Abstract
The global control of tuberculosis (TB) is at risk by the spread of multidrug-resistant TB (MDR TB). Treatment of MDR TB is lengthy and involves injected drugs, such as capreomycin, that have severe side effects. It was previously reported that a single daily dose of inhaled capreomycin had a positive effect on the bacterial burden of TB-infected guinea pigs. The modest effect observed was possibly due to a dose that resulted in insufficient time of exposure to therapeutic systemic and local levels of the drug. In order to determine the length of time that systemic and local drug concentrations are above therapeutic levels during the treatment period, the present study investigated the disposition of capreomycin powders after sequential pulmonary administration of doses of 20 mg/kg of body weight. Capreomycin concentrations in bronchoalveolar lavage fluid and lung tissue of animals receiving a series of one, two, or three doses of capreomycin inhalable powder were significantly higher (50- to 100-fold) at all time points than plasma concentrations at the same time points or those observed in animals receiving capreomycin solution by intramuscular (i.m.) injection (10- to 100-fold higher). Notably, at the end of each dosing period, capreomycin concentrations in the lungs were approximately 100-fold higher than those in plasma and severalfold higher than the MIC, suggesting that sufficient capreomycin remains in the lung environment to kill Mycobacterium tuberculosis. No accumulation of capreomycin powder was detected in the lungs after 3 pulmonary doses. These results indicate that the systemic disposition of capreomycin after inhalation is the same as when injected i.m. with the advantage that higher drug concentrations are present at all times in the lungs, the primary site of infection.
INTRODUCTION
Multidrug-resistant tuberculosis (MDR TB) is an increasing challenge for the treatment and control programs of TB, a global deadly disease. Inappropriate treatment regimens with “first-line” drugs (mainly isoniazid and rifampin) and poor patient adherence to treatment have led to the increase in the number of MDR TB cases and, lately, the emergence of extensively drug-resistant cases (XDR TB) (8). Current international guidelines for the treatment of MDR TB recommend a treatment course of at least 18 months of “second-line” agents, including at least one of the following: capreomycin, ethionamide, kanamycin, or amikacin (22). With the exception of ethionamide, all these are administered by injection. These agents are more toxic, than generally not well tolerated, and less effective than first-line agents (19). While MDR TB is difficult to cure, XDR TB is often fatal; therefore, the judicious use of second-line drugs must be ensured to cure MDR TB or decrease its transmission and prevent emergence of XDR TB. Despite new drugs being evaluated, the present situation requires action to improve existing drug efficacy, delivery strategies, and mechanisms that support patient adherence to the prescribed treatment (11).
The major limiting factor for the use of capreomycin in the treatment of MDR TB is its severe systemic side effects. These include anorexia, thirst, anemia, hearing loss, and, notably, nephrotoxicity (proteinuria, hematuria, nitrogen metabolism, and electrolyte disturbances) (3, 7, 18). It was previously postulated that formulation of capreomycin into solid particles for inhalation would increase drug concentrations at the site of infection, the lungs, while decreasing systemic toxicity, thus rendering a more effective approach for the treatment of pulmonary TB. Previous studies by our group reported the disposition of a single dose of capreomycin solution administered by the intravenous (i.v.) and intramuscular (i.m.) routes and of solid particles delivered by the pulmonary route in guinea pigs (5). Pharmacokinetic (PK) parameters after administration of capreomycin solutions or suspensions have been determined in only mice and humans (2, 3, 7, 9, 14–16, 20), and there are no reports of these studies in guinea pigs, a species known to have drug disposition characteristics that are different from those of other rodents. Our study showed that capreomycin plasma concentrations were comparable in animals receiving capreomycin by the systemic and pulmonary routes after 2 h of drug administration (5). PK parameters indicated that capreomycin was eliminated at a lower rate when delivered by the pulmonary route than when delivered i.v. and i.m., resulting in a significantly longer half-life. Subsequent studies evaluated the effect of daily doses of these capreomycin formulations on the extent of infection in the guinea pig model of TB (5). Animals receiving capreomycin dry powder aerosols showed a significantly smaller degree of inflammation, bacterial burden (CFU/ml), and percentage of lung tissue affected by granulomas and caseous necrosis (by histopathology) compared to those with the conventional treatment. However, in this efficacy study, the doses of capreomycin were delivered as an aerosol by passive, nose-only exposure to awake animals as required for a 1-month daily dosing regimen. Since guinea pigs are obligate nose breathers, this method of aerosol delivery resulted in a fraction (2 mg/kg of body weight) of the nominal dose (14.5 mg/kg) reaching the periphery of the lungs, unlike the anticipated situation in humans in which inhalation through the mouth would result in a larger proportion of the dose reaching the periphery of the lungs. A PK study design in which particles were insufflated by the endotracheal tube in sequential dosing more accurately reflects human dosing and was considered a prerequisite for understanding dosing in the phase IA clinical studies in healthy volunteers.
