Abstract
Resistance-nodulation-division efflux system AdeIJK contributes to intrinsic resistance to various drug classes in Acinetobacter baumannii. By whole-genome sequencing, we have identified in clinical isolate BM4587 the adeN gene, located 813 kbp upstream from adeIJK, which encodes a TetR transcriptional regulator. In one-step mutant BM4666 overexpressing adeIJK, the deletion of cytosine 582 (C582) in the 3′ portion of this gene was responsible for a frameshift mutation resulting in the deletion of the seven C-terminal residues. trans-Complementation of this BM4587 derivative with a plasmid expressing adeN restored antibiotic susceptibility to the host associated with the loss of adeJ overexpression. The inactivation of adeN in BM4587 led to a diminished susceptibility to antibiotics that are substrates for AdeIJK and to a 5-fold increase in adeJ expression. Taken together, these results indicate that AdeN represses AdeIJK expression. Quantitative reverse transcription-PCR (qRT-PCR) demonstrated that AdeN is constitutively expressed in BM4587 and does not regulate its own expression. Deletion of cytosine 582 and a 394-bp deletion of the 3′ part of adeN were found in independent one-step adeIJK-overexpressing mutants selected from clinical isolates BM4667 and BM4651, respectively. The corresponding alterations were located in the α9 helix, which is known to be involved in dimerization, a process essential for the activity of TetR regulators. The adeN gene was detected in all of the 30 A. baumannii strains tested and in Acinetobacter calcoaceticus, Acinetobacter nosocomialis, and Acinetobacter pittii.
INTRODUCTION
Acinetobacter baumannii, a nonfermentative Gram-negative coccobacillus, is a major nosocomial pathogen responsible for epidemic infections, such as pneumonia, meningitis, and septicemia, as well as urinary tract, skin and soft tissue, and bloodstream infections with a high morbidity and mortality. These infections are often difficult to treat due to intrinsic resistance of this species, mainly due to a chromosomally encoded cephalosporinase and low membrane permeability, as well as a high propensity to acquire resistance to numerous drugs. Common in bacteria, active efflux systems play, when overexpressed, a substantial role in acquired multidrug resistance (MDR). Among the efflux systems, resistance-nodulation-cell division (RND) pumps are the most prevalent systems in Gram-negative bacteria (19).
Three RND systems, AdeABC, AdeIJK, and AdeFGH, have been characterized and reported to be responsible for MDR in A. baumannii (5, 6, 16). AdeABC, found in ca. 80% of clinical isolates and probably the system most often implicated in MDR (3), is responsible for high-level resistance to aminoglycosides, β-lactams, fluoroquinolones, tetracyclines, tigecycline, macrolides, chloramphenicol, and trimethoprim (16). Its expression is tightly regulated by the two-component system AdeRS (18). Point mutations in this system or the presence of an insertion sequence upstream from the operon can lead to overexpression of the adeABC operon (18). However, since adeABC-overexpressing mutants with no mutations in adeRS have been reported (12, 21), other mechanisms are probably involved in the regulation of adeABC expression.
AdeFGH is found in ca. 90% of clinical isolates, and its overexpression is responsible for high-level resistance to chloramphenicol, clindamycin, fluoroquinolones, and trimethoprim and for decreased susceptibility to tetracycline-tigecycline and sulfonamides (5). Increased expression of the pump is due to mutations in the adeL gene, located upstream from adeFGH, that encodes a putative LysR-type transcriptional regulator (5). Analysis of the expression of the efflux system established that overexpression of AdeFGH is prevalent in clinical isolates of A. baumannii in Canada (2).
As opposed to AdeABC and AdeFGH, AdeIJK is present in all strains and contributes to intrinsic resistance to various drugs, including β-lactams, such as ticarcillin, cephalosporins and aztreonam, fluoroquinolones, tetracyclines, tigecycline, lincosamides, rifampin, chloramphenicol, co-trimoxazole, novobiocin, and fusidic acid (6). High-level expression of AdeIJK was experimentally shown to be toxic for A. baumannii (6), but one-step mutants overexpressing the pump have been obtained in vitro (4). Transcriptomic microarray experiments and quantitative real-time reverse transcription-PCR (qRT-PCR) have shown that the levels of adeIJK overexpression were low compared to those of adeABC, suggesting a threshold for toxicity for the host (4) and that AdeIJK is tightly regulated.
No regulatory genes were found in the vicinity of adeIJK, and analysis of the putative promoter region of in vitro overexpressing mutants did not reveal any mutations (6). We report identification, by a whole-genome sequencing approach, of AdeN, a transcription regulator belonging to the TetR family which represses expression of the adeIJK operon.
MATERIALS AND METHODS
Bacterial strains, plasmids, growth conditions, and antibiotic susceptibility testing.
The bacterial strains and plasmids used in this study are listed in Table 1. Cells were grown at 37°C in brain heart infusion (BHI) broth and agar (Difco Laboratories, Detroit, MI). Antibiotic susceptibility was tested by disk diffusion on Mueller-Hinton agar (Bio-Rad, Marnes-la-Coquette, France), and MICs were determined by the Etest procedure (bioMérieux, Marcy l'Etoile, France).
