 10-fold more potent than linezolid intracellularly when used at the same weight concentration (15). Given the high AM concentrations, it is also possible that these cells carry tedizolid into the ELF to some extent.
We also employed a population pharmacokinetic analysis of the ELF data followed by a Monte Carlo simulation to determine the variability in penetration ratio estimates. The results of the population pharmacokinetics and the subsequent Monte Carlo simulations are remarkably similar to those of the noncompartmental analysis. Of equal importance, the between-subject variance was remarkably small, in contrast to previous ELF drug penetration analyses with healthy volunteers (8, 18, 19). Previous pulmonary disposition studies incorporating population pharmacokinetic analyses have reported considerable variability among penetration ratios. Ratios of vancomycin penetration into the ELF between 0.160 and 1.398 have been reported for the 10th and 90th percentiles, respectively (18). Further, levofloxacin population analyses have reported that 61% of the simulations had a penetration ratio that was greater than 1, with a mean penetration ratio of 3.18 ± 5.71. This resulted in a 95% confidence interval from 0.143 to 19.12 (8). Telavancin has also been studied for pulmonary disposition. In the population analyses, the 25th and 75th percentiles for ELF penetration ratios were 0.43 and 1.24 (19). In our Monte Carlo simulation, we observed a small amount of variability, with 5th and 95th percentiles ranging from 23.9 to 54.6 and an ELF AUC0-24 of 40.9 to 213.0. Given that the pharmacodynamic target for this oxazolidinone is AUC/MIC, this speaks well for the use of tedizolid phosphate in the setting of pneumonia.
As with all pulmonary disposition studies, a few assumptions were made that could impact the results of this study. First, penetration ratios compared total drug in the ELF and AM with free drug in the plasma. While protein binding in the pulmonary compartment has not been studied for tedizolid or any other antibiotic to our knowledge, it is believed to be minimal in the ELF and AM (12). Therefore, the difference between free and total drug in the pulmonary compartments should be negligible. Second, when AM concentrations were calculated, only the percentage of monocytes and histiocytes was used. These cell lineages represent the majority of immune cells found in the BAL fluid, but it is possible that drug from other cells was recovered. Further, we corrected for LLQ toward the end of the sampling interval when peaks were present on the chromatogram. While this may have increased drug concentrations, the change in AUC would be minimal. Additionally, it is possible that contamination of the ELF by AM cell lysis in the processing of the BAL sample could alter the ELF penetration ratio. As with other ELF penetration studies, this is minimized as much as possible by processing the BAL sample immediately upon collection. Such a phenomenon has been thought to happen with macrolide antibiotics such as azithromycin and clarithromycin (12). Because these antibiotics have extremely high concentrations in the AM and low concentrations in the plasma and ELF, cell lysis can skew penetration ratios to a large degree. However, azithromycin and clarithromycin penetration ratios range from approximately 1,000 to 4,500 in the AM and from 9 to 150 in the ELF (5, 20, 26–28). The vast difference between these values and the ratios determined in the current study suggest that AM contamination is unlikely to have had a substantial effect on ELF concentrations. Further, we estimated the maximum amount of contamination possible from the AM based on the absolute concentration of tedizolid in AM relative to the amount in ELF and determined the percent difference. This resulted in a mean difference of 1.16% ± 0.006% if all AM had been lysed, suggesting that if any contamination from the AM was present in the current study, it would be insignificant. Lastly, this study assessed pulmonary disposition of tedizolid in healthy participants. Future studies should evaluate the pulmonary concentrations in infected patients to determine if the pulmonary disposition of tedizolid is dependent upon patient factors, including the presence of infection and patient comorbidities.
In conclusion, tedizolid phosphate was well tolerated, and tedizolid penetrated into both the ELF and AM compartments of the lung with AUC0-24/MIC90 exposures similar to, if not greater than, those observed previously with linezolid. These data support further clinical investigation of tedizolid phosphate for the treatment of pulmonary infections caused by Gram-positive organisms, including MRSA.
ACKNOWLEDGMENTS
We thank Mary Anne Banevicius for her efforts in conducting the study, Lee Steere for his efforts involving venous access and drug administration, and David Marshall for the provision of the bronchoscopes. We also thank George Drusano for his assistance with the population pharmacokinetic analysis and Monte Carlo simulation. Lastly, we also express gratitude to the entire staff of the Center for Anti-Infective Research and Development for their efforts in the performance of this study.