The present study was designed to build upon the findings described above to evaluate the effect of directly administering sequential known doses of capreomycin powders by the pulmonary route on local and systemic levels of the drug. The goal of this study was to determine the disposition of sequential doses of inhaled capreomycin and the ability to sustain local and systemic therapeutic levels for TB treatment. PK parameters were determined to characterize the disposition of sequential doses of capreomycin powder for inhalation. Capreomycin plasma levels, peak concentration, and time to achieve that concentration will aid in the calculation of a possible dose size to attain appropriate therapeutic levels of the drug, whereas mean residence time, half-life, and clearance will dictate the dosing regimen required to maintain therapeutic drug levels.
MATERIALS AND METHODS
Materials.
Capreomycin sulfate powder drug product (98% purity; Zhejiang Hisun Pharmaceutical Co., China) was employed to prepare the solution for injection. Capreomycin powder for inhalation (batch MS08MED035 BR7) was manufactured by Micro-Sphere SA (Madonna del Piano, Switzerland) by procedures described previously (4, 5) and used as supplied. Capreomycin powder for inhalation was characterized in terms of drug content, volume, aerodynamic diameters, and fine particle fractions (FPF). Capreomycin content in the powder for inhalation was 80%, as determined by high-performance liquid chromatography (HPLC).
Powders had a volume diameter of 2.71 μm and a geometric standard deviation (GSD) of 1.74, as determined by the HELOS system with a RODOS dry dispersing unit (Sympatec Inc., Lawrenceville, NJ). Analysis of the powders by cascade impaction (Andersen eight-stage Mark II cascade impactor; Thermo-Electron, Waltham, MA) indicated that the powders had appropriate aerodynamic properties for inhalation, with a mass median aerodynamic diameter (MMAD) of 3.7 μm and fine particle fractions for FPFTD values of <5.8 μm and <3.3 μm of 74.6% and 31.5%, respectively, where TD is total dose.
Animals.
Male guinea pigs (n = 86) weighing an average of 598.5 ± 74.0 g were employed in these studies. The animals were housed in a 12-h light/12-h dark cycle and a constant temperature environment of 22°C. A standard diet and water were supplied ad libitum. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of North Carolina.
Twenty-four hours before the study, each animal underwent cannulation of the right external jugular vein for continuous blood sampling. The cannula was routed subcutaneously, externalized at the neck, and secured to musculature.
Treatments.
The animals were randomly divided into three groups, with each receiving a different number of pulmonary doses (20 mg/kg per dose) of capreomycin powder per day as outlined in Table 1. Animals receiving a single dose of capreomycin solution by the i.m. route were employed as controls. The dose employed in this study was selected based on previous studies performed in our laboratory (5). Capreomycin solutions were prepared by dissolving a weighed amount of capreomycin sulfate powder drug in water for injection and filtering the solution through a 0.22-μm Millex GS filter (Millipore, Cork, Ireland). Capreomycin powders for inhalation were preweighed in a gelatin capsule (V-Caps; Capsugel, Greenwood, SC) and administered to the animals by insufflation. For this procedure, each animal was anesthetized and the trachea was visualized with a laryngoscope. A small-animal insufflator (Penn Century Inc., Philadelphia, PA) was inserted into the trachea and placed at a distance of 1 cm from the carina. Capreomycin powders were aerosolized from the reservoir of the insufflator into the animal's airways with the help of 5 ml of air from an empty syringe. The insufflator chamber and the tube containing the capreomycin powder were weighed carefully before and after delivery to the animal to verify that the entire dose was delivered.
Table 1.
Study design for the disposition of capreomycin in guinea pigs
| Group | Formulation (route of administration) | No. of dosesa/day | Dosing time(s) (h) | Time(s) of BAL (h) | No. of guinea pigs per respective BAL time point (total no. of guinea pigs per group) |
|---|---|---|---|---|---|
| 1 | Powder (pulmonary) | One | 0 | 0.5, 2, 4, 8 | 8, 8, 9, 7 (32) |
| 2 | Powder (pulmonary) | Two | 0, 8 | 8.5, 16 | 6, 8 (14) |
| 3 | Powder (pulmonary) | Three | 0, 8, 16 | 16.5, 24 | 8, 8 (16) |
| 4 | Solution (i.m.) | One | 0 | 0.5, 2, 4, 8 | 6, 6, 6, 6 (24) |
20 mg/kg each.
Blood samples (0.25 ml) were collected from each animal into heparinized microcentrifuge tubes at 0, 0.08, 0.25, 0.50, 1, 1.5, 2, 3, 4, 5, 6, and 8 h after administration of capreomycin powder or solutions. Sterile saline solution was used to replace the volume of blood drawn at each sample collection. Samples were then centrifuged, and plasma was separated and stored at −80°C until analysis. For animals receiving a single dose, lung tissues were collected and a bronchoalveolar lavage (BAL) was performed at 0.5, 2, 4, and 8 h to determine capreomycin local concentrations in tissue and airways. Since BAL is a terminal procedure for guinea pigs, extra animals were employed for each time point as indicated in Table 1. For animals receiving two or three doses, lung tissue and BAL fluid were collected at 0.5 and 8 h after each dose as shown in Table 1. After collection of the last blood sample, animals were euthanized by exsanguination and BAL was performed by instilling 5 ml of sterile saline as described before (6). Tissues were visually inspected for any signs of toxicity. Lungs and tracheae were resected and frozen immediately at −80°C until analysis.