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Reference or source |
|---|---|---|
| Strains | ||
| A. baumannii | ||
| BM4587 | Wild-type clinical isolate | 4 |
| BM4666 | BM4587 adeNΔC582 spontaneous mutant obtained on cefotaxime | 4 |
| BM4667 | Wild-type clinical isolate | 4 |
| BM4668 | BM4667 adeNΔC582 spontaneous mutant obtained on tetracycline | 4 |
| BM4651 | BM4454 ΔadeABC | 6 |
| BM4669 | BM4651 adeNΔ258-651 spontaneous mutant obtained on tetracycline | This study |
| BM4685 | BM4587 ΔadeN | This study |
| E. coli | ||
| NEB5α | fhuA2 Δ(argF-lacZ)U169 phoA glnV44 Φ80(lacZ)ΔM15 gyrA96 recA1 relA1 endA1 thi-1 hsdR17 | New England Biolabs |
| CC118 | A(ara-leu) araD ΔlacX74 galE galK phoA20 thi-1 rpsE rpoB argE(Am) recAl | 17 |
| CC118λpir | CC118 lysogenized with λpir | 11 |
| HB101 | supE44 hsdS20(rB− mB−) recA13 ara-14 leuB6 proA2 lacY1 galK2 rpsL20 xyl-5 mtl-1 F−thi-1 | 14 |
| Plasmids | ||
| pCR2.1 | lacZα ColE1oriR Apr Kmr | Invitrogen |
| pCRBlunt | Kmr Zeocinr ColE1oriR lacZα ccdB | Invitrogen |
| pUC18 | Tra− Mob− AprlacZα | 26 |
| pAT801 | Apr Tcr | 10 |
| pAT522 | 702-bp adeN fragment of BM4587 cloned in pAT801 | This study |
| pAT523 | 701-bp adeNΔC582 fragment of BM4666 cloned in pAT801 | This study |
| pKNG101 | Suicide vector, sacB Smr | 13 |
| pRK2013 | Helper plasmid; ColE1oriR Tra Mob Kmr | 8 |
| pAT524 | 1,154-bp truncated adeN cloned in pKNG101 | This study |
Ap, ampicillin; Km, kanamycin; Mob, mobilizable; r, resistant; Sm, streptomycin; Tc, tetracycline; Tra, self-transferable.
Production of mutants overexpressing adeIJK.
Spontaneous mutants overexpressing adeIJK were obtained from wild-type susceptible strains BM4587 and BM4667 (4) and from laboratory strain BM4651 (6) on BHI gradient plates (25) containing 0 to 16 μg/ml of either cefotaxime or tetracycline (Table 1). Briefly, colonies growing at concentrations higher than the MIC were tested for antibiotic resistance by agar diffusion, and those resistant to several drug classes, such as tetracyclines, phenicols, fluoroquinolones, sulfonamides, and macrolides, were selected for quantitative real-time reverse-transcription PCR (qRT-PCR) analysis to quantify the expression levels of AdeIJK.
DNA manipulations.
A. baumannii genomic DNA was extracted as described previously (24) or by using a Wizard genomic DNA purification kit (Promega, Madison, WI). DNA amplification was performed in a GeneAmp PCR system 9700 (Perkin-Elmer Cetus, Norwalk, CT) with Taq (MPbio, Illkirch, France) or Phusion (Finnzymes, Espoo, Finland) DNA polymerase. PCR elongation times and temperatures were adjusted according to the expected sizes of the PCR products and the nucleotide sequences of the primers. PCR products were purified with a QIAquick PCR purification kit (Qiagen, Inc., Chatsworth, CA). DNA fragments were extracted from agarose gels with a QIAquick gel extraction kit (Qiagen). Nucleotide sequencing was carried out with a CEQ 8000 DNA analysis system automatic sequencer (Beckman Instruments, Inc., Palo Alto, CA).
Recombinant DNA techniques.
DNA isolation, digestion with restriction endonucleases (New England BioLabs, Ipswich, MA), ligation with T4 DNA ligase (New England BioLabs), and transformation of NEB5α competent Escherichia coli (New England BioLabs) with recombinant plasmid DNA were performed by standard methods (24).
TAIL-PCR amplification.
The sequence downstream from the first 200 nucleotides of adeN in BM4669 was determined by using thermal asymmetric interlaced PCRs (TAIL-PCRs) (15) using the primers listed in Table 2. The primary PCR mixture was prepared as described previously (7). For the secondary reaction, the primary reaction mixture was diluted 50-fold, and for the tertiary reaction, a 1/10-diluted secondary reaction was used.
Table 2.
