Abstract
Many Pseudomonas aeruginosa isolates from the airways of patients with cystic fibrosis (CF) are sensitive to antibiotics in susceptibility testing, but eradication of the infection is difficult. The main reason is the biofilm formation in the airways of patients with CF. The pharmacokinetics (PKs) and pharmacodynamics (PDs) of antimicrobials can reliably be used to predict whether antimicrobial regimens will achieve the maximum bactericidal effect against infections. Unfortunately, however, most PK/PD studies of antimicrobials have been done on planktonic cells and very few PK/PD studies have been done on biofilms, partly due to the lack of suitable models in vivo. In the present study, a biofilm lung infection model was developed to provide an objective and quantitative evaluation of the PK/PD profile of antimicrobials. Killing curves were set up to detect the antimicrobial kinetics on planktonic and biofilm P. aeruginosa cells in vivo. Colistin showed concentration-dependent killing, while imipenem showed time-dependent killing on both planktonic and biofilm P. aeruginosa cells in vivo. The parameter best correlated to the elimination of bacteria in lung by colistin was the area under the curve (AUC) versus MIC (AUC/MIC) for planktonic cells or the AUC versus minimal biofilm inhibitory concentration (MBIC; AUC/MBIC) for biofilm cells. The best-correlated parameter for imipenem was the time that the drug concentration was above the MIC for planktonic cells (TMIC) or time that the drug concentration was above the MBIC (TMBIC) for biofilm cells. However, the AUC/MIC of imipenem showed a better correlation with the efficacy of imipenem for biofilm infections (R2 = 0.89) than planktonic cell infections (R2 = 0.38). The postantibiotic effect (PAE) of colistin and imipenem was shorter in biofilm infections than planktonic cell infections in this model.
INTRODUCTION
Although progress on biofilm research has been obtained during the past decades (48), the treatment of biofilm infections with antibiotics remains an important clinical challenge. The pharmacokinetic (PK) and pharmacodynamic (PD) profiles of an antimicrobial agent provide important information helping to establish an efficient dosing regimen (49). Therefore, PK and PD information on the activities of antimicrobial agents against biofilm-associated bacteria can contribute to better clinical treatment of biofilm-associated diseases. Unfortunately, most previous PK/PD studies of antibiotics have been done on planktonic cells, and extrapolation of the results to biofilm cells is problematic, as bacterial biofilm cells differ from cells growing in planktonic form in growth rate (36), gene expression (10, 12), and metabolism (15, 52).
We have recently shown that colistin has a concentration-dependent killing and imipenem has a time-dependent killing of Pseudomonas aeruginosa biofilm cells in vitro (30). The rationale for the present study is to validate the results obtained in vitro with an in vivo model of P. aeruginosa biofilm infection in neutropenic mice.
(This in vivo PK/PD study was presented in part at the 110th General Meeting of the American Society for Microbiology, San Diego, CA, May 2010 [abstr. A-2973].)
MATERIALS AND METHODS
Strains, chemicals, and susceptibility assay.
Wild-type P. aeruginosa PAO1 (45) was used in this study. Colistin and imipenem were pharmaceutical grade. The MIC was detected by Etest (AB Biodisk, Solna, Sweden) and by a microtiter method; the minimal bactericidal concentration (MBC) for planktonic cells was detected by the microtiter method (1). The MICs and MBCs of colistin for PAO1 were 2 to 4 mg/liter and 8 mg/liter, respectively; the MICs and MBCs of imipenem were 1 mg/liter and 4 mg/liter, respectively. The minimal biofilm inhibitory concentration (MBIC) and minimal biofilm eradication concentration (MBEC) of colistin and imipenem were determined by use of a modified Calgary biofilm device as previously reported (30). In short, the biofilms are formed on pegs, treated by antibiotics, and detached by sonication for the assessment of bacterial killing. The MBIC and MBEC of colistin were 8 mg/liter and 64 mg/liter, respectively; the MBIC and MBEC of imipenem were 8 mg/liter and 128 mg/liter, respectively (30).
Animals.
Ten-week-old female NMRI mice (Taconic, Denmark) weighing 32 to 34 g were used. The mice were maintained on standard mouse chow and water ad libitum for 1 week before challenge. All animal experiments were performed under authorization from the National Animal Ethics Committee of Denmark.
Neutropenic mouse model of biofilm lung infection.
Mice were rendered neutropenic by injecting three doses of cyclophosphamide (Sendoxan; Baxter A/S, Denmark) intraperitoneally on day 1 (150 mg/kg of body weight), day 3 (100 mg/kg), and day 4 (100 mg/kg) prior to experimental infection. Optimization of the cyclophosphamide dose for NMRI mice was performed in pilot studies. Blood was drawn from the tail, and leukocytes were counted with a NucleoCassette device (NucleoCounter system; ChemoMetec A/S, Denmark). Blood smears were checked for the presence of granulocytes (22). Mice were severely granulocytopenic (absolute granulocyte count, <50/mm3) by day 4 and remained so through day 5 and day 6 after the first injection of cyclophosphamide. The bacterial infection was performed on day 5. The neutropenic mice were raised in a microisolation cage system in a sterile environment. Mouse operations were performed in a ventilated cabinet while the mice were under anesthesia (29). Optimization of the inocula of planktonic and biofilm bacteria was performed in pilot studies. To prepare the biofilm bacteria, planktonic P. aeruginosa cells were immobilized in spherical alginate beads, as previously described (33, 45, 54). The anesthetized mice were tracheostomized, and a 0.04-ml inoculum of planktonic or biofilm bacteria adjusted to yield approximately 5 × 105 CFU/ml was instilled in the lower left lung using a curved bead-tipped needle. The incision was sutured.
PKs of colistin and imipenem.