Funding for this study was supplied by Trius Therapeutics, San Diego, CA.
Footnotes
Published ahead of print 13 February 2012
REFERENCES
- 1. Bien P, et al. 2008. Human pharmacokinetics of TR-700 after ascending single oral doses of the prodrug TR-701, a novel oxazolidinone antibiotic, poster F1-2063. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother. and the Infect. Dis. Soc. Am. 46th Conf., Washington, DC, 25 to 28 October, 2008 American Society for Microbiology, Washington, DC [Google Scholar]
- 2. Bien P, Prokocimer P, Munoz KA, Bethune C. 2010. Absolute bioavailability of TR-701 FA and pharmacokinetics after single and multiple dose intravenous administration in healthy adult subjects, poster A1-013. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother., Boston, MA, 12 to 15 September, 2010 American Society for Microbiology, Washington, DC [Google Scholar]
- 3. Capitano B, et al. 2004. Steady-state intrapulmonary concentrations of moxifloxacin, levofloxacin, and azithromycin in older adults. Chest 125:965–973 [DOI] [PubMed] [Google Scholar]
- 4. Choi S, Son T, Im W, Rhee J. 2007. In vitro and in vivo antibacterial activity of TR-701 (DA-7218) against penicillin-resistant Streptococcus pneumoniae, poster F1-1689. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL, 17 to 20 September, 2007 American Society for Microbiology, Washington, DC [Google Scholar]
- 5. Conte JE, Jr, Golden JA, Kelley MJ, Zurlinden E. 1995. Intrapulmonary pharmacokinetics of clarithromycin and erythromycin. Antimicrob. Agents Chemother. 39:334–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conte JE, Jr, Golden JA, Kipps J, Zurlinden E. 2002. Intrapulmonary pharmacokinetics of linezolid. Antimicrob. Agents Chemother. 46:1475–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. D'Argenio DZ, Schumitzky A. 1997. ADAPT IIA program for simulation, identification, and optimal experimental design. Biomedical Simulations Resource user manual. University of Southern California, Los Angeles, CA [Google Scholar]
- 8. Drusano GL, Preston SL, Gotfried MH, Danziger LH, Rodvold KA. 2002. Levofloxacin penetration into epithelial lining fluid as determined by population pharmacokinetic modeling and Monte Carlo simulation. Antimicrob. Agents Chemother. 46:586–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. File TM. 2003. Community-acquired pneumonia. Lancet 362:1991–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jones RN. 2010. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin. Infect. Dis. 51(Suppl. 1):S81–S87 [DOI] [PubMed] [Google Scholar]
- 11. Jones RN, Moet GJ, Sader HS, Mendes RE, Castanheira M. 2009. TR-700 in vitro activity against and resistance mutation frequencies among Gram-positive pathogens. J. Antimicrob. Chemother. 63:716–720 [DOI] [PubMed] [Google Scholar]
- 12. Kiem S, Schentag JJ. 2008. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob. Agents Chemother. 52:24–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klevens RM, et al. 2006. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992–2003. Clin. Infect. Dis. 42:389–391 [DOI] [PubMed] [Google Scholar]
- 14. Leary R, Jelliffe R, Schumitzky A, Van Guilder M. 2001. An adaptive grid non-parametric approach to pharmacokinetic and dynamic (PK/PD) models, p 389–394 In Proceedings of the 14th IEEE Symposium on Computer-Based Medical Systems IEEE Computer Society, Bethesda, MD [Google Scholar]
- 15. Lemaire S, Van Bambeke F, Appelbaum PC, Tulkens PM. 2009. Cellular pharmacokinetics and intracellular activity of torezolid (TR-700): studies with human macrophage (THP-1) and endothelial (HUVEC) cell lines. J. Antimicrob. Chemother. 64:1035–1043 [DOI] [PubMed] [Google Scholar]
- 16. Liu C, et al. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52:e18–e55 [DOI] [PubMed] [Google Scholar]
- 17. Livermore DM, Mustaq S, Warner M, Woodford N. 2009. Activity of oxazolidinone TR-700 against linezolid-susceptible and -resistant staphylococci and enterococci. J. Antimicrob. Chemother. 63(4):713–715 [DOI] [PubMed] [Google Scholar]