Oligonucleotides
| Primer | Sequence (5′ → 3′)a | Positionb |
|---|---|---|
| adeN1 | ggatccGCTGTTAGGTTGGGGTCGTA | −387 to −368 |
| adeN2c | ggagatgTTTTTGAACCACCAGCATGA | 172–153 |
| adeN3d | tcaaaaaCATCTCCCGATCATTCCTCT | 497–516 |
| adeN4 | gggcccCGTGACCAAAAGTACGAATCA | 1091–1071 |
| adeN5v | TCGTGAGGTTTATCCGGAAG | −645 to −636 |
| adeN6v | TGCATTTCAACCAGAGCAAA | 264–283 |
| adeN7v | GAAACCCATCATGTGTGCAG | 1294–1275 |
| adeN9 | AACGACGGCTAACACTGCTT | −11 to −30 |
| adeNM3 | TGCATGATCCAGTCCTTGAG | 2–21 |
| adeNM4 | ACCACCAGCATGATTAACGA | 165–146 |
| adeNAYE3 | CATTTCAAAATGCAAAAGAATT | 674–653 |
| adeNAYE4 | GCAGTGTTAGCCGTCGTTAT | −28 to −9 |
| adeNAYE5 | AAAACATTTCACGGCGATAA | 247–228 |
| AdeNAYE7 | TTATCGCCGTGAAATGTTTT | 228–247 |
| adeNAYE8 | ATTTTGGACATCCAGAGCAC | 444–425 |
| TailAdeN1 | TCATGCTGGTGGTTCAAAAA | −122 to −102 |
| TailAdeN2 | ACACGCCGCGGTATAGAAC | 51–70 |
| TailAdeN3 | TTTTCCCCAAAACTATGCTCT | 152–171 |
| adeJ For | GGTCATTAATATCTTTGGC | 1143–1161 |
| adeJ Rev | GGTACGAATACCGCTGTCA | 1364–1346 |
| rpoB For | TCCGCACGTAAAGTAGGAAC | 2114–2095 |
| rpoB Rev | ATGCCGCCTGAAAAAGTAAC | 1959–1979 |
The restriction sites introduced in the oligonucleotides are underlined.
Coordinates refer to the first base of the gene.
The first 7 nucleotides are complementary to those of adeN3 indicated in italics.
The first 7 nucleotides are complementary to those of adeN2 indicated in italics.
Whole-genome sequencing.
Whole-genome sequencing of strains was performed with Solexa single-reads sequencing technology. Illumina library preparation and sequencing followed standard protocols developed by the supplier. Briefly, genomic DNA was sheared by nebulization, and the fragments were end repaired and phosphorylated. Blunt-end fragments were A-tailed and ligated to sequencing adapters. Fragments with an insert size of approximately 400 bp were gel extracted and enriched with 14 cycles of PCR before library quantification and validation. Hybridization of the library to the flow cell and bridge amplification were performed to generate clusters, and single reads of 100 cycles were collected on a HiSeq 2000 (Illumina, San Diego, CA). After sequencing was completed, image analysis, base calling, and error estimation were performed using Illumina Analysis Pipeline version 1.7.
Insertion-inactivation of adeN.
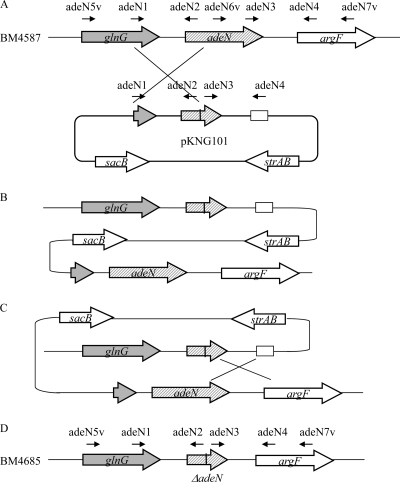
Deletion of adeN in BM4587 was carried out following the sacB-based strategy (13). Briefly, the 5′ and 3′ flanking regions of the gene were amplified by PCR with specific primers adeN1/adeN2 and adeN3/adeN4 (Table 2; Fig. 1) designed such that each fragment had, at its end closest to adeN, a short sequence of 7 nucleotides complementary to the corresponding sequence in the other fragment. The PCR products were then linked by overlapping PCR using primers adeN1/adeN4 to form an insert that was cloned in pCR2.1 (Invitrogen, San Diego, CA). The recombinant plasmid was digested by BamHI and ApaI, and the resulting fragment was ligated to BamHI/ApaI-linearized pKNG101 and introduced into competent E. coli CC118λpir (Table 1). The recombinant plasmid obtained, pAT524 (ΔadeN), was then transferred into A. baumannii BM4587 by tripartite conjugation using E. coli CC118λpir(pAT524) as the donor and E. coli HB101 containing the “helper” plasmid pRK2013 (Table 1). Deletion mutants were selected on M9 minimal medium plates containing 5% sucrose, 200 μg/ml streptomycin, and 60 μg/ml chloramphenicol. Deletion of 324 bp in adeN following two recombination events was confirmed in the resulting BM4685 mutant by PCR with external primers adeN5v and adeN7v (Table 2; Fig. 2) and sequencing.
Fig 1.