While the infected animals were under anesthesia, they were treated intraperitoneally 2 h after infection with 0.2 ml of different doses of colistin (16 mg/kg, 64 mg/kg, 256 mg/kg; 6 mice/regimen; total, 18 mice) or imipenem (4 mg/kg, 8 mg/kg, 16 mg/kg, 32 mg/kg, 64 mg/kg; 6 mice/regimen; total, 30 mice) as a single administration. The control groups received equal volumes of 0.15 M NaCl intraperitoneally. An approximately 0.08-ml blood sample was collected from the tail (28) at 5 min, 15 min, 30 min, 60 min, 120 min, 180 min, and 240 min after antibiotic administration. At the end of the experiment, the mice were euthanized with pentobarbital/lidocaine (29). Blood samples were centrifuged at 3,000 rpm, and serum was collected for measurement of antibiotic concentration by a biologic method (agar diffusion), as reported previously, employing Streptococcus sp. strain EB68 (imipenem) or Bordetella bronchiseptica ATCC 4617 (colistin) (4, 35, 39). The detection limits were 1 μg/ml (colistin) and 0.2 μg/ml (imipenem). Data about the variability of the assay are presented in the supplementary material. Time-concentration curves of colistin and imipenem were established.
Time-kill study of colistin and imipenem in planktonic and biofilm bacteria and PAE.
To establish killing curves of colistin and imipenem, anesthetized neutropenic mice infected with planktonic bacteria (4 mice/point; total, 176 mice) or biofilm bacteria (4 mice/point; total, 176 mice) were treated at 2 h after infection with a single intraperitoneal dose of colistin or imipenem (4× MIC, 16× MIC, and 64× MIC; 4 to 256 mg/kg). Control mice received the same volume of saline. The mice were euthanized, and lungs were collected aseptically at −2, 0, 2, 4, 8, 12, and 24 h after bacterial challenge and homogenized in 5 ml of sterilized saline. Humane endpoints were applied during the period. The numbers of CFU were counted for plotting of the killing curves. The duration of the postantibiotic effect (PAE) was calculated by the formula T − C (21, 43), where T is the time required for the mean count of CFU in the lung of treated mice to increase by 1 log10 unit above its value at the time that the antibiotic concentration in serum fell below the MIC or MBIC, and C is the time required for the mean count of CFU in the lungs of control mice to increase by 1 log unit above the viable count at time zero.
PK/PD indices of colistin and imipenem in planktonic and biofilm bacteria in vivo.
To establish PK/PD indices of colistin and imipenem, anesthetized neutropenic mice infected with planktonic bacteria (total, 60 mice) or biofilm bacteria (total, 60 mice) were treated from the time point of 2 h after infection with multiple intraperitoneal doses of colistin (range, 16 to 256 mg/kg, representing 2× MBIC to 32× MBIC/4× MBEC) or imipenem (range, 8 to 64 mg/kg, representing 1× MBIC to 8× MBIC). Due to the toxicity of imipenem, it was not possible to administer higher dosages. The multiple dosages were administered at time intervals ranging from 2 h to 16 h after infection for periods of 12 h (colistin) and 24 h (imipenem) (23). The mice were euthanized, and lungs were collected at the end of the experiment and homogenized in 5 ml of sterilized saline. The numbers of CFU were counted for each lung and expressed as the log10 number of CFU per lung. The counts of viable bacteria for each regimen were plotted with the PK parameters.
Data analysis and statistical methods.
The serum concentrations of colistin and imipenem arising from each single dose were used to generate the time course and fit the curve. The values of the PK parameters were calculated with noncompartmental analysis by the Kinetica (version 5.0) program (Thermo Fisher Scientific/Cypress Software Inc., Langley, WA), including the maximum concentration (Cmax), time to reach the maximum concentration (Tmax), total area under the curve (AUCtot), apparent volume of distribution during the terminal phase (Vz/F), apparent volume of distribution at steady-state (Vss/F), total clearance (CL/F), half-life of elimination (t1/2), and mean residence time (MRT); F is the bioavailability. PK parameters for antibiotics were calculated using the following equations: t1/2 = ln 2/kel, CL/F = dose/AUC, Vz/F = dose/(AUC · kel), Vss/F = dose · AUMC/AUC2, and MRT = AUMC/AUC. kel is the elimination rate constant, and AUMC is the partial area under the moment curve. The superposition principle (23) was applied to the single-dose serum concentration-time data for generating the corresponding time course of multiple administrations in the PK/PD studies. The PK/PD analysis was also performed by using the inhibitory sigmoid dose-effect model (Origin, version 8.0; OriginLab, Northampton, MA). The formula is E = E0 − Emax · Xγ/ (Xγ + EC50γ), where E is the measure of effect (log10 numbers of CFU/lung), X is the value of the relevant PK/PD index (Cmax/MIC, AUC/MIC, or time that the drug concentration was above the MIC [TMIC]), E0 is the effect in the absence of drug, Emax is the maximal drug effect, EC50 is the value of the target PK/PD index required to achieve 50% of Emax, and γ is the Hill coefficient of the PK/PD index-effect curve. The correlation between the efficacy and each of the three PK/PD indices was determined by unweighted nonlinear least-squares regression. R2 was used to evaluate the variance of regression for each of the PK/PD indices; the goodness of fit was also assessed by visual examination.
RESULTS
PKs of colistin and imipenem.
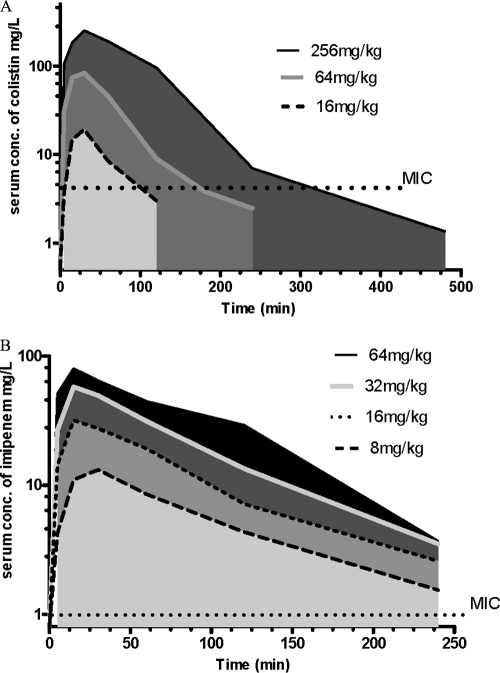
The time-concentration curves of colistin and imipenem in the serum of neutropenic mice with lung infection after an intraperitoneal dose of 16 to 256 mg/kg of colistin and 8 to 64 mg/kg of imipenem are presented in Fig. 1, and the PK parameters are presented in Table 1. The Tmax of colistin was dependent on the dose administered and ranged from 25 to 35 min. The Tmax of imipenem was 15 to 21 min. t1/2 was 31 to 54 min for colistin and 43 to 67 min for imipenem. A dose dependency was observed for the AUCtot value for both colistin and imipenem, with ranges of from 1,119 to 24,527 mg · min/liter for colistin and 1,470 to 6,037 mg · min/liter for imipenem. The variation of the PK parameters could be due to the intraperitoneal mode of administration.