Sample analysis.
Capreomycin was extracted from plasma samples by acid extraction using perchloric acid solution followed by vortex mixing and centrifugation. The acid was then neutralized with a basic mix of 1:10 KOH/K2HPO4 solution. Supernatant was transferred to glass injection vials for HPLC analysis. A standard curve was constructed by spiking known concentrations of capreomycin (0.5 to 35 μg/ml) into blank plasma and treating them as described before (5). To determine capreomycin levels in lung tissue, the right lower lobe for each animal was weighed and homogenized in phosphate buffer, pH 6, and capreomycin was extracted from the homogenate as described for plasma samples. A standard curve was constructed by spiking known concentrations of capreomycin (0.5 to 35 μg/ml) into blank lung homogenates and treating them in the same manner as the plasma samples. Capreomycin in BAL fluid was determined by first sonicating BAL fluid samples to disrupt cell contents and then vortex mixing and centrifuging them. The supernatant was analyzed by HPLC for drug content.
Capreomycin concentrations in plasma, BAL fluid, and tissues were determined by HPLC as follows. The system (Waters Corp., Milford, MA) consisted of a model 510 pump, a model 717 plus autosampler, and a model 480 UV detector set at a wavelength of 268 nm. The system was equipped with a Nova-Pak C18 guard column and a Waters Spherisorb ODS2 analytical column (5-μm particle size; 4.6-mm diameter; 150-mm length). The mobile phase consisted of 92% phosphate buffer, pH 6, 8% acetonitrile, and 0.3% heptafluorobutyric acid. The limit of capreomycin detection by this assay in all samples was 0.05 μg/ml. The concentration reported in BAL fluid was calculated taking into account the volume of epithelial lining fluid in guinea pigs (10) and the volume of saline solution used to perform the lavage.
Data analysis. (i) Pharmacokinetic analysis.
Capreomycin plasma concentration-versus-time data were analyzed by noncompartmental methods (WinNonlin; Pharsight Corporation, Mountain View, CA) to obtain the area under the curve (AUC), the apparent total body clearance (CL), the mean residence time (MRT), the half-life (t1/2), and the elimination rate constant (kel). The maximum capreomycin concentration (Cmax) and time to obtain the maximum capreomycin concentration (Tmax) were determined directly from the plasma-versus-time profiles for each individual animal. Relative bioavailability (F′) was calculated using the area under the curve from 0 h to t (AUC0-t) values with the i.m. administration as a reference.
(ii) Statistical analysis.
Data from plasma, BAL fluid, lung tissue, and PK parameters were subjected to analysis of variance (ANOVA) and the least-squares significant-differences multiple-comparison method. A probability level of 5% (P < 0.05) was considered to be statistically significant.
RESULTS
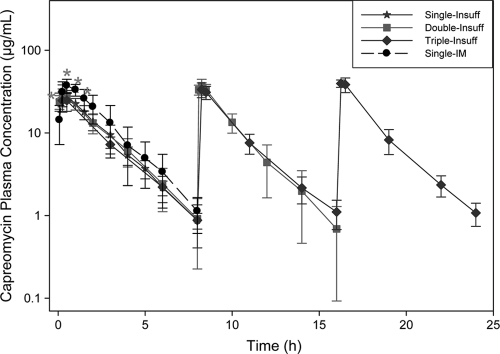
Capreomycin plasma concentration-versus-time curves are shown in Fig. 1. Similar capreomycin plasma concentrations were observed for all animals after receiving capreomycin powders by the pulmonary route. Compared to i.m. injection, capreomycin was absorbed faster when administered by the pulmonary route, as evidenced by the significantly higher (P = 0.0003) plasma concentrations at initial time points (0.08 and 0.25 h) (Fig. 1). At later time points (0.5, 1.0, and 2 h), plasma concentrations were significantly higher (P = 0.005) in animals dosed by the i.m. route, but from the 2-h time point onward, plasma concentrations were similar (P = 0.5) for both i.m. and pulmonary routes of administration. These parallel terminal phases indicated the same first-order elimination mechanism for both routes of administration.
Fig 1.
Average-plasma-concentration-versus-time curves after administration of capreomycin solution or powder for inhalation for the following different dosing regimens (average ± standard deviation, n = 8 to 14): one dose of capreomycin powder for inhalation by insufflation (Single-Insuff), two doses of capreomycin powder for inhalation by insufflation (Double-Insuff), three doses of capreomycin powder for inhalation by insufflation (Triple-Insuff), and one dose of capreomycin solution by intramuscular administration (Single-IM). Asterisks indicate significant differences in concentration at that time point.