- 18. Lodise TP, et al. 2011. Penetration of vancomycin into epithelial lining fluid in healthy volunteers. Antimicrob. Agents Chemother. 55:5507–5511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lodise TP, Gotfried M, Barriere S, Drusano GL. 2008. Telavancin penetration into human epithelial lining fluid determined by population pharmacokinetic modeling and Monte Carlo simulation. Antimicrob. Agents Chemother. 52:2300–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Olsen KM, et al. 1996. Intrapulmonary pharmacokinetics of azithromycin in healthy volunteers given five oral doses. Antimicrob. Agents Chemother. 40:2582–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ong CT, Dandekar PK, Sutherland C, Nightingale CH, Nicolau DP. 2005. Intrapulmonary concentrations of telithromycin: clinical implications for respiratory tract infections due to Streptococcus pneumoniae. Chemotherapy 51:339–346 [DOI] [PubMed] [Google Scholar]
- 22. Pichereau S, Bohrmuller J, Marchillo K, Stamstad T, Craig W, Andes D. 2009. Comparative pharmacodynamics of a novel oxazolidinone, torezolid phosphate (TR-701), against S. aureus in a neutropenic murine pneumonia infection model, poster A1-1939. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA, 12 to 15 September, 2009 American Society for Microbiology, Washington, DC [Google Scholar]
- 23. Prokocimer P, et al. 2008. Human pharmacokinetics of the prodrug TR-701 and TR-700, its active moiety, after multiple oral doses of TR-701, a novel oxazolidinone, poster F1-2064. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother., Washington, DC, 25 to 28 October, 2008 American Society for Microbiology, Washington, DC [Google Scholar]
- 24. Prokocimer P, et al. 2011. Phase 2, randomized, double-blind, dose ranging study evaluating the safety, tolerability, population pharmacokinetics, and efficacy of oral torezolid phosphate in patients with complicated skin and skin structure infections. Antimicrob. Agents Chemother. 55:583–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rennard SI, et al. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as a marker of dilution. J. Appl. Physiol. 60:532–538 [DOI] [PubMed] [Google Scholar]
- 26. Rodvold KA. 1999. Clinical pharmacokinetics of clarithromycin. Clin. Pharmacokinet. 37:385–398 [DOI] [PubMed] [Google Scholar]
- 27. Rodvold KA, Danziger LH, Gotfried MH. 2003. Steady-state plasma and bronchopulmonary concentrations of intravenous levofloxacin and azithromycin in healthy adults. Antimicrob. Agents Chemother. 47:2450–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rodvold KA, Gotfried MH, Danziger LH, Servi RJ. 1997. Intrapulmonary steady-state concentrations of clarithromycin and azithromycin in healthy adult volunteers. Antimicrob. Agents Chemother. 41:1399–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rubinstein E, Cammarata S, Oliphant T, Wunderink R, and Linezolid Nosocomial Pneumonia Study Group 2001. Linezolid (PNU-100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double-blind, multicenter study. Clin. Infect. Dis. 32:402–412 [DOI] [PubMed] [Google Scholar]
- 30. Schaadt R, Sweeney D, Shinabarger D, Zurenko G. 2009. In vitro activity of TR-700, the active ingredient of the antibacterial prodrug TR-701, a novel oxazolidinone antibacterial agent. Antimicrob. Agents Chemother. 53:3236–3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shaw KJ, et al. 2008. In vitro activity of TR-700, the antibacterial moiety of the prodrug TR-701, against linezolid-resistant strains. Antimicrob. Agents Chemother. 52:4442–4447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wunderink RG, Rello J, Cammarata SK, Croos-Dabrera RV, Kollef MH. 2003. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest 124:1789–1797 [PubMed] [Google Scholar]
- 33. Yamaoka K, Nakagawa T, Uno T. 1978. Application of Akaike's Information Criterion (AIC) in the evaluation of linear pharmacokinetic equations. J. Pharmacokinet. Biopharm. 6:165–175 [DOI] [PubMed] [Google Scholar]