Insertion-inactivation of adeN. (A) Plasmid pAT524 (ΔadeN), possessing a truncated portion of adeN, underwent homologous recombination in the glnG-adeN-argF fragment, leading to (B) integration in the A. baumannii chromosome. (C) A second crossover event led to loss of the entire adeN and of the plasmid, resulting in a truncated adeN gene (D).
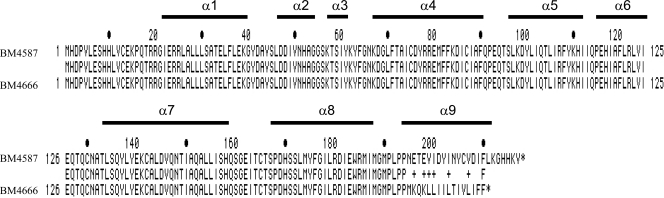
Fig 2.
Alignment of wild-type and mutated AdeN. AdeN from BM4587 and that from BM4666 (BM4587 adeNΔC582) are aligned. Cytosine deletion at position 582 of adeN is responsible for a frameshift leading to the replacement of 15 amino acids in the C-terminal part of AdeN (corresponding to positions 195 to 210) and to the presence of a premature stop codon at position 211. α-Helices predicted by using the http://www.predictprotein.org website are indicated.
trans-Complementation experiments.
Low-copy-number plasmid pAT801 (10), conferring resistance to ampicillin and in which gene expression is under the control of the IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible promoter Plac, was used as an A. baumannii-E. coli shuttle vector. A fragment containing the putative ribosome-binding site (RBS) region and either the entire adeN gene of BM4587 or the mutated adeNΔC582 gene of BM4666 was amplified with primers adeNAYE3 and adeNAYE4 (Table 2) and cloned in pCR2.1 (Table 1). After BamHI/XbaI restriction, the insert was ligated in BamHI/XbaI-linearized pAT801, giving rise to pAT522 (adeN) and to pAT523 (adeNΔC582) (Table 1), which were electrotransformed into A. baumannii BM4666 (BM4587 adeNΔC582) and BM4587, respectively. Cultures were then incubated at 37°C in BHI containing 100 μg/ml of ampicillin until the optical density at 600 nm reached 0.5 to 0.6 and expression of adeN or adeNΔC582 was induced for 4 h at 37°C by addition of 0.2 or 1 mM IPTG.
RNA isolation and qRT-PCR.
A. baumannii total RNA was isolated from exponentially growing cells (optical density at 600 nm of 0.8 to 0.9) with a FastRNA Blue kit (MPbio). cDNA synthesis and qRT-PCR were performed with a LightCycler RNA amplification kit SYBR green I (Roche Diagnostic GmbH, Mannheim, Germany) and 0.5 μM gene-specific primers (adeN6v/adeNAYE8 for adeN, adeNM3/adeNM4 for truncated adeN, adeJfor/adeJrev for adeJ, and rpoBfor/rpoBrev for rpoB) (Table 2), according to the manufacturer's instructions. Amplification and detection of specific products were performed using the LightCycler sequence detection system (Roche) with the following cycle profile: 1 cycle at 95°C for 30 s, followed by 45 cycles at 95°C for 5 s, 54°C for 10 s, and 72°C for 20 s. Each experiment was performed in duplicate.
Determination of the TSP.
To determine the transcription start point (TSP) of adeN, 5′ rapid amplification of cDNA ends-PCR (5′ RACE-PCR) was carried out with the 5′-RACE system for rapid amplification of cDNA ends, version 2.0 (Invitrogen), according to the manufacturer's instructions. Briefly, total RNA (3 μg) was used to generate double-stranded cDNA with adeNAYE8, an adeN-specific antisense oligonucleotide (Table 2). A homopolymeric tail was then added to the 3′ end of the cDNA (corresponding to the 5′ end of the specific mRNA) using TdT (terminal deoxynucleotidyltransferase) and dCTP. Finally, tail-cDNA was used as a template for two amplification steps, using a nested gene-specific primer (adeNAYE5 then adeN2) (Table 2) and a combination of a complementary homopolymer-containing anchor primer and the corresponding adapter primer which allows amplification from the homopolymeric tail (abridged anchor primer [AAP], then abridged universal amplification primer [AUAP]). The resulting amplified fragments were purified by low-melting-point agarose gel electrophoresis, cut with appropriate restriction enzymes, cloned into pCRblunt (Invitrogen), and sequenced.
RESULTS AND DISCUSSION
Identification of AdeN, a TetR-like regulator involved in AdeIJK regulation.
Sequencing of 300 bp upstream from adeI, including a putative promoter for adeIJK, did not reveal any mutations between susceptible BM4587 and one-step mutant BM4666 overexpressing the pump (6). Furthermore, no open reading frames (ORFs) coding for regulatory proteins were found in the vicinity of the adeIJK operon. To detect a putative transcriptional regulator of adeIJK, whole-genome sequencing of the two strains was carried out.