Fig 1.
PK studies. Serum concentrations (conc.) of colistin (A) and imipenem (B) in mice after intraperitoneal treatment with doses from 8 mg/kg to 256 mg/kg are shown. The y axis in both panels is log10. The MICs against P. aeruginosa PAO1 were 4 mg/liter for colistin and 1 mg/liter for imipenem.
Table 1.
Values of pharmacokinetic parameters for colistin and imipenem following intraperitoneal administration of a single dose in mice with biofilm bacterial lung infectiona
| Drug and dose (mg/kg) | Cmax (mg/liter) | Tmax (min) | AUCtot (mg · min/liter) | Vz/F (ml/kg) | Vss/F (ml/kg) | CL/F (ml/min/kg) | t1/2 (min) | MRT (min) |
|---|---|---|---|---|---|---|---|---|
| Colistin | ||||||||
| 16 | 21 (3.1) | 25 (7.8) | 1,119 (240) | 676 (300) | 868 (314) | 15 (3.3) | 31 (6.6) | 58 (10) |
| 64 | 83 (5.6) | 28 (6.1) | 5,766 (542) | 875 (108) | 761 (84) | 11 (1) | 54 (2.6) | 68 (2.3) |
| 256 | 264 (31) | 35 (12) | 24,527 (2,679) | 803 (189) | 871 (84) | 11 (1.1) | 53 (13) | 83 (7.7) |
| Imipenem | ||||||||
| 8 | 15 (7.1) | 21 (11) | 1,470 (777) | 648 (330) | 721 (343) | 6.7 (3) | 67 (11) | 108 (12) |
| 16 | 34 (6) | 28 (18) | 2,857 (559) | 507 (140) | 543 (121) | 5.8 (1) | 60 (9.1) | 94 (10) |
| 32 | 54 (11) | 18 (6.1) | 4,895 (635) | 516 (75) | 566 (83) | 6.6 (0.8) | 54 (6.5) | 86 (11) |
| 64 | 69 (37) | 15 (9.5) | 6,037 (2,976) | 547 (274) | 617 (308) | 7.4 (3.6) | 43 (22) | 70 (35) |
The doses are 16 to 256 mg/kg of colistin and 8 to 64 mg/kg of imipenem. Data in parentheses are the standard deviation (SD).
Time-kill study of colistin and imipenem in planktonic and biofilm bacteria and PAEs.
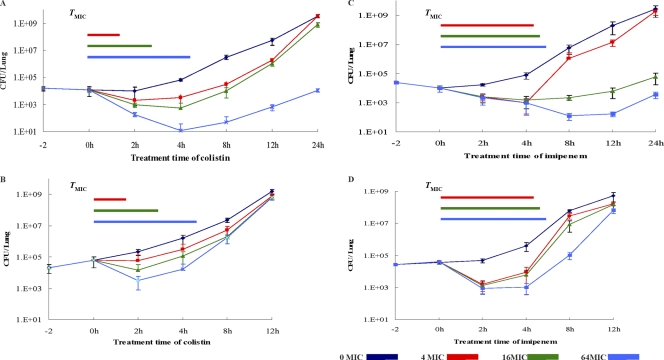
The killing curves of colistin and imipenem in the in vivo lung infection model with P. aeruginosa cells grown in the planktonic or biofilm form are shown in Fig. 2. The bactericidal activity of colistin in vivo appeared to be concentration dependent, while imipenem showed time-dependent killing of both planktonic and biofilm cells. Treatment for 2 h with 256 mg/liter colistin, representing 64× MIC or 32× MBIC, led to 2-log10-CFU reductions in planktonic bacteria but only a 1-log10-CFU decrease in biofilm bacteria. The colistin concentration that inhibited biofilm cell growth was 4 times higher than the concentration needed to inhibit planktonic growth. The killing effect of imipenem showed a correlation with the treatment time for cells growing both in planktonic form and as biofilms, but a higher concentration was required to control the regrowth of biofilm cells in vivo. One imipenem dose of 16 mg/kg suppressed the planktonic cell infection for 12 h but suppressed the biofilm infection for only 2 h. The PAEs of colistin and imipenem on planktonic and biofilm P. aeruginosa cells are shown in Table 2. The PAEs of colistin and imipenem were shorter on biofilm cells than on planktonic cells. On the basis of the parameter of TMIC, the PAE values of colistin were 0.9 to 1.5 h for planktonic cells versus −0.9 to −2.1 h for biofilm cells; the PAEs of imipenem were −2.8 to 12 h for planktonic cells versus −2.4 to −3 h for biofilm cells. The PAE of imipenem showed a concentration dependence on planktonic cells, −2.8 h for 4 mg/kg and 12 h for 64 mg/kg, but not on biofilm P. aeruginosa cells. Based on the parameter TMBIC, the PAE values of colistin were −0.1 to −2 h for biofilms, and those of imipenem were −1.2 to −1.5 h for biofilms.
Fig 2.
Growth curves of control and antibiotic-exposed planktonic and biofilm P. aeruginosa PAO1 cells in the neutropenic mouse lung after a single intraperitoneal dose of colistin and imipenem. MIC of colistin, 4 μg/ml; MIC of imipenem, 1 μg/ml. Color bars denote the interval that serum levels of antibiotic concentrations exceeded the MIC (TMIC). (A) Colistin versus planktonic bacteria; (B) colistin versus biofilms; (C) imipenem versus planktonic bacteria; (D) imipenem versus biofilms.
Table 2.
