Abstract
The in vitro antibacterial effects of diallyl sulfide (DAS) against the Gram-negative periodontopathogen Aggregatibacter actinomycetemcomitans, the key etiologic agent of the severe form of localized aggressive periodontitis and other nonoral infections, were studied. A. actinomycetemcomitans was treated with garlic extract, allicin, or DAS, and the anti-A. actinomycetemcomitans effects of the treatment were evaluated. Garlic extract, allicin, and DAS significantly inhibited the growth of A. actinomycetemcomitans (greater than 3 log; P < 0.01) compared to control cells. Heat inactivation of the garlic extracts significantly reduced the protein concentration; however, the antimicrobial effect was retained. Purified proteins from garlic extract did not exhibit antimicrobial activity. Allicin lost all its antimicrobial effect when it was subjected to heat treatment, whereas DAS demonstrated an antimicrobial effect similar to that of the garlic extract, suggesting that the antimicrobial activity of garlic extract is mainly due to DAS. An A. actinomycetemcomitans biofilm-killing assay performed with DAS showed a significant reduction in biofilm cell numbers, as evidenced by both confocal microscopy and culture. Scanning electron microscopy (SEM) analysis of DAS-treated A. actinomycetemcomitans biofilms showed alterations of colony architecture indicating severe stress. Flow cytometry analysis of OBA9 cells did not demonstrate apoptosis or cell cycle arrest at therapeutic concentrations of DAS (0.01 and 0.1 μg/ml). DAS-treated A. actinomycetemcomitans cells demonstrated complete inhibition of glutathione (GSH) S-transferase (GST) activity. However, OBA9 cells, when exposed to DAS at similar concentrations, showed no significant differences in GST activity, suggesting that DAS-induced GST inhibition might be involved in A. actinomycetemcomitans cell death. These findings demonstrate that DAS exhibits significant antibacterial activity against A. actinomycetemcomitans and that this property might be utilized for exploring its therapeutic potential in treatment of A. actinomycetemcomitans-associated oral and nonoral infections.
INTRODUCTION
Aggregatibacter actinomycetemcomitans is a Gram-negative bacterium that has been implicated as the primary etiologic agent of a particularly severe form of periodontal disease, localized aggressive periodontitis (LAP) (81). Recent studies showed that A. actinomycetemcomitans has been associated with an increased risk of atherosclerosis (22, 67) and other cardiovascular diseases (CVD) (82). Other infections caused by A. actinomycetemcomitans include urinary tract infections, osteomyelitis, and brain abscesses (39, 64). A. actinomycetemcomitans can potentially be a very destructive pathogen due to its many virulence factors, including catalase, IgA protease, cytolethal distending toxin, leukotoxin, bone resorption-inducing toxin, fibroblast-inhibiting factor, collagenase, and epitheliotoxin (29, 32, 81). These factors further assist in colonization and survival of A. actinomycetemcomitans in the oral cavity. A. actinomycetemcomitans exhibits extremely tenacious adherence to inert surfaces in vitro (27) and is capable of binding to and invading epithelial cells (56).