Comparative analysis of the sequence of the contigs with the seven A. baumannii sequences deposited in GenBank indicated that the genome of BM4587 was most closely related to that of strain AB307-0294 (1). The latter possesses 199 genes annotated as “putative transcriptional regulators,” most of them belonging to the LysR (31%) or TetR (23%) family. These putative regulators were all found in both strains, and the sequence of every gene was compared with those in BM4587 and BM4666. A mutation was found in the 651-bp gene corresponding to ABBFA_001467 of strain AB307-0294, annotated as “bacterial regulatory protein, TetR family protein.” A cytosine deletion at position 582 in BM4666 introduced an alteration in the amino acid sequence corresponding to positions 195 to 210 and a premature stop codon at position 211, resulting in deletion of the 7 C-terminal residues (Fig. 2). Resequencing of the PCR fragments obtained with oligonucleotides adeN1/adeN2 (Table 2) from BM4587 and BM4666 confirmed cytosine deletion at position 582. The gene, located 813 kbp from adeIJK in AB307-0294, was named adeN.
Inactivation of adeN in susceptible BM4587.
To test the role of adeN in the regulation of adeIJK expression, the gene was partially deleted by insertion-inactivation in BM4587 using pKNG101 (13) (Fig. 1). Deletion in the resulting strain, BM4685 (BM4587 ΔadeN), which was more resistant to AdeIJK substrates erythromycin, chloramphenicol, tetracycline, β-lactams, sulfonamides, and quinolones (Table 3), was confirmed by PCR and sequencing. Determination of adeJ expression levels by qRT-PCR indicated an increase of approximately 5-fold (ranging from 3.8- to 6.5-fold) in BM4685 compared with that of BM4587. This is similar to the adeJ expression increase observed in BM4666 (BM4587 adeNΔC582) relative to BM4587 (4). These data confirm the contribution of AdeN to the regulation of AdeIJK.
Table 3.
Antibiotic susceptibility of A. baumannii strains
| Antimicrobial agent | MICa (μg/ml) for strain: |
||||
|---|---|---|---|---|---|
| BM4587b | BM4666 (BM4587 adeNΔC582)c | BM4666/pAT522 (adeN)d | BM4685 (BM4587 ΔadeN)e | BM4685/pAT522 (adeN)d | |
| Erythromycin | 6 | 32 | 6 | 24 | 4 |
| Chloramphenicol | 64 | >256 | 32 | 128 | 24 |
| Tetracycline | 3 | 8 | 1.5 | 4 | 0.75 |
| Minocycline | 0.094 | 0.38 | 0.094 | 0.38 | 0.094 |
| Tigecycline | 0.064 | 0.25 | 0.094 | 0.25 | 0.064 |
| Rifampin | 1.5 | 3 | 3 | 8 | 4 |
| Ciprofloxacin | 0.125 | 0.38 | 0.25 | 0.5 | 0.125 |
| Norfloxacin | 1.5 | 6 | 3 | 6 | 3 |
| Trimethoprim | 24 | >32 | 16 | >32 | 24–32 |
| Sulfamethoxazole | 4 | 8 | 3 | 16 | 6 |
| Co-trimoxazole | 0.19 | 0.38 | 0.125 | 0.75 | 0.25 |
| Ticarcillin | 8 | 16 | NAf | 64 | NA |
| Aztreonam | 12 | 48 | 16 | 32 | 12 |
| Cefepime | 1 | 3 | 2 | 3 | 1.5 |
| Ceftazidime | 2 | 6 | 2 | 3 | 1.5 |
| Imipenem | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Ertapenem | 3 | 12 | 3 | 6 | 1.5 |
| Meropenem | 0.25 | 1 | 0.38 | 0.75 | 0.19 |
MICs determined by Etest.
Wild-type clinical isolate.
Mutant of BM4587 overexpressing adeIJK.
Strain trans-complemented with plasmid pAT522 (adeN).
Derivative of BM4587 deleted for adeN and overexpressing adeIJK.
NA, not assessed because of the presence of an ampicillin resistance cassette.
trans-Complementation experiments.
To study if AdeN is sufficient to restore antibiotic susceptibility to BM4666 (BM4587 adeNΔC582), plasmid pAT522 (adeN) was introduced into that strain by electrotransformation. In the presence of IPTG (0.2 or 1 mM), the susceptibility of transformant BM4666/pAT522(adeN) to erythromycin, chloramphenicol, tetracycline, β-lactams, sulfonamides, and quinolones was restored to close to the wild-type level (Table 3). In addition, there was no difference in expression of adeJ between BM4666/pAT522(adeN) and BM4587, as determined by qRT-PCR (ratio = 1.16 ± 0.36). These results demonstrate that AdeN is sufficient to prevent overexpression of AdeIJK. trans-Complementation of BM4685 (BM4587 ΔadeN) with pAT522(adeN) increased the susceptibility to the host to close to the level of BM4587 (Table 3). Furthermore, comparison by qRT-PCR of the expression levels of adeJ in BM4685/pAT522(adeN) and in BM4587 showed that they were similar (ratio = 1.26 ± 0.16), confirming the loss of adeJ overexpression in BM4685/pAT522. Electrotransformation of pAT523 (adeNΔC582) in mutant strain BM4685 (BM4587 ΔadeN) and in wild-type BM4587 did not alter the phenotype, despite the fact that the plasmid-borne adeNΔC582 was highly expressed (more than 104-fold) (Table 3). These data confirm that AdeN is required for the repression of AdeIJK expression.