PAEs of colistin and imipenem after single-dose therapy against P. aeruginosa PAO1 in the lung infection model of neutropenic mice
| Drug and dose (mg/kg) | TMIC (h) | TMBIC (h) | PAE (h)a |
||
|---|---|---|---|---|---|
| Planktonic cells (TMIC) | Biofilm cells |
||||
| TMIC | TMBIC | ||||
| Colistin | |||||
| 16 | 1.6 | 0.9 | 0.9 | −0.9 | −0.1 |
| 64 | 3 | 2 | 1 | −1.5 | −1.2 |
| 256 | 5 | 4 | 1.5 | −2.1 | −2 |
| Imipenem | |||||
| 4 | 5 | 0 | −2.8 | −3 | |
| 16 | 5.7 | 2 | 6.8 | −2.7 | −1.5 |
| 64 | 5.9 | 3 | 12 | −2.4 | −1.2 |
The indicated parameters (TMIC and TMBIC) were involved in the PAE calculation.
PK/PD index determination.
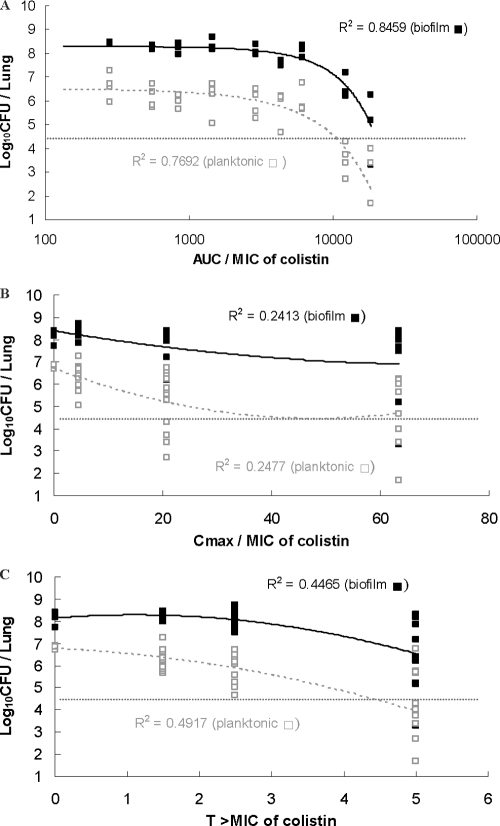
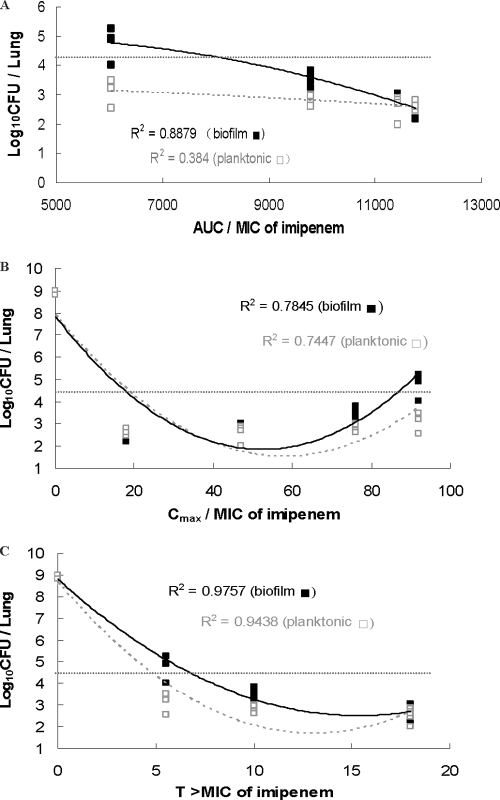
The Cmax/MIC, AUC/MIC, and TMIC for colistin and imipenem are presented in Fig. 3 and 4, respectively. The PK/PD indices that best correlated with the in vivo efficacy were AUC/MIC for colistin (R2 = 0.77 in planktonic cell infection and 0.85 in biofilm infection) and TMIC for imipenem (R2 = 0.94 for planktonic cell infection and 0.98 for biofilm infection). The planktonic and biofilm bacterial loads in the lungs of mice were 2.0 × 104 CFU/lung in the beginning and reached 109 CFU/lung after 24 h in the untreated control mice. The AUC/MIC of imipenem showed a good correlation with the efficacy of imipenem treatment of biofilm infections (R2 = 0.89) but a poor correlation for planktonic cell infections (R2 = 0.38). The AUC/MBIC and TMBIC of imipenem and colistin are presented in figures in the supplemental material, which show the time-dependent killing of imipenem (R2 = 0.98) and the AUC-dependent killing of colistin (R2 = 0.85).
Fig 3.
Relationships for P. aeruginosa between the log10 numbers of CFU per lung and PK/PD indices AUC/MIC, Cmax/MIC, and TMIC of colistin. Each symbol represents the data from a single lung. The horizontal dotted lines represent the mean bacterial burden in the lungs at the start of treatment.
Fig 4.
Relationships for P. aeruginosa between the log10 numbers of CFU per lung and PK/PD indices AUC/MIC, Cmax/MIC, and TMIC of imipenem. Each symbol represents the data from a single lung. The horizontal dotted lines represent the mean bacterial burden in the lungs at the start of treatment.
Relationship between AUC/MIC and TMIC with antibacterial effect.
The influences of PK and PD parameters on the antimicrobial effect are presented in Tables 3 and 4. Dose-response data were analyzed to examine the impact of the PK/PD parameters by relating the number of bacteria in lung to AUC/MIC (colistin) and TMIC (imipenem). To determine the PK/PD relationships, the correlations (Hill's equation) in both planktonic and biofilm cells were calculated, and the effect was defined as the decrease in the number of CFU in the lung after the first treatment compared to the number of CFU in untreated control mice (at the 2-h time point). Emax values were estimated to be −4.4 (planktonic cells) versus −2.2 (biofilms) log CFU for colistin and −6.6 (planktonic cells) versus −6.6 (biofilms) log CFU for imipenem. The EC50s (AUC/MIC) were 3,840 (planktonic cells) versus >18,420 (biofilms) for colistin and 3.4 (planktonic cells) versus 4.6 (biofilms) for imipenem (TMIC). The depression of the numbers of CFU in the lung with the PK/PD model parameters of AUC/MIC (colistin) and TMIC (imipenem) is presented in Table 4. The 2-log10-kill effect of colistin required AUC/MIC ratios of 61,980 for biofilm cells and 25,980 for planktonic cells and at least 18 h imipenem treatment for biofilm cells and 10 h for planktonic cells (TMIC).
Table 3.