BAL was performed in all animals at different time points, as indicated in Table 1, and lung tissue was collected to determine local capreomycin concentrations. As expected in animals dosed by i.m. injection, capreomycin concentrations were higher in plasma than in BAL fluid or lung tissue at all time points (Fig. 1 and Table 2). In these animals, BAL fluid and lung capreomycin concentrations (Table 2) were highest (P < 0.0001) at the 0.5-h time point and decreased gradually, falling under detection limits in most animals 8 h after receiving their dose. A similar concentration-time trend was observed in animals dosed with capreomycin powder by the pulmonary route, with maximum drug concentrations in BAL fluid and lung tissue observed at the 0.5-h time point (Table 2) and decreasing gradually until 8 h. However, unlike those in the i.m. group, capreomycin concentrations remained more than 15-fold above the detection limit in BAL fluid and more than 100-fold above the detection limit in lung tissue (Table 2) in animals dosed by the pulmonary route. Moreover, while local drug concentrations were of magnitudes similar to those in plasma at all time points for i.m.-dosed animals, capreomycin concentrations were significantly higher in BAL fluid (5- to 10-fold; P < 0.0001) and lung tissue (10- to 100-fold; P < 0.0001) than in plasma (Table 2 and Fig. 1) at all time points for animals dosed by the pulmonary route.
Table 2.
Average capreomycin concentrations in BAL fluid and lung tissue of animals receiving a single dose of capreomycin by intramuscular injection or pulmonary administration at different time points after dose administration
| Route of administration and time point (h) | Capreomycin concn ina: |
|
|---|---|---|
| BAL fluid (μg/ml) | Lung (μg/g) | |
| i.m. | ||
| 0.5 | 2.9 ± 1.1 | 16.9 ± 5.4 |
| 2 | 0.4 ± 0.0 | 14.9 ± 3.7 |
| 4 | 0.6 ± 0.3 | 11.2 ± 2.8 |
| 8 | 0.1 ± 0.1 | 1.9 ± 2.0 |
| Pulmonary | ||
| 0.5 | 264.6 ± 82.2* | 475.2 ± 243.64* |
| 2 | 83.1 ± 72.2* | 194.9 ± 51.1* |
| 4 | 7.1 ± 3.4* | 77.4 ± 23.1* |
| 8 | 7.2 ± 6.6* | 106.8 ± 28.9* |
Average ± standard deviation. n = 6 to 9.
, significantly higher than i.m. concentration at that time point.
Furthermore, when comparing concentrations at each time point for both routes of administration, capreomycin concentrations in the BAL fluid of animals dosed by the pulmonary route were 70- to 170-fold higher (P < 0.0001) than those observed in i.m.-dosed animals (Table 2). Likewise, capreomycin lung tissue concentrations were 10- to 63-fold higher (P < 0.0001) in animals dosed by the pulmonary route than in those receiving i.m. injection (Table 2).
The capreomycin concentration trends observed in plasma, BAL fluid, and lung tissue of animals dosed by the pulmonary route (Fig. 1 and Table 2) indicate that capreomycin absorption was limited by dissolution of the powder in the airways. The higher initial concentrations in lung tissue (Table 2) and in plasma (Fig. 1) indicated that after dissolution, absorption into lung tissue and plasma occurred very quickly. However, the advantage of pulmonary administration of capreomycin is that lung tissue concentrations remained above the MIC even after plasma levels were at or below detection limits (8 h) (Fig. 1 and Table 2).
Table 3 shows BAL fluid and lung tissue concentrations at the maximum and minimum plasma concentrations (Cmax at 0.5 h and Cmin at 8 h after administration of each dose). At the time of Cmax, animals receiving a single dose of capreomycin powder by the pulmonary route exhibited BAL fluid concentrations that were significantly higher (264.6 μg/ml; P < 0.0001) than those observed in the i.m. group (2.9 μg/ml) (Table 3). It is important to note that in animals dosed by the pulmonary route, BAL fluid concentrations were significantly higher (3- to 6-fold; P < 0.0001) than those observed in plasma at that time point (Fig. 1 and Table 3). Lung tissue concentrations were also the highest (P < 0.0001) after pulmonary administration of a single dose of capreomycin, and they were similar (P < 0.0001) for two and three administered doses of powder (Table 3). Notably, lung tissue concentrations were 200- to 300-fold higher in animals dosed by the pulmonary route than in those in the i.m. group. Capreomycin concentrations were also approximately 2-fold higher in lung tissue than in BAL fluid (Table 3) and 4- to 10-fold higher in lung tissue (P < 0.0001) than in plasma at those time points (0.5, 8.5, and 16.5 h) (Fig. 1 and Table 3).
Table 3.
Average capreomycin concentrations in BAL fluid and lung tissue of animals receiving different numbers of sequential doses of capreomycin by intramuscular injection or pulmonary administration at the times of Cmax and Cmin
| Route of administration (dose no. within 1 day) and time point (h) | Capreomycin concn ina: |
|
|---|---|---|
| BAL fluid (μg/ml) | Lung (μg/g) | |
| i.m. (one) | ||
| 0.5 | 2.9 ± 1.1 | 16.9 ± 5.4 |
| 8 | 0.1 ± 0.1 | 1.9 ± 2.0 |
| Pulmonary (one) | ||
| 0.5 | 264.6 ± 82.2** | 475.2 ± 243.64** |
| 8 | 7.2 ± 6.6** | 106.8 ± 28.9* |
| Pulmonary (two) | ||
| 8.5 | 105.9 ± 51.3* | 294.4 ± 129.3* |
| 16 | 2.5 ± 1.2* | 100.8 ± 37.4* |
| Pulmonary (three) | ||
| 16.5 | 153.4 ± 50.4* | 275.5 ± 61.1* |
| 24 | 4.6 ± 1.9* | 102.4 ± 36.8* |
Average ± standard deviation. n = 6 to 8.