Many different antimicrobial agents and antibiotics have been used to treat LAP; however, the ability of this organism to invade tissues and cells makes it very difficult to eradicate the organism from the oral cavity. Also, the constant emergence of resistant strains makes it difficult to treat A. actinomycetemcomitans infections, resulting in a greater number of cases that recur following treatment. Various chemical agents have been evaluated over the years for their antimicrobial effects in the oral cavity; however, all are associated with side effects that prohibit regular long-term use (6, 20, 36, 41, 55, 57, 61, 75). Therefore, an alternative therapy is essential to prevent the disease. With the rise in bacterial resistance to antibiotics, there is considerable interest in the development of other classes of antimicrobials for the control of infection. Garlic has been used as a medicine since ancient times and has long been known to have antibacterial, antiviral, antifungal, antialgal, antiproteolytic, anticancer, antithrombotic, antiatherosclerotic, antioxidant, and immune modulation properties (4, 5, 12, 16, 45, 58, 59, 60, 66, 69, 70, 77). Recently, garlic extract was shown to have effective broad-spectrum antimicrobial activity (11, 15, 21, 44, 62, 74), including activity against oral bacteria (8). The main antimicrobial constituent of garlic has been identified as the oxygenated sulfur compound allicin. It is not present in raw garlic and is formed rapidly by the action of an enzyme, alliinase, when garlic is crushed. Although allicin has been shown to be an effective antimicrobial agent in vitro, its effects in vivo are obscure and questionable (47). Another study showed that alliinase is inactivated by heat, thus preventing allicin formation (9). Garlic oils contain major organosulfur compounds, including diallyl sulfide (DAS; 112 μg/g), diallyl disulfide (DADS; 1,183 μg/g), and diallyl trisulfide (DATS; 751 μg/g), and minor amounts of many other volatile compounds (49). These compounds possess potent anticancer, antioxidant, and anti-inflammatory immune modulation activities and antimicrobial properties (2, 30, 71, 72, 79, 83). The inhibitory effect of DASs against Bacillus cereus, Salmonella sp., and Vibrio cholerae (7, 62, 63), Staphylococcus aureus and Candida albicans (42, 51), Helicobacter pylori (52, 58), and Klebsiella pneumoniae and Pseudomonas aeruginosa (17, 37, 53, 73) were studied, and a recent report has shown that DASs were synergistically effective when combined with antibiotics against antibiotic-resistant strains of Escherichia coli, Enterobacter cloacae, E. faecalis, and Citrobacter freundii (80). On the basis of the results of previous studies, we hypothesize that DAS is effective against A. actinomycetemcomitans and that it retains its activity even after heat treatment. We address the following questions. (i) Does garlic kill A. actinomycetemcomitans, and is the effect retained after heat treatment? (ii) Does allicin demonstrate anti-A. actinomycetemcomitans activity, and does heat treatment reduce its effect? (iii) Does DAS demonstrate anti-A. actinomycetemcomitans activity, and is it heat stable? (iv) Does DAS retain its activity in the presence of saliva? (v) Do proteins of garlic extract demonstrate anti-A. actinomycetemcomitans activity? (vi) Does DAS demonstrate any cytotoxic effect against oral epithelial cells?
To our knowledge, there have been no reports to date on the effects of these sulfides against A. actinomycetemcomitans. The aims of this study were to evaluate the antimicrobial effects of diallyl sulfide (DAS) treatment on A. actinomycetemcomitans and to understand their potential as therapeutic agents for treatment of periodontal disease.
MATERIALS AND METHODS
Allicin and DAS (LKT Laboratories Inc.) were mixed with 0.1% dimethyl sulfoxide (DMSO), which was used as the solvent (72). Garlic bulbs were obtained from a supermarket and homogenized with sterile distilled water. The homogenized extract was then filtered through Whatman filter paper (number 1), centrifuged at 3,400 × g for 30 min, and stored at −20°C until use according to a previously published method (16). The extract was purified (see below) and tested for anti-A. actinomycetemcomitans activity.
Isolation of proteins from garlic extract.
To study the independent effects of proteins in the garlic extract, we removed the low-molecular-weight organosulfur compounds by the use of a Centriprep system with a 3-kDa-cutoff membrane disc filter (Millipore 3K; Millipore Corporation, Bedford, MA) and performed dialysis using 3.5-kDa-cutoff dialysis membranes (16). Fractionation of proteins from the extract was carried out by SP Sepharose column chromatography according to a previously published method (76).
Partial purification of garlic proteins by SP Sepharose column chromatography.
Partial purification of proteins in garlic extract was carried out as described earlier (16, 76). Briefly, 100 ml of garlic extract was subjected to SP Sepharose column chromatography (Bio-Rad). The column had been preequilibrated with 10 mM Tris-HCl buffer, pH 8.0. After adsorption of the sample and washing with 150 ml of the buffer described above, the bound proteins were eluted by stepwise elution using the same buffer containing different concentrations of NaCl (0.2 to 1 M). The presence of protein in different step-eluate fractions was monitored by absorbance at 280 nm (Shimadzu Corp.). The major components were then dialyzed and lyophilized and stored at −20°C until use.