PK/PD model parameter estimates for prediction of viable counts for AUC/MIC, MBIC index for colistin, and TMIC and TMBIC for imipenem against P. aeruginosa PAO1 in the lung infection model of neutropenic mice
| Antibiotic and cell type (parameter) | Emax (log10 no. of CFU/lung) | E0 (log10 no. of CFU/lung) | EC50 | γ |
|---|---|---|---|---|
| Colistina | ||||
| Planktonic (AUC/MIC) | −4.4 (28)b | 6.2 (2.8) | 3,840 (17) | 4.1 (57) |
| Biofilm (AUC/MIC) | −2.2 (61) | 8.3 (1.7) | >18,420 | 1.7 (60) |
| Biofilm (AUC/MBIC) | −2.2 (61) | 8.3 (1.7) | >9,180 | 1.7 (42) |
| Imipenemc | ||||
| Planktonic (TMIC) | −6.6 (0.3) | 8.6 (0.1) | 3.4 (0.3) | 2 (0.5) |
| Biofilm (TMIC) | −6.6 (0.3) | 9 (0.1) | 4.6 (0.2) | 1.2 (0.9) |
| Biofilm (TMBIC) | −6.8 (0.2) | 9 (0.1) | 2.4 (0.1) | 2 (0.2) |
AUC, mg · min/liter.
Data in parentheses are percent relative standard error.
In hours.
Table 4.
Target values of colistin (AUC/MIC, MBIC) and imipenem (TMIC, TMBIC) for stasis and 1- and 2-log10-unit kill against P. aeruginosa PAO1 in the lung infection model of neutropenic mice
| Kill effect | Target value of antibiotics |
|||||
|---|---|---|---|---|---|---|
| Colistina |
Imipenemb |
|||||
| Planktonic (AUC/MIC) | Biofilms |
Planktonic (TMIC) | Biofilms |
|||
| AUC/MIC | AUC/MBIC | TMIC | TMBIC | |||
| Static | 10,020 | 30,000 | 3,780 | 5.5 | 7.5 | 3.2 |
| 1 log10 | 17,820 | 52,020 | 11,100 | 7.5 | 11 | 5.2 |
| 2 log10 | 25,980 | 61,980 | 13,080 | 10 | 18 | 8 |
AUC, mg · min/liter.
In hours.
PK-PD simulation in the biofilm infection model of neutropenic mouse.
The PK-PD simulation with planktonic and biofilm cells is presented in Table 5. The colistin doses were 16 to 256 mg/kg, and the imipenem doses were 8 to 64 mg/kg. The Cmax/MIC values were 5.4 to 66 for colistin and 15 to 69 for imipenem, but the Cmax/MBEC values were 0.3 to 4.1 for colistin and 0.12 to 0.54 for imipenem. The AUC/MIC values of colistin were 280 to 6,132, while the AUC/MBEC values were 18 to 383. The time that the drug concentration was above the MBEC (TMBEC) was 0 when imipenem was administered at doses of 16 mg/kg to 64 mg/kg.
Table 5.
Values of pharmacokinetics and pharmacodynamics modeling in P. aeruginosa biofilm infection model of leucopenia mice
| Drug and dose (mg/kg) | Cmax/MIC | Cmax/MBC | Cmax/MBIC | Cmax/MBEC | AUC/MICa | AUC/MBC | AUC/MBIC | AUC/MBEC | TMBC (h)b | TMBEC (h) |
|---|---|---|---|---|---|---|---|---|---|---|
| Colistin | ||||||||||
| 16 | 5.4 | 2.7 | 2.7 | 0.3 | 280 | 140 | 140 | 18 | 0.9 | 0 |
| 64 | 21 | 10 | 10 | 1.3 | 1,442 | 721 | 721 | 90 | 2 | 0.7 |
| 256 | 66 | 33 | 33 | 4.1 | 6,132 | 3,066 | 3,066 | 383 | 4 | 2.3 |
| Imipenem | ||||||||||
| 8 | 15 | 3.9 | 1.9 | 0.12 | 1,470 | 368 | 184 | 11 | 1.8 | 0 |
| 16 | 34 | 8.5 | 4.3 | 0.27 | 2,857 | 714 | 357 | 22 | 3 | 0 |
| 32 | 54 | 14 | 6.8 | 0.43 | 4,895 | 1,224 | 612 | 38 | 3.8 | 0 |
| 64 | 69 | 17 | 8.6 | 0.54 | 6,037 | 1,509 | 755 | 47 | 4.2 | 0 |
AUC, mg · min/liter.
TMIC and TMBIC in Table 2.
DISCUSSION
The majority of PK/PD antibiotic studies have been described with planktonic cells (2, 17, 19, 51, 53). As the PK/PD results obtained from cells growing in planktonic form cannot be extrapolated to biofilm cells, we proposed to conduct a study of PKs/PDs with biofilm cells. Few in vivo models of biofilms have been developed to test the PKs and PDs of antimicrobials. Blaser et al. described an animal model of device-related infection, but they found that pharmacodynamic ratios Cmax/MIC and AUC/MIC and also TMIC were not predictive of the therapeutic outcome in that model (7). To investigate the PK/PD profile of antimicrobials on biofilm P. aeruginosa, a biofilm lung infection model was developed in the neutropenic mouse. We demonstrated in this model the impact of doses and dosing schedules on the time course of the pharmacologic response to two antipseudomonas drugs and determined the magnitude of reduction of planktonic and biofilm cell CFU counts. To dissociate between the effect of antibiotics and those of the immune system, the PK/PD studies of antimicrobials are traditionally conducted in neutropenic mice (22). Although we appreciate the role played by the host response in the eradication of biofilm cells, it was beyond the purpose of this study to investigate the killing dynamics of antibiotics in animals with immune systems with normal function. Knowledge of both the kinetics of antimicrobial activity and the kinetics of drug concentrations in serum and tissues allows us to establish dosage regimens for most drugs that might be used for therapy (13, 14).