, significantly different from i.m. concentration at same time point;
, significantly different from i.m. concentration and concentrations after two doses and three doses at same time point.
Capreomycin concentrations in BAL fluid and lung tissue were also determined at the end of the dosing period for each regimen (time of Cmin = 8, 16, or 24 h) to compare local drug concentrations with systemic concentrations at the same time points (Fig. 1 and Table 3). Consistent with other time points, BAL fluid concentrations in animals dosed by the pulmonary route at the time of Cmin were 35- to 105-fold higher (P < 0.0001) than those in i.m.-dosed animals. Remarkably, these BAL fluid concentrations in animals dosed by the pulmonary route were 2- to 3-fold higher (P = 0.003) than the MIC (MIC = 2.5 μg/ml) for capreomycin and, consequently, the drug was still available to act on bacteria in the airways, whereas plasma concentrations were below MIC levels at this time point (Fig. 1 and Table 3), highlighting the advantage of administering drugs by the pulmonary route.
Likewise, lung tissue concentrations in animals dosed by the pulmonary route were 60- to 80-fold higher (P < 0.0001) at the time of Cmin than those in i.m.-dosed animals and approximately 100-fold higher than plasma concentrations (P < 0.0001) at that time point. Most importantly, capreomycin levels in lung tissue were 40- to 50-fold times higher than the MIC (P < 0.0001), a result which suggests that some drug remained present in the lung to kill bacteria at the site of colonization even after systemic levels fell below the MIC.
Table 3 also shows that capreomycin levels in lung tissue were comparable (P = 0.2, no statistical difference) in animals receiving one, two, and three doses of capreomycin powder in a day, indicating that there is no drug accumulation in the lungs which may have had undesired effects on lung function.
PK parameters obtained by noncompartmental methods for capreomycin disposition are presented in Table 4. A comparison of the single/first doses among all treatments (lines 1 to 9) indicates that the AUCs and Cmax (lines 1, 2, and 7) were significantly larger for i.m.-dosed animals than for animals dosed by the pulmonary route (P < 0.0001) and similar among animals dosed by the pulmonary route. Clearance (CL; line 3) was significantly smaller (P = 0.0008) and the Tmax (line 8) was significantly longer (P < 0.0001) in i.m.-dosed animals than in animals dosed by the pulmonary route, indicating that capreomycin may be absorbed faster into systemic circulation from the lungs than from muscle. No significant differences (P = 0.1112) were observed in bioavailability (F′) following the first dose among groups receiving capreomycin by the pulmonary route.
Table 4.
Pharmacokinetic parameters obtained by noncompartmental analysis after administration of capreomycin formulations by the i.m. or pulmonary route
| Dose and line no. | Parametera | Value forb: |
|||
|---|---|---|---|---|---|
| i.m. single dose | Insufflation |
||||
| Single dose | Double dose | Triple dose | |||
| First dose | |||||
| 1 | AUC0-t (μg · h/ml) | 104.16 ± 25.561 | 75.54 ± 16.202 | 74.42 ± 11.982 | 64.92 ± 11.422 |
| 2 | AUC0-∞ (μg · h/ml) | 116.29 ± 47.631 | 79.04 ± 15.812 | 76.55 ± 12.832 | 67.27 ± 11.282 |
| 3 | CL (ml/h · kg) | 0.21 ± 0.033 | 0.25 ± 0.052 | 0.27 ± 0.051,2 | 0.31 ± 0.051 |
| 4 | kel (h−1) | 0.45 ± 0.10 | 0.50 ± 0.10 | 0.53 ± 0.16 | 0.44 ± 0.09 |
| 5 | t1/2 (h) | 1.51 ± 0.39 | 1.45 ± 0.33 | 1.39 ± 0.34 | 1.62 ± 0.32 |
| 6 | MRT (h) | 1.87 ± 0.44 | 2.01 ± 0.38 | 1.92 ± 0.31 | 1.84 ± 0.31 |
| 7 | Cmax (μg/ml) | 38.47 ± 4.201 | 30.16 ± 7.142 | 31.38 ± 4.812 | 26.98 ± 5.262d |
| 8 | Tmax (h) | 0.68 ± 0.251 | 0.35 ± 0.132 | 0.27 ± 0.112 | 0.31 ± 0.122 |
| 9 | F′ | 0.73 ± 0.16 | 0.71 ± 0.11 | 0.62 ± 0.11 | |
| Second dose | |||||
| 10 | AUC0-t (μg · h/ml) | 74.97 ± 14.78 | 78.09 ± 16.64 | ||
| 11 | AUC0-∞ (μg · h/ml) | 77.23 ± 15.25 | 80.74 ± 17.24 | ||
| 12 | CL (ml/h · kg) | 0.27 ± 0.06 | 0.27 ± 0.07 | ||