Estimation of protein concentration in garlic extract.
The concentration of total proteins in garlic extract was measured by spectrophotometric analysis using a Benchmark Microplate reader (Bio-Rad) at 595 nm according to Bradford's assay (13). Equal amounts of total proteins from garlic extract and eluted fractions from SP Sepharose beads were denatured in 5× sample buffer (0.31 M Tris-HCl [pH 6.8], 20% sodium dodecyl sulfate [SDS], 25% glycerol, 0.01% bromophenol blue, 25% 2-β-mercaptoethanol) by heating at 100°C for 8 min. Proteins were separated on 4% to 20% gradient gels according to the instructions of the manufacturer (Thermo Scientific). The gels were stained with Coomassie brilliant blue R250 and photographed (43).
Anti-A. actinomycetemcomitans assay.
We tested 5 different serotypes of A. actinomycetemcomitans, including the following clinical isolates: DF2200 (serotype a), NJ 1000 (serotype b), and CU1000 (serotype f) (38), NJ9500 (serotype e) (28), and IDH781 (serotype d) (35) and found similar results with all of them. Therefore, for all the remaining experiments, we chose to use IDH781 as the representative strain. IDH781 is a rough strain of A. actinomycetemcomitans that belongs to serotype d and had been isolated clinically from a patient. A. actinomycetemcomitans (IDH781) was grown in Trypticase soy broth (BD Biosciences) supplemented with 6 g of yeast extract and 8 g of glucose per liter at 37°C in 10% CO2; this medium was designated AAGM (A. actinomycetemcomitans growth medium). The A. actinomycetemcomitans killing assay was carried out in 1.5-ml microcentrifuge tubes containing 1 ml of a single-cell bacterial suspension of A. actinomycetemcomitans (37) (108 CFU/ml) with different concentrations of the agents described below (8). Briefly, after gentle agitation, cell suspensions were incubated at 37°C for 2 h. The cells were then harvested by brief centrifugation, washed three times with phosphate-buffered saline (PBS), serially diluted, and plated on Trypticase soy agar plates (AAGM plates). The plates were then incubated for 2 days at 37°C in 10% CO2, and colonies were enumerated. The anti-A. actinomycetemcomitans assay was carried out for the following agents: garlic extract, purified garlic proteins, allicin, and DAS. To compare the efficacies of DAS on planktonic cells and preformed biofilms, the A. actinomycetemcomitans biofilms were grown as described elsewhere in Materials and Methods.
Garlic extract-A. actinomycetemcomitans killing assay.
The effect of temperature on the efficacy of garlic extract for A. actinomycetemcomitans inhibition was determined by heating the extract at 100°C for different time intervals (15, 30, 45, and 60 min) and subjecting the extract to different temperatures (40, 60, 80, and 100°C) for 20 min in a water bath. The samples were cooled to room temperature before use. The effect of heat treatment on allicin or DAS against A. actinomycetemcomitans was determined by heating the compounds for 20 min at 100°C and then cooling the compounds. The anti-A. actinomycetemcomitans activity was determined as mentioned above. Also, the garlic extract was subjected to proteinase K treatment by incubating it with either proteinase K or PBS at 55°C for 2 h followed by incubation at 65°C for 10 min to stop proteinase K activity if present (26).
Anti-A. actinomycetemcomitans activity of purified garlic proteins.
After purification of the garlic proteins as described above, A. actinomycetemcomitans was treated with the purified protein fractions (100 μg/ml) to investigate their anti-A. actinomycetemcomitans activity.
Anti-A. actinomycetemcomitans activity of allicin and DAS.
A. actinomycetemcomitans cells were incubated with different concentrations (10, 1, 0.1, and 0.01 μg/ml) of allicin or DAS in an anti-A. actinomycetemcomitans assay as described above.