This study presents the pharmacodynamics of antibiotics on biofilm infection in vivo and shows for the first time the concentration-dependent killing of colistin and time-dependent killing of imipenem in vivo (Fig. 2), confirming our previous findings on in vitro biofilm cells. However, higher concentrations and longer treatment times are required to eradicate biofilm cells than planktonic cells. As a difference in colistin concentrations that inhibit biofilm and planktonic cell growth of four times was seen and as we have previously shown that both MBIC and MBC are four times the MIC, we propose that the MBC might be used as the concentration that temporarily inhibits the growth of biofilm cells. However, our in vivo data show that biofilm cell eradication is not possible in our model, even with a concentration of 4× MBEC (Fig. 2B). We are also presenting for the first time the PAEs of antimicrobials on biofilm cells in vivo (Fig. 2; Table 2) and show that the PAE is shorter for biofilm cells than planktonic cells. The mechanisms of the PAE on biofilm cells are still unknown. PAE provides a rationale for modification of the dosing interval of antimicrobials and could be significant for optimization of the treatment regimen and minimization of drug-induced adverse effects (46).
As imipenem treatment of biofilm cells requires a longer treatment in terms of the TMIC than imipenem treatment of planktonic cells and as the PAE of imipenem on biofilm cells was shorter than that on planktonic cells, it can be proposed that the best effect of imipenem (and β-lactams in general) on biofilm cells could be achieved by continuous administration. The continuous administration of β-lactams may contribute to the decrease in the development of resistant cells of P. aeruginosa (41, 42). For a better therapeutic outcome in biofilms, development of antibiotics with longer half-life profiles or the use of controlled-reagent-release technology is proposed.
As colistin treatment of biofilm cells requires high drug concentrations, the level of 16 mg/liter of colistin, representing the MBIC of mature biofilm cells (30), is possible to reach in the lung by inhalation, as sputum concentrations of polymyxin E were measured to be almost 40 mg/liter after 1 h of a single inhalation of 2 million units (47). However, the very high concentration of 128 mg/liter, representing the MBEC of colistin on mature biofilm cells (30), is impossible to reach. Therefore, the treatment principle for established biofilm infection is to repeatedly decrease the bacterial load, with a subsequent decreased inflammatory response, but a total eradication of mature biofilm cells in one administration is not possible.
Another treatment strategy is to take advantage of the synergistic effect of combination therapy, which has been proven to be useful in the control of biofilm infections (31, 50). In addition, in planktonic cell infections, the PAE was shown to be prolonged by combining antibiotics (24), and this finding is also probably to be expected in biofilm infections.
The important antimicrobial strategy in CF patients is to prevent biofilm formation and the progression of biofilm infections to chronic infections in the lung. We have shown that colistin inhalation and oral ciprofloxacin are successful in postponing or preventing chronic P. aeruginosa infection in CF patients (20). A synergistic effect of these two antipseudomonal drugs in the treatment of biofilm cells was observed in vitro and was also observed for tobramycin and colistin in animal experiments (31, 44).
In the present study, we were also able to investigate the PK/PD indices that were the most predictive of activity against planktonic and biofilm P. aeruginosa cells with multiple-dose administration (Fig. 3. and 4). Although the PK/PD index that showed the best correlation was similar in planktonic and biofilm cells (Fig. 3 and 4)—AUC/MIC for colistin (R2 = 0.85 in biofilms and 0.77 in planktonic cells) and TMIC for imipenem (R2 = 0.98 in biofilms and 0.94 in planktonic cells)—the tolerance of biofilm cells to colistin and imipenem is shown in Tables 3 to 5. Higher colistin concentrations and a longer treatment period with imipenem were therefore required to treat biofilm infections than planktonic cell infections. So, we proposed to use the MBIC or MBEC as the parameters for biofilm infections in vivo (Table 5). In Fig. 3A, the colistin killing profiles of planktonic or biofilm cells show a parallel pattern with AUC/MIC. This finding is not surprising, taking into account the heterogeneity of biofilms (52) and the fact that metabolically active cells in biofilms might respond to antibiotics similarly to planktonic cells. Interestingly, the same PK/PD index for colistin treatment of planktonic cell infection was reported in other animal models of P. aeruginosa infection, and AUC/MIC correlated the best with elimination efficacy in both the thigh infection model and the lung infection model of planktonic P. aeruginosa infection (18).
In particular, the AUC/MIC of imipenem correlated much better with the efficacy of killing biofilm infections than the efficacy of killing planktonic cell infections (Fig. 4). One of the mechanisms may be the induction of β-lactamase production in P. aeruginosa growing as a biofilm after exposure to imipenem (3–5). As a strong inducer of β-lactamase production, imipenem could induce passage of the β-lactamase through all the bacterial layers of biofilms (4, 34). This result may explain the dose-dependent killing profile of imipenem in P. aeruginosa biofilm infections. This suggests that stable β-lactamase compounds, such as meropenem (9), or combinations of β-lactams with β-lactamase inhibitors (37) could be more effective in the treatment of biofilm cell infections than other compounds containing the β-lactam ring (11). Studies investigating this hypothesis are in progress in our labs.
The advantage of determining PK parameters in an in vivo model is that the whole-body system and the dynamic nature of infection are taken into account (8). After establishing the best correlation index for PKs/PDs, a precise calculation was performed in the inhibitory sigmoid dose-effect model (Table 3). Colistin showed a high tolerance to biofilm cells in vivo, with an EC50 of >18,420 (AUC/MIC). These data showed that eradication of P. aeruginosa biofilm cells is difficult to obtain in vivo. Both higher-dose and longer-term treatments are also required to reach the clinical target for biofilm infections compared with planktonic cell infections (30). Tolerance to colistin on biofilms was proposed to be linked to induced lipopolysaccharide modification after surface attachment and to subpopulation-associated tolerance (25). The possible mechanisms of biofilm resistance to antimicrobials might correlate with slow growth and stationary-phase physiology (27, 32), low oxygen concentration (26), matrix components and chromosomal β-lactamase (4, 27), mutators (16, 40), quorum sensing (6, 38, 54), and efflux pumps (44). Furthermore, the PK and PD profiles of antimicrobials on biofilms can be applied to minimize selective pressure for developing antimicrobial resistance in biofilms and determine an ideal dosing regimen for biofilm infections.