| 13 | kel (h−1) | 0.51 ± 0.19 | 0.46 ± 0.06 | ||
| 14 | t1/2 (h) | 1.53 ± 0.53 | 1.54 ± 0.18 | ||
| 15 | MRT (h) | 1.68 ± 0.53 | 1.67 ± 0.20 | ||
| 16 | Cmax (μg/ml) | 33.26 ± 3.24 | 34.06 ± 6.77 | ||
| 17 | Tmax (h) | 8.21 ± 0.08 | 8.29 ± 0.09 | ||
| 18 | F′ | 0.72 ± 0.14 | 0.75 ± 0.16 | ||
| Third dose | |||||
| 19 | AUC0-t (μg · h/ml) | 92.69 ± 18.90c | |||
| 20 | AUC0-∞ (μg · h/ml) | 103.66 ± 24.34c | |||
| 21 | CL (ml/h · kg) | 0.22 ± 0.06d | |||
| 22 | kel (h−1) | 0.39 ± 0.06 | |||
| 23 | t1/2 (h) | 1.80 ± 0.31 | |||
| 24 | MRT (h) | 1.50 ± 0.22d | |||
| 25 | Cmax (μg/ml) | 41.98 ± 7.83c | |||
| 26 | Tmax (h) | 16.34 ± 0.13 | |||
| 27 | F′ | 0.89 ± 0.18c | |||
AUC0-t, area under the curve from 0 h to t; AUC0-∞, area under the curve from 0 h to infinity; kel, elimination rate constant; t1/2, half-life; MRT, mean residence time; Cmax, maximum concentration; Tmax, time at which Cmax occurs; F′, relative bioavailability compared to that of the i.m. injection.
Mean ± standard deviation. n = 8 to 14. Each dose contained 20 mg/kg capreomycin. Numeric superscripts show the relative ranks of values. When the means are not significantly different, the same superscript is used.
Significantly larger for this dose than any other dose in this treatment.
Significantly smaller for this dose than any other dose in this treatment.
A comparison of PK parameters in animals receiving more than one dose per day indicated that in animals receiving two sequential doses in a day, there were no significant differences in PK parameters between the first and second doses (lines 1 to 18).
However, significant differences were observed in PK parameters among doses in animals receiving three sequential doses. The AUCs and Cmax (lines 19, 20, and 25) were significantly larger for the third dose than for the other two doses (P = 0.0027 and P = 0.0002, respectively), possibly due to a small quantity of capreomycin still remaining in systemic circulation from the previous two doses in two animals of that group. CL and the MRT (lines 21 and 24) were significantly smaller (P = 0.005 and P = 0.017, respectively) after the first dose, an outcome which is reflected in a significantly higher bioavailability (line 27; P = 0.002) and correlates well with higher concentrations in BAL fluid and lung tissue for this group at the end of the dosing period. Interestingly, capreomycin bioavailability after the third dose of aerosolized powder was higher (P = 0.0025) than that after any other dosing regimen.
DISCUSSION
Capreomycin, an existing second-line antibiotic for the treatment of TB, has received renewed interest because it is effective against MDR TB. There are few drugs active against nonreplicating bacteria, and capreomycin is the only one that has potential against bacilli persistent in human tissues (12). However, its use has been limited due to the associated toxicities to the auditory and visual nerves as well as to renal toxicity (3, 7, 17, 18). Furthermore, it has been reported that capreomycin absorption after i.m. injection can be incomplete or delayed when the same site of injection is used repeatedly (1).
Previous studies conducted by our group administering a daily single dose of inhaled capreomycin powder to TB-infected guinea pigs showed a significant decrease in the lung bacterial burden compared to that of the conventional parenteral treatment (5). Histopathological analysis showed a positive effect of the inhaled treatment on the lungs and spleen. This treatment also resulted in a significant decrease in the bacterial burden in the lungs, but not the spleen, of infected animals, possibly due to systemic drug levels that were transiently above the MIC (5). Therefore, this study determined the disposition of sequential doses of inhaled capreomycin and the ability of pulmonary administration to sustain local and systemic therapeutic levels for TB treatment and addressed the concern of powder accumulation in the lungs, which may be detrimental for the patient.