Taken together, these inactivation and trans-complementation experiments indicate that AdeN acts as a repressor of efflux pump AdeIJK expression in BM4587.
Overexpression of AdeIJK in other mutants.
Two other adeIJK-overexpressing one-step mutants, BM4668 and BM4669, selected from susceptible strains BM4667 and BM4651 (ΔadeABC), respectively, were studied (Table 2). Sequence analysis of adeN from BM4668 revealed the same mutation as that in BM4666 (BM4587 adeNΔC582). The deletion occurred in a highly C-rich (75%) 12-bp sequence (from position 571 to 582) which could be a potential mutational hot spot due to a slippage of the DNA polymerase (23). Amplification of the entire adeN gene could not be obtained from BM4669 total DNA. A PCR product containing 645 bp upstream from adeN and the first 247 bp of the gene was obtained, whereas attempts with various primers located in the 3′ part of the gene were unsuccessful. The sequence of this portion of adeN was determined by TAIL-PCR (15) and showed a deletion encompassing the 394 last nucleotides of adeN, the intergenic region, and the 470 first nucleotides of argF for an ornithine carbamoyltransferase located downstream from adeN. Determination of adeJ expression levels by qRT-PCR showed that adeJ was overexpressed in the mutant compared to the parent (from 2.5- to 5-fold for BM4668/BM4667 [4] and from 3- to 4.8-fold for BM4669/BM4651). The presence of mutation/deletion events in the three one-step in vitro mutants overexpressing adeIJK constitutes additional proof for the role of AdeN in the regulation of AdeIJK expression.
Promoter analysis of adeN.
The transcription start point (TSP) of adeN was determined by 5′ rapid amplification of cDNA ends-PCR (RACE-PCR) and was identified 27 (C) or 28 (G) bp upstream from the start codon of the gene. Upstream from the TSP, and based on the Escherichia coli σ70 consensus sequences (9), putative −10 (TATACT) and −35 (ATGACC) regions separated by 20 bp were identified.
Sequencing of a 300-bp fragment upstream from adeN that includes the TSP and putative −35 and −10 motifs in mutants BM4666, BM4668, and BM4669 overexpressing AdeIJK did not reveal mutations relative to the respective parental strains. Quantitative RT-PCR experiments with primers adeNM3/adeNM4 (Table 2) showed that the levels of adeN expression were similar in BM4666 (BM4587 adeNΔC582)/BM4587, as well as in BM4668 (BM4667 adeNΔC582)/BM4667 and BM4669 (BM4651 adeNΔ258-651)/BM4651 (from 1.1- to 1.4-fold). Furthermore, analysis of the expression level of the truncated adeN in BM4685 (BM4587 ΔadeN) was also similar to that of adeN in BM4587 (ratio = 0.4 ± 0.17). Thus, there was neither positive nor negative regulation of adeN expression in BM4666 and BM4685. These results demonstrate that AdeN is constitutively expressed and does not regulate its own expression.
Relationship between AdeN and other TetR regulators.
The conventional model of TetR secondary structures shows that the protein consists of 9 or 10 conserved α-helices, each having a specific function (22, 27). The core DNA-binding domain (DBD) is usually composed of the α1-α3 N-terminal helices, with α2 and α3 forming the helix-turn-helix (HTH) motif. The C-terminal domain, composed of helices α4 to α9 or α10, forms the ligand-binding and dimerization domain (LBD), which contains a ligand-binding pocket of varying size and properties, with helices α8 and α9 usually constituting the dimerization interface to form a four-helix bundle with the same helices (α8′ and α9′) from the other monomer (22, 27). The DBD, especially the HTH motif, is well conserved in the TetR family members, in contrast to the LBD; the latter allows the TetR protein to interact with a wide range of structurally unrelated signals.
Computational secondary structure predictions using the website http://www.predictprotein.org showed that AdeN was composed of 9 α-helices (Fig. 2). The cytosine deletion in adeN of the adeIJK-overexpressing strain BM4666 is responsible for impairment of the amino acid sequence of the α9 helix (Fig. 2), suggesting that this mutation can prevent dimerization and thus the activity of AdeN.
The vast majority of the TetR-like proteins are active as homodimers, recognizing a specific operator sequence composed of a palindromic inverted repeat sequence (22, 27). We have located, 11 bp upstream from adeIJK, a 28-bp sequence, CAAATATATTTTtagaTTTTATCTAAAC, containing an imperfect 12-bp sequence repeated in inverse orientation (uppercase). This 28-bp motif, which also contains a 14-bp inverted repeat sequence (TTTAGATtttATCTAAA), could play a role in the regulation of adeIJK expression.