In conclusion, the kinetics of colistin and imipenem in vivo showed concentration-dependent and time-dependent killing, respectively, on P. aeruginosa cells growing as a biofilm, and the elimination of the biofilm bacteria in the lung was best correlated to the AUC/MIC (AUC/MBIC) of colistin and TMBIC of imipenem. Imipenem showed more relationships to the AUC/MIC parameter in biofilm infection than planktonic cell infection, which implied the dose-dependent killing profile of imipenem in the P. aeruginosa biofilm infections. PK/PD studies of β-lactams in biofilms formed by β-lactamase hyperproducer strains as well as combination therapies are in progress in our lab. PAEs of colistin and imipenem are shorter on biofilm cells than on planktonic P. aeruginosa cells in the in vivo model. The results of this PK/PD study can probably be used to improve the antibiotic dose regimen for P. aeruginosa biofilm infections.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by a grant from the European Union (Project NPARI, contract no. 037692).
Footnotes
Published ahead of print 21 February 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Amsterdam D. 2005. Susceptibility testing of antimicrobials in liquid media, p 61–143 In Lorian V. (ed), Antibiotics in laboratory medicine, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 2. Andes D, Craig WA. 2002. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int. J. Antimicrob. Agents 19:261–268 [DOI] [PubMed] [Google Scholar]
- 3. Bagge N, et al. 2002. Constitutive high expression of chromosomal {beta}-lactamase in Pseudomonas aeruginosa caused by a new insertion sequence (IS1669) located in ampD. Antimicrob. Agents Chemother. 46:3406–3411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bagge N, et al. 2004. Dynamics and spatial distribution of beta-lactamase expression in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 48:1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bagge N, et al. 2004. Pseudomonas aeruginosa biofilms exposed to imipenem exhibit changes in global gene expression and {beta}-lactamase and alginate production. Antimicrob. Agents Chemother. 48:1175–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bjarnsholt T, et al. 2005. Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology 151:373–383 [DOI] [PubMed] [Google Scholar]
- 7. Blaser J, Vergeres P, Widmer AF, Zimmerli W. 1995. In vivo verification of in vitro model of antibiotic treatment of device-related infection. Antimicrob. Agents Chemother. 39:1134–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brinch KS, et al. 2009. Plectasin shows intracellular activity against Staphylococcus aureus in human THP-1 monocytes and in a mouse peritonitis model. Antimicrob. Agents Chemother. 53:4801–4808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ciofu O, et al. 1996. Meropenem in cystic fibrosis patients infected with resistant Pseudomonas aeruginosa or Burkholderia cepacia and with hypersensitivity to beta-lactam antibiotics. Clin. Microbiol. Infect. 2:91–98 [DOI] [PubMed] [Google Scholar]
- 10. Ciofu O, Mandsberg LF, Bjarnsholt T, Wassermann T, Høiby N. 2010. Genetic adaptation of Pseudomonas aeruginosa during chronic lung infection of patients with cystic fibrosis: strong and weak mutators with heterogeneous genetic backgrounds emerge in mucA and/or lasR mutants. Microbiology 156:1108–1119 [DOI] [PubMed] [Google Scholar]
- 11. Ciofu O, Tolker-Nielsen T. 2011. Antibiotic tolerance and resistance in biofilms, p 215–229 In Bjarnsholt T, Jensen PØ, Moser C, Høiby N. (ed), Biofilm infections. Springer, Berlin, Germany [Google Scholar]
- 12. Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322 [DOI] [PubMed] [Google Scholar]
- 13. Craig WA. 1995. Kinetics of antibiotics in relation to effective and convenient outpatient parenteral therapy. Int. J. Antimicrob. Agents 5:19–22 [DOI] [PubMed] [Google Scholar]
- 14. Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1–10 [DOI] [PubMed] [Google Scholar]
- 15. Davies DG, et al. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295–298 [DOI] [PubMed] [Google Scholar]
- 16. Driffield K, Miller K, Bostock JM, O'Neill AJ, Chopra I. 2008. Increased mutability of Pseudomonas aeruginosa in biofilms. J. Antimicrob. Chemother. 61:1053–1056 [DOI] [PubMed] [Google Scholar]
- 17. Drusano GL. 1991. Human pharmacodynamics of beta-lactams, aminoglycosides and their combination. Scand. J. Infect. Dis. Suppl. 74:235–248 [PubMed] [Google Scholar]
- 18. Dudhani RV, et al. 2010. Elucidation of the pharmacokinetic/pharmacodynamic determinant of colistin activity against Pseudomonas aeruginosa in murine thigh and lung infection models. Antimicrob. Agents Chemother. 54:1117–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fiekers JF. 1983. Effects of the aminoglycoside antibiotics, streptomycin and neomycin, on neuromuscular-transmission. 1. Pre-synaptic considerations. J. Pharmacol. Exp. Ther. 225:487–495 [PubMed] [Google Scholar]
- 20. Frederiksen B, Koch C, Høiby N. 1997. Antibiotic treatment of initial colonization with Pseudomonas aeruginosa postpones chronic infection and prevents deterioration of pulmonary function in cystic fibrosis. Pediatr. Pulmonol. 23:330–335 [DOI] [PubMed] [Google Scholar]
- 21. Fuentes F, Martin M, Izquierdo J, Gomez-Lus M, Prieto J. 1995. In vivo and in vitro study of several pharmacodynamic effects of meropenem. Scand. J. Infect. Dis. 27:469–474 [DOI] [PubMed] [Google Scholar]
- 22. Gerber A, et al. 1983. Impact of dosing intervals on activity of gentamicin and ticarcillin against Pseudomonas aeruginosa in granulocytopenic mice. J. Infect. Dis. 147:910–917 [DOI] [PubMed] [Google Scholar]
- 23. Gibaldi M, Perrier D. 1982. Pharmacokinetics, 2nd ed, p 451–457 Marcel Dekker, Inc., New York, NY [Google Scholar]
- 24. Gudmundsson S, Erlendsdottir H, Gottfredsson M, Gudmundsson A. 1990. The postantibiotic effect induced by antimicrobial combinations. Scand. J. Infect. Dis. Suppl. 74:80–93 [PubMed] [Google Scholar]