The disposition of each capreomycin powder dose was assessed and characterized by the respective PK parameters. In the present study, capreomycin powders with particle sizes, drug content, and aerodynamic properties similar to those used in the previous study (5) were employed. Yet the disposition of capreomycin that was determined in previous studies (employing capreomycin from Eli Lilly and Co.), was different from the disposition of capreomycin in the present studies (employing capreomycin from Zhejian Hisun Pharmaceutical Co.) for single-dose/first-dose animals. In previous studies, capreomycin concentrations were undetectable in plasma 6 h after dose administration, whereas these levels remain detectable 8 h after dosing in the present studies. In previous studies, no capreomycin was detected in the BAL fluid of animals dosed by the pulmonary route at the end of the dosing period, whereas in the present study, capreomycin concentrations in the BAL fluid at the end of the dosing period were well above detection limits. The AUCs and Cmax were significantly higher, clearance was faster, and the MRT was shorter for capreomycin in the first study (5) than in the present study. Differences in these parameters were reflected in the higher bioavailability of capreomycin powders for inhalation that was observed in the present study. These differences in capreomycin disposition may be attributed to differences in the composition of the drug substance (chemical entity). Capreomycin is isolated as 4 isomers from fermentation broths (IA, IB, IIA, IIB), and drug substances primarily contain isomers IA and IB. Based on the proportions of the areas for each isomer analyzed by HPLC, we determined that there was approximately 40% of IA and 60% of IB in the capreomycin drug substance from Eli Lilly and Co., whereas there was approximately 65% of IA and 35% of IB in the capreomycin drug substance from Zhejian Hisun Pharmaceutical Co. Even though it would be desirable to study the disposition of each capreomycin isomer and its influence on drug bioavailability, it is beyond the scope of the present study.
Disposition of capreomycin in guinea pigs was different from that observed in other species, as expected. In this study, the half-life after i.m. injection of capreomycin to guinea pigs was 1.51 h, whereas a half-life of 0.18 h is reported in mice (14) and it ranges between 2 to 3 h (2) and 4 to 6 h (1) in humans. The Tmax after capreomycin i.m. injection was 0.68 h in guinea pigs and 1 to 2 h in humans, and capreomycin is also cleared (CL) faster in guinea pigs (0.21 ml/h · kg) than in humans (0.011 ml/h · kg). Interestingly, the Cmax after administration of a 20-mg/kg dose in guinea pigs (38.47 μg/ml) is comparable to that after administration of 1 g of capreomycin in humans (20 to 47 μg/ml) (13, 21).
Le Conte et al. (14) determined capreomycin concentrations in tissues (lung, spleen, and kidney) at the same time point after i.v. administration of free and liposomal capreomycin injections, but to the best of our knowledge, the present study is the first in which local (airways and lung tissue) and systemic concentrations were evaluated and compared at different time points after sequential pulmonary administrations of an antitubercular drug. Despite differences in route of administration, formulations, dosing regimen, and animal species being employed, the comparison of results from the present study with those of Le Conte et al. emphasizes the advantage of delivering capreomycin by the pulmonary route. In the study by Le Conte et al., at a Tmax of 0.25 h, the capreomycin concentration in lung tissue was approximately half that in serum after free drug administration and about twice that in serum after liposomal administration of a 120-mg/kg dose by the i.v. route (14). This concentration was also the highest concentration observed in lung tissue after administration of the dose. In contrast, in the present study, at a Tmax of 0.5 h, capreomycin concentrations were 7- to 10-fold higher in BAL fluid and 10- to 20-fold higher in lung tissue than in plasma following administration of a 20-mg/kg dose by the pulmonary route. Furthermore, while serum levels fell below detection limits 2 h after free drug administration and 4 h after liposomal i.v. administration of a 120-mg/kg dose in the study by Le Conte et al., capreomycin plasma concentrations remained detectable up to 8 h after pulmonary administration of 20 mg/kg capreomycin powder. Most importantly, while capreomycin concentrations in lung tissue were only 2- to 3-fold higher than the MIC for capreomycin 6 h after i.v. administration, capreomycin concentrations remained 40- to 50-fold higher than the MIC in lung tissue 8 h after pulmonary administration of one-sixth of the dose in the present study, indicating that sufficient amounts of the drug are still present at the site of delivery to kill bacteria. The dose size and number of administrations did not cause any adverse events or drug accumulation in the lungs of guinea pigs, and no histopathological changes were observed as a result of powder administration.
The higher local concentrations achieved in the present study and the similarity of the capreomycin disposition after pulmonary administration to that after i.m. injection suggest that it may be possible to obtain positive effects in TB treatment by using a smaller dose of capreomycin administered by inhalation, thus reducing the likelihood of systemic adverse effects. Beyond the efficacy of an inhaled TB treatment with capreomycin, it may be possible that pulmonary administration of capreomycin powders would prevent transmission of MDR TB, since the number of viable bacteria in the lungs of infected patients should be significantly reduced by the inhaled treatment. Additional experiments may be required to address the disposition of the drug in diseased rather than healthy lungs before translating these observations into a therapeutic approach.
Conclusions.
The results of the present studies support the use of the pulmonary route to administer capreomycin for TB or MDR TB treatment, since a systemic disposition similar to that of i.m. administration can be obtained with the advantage of achieving significantly higher drug concentrations in the lung environment. The fact that these concentrations are sustained above the MIC implies that the drug may be still available in the lung for prolonged periods of time to act on local Mycobacterium tuberculosis even after systemic concentrations are below the MIC. Thus, it may be possible to use a smaller dose of capreomycin by inhalation and limit its systemic toxicity. Further studies are required to address the efficacy of such a dosing regimen and its safety in humans.
ACKNOWLEDGMENTS
We are grateful to David Edwards of the Department of Engineering and Applied Science at Harvard University and members of his group for developing the technology on which the particles employed in this study were based. In addition, we thank Bernard Fourie for discussions pertaining to the use of capreomycin in a clinical setting.