A. actinomycetemcomitans biofilm preparation.
A. actinomycetemcomitans cells were grown on a Fisher brand coverslip (Fisher Scientific Inc.) for 48 h in 6-well cell culture plates (Cellstar; Greiner Bio One, Germany) to allow mature biofilm formation. After the biofilm was rinsed with PBS, it was treated with either PBS or DAS (0.01 μg/ml) for 2 h at 37°C. After the DAS was removed and washed with PBS, the biofilms were used for confocal microscopy. The biofilm also scraped, resuspended in the AAGM, serially diluted, and plated for enumeration.
Penetration of DAS into an A. actinomycetemcomitans biofilm.
To determine the efficacy of DAS treatment using preformed A. actinomycetemcomitans biofilms, the biofilms were stained with a LIVE/DEAD BacLight bacterial viability kit (Invitrogen), which utilizes SYTO9 and propidium iodide (PI) to differentiate between live and dead cells. After staining was performed for 45 min, the stain was washed away and the cells were visualized using an inverted confocal microscope (LSM 510; Carl Zeiss, Thornwood, NY) on a Zeiss Axiovert 100 M Base at a confocal imaging facility (University of Medicine and Dentistry of New Jersey [UMDNJ]) using wavelengths of 543 nm (HeNe laser) and 488 nm (argon laser) and chroma filters (Ch 1 detector, bp 560 to 615; Ch 2 detector, bp 505 to 550) and a stack size of 225.0 μm by 225.0 μm by 0.0 μm, which allows simultaneous collection of two fluorescent signals (14, 38).
Anti-A. actinomycetemcomitans activity of DAS in the presence of saliva.
The protocol was reviewed and approved by the Institutional Review Board (IRB) of the University of Medicine and Dentistry of New Jersey. All participants were provided with consent forms that they signed prior to participation in the study. We collected saliva from three subjects according to a previously published method (26). Subjects were asked to contribute 5 ml of unstimulated whole saliva, which was collected in a 50-ml wide-mouthed plastic tube and kept on ice. Saliva was centrifuged at 10,000 × g for 20 min. The supernatant was decanted and used for the experiments.
SEM.
Scanning electron microscopy (SEM) analysis was performed to examine morphological changes of A. actinomycetemcomitans cells before and after treatment with DAS (0.01 μg/ml). The bacterial cells were treated either with PBS or with DAS (0.01 μg/ml) for 2 h as described for the anti-A. actinomycetemcomitans assay. After washing was performed, the cells were grown on a plastic coverslip in 6-well cell culture plates for 48 h. The cells were washed with PBS, and the biofilms were fixed with 2% glutaraldehyde for 2 h, dehydrated, and processed for SEM according to a previously published method using an accelerating voltage of 30 kV (Hitachi SEM S2500; Hitachi High Technologies America, Inc.) (54).
Cell culture.
An immortalized human gingival epithelial cell line (OBA9) (23) was kindly provided by Gill Diamond (UMDNJ). OBA9 cells were grown in serum-free keratinocyte growth medium (KSFM; Gibco Life Technologies) containing insulin, epidermal growth factor, and fibroblast growth factor (Invitrogen) and supplemented with streptomycin (100 μg/ml) and penicillin (100 U/ml) (Sigma, St. Louis, MO) and cultured at 37°C in 5% CO2.
Trypan blue exclusion test of OBA9 cell viability.
The cells were seeded into six-well plates and grown to 80% confluence in the growth medium (KSFM). The cells were then rinsed and incubated with different concentrations (0.01, 0.1, and 1 μg/ml) of DAS for 12 h. The cells were washed two times with PBS and suspended in 1 ml of PBS, and the viable cells were counted by the trypan blue method using a cell counter as stated in the manufacturer's instructions (Vi-CELL series; Beckman Coulter). The proportions of live cells determined in independent triplicate experiments were expressed in percentages (68). The morphological changes were also observed using an upright Olympus BX50W1 microscope (Olympus, Tokyo, Japan).