- 25. Haagensen JAJ, et al. 2007. Differentiation and distribution of colistin- and sodium dodecyl sulfate-tolerant cells in Pseudomonas aeruginosa biofilms. J. Bacteriol. 189:28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hansen MC, Palmer RJ, Jr, Udsen C, White DC, Molin S. 2001. Assessment of GFP fluorescence in cells of Streptococcus gordonii under conditions of low pH and low oxygen concentration. Microbiology 147:1383–1391 [DOI] [PubMed] [Google Scholar]
- 27. Harmsen M, Lappann M, Knochel S, Molin S. 2010. Role of extracellular DNA during biofilm formation by Listeria monocytogenes. Appl. Environ. Microbiol. 76:2271–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hau J, Hoosier GLV. 2003. Handbook of laboratory animal science, vol. I. Essential principles and practices, p 351–389 CRC Press LLC, Boca Raton, FL [Google Scholar]
- 29. Hau J, Hoosier GLV. 2003. Handbook of laboratory animal science, vol. I. Essential principles and practices, p 413–455 CRC Press LLC, Boca Raton, FL [Google Scholar]
- 30. Hengzhuang W, Wu H, Ciofu O, Song Z, Høiby N. 2011. Pharmacokinetics/pharmacodynamics of colistin and imipenem on mucoid and nonmucoid Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 55:4469–4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Herrmann G, et al. 2010. Colistin-tobramycin combinations are superior to monotherapy concerning the killing of biofilm Pseudomonas aeruginosa. J. Infect. Dis. 202:1585–1592 [DOI] [PubMed] [Google Scholar]
- 32. Heydorn A, et al. 2002. Statistical analysis of Pseudomonas aeruginosa biofilm development: impact of mutations in genes involved in twitching motility, cell-to-cell signaling, and stationary-phase sigma factor expression. Appl. Environ. Microbiol. 68:2008–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoffmann N, et al. 2007. Azithromycin blocks quorum sensing and alginate polymer formation and increases the sensitivity to serum and stationary-growth-phase killing of Pseudomonas aeruginosa and attenuates chronic P. aeruginosa lung infection in Cftr(−/−) mice. Antimicrob. Agents Chemother. 51:3677–3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. 2010. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 35:322–332 [DOI] [PubMed] [Google Scholar]
- 35. Høiby N, Johansen H. 1995. Ciprofloxacin in sweat and antibiotic resistance. The Copenhagen Study Group on Antibiotics in Sweat. Lancet 346:1235. [DOI] [PubMed] [Google Scholar]
- 36. Høiby N, et al. 2001. Pseudomonas aeruginosa and the in vitro and in vivo biofilm mode of growth. Microbes Infect. 3:23–35 [DOI] [PubMed] [Google Scholar]
- 37. Jaccard C, et al. 1998. Prospective randomized comparison of imipenem-cilastatin and piperacillin-tazobactam in nosocomial pneumonia or peritonitis. Antimicrob. Agents Chemother. 42:2966–2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jensen PO, et al. 2007. Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology 153:1329–1338 [DOI] [PubMed] [Google Scholar]
- 39. Leroy P, Decolin D, Nicolas S, Archimbault P, Nicolas A. 1989. Residue determination of two co-administered antibacterial agents—cephalexin and colistin—in calf tissues using high-performance liquid chromatography and microbiological methods. J. Pharm. Biomed. Anal. 7:1837–1846 [DOI] [PubMed] [Google Scholar]
- 40. Molin S, Tolker-Nielsen T. 2003. Gene transfer occurs with enhanced efficiency in biofilms and induces enhanced stabilisation of the biofilm structure. Curr. Opin. Biotechnol. 14:255–261 [DOI] [PubMed] [Google Scholar]
- 41. Mouton JW, Den Hollander J. 1994. Killing of Pseudomonas aeruginosa during continuous and intermittent infusion of ceftazidime in an in vitro pharmacokinetic model. Antimicrob. Agents Chemother. 38:931–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mouton JW, Vinks AA, Punt NC. 1997. Pharmacokinetic-pharmacodynamic modeling of activity of ceftazidime during continuous and intermittent infusion. Antimicrob. Agents Chemother. 41:733–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Munckhof WJ, Giles C, Turnidge JD. 2001. Post-antibiotic growth suppression of linezolid against Gram-positive bacteria. J. Antimicrob. Chemother. 47:879–883 [DOI] [PubMed] [Google Scholar]
- 44. Pamp SJ, Gjermansen M, Johansen HK, Tolker-Nielsen T. 2008. Tolerance to the antimicrobial peptide colistin in Pseudomonas aeruginosa biofilms is linked to metabolically active cells, and depends on the pmr and mexAB-oprM genes. Mol. Microbiol. 68:223–240 [DOI] [PubMed] [Google Scholar]
- 45. Pedersen SS, Shand GH, Hansen BL, Hansen GN. 1990. Induction of experimental chronic Pseudomonas aeruginosa lung infection with P. aeruginosa entrapped in alginate microspheres. APMIS 98:203–211 [PubMed] [Google Scholar]
- 46. Plachouras D, et al. 2007. In vitro postantibiotic effect of colistin on multidrug-resistant Acinetobacter baumannii. Diagn. Microbiol. Infect. Dis. 57:419–422 [DOI] [PubMed] [Google Scholar]
- 47. Ratjen F, et al. 2006. Pharmacokinetics of inhaled colistin in patients with cystic fibrosis. J. Antimicrob. Chemother. 57:306–311 [DOI] [PubMed] [Google Scholar]
- 48. Rogers GB, Hoffman LR, Doring G. 2011. Novel concepts in evaluating antimicrobial therapy for bacterial lung infections in patients with cystic fibrosis. J. Cyst. Fibros. 10:387–400 [DOI] [PubMed] [Google Scholar]
- 49. Safdar N, Andes D, Craig WA. 2004. In vivo pharmacodynamic activity of daptomycin. Antimicrob. Agents Chemother. 48:63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stewart PS. 1996. Theoretical aspects of antibiotic diffusion into microbial biofilms. Antimicrob. Agents Chemother. 40:2517–2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Turnidge JD. 1998. The pharmacodynamics of beta-lactams. Clin. Infect. Dis. 27:10–22 [DOI] [PubMed] [Google Scholar]
- 52. Tyson GW, et al. 2004. Community structure and metabolism through reconstruction of microbial genomes from the environment. Nature 428:37–43 [DOI] [PubMed] [Google Scholar]
- 53. Vogelman B, Craig WA. 1986. Kinetics of antimicrobial activity. J. Pediatr. 108:835–840 [DOI] [PubMed] [Google Scholar]
- 54. Wu H, et al. 2000. Detection of N-acylhomoserine lactones in lung tissues of mice infected with Pseudomonas aeruginosa. Microbiology 146:2481–2493 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.