These studies were sponsored by Medicine in Need with funds from the Bill and Melinda Gates Foundation to address the Grand Challenges in Drug Delivery and Global Health, Goal C.
Footnotes
Published ahead of print 13 February 2012
REFERENCES
- 1. Arbex MA, Varella Mde C, Siqueira HR, Mello FA. 2010. Antituberculosis drugs: drug interactions, adverse effects, and use in special situations. Part 2. Second line drugs. J. Bras. Pneumol. 36:641–656 [DOI] [PubMed] [Google Scholar]
- 2. Black HR, Griffith RS, Peabody AM. 1966. Absorption, excretion, and metabolism of capreomycin in normal and diseased states. Ann. N. Y. Acad. Sci. 135:974–982 [DOI] [PubMed] [Google Scholar]
- 3. Donomae I. 1966. Capreomycin in the treatment of pulmonary tuberculosis. Ann. N. Y. Acad. Sci. 135:1011–1038 [DOI] [PubMed] [Google Scholar]
- 4. Fiegel J, et al. 2008. Preparation and in vivo evaluation of dry powder for inhalation of capreomycin. Pharm. Res. 25:805–811 [DOI] [PubMed] [Google Scholar]
- 5. Garcia-Contreras L, et al. 2007. Inhaled large porous particles of capreomycin for the treatment of tuberculosis in a guinea pig model. Antimicrob. Agents Chemother. 51:2830–2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garcia-Contreras L, et al. 2001. Immediate and short-term cellular and biochemical responses to pulmonary single-dose studies of insulin and H-MAP. Pharm. Res. 18:1685–1693 [DOI] [PubMed] [Google Scholar]
- 7. Garfield J, Jones J, Cohen N, Daly J, McClement J. 1966. The auditory, vestibular and renal effects of capreomycin in humans. Ann. N. Y. Acad. Sci. 135:1039–1046 [DOI] [PubMed] [Google Scholar]
- 8. Gothi D, Joshi JM. 2011. Resistant TB: newer drugs and community approach. Recent Pat. Antiinfect. Drug Discov. 6:27–37 [DOI] [PubMed] [Google Scholar]
- 9. Gyselen A, Verbist L, Prignot J, Cosemans J. 1965. Capreomycin in the re-treatment of patients with pulmonary tuberculosis. Tubercle 46:243–249 [DOI] [PubMed] [Google Scholar]
- 10. Harkness JE, Wagner JE. 1995. The biology and medicine of rabbits and rodents. Williams and Wilkins, Media, PA [Google Scholar]
- 11. Hedt BL, Laufer MK, Cohen T. 2011. Drug resistance surveillance in resource-poor settings: current methods and considerations for TB, HIV, and malaria. Am. J. Trop. Med. Hyg. 84:192–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heifets L, Simon J, Pham V. 2005. Capreomycin is active against non-replicating M. tuberculosis. Ann. Clin. Microbiol. Antimicrob. 4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holdiness MR. 1984. Clinical pharmacokinetics of the antituberculosis drugs. Clin. Pharmacokinet. 9:511–544 [DOI] [PubMed] [Google Scholar]
- 14. Le Conte P, et al. 1994. Pharmacokinetics, toxicity, and efficacy of liposomal capreomycin in disseminated Mycobacterium avium beige mouse model. Antimicrob. Agents Chemother. 38:2695–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee SH, et al. 2003. The impurities of capreomycin make a difference in the safety and pharmacokinetic profiles. Int. J. Antimicrob. Agents 22:81–83 [DOI] [PubMed] [Google Scholar]
- 16. Lehmann CR, et al. 1988. Capreomycin kinetics in renal impairment and clearance by hemodialysis. Am. Rev. Respir. Dis. 138:1312–1313 [DOI] [PubMed] [Google Scholar]
- 17. Magazine R, Pal M, Chogtu B, Nayak V. 2010. Capreomycin-induced optic neuritis in a case of multi-drug resistant pulmonary tuberculosis. Indian J. Pharmacol. 42:247–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller J, Popplewell A, Landwehr A, Greene M. 1966. Toxicology studies in patients on prolonged therapy with capreomycin. Ann. N. Y. Acad. Sci. 135:1047–1056 [DOI] [PubMed] [Google Scholar]
- 19. O'Brien RJ. 1993. The treatment of tuberculosis, p 207–240 In Reichman LB, Hershfield ES. (ed), Tuberculosis: a comprehensive international approach. Marcel Dekker, New York, NY [Google Scholar]
- 20. Organick AB, Wilson EM. 1968. Multiple drugs in retreatment of chronic pulmonary tuberculosis. Results with capreomycin and ethambutol. Dis. Chest 53:560–570 [DOI] [PubMed] [Google Scholar]
- 21. Tuberculosis (Edinburgh, Scotland) 2008. Capreomycin. Tuberculosis (Edinb.) 88:89–91 [DOI] [PubMed] [Google Scholar]
- 22. WHO 2011. Guidelines for the programmatic management of drug-resistant tuberculosis. 2011 update. WHO Document Production Services, Geneva, Switzerland [Google Scholar]