Flow cytometry.
Cells exposed to different concentrations (0.01, 0.1, and 1 μg/ml) of DAS were collected and resuspended in KSFM medium. The cells were then washed with PBS two times, and the final cell pellet (1 × 109 cells) was suspended in 0.5 ml of PI-hypotonic lysis buffer and 10 μl of PI. The cells were placed on ice and kept in the dark, and G0 apoptosis and cell cycle arrest were analyzed by flow cytometry at 488 nm (50).
Nucleotide sequence analysis of A. actinomycetemcomitans gst gene.
Glutathione (GSH) S-transferase (GST) from serotypes a, b, c, d, e, and f of A. actinomycetemcomitans was PCR amplified using genomic DNA templates (5 ng) from the respective serotype strains and primers gst F (ATGAAACTATATTTTAAACCG) and gst R (AATCAACCCTTCGG CTTTTTG) with initial denaturation at 94°C for 5 min followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 59°C for 30 s, extension at 72°C for 1 min, and an additional final extension step at 72°C for 10 min. The PCR products (600 bp) were purified using a QIAquick gel extraction kit (Qiagen), and then gst was sequenced with T3 and T7 universal primers in the UMDNJ MRF (Molecular Resource Facility) using a DNA sequencer (ABI 3130xl Sequencer). The sequence was analyzed with BioEdit (http://www.mbio.ncsu.edu/bioedit/page2.html), and the sequence for IDH781 was deposited in GenBank and NCBI.
GST colorimetric assay.
CDNB-GST (1-chloro-2,4-dinitro-benzene–GST) assays were performed using 1-cm cuvettes as described previously (33). The reaction mixture was composed of 2 μmol of CDNB, 1 μmol of GSH, and 0.1 ml of A. actinomycetemcomitans cell extract or OBA9 cell extract at a final volume of 1 ml. All the assay components were prepared in PBS (Sigma-Aldrich, St. Louis) (pH 7.9). The reaction was initiated with addition of GSH and 1 min of equilibration prior to recording absorbance changes. CDNB-GSH conjugation (formation of a 2,4-dinitrophenol–glutathione conjugate [DNP-SG] via nucleophilic displacement of Cl with GSH-thiol) was monitored spectrophotometrically at 340 nm for 5 min (kinetic mode for 30 s) with a Shimadzu UV160U spectrophotometer (Shimadzu Corp.). DNP-SG concentrations were calculated using an extinction coefficient of 9.6 mM/cm. Enzyme preparations were assayed in triplicate, and control reactions (without enzyme and without substrate) were included to determine nonenzymatic CDNB-GSH conjugation.
Statistical analyses.
All experiments were performed in duplicate on at least three different occasions. The results for the antibacterial assays were tested for statistical significance using a Student's paired t test and one-way analysis of variance (ANOVA) wherever applicable. Data represent means ± standard errors of the means (SE).
Nucleotide sequence accession number.
The gst gene sequence of IDH781 was deposited in GenBank and NCBI under accession number JN625753.
RESULTS
Garlic extract demonstrates an antibacterial effect against A. actinomycetemcomitans.
A 2-h cell-killing assay was performed as described in Materials and Methods to examine the anti-A. actinomycetemcomitans activity of garlic extract. Garlic extract showed a dose-dependent killing effect against A. actinomycetemcomitans, with the highest concentration showing complete killing (Fig. 1A; P < 0.01). In an attempt to elucidate whether proteins in garlic extract contributed to the anti-A. actinomycetemcomitans effect, garlic proteins were treated with proteinase K or PBS for 2 h at 55°C, cooled, and incubated with A. actinomycetemcomitans cells for 2 h to determine the anti-A. actinomycetemcomitans activity of the extract. The treatment of garlic extract with proteinase K did reduce the antimicrobial efficacy of DAS (approximately 1 log) compared to control heated garlic extract, but the difference was not statistically significant (Fig. 1B). The heat treatment of garlic extract with PBS also resulted in some loss of anti-A. actinomycetemcomitans activity (Fig. 1B) compared to non-heat-treated garlic extract (Fig. 1A).