Comparison of the 217-amino-acid sequence of AdeN with those in the Swiss-Prot protein database revealed that AdeN exhibits low-level identity with other TetR proteins in various genera. The highest degree of identity was found with TetR from Ralstonia eutropha (29%), Cupriavidus metallidurans (29%), Alcanivorax borkumensis (28%), and Sphingobium japonicum (26%). However, the DBDs of these proteins are well conserved and exhibit 44 to 61% identity.
Homologues of AdeIJK and AdeN in other Acinetobacter species.
In silico analysis revealed that AdeN is present and conserved (amino acid identity of >99%) in the seven A. baumannii strains in the GenBank database. The adeN gene was also detected by PCR, using primers adeNAYE3/adeNAYE4 and adeNAYE7/adeNAYE8 (Table 2), in 30 A. baumannii strains from our laboratory collection. By using the same primers, no PCR products corresponding to adeN were obtained using DNA from Acinetobacter baylyi, Acinetobacter haemolyticus, Acinetobacter radioresistens, and Acinetobacter lwoffii strains as templates. This suggests either that adeN is absent in these species or that the nucleotide sequences of putative homologues differ significantly. In silico analysis of the genomes of various Acinetobacter species available in the Acinetobacter Group Sequencing Project of the Broad Institute of Harvard and MIT (http://www.broadinstitute.org/) and in the XBase database (http://www.xbase.ac.uk/) revealed that both an operon homologous to adeIJK and a gene homologous to adeN were present in other Acinetobacter species, such as Acinetobacter calcoaceticus, Acinetobacter nosocomialis, and Acinetobacter pittii (Table 4). The level of identity between AdeI, AdeJ, and AdeK of A. baumannii and those of A. calcoaceticus, A. nosocomialis and A. pittii was greater than 96%. For the other species tested, the sequences were less conserved (from 74% to 93% identity) (Table 4). Analysis of AdeN showed similar results: amino acid sequences of A. baumannii, A. calcoaceticus, A. nosocomialis, and A. pittii were highly conserved (identity comprised between 79% and 92%), whereas identity was less than 63% between A. baumannii and the other species (Table 4). In all the species tested, adeN was always located upstream from the argF gene. These results confirm that the AdeIJK-like efflux system, present in various Acinetobacter species, is likely to be regulated by an AdeN-like TetR regulator.
Table 4.
Amino acid identity (%) between Ade proteins of A. baumannii and other Acinetobacter species
| A. baumannii protein | % Amino acid identity with corresponding protein of: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A. calcoaceticus | A. nosocomialis | A. pittii | A. junii | A. haemolyticus | A. parvus | A. radioresistens | A. ursingii | A. baylyi | A. johnsonnii | A. lwoffii | |
| AdeN | 91 | 92 | 79 | 63 | 61 | 61 | 60 | 59 | 57 | 53 | 53 |
| AdeI | 96 | 98 | 99 | 78 | 87 | 74 | 76 | 82 | 81 | 78 | 72 |
| AdeJ | 97 | 99 | 98 | 86 | 91 | 84 | 86 | 77 | 89 | 86 | 84 |
| AdeK | 96 | 97 | 98 | 85 | 89 | 74 | 82 | 87 | 88 | 81 | 77 |
Gene expression is mostly controlled at the transcriptional level by several families of transcriptional regulators. Interestingly, each of the three multidrug efflux systems characterized so far in A. baumannii is regulated in a different way: AdeABC by a two-component regulatory system (AdeRS) (18), AdeFGH by a LysR-type transcriptional regulator (AdeL) (5), and AdeIJK by a TetR regulator (AdeN). The TetR family constitutes the third most common transcriptional regulator family in bacteria (20). It is now well established that these proteins are often involved in the regulation of the expression of efflux systems by acting as repressors (22). It has been demonstrated that interaction of a ligand with the LBD domain of the regulator leads to a conformational change that interferes with the DNA-binding ability of the protein, thus relieving transcription repression (22, 27). The adeN gene is most likely responsible for the repression of the adeIJK operon in susceptible A. baumannii strains. In BM4666 and in BM4668, the same mutation (C582) in adeN, introducing important modifications in the α9 helix sequence within the putative dimerization domain of the regulator, is probably involved in the loss of activity of AdeN, likely by impairing protein dimerization. In BM4669, deletion of a large C-terminal fragment is probably responsible for the inactivation of AdeN.
Finally, TetR-like genes can be either global or local regulators, sometimes acting with several intermediate regulatory genes (22). The possibility that AdeN could be involved in the regulation of expression of other genes cannot be excluded.
ACKNOWLEDGMENTS
We thank K. Jeannot for the gift of plasmid pKNG101, L. Ma for help in genome sequencing, and P. Trieu-Cuot and C. Grillot-Courvalin for helpful discussions.
This work was supported in part by the European Union FP7-PAR that included a fellowship in support of N.R. and by unrestricted grants from Reckitt-Benkiser.