Fig 1.
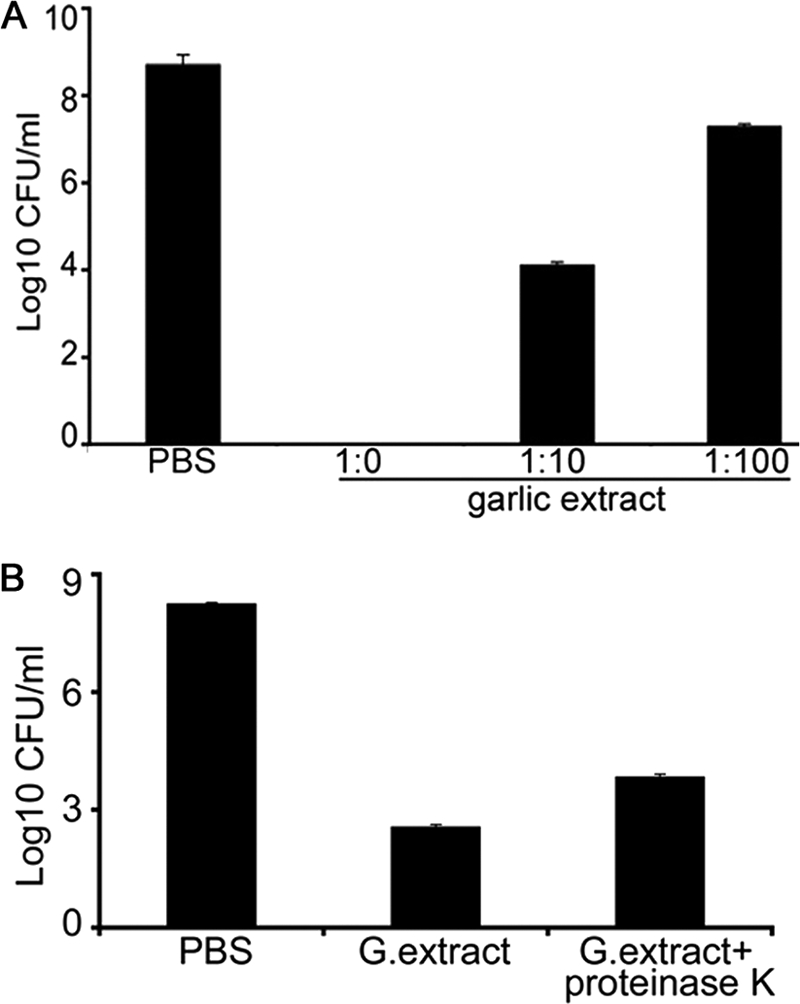
Antibacterial effect of garlic extract against A. actinomycetemcomitans. (A) The anti-A. actinomycetemcomitans activity of garlic extract was determined by incubation of bacterial cells at a concentration of garlic extract of 1:0 for 2 h at 37°C. Subsequently, the cells were serially diluted and plated on AAGM agar plate. The viable bacteria were enumerated after 48 h. (B) Garlic (G.) extract (1:0) was treated with proteinase K for 2 h at 55°C and its anti-A. actinomycetemcomitans activity compared to that of PBS heat-treated extract. Data represent means ± SD and were significantly different from PBS-treated control results (P < 0.01).
Effect of heat treatment on garlic extract.