Footnotes
Published ahead of print 27 February 2012
REFERENCES
- 1. Adams MD, et al. 2008. Comparative genome sequence analysis of multidrug-resistant Acinetobacter baumannii. J. Bacteriol. 190:8053–8064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cortez-Cordova J, Kumar A. 2011. Activity of the efflux pump inhibitor phenylalanine-arginine beta-naphthylamide against the AdeFGH pump of Acinetobacter baumannii. Int. J. Antimicrob. Agents 37:420–424 [DOI] [PubMed] [Google Scholar]
- 3. Coyne S, Courvalin P, Perichon B. 2011. Efflux-mediated antibiotic resistance in Acinetobacter spp. Antimicrob. Agents Chemother. 55:947–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coyne S, Guigon G, Courvalin P, Perichon B. 2010. Screening and quantification of the expression of antibiotic resistance genes in Acinetobacter baumannii with a microarray. Antimicrob. Agents Chemother. 54:333–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coyne S, Rosenfeld N, Lambert T, Courvalin P, Perichon B. 2010. Overexpression of resistance-nodulation-cell division pump AdeFGH confers multidrug resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 54:4389–4393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Damier-Piolle L, Magnet S, Bremont S, Lambert T, Courvalin P. 2008. AdeIJK, a resistance-nodulation-cell division pump effluxing multiple antibiotics in Acinetobacter baumannii. Antimicrob. Agents Chemother. 52:557–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Depardieu F, Bonora MG, Reynolds PE, Courvalin P. 2003. The vanG glycopeptide resistance operon from Enterococcus faecalis revisited. Mol. Microbiol. 50:931–948 [DOI] [PubMed] [Google Scholar]
- 8. Ditta G, Stanfield S, Corbin D, Helinski DR. 1980. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc. Natl. Acad. Sci. U. S. A. 77:7347–7351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harley CB, Reynolds RP. 1987. Analysis of E. coli promoter sequences. Nucleic Acids Res. 15:2343–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heritier C, Poirel L, Lambert T, Nordmann P. 2005. Contribution of acquired carbapenem-hydrolyzing oxacillinases to carbapenem resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 49:3198–3202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herrero M, de Lorenzo V, Timmis KN. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 172:6557–6567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hornsey M, et al. 2010. AdeABC-mediated efflux and tigecycline MICs for epidemic clones of Acinetobacter baumannii. J. Antimicrob. Chemother. 65:1589–1593 [DOI] [PubMed] [Google Scholar]
- 13. Kaniga K, Delor I, Cornelis GR. 1991. A wide-host-range suicide vector for improving reverse genetics in gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene 109:137–141 [DOI] [PubMed] [Google Scholar]
- 14. Lacks S, Greenberg B. 1977. Complementary specificity of restriction endonucleases of Diplococcus pneumoniae with respect to DNA methylation. J. Mol. Biol. 114:153–168 [DOI] [PubMed] [Google Scholar]
- 15. Liu YG, Whittier RF. 1995. Thermal asymmetric interlaced PCR: automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking. Genomics 25:674–681 [DOI] [PubMed] [Google Scholar]
- 16. Magnet S, Courvalin P, Lambert T. 2001. Resistance-nodulation-cell division-type efflux pump involved in aminoglycoside resistance in Acinetobacter baumannii strain BM4454. Antimicrob. Agents Chemother. 45:3375–3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Manoil C, Beckwith J. 1985. TnphoA: a transposon probe for protein export signals. Proc. Natl. Acad. Sci. U. S. A. 82:8129–8133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marchand I, Damier-Piolle L, Courvalin P, Lambert T. 2004. Expression of the RND-type efflux pump AdeABC in Acinetobacter baumannii is regulated by the AdeRS two-component system. Antimicrob. Agents Chemother. 48:3298–3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nikaido H. 2011. Structure and mechanism of RND-type multidrug efflux pumps. Adv. Enzymol. Relat. Areas Mol. Biol. 77:1–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pareja E, et al. 2006. ExtraTrain: a database of extragenic regions and transcriptional information in prokaryotic organisms. BMC Microbiol. 6:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peleg AY, Adams J, Paterson DL. 2007. Tigecycline efflux as a mechanism for nonsusceptibility in Acinetobacter baumannii. Antimicrob. Agents Chemother. 51:2065–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ramos JL, et al. 2005. The TetR family of transcriptional repressors. Microbiol. Mol. Biol. Rev. 69:326–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rocha EP, Pradillon O, Bui H, Sayada C, Denamur E. 2002. A new family of highly variable proteins in the Chlamydophila pneumoniae genome. Nucleic Acids Res. 30:4351–4360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sambrook J, Russell W. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 25. Szybalski W, Bryson V. 1952. Genetic studies on microbial cross resistance to toxic agents. I. Cross resistance of Escherichia coli to fifteen antibiotics. J. Bacteriol. 64:489–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yanisch-Perron C, Vieira J, Messing J. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119 [DOI] [PubMed] [Google Scholar]
- 27. Yu Z, Reichheld SE, Savchenko A, Parkinson J, Davidson AR. 2010. A comprehensive analysis of structural and sequence conservation in the TetR family transcriptional regulators. J. Mol. Biol. 400:847–864 [DOI] [PubMed] [Google Scholar]