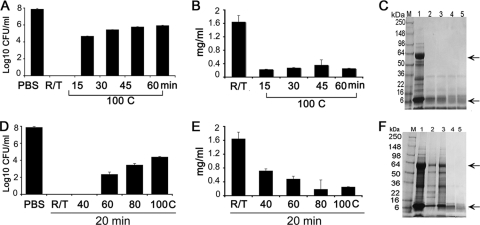
The effect of heat treatment on the anti-A. actinomycetemcomitans activity of garlic extract was determined. The antimicrobial activity was reduced when garlic extract was heat treated at 100°C for 15 min but still resulted in a statistically significant (3 logs; P < 0.01) reduction in bacterial counts (Fig. 2A). Heat treatment of garlic extract for up to 60 min at 100°C did not show any further negation of anti-A. actinomycetemcomitans efficacy. Cell-killing experiments at room temperature and at 40°C demonstrated no bacterial growth, whereas higher temperatures reduced the antimicrobial efficacy against A. actinomycetemcomitans (Fig. 2D). However, even at 100°C, the reduction was approximately 3 logs (P < 0.01), suggesting that the heat-stable components of garlic extract play a major role in the antimicrobial activity of garlic extract.
Fig 2.
Effect of heat on the antibacterial effect of garlic extract. (A) Garlic extract was incubated at 100°C for different time intervals (0, 15, 30, 45, and 60 min). A. actinomycetemcomitans cells were then incubated with the extract for 2 h at 37°C. After serial dilution, the samples were plated on AAGM agar plates and incubated at 37°C and viable bacteria were enumerated after 48 h. R/T, room temperature. (B) Estimation of total garlic protein after incubation at 100°C for different time intervals. (C) SDS-PAGE analysis of garlic proteins incubated at 100°C for different time intervals. Arrows indicate degradation of proteins in lanes 2, 3, 4, and 5 compared to lane 1. Lanes: M, marker; 1, untreated garlic extract; 2, 3, 4, and 5, heat treatment of garlic extract for 15, 30, 45, and 60 min, respectively. (D) Garlic extract demonstrates significant antibacterial effect against A. actinomycetemcomitans after incubation at different temperatures (40, 60, 80, and 100°C) for 20 min. (E) Estimation of total garlic protein after incubation at different temperatures for 20 min. (F) SDS-PAGE analysis of garlic proteins after incubation at different temperatures for 20 min. Lanes: M, marker; 1, room temperature garlic extract; 2, 3, 4, and 5, heat treatment of garlic extract at 40, 60, 80, and 100 min, respectively. Arrows indicate degradation of proteins in lanes 2, 3, 4, and 5 compared to lane 1.
Garlic proteins do not demonstrate anti-A. actinomycetemcomitans activity.
Protein concentrations of garlic extract were tested after heat treatment. The heat treatment of the garlic extract at 100°C for different time periods (15, 30, 45, and 60 min) resulted in a decrease in the total protein concentration, with a minimal amount persistent after 60 min. The total protein concentration at room temperature was 1.5 mg/ml, and the concentration declined to 0.3 mg/ml after heating at 100°C for 15 min; prolonged heating did not result in any further decrease (Fig. 2B). Heating garlic extract at increasing temperatures (40, 60, 80, and 100°C) for a fixed period of 20 min showed concomitant decreases in protein concentrations (Fig. 2E). SDS-polyacrylamide gel electrophoresis (SDS-PAGE) analyses of the garlic extract after heat treatment showed that two prominent bands of 59 and 13.5 kDa were completely degraded (Fig. 2C and F).
Purification of garlic protein by SP Sepharose column chromatography.
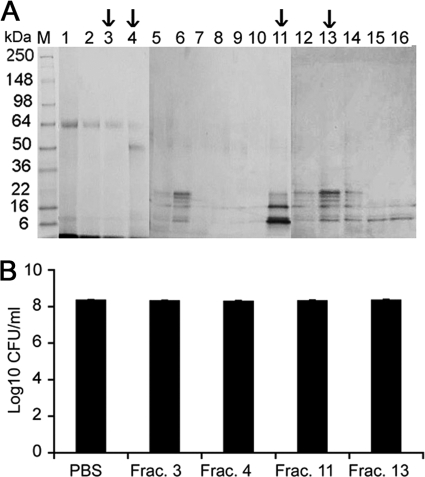
Garlic proteins were purified using SP Sepharose affinity column chromatography (Fig. 3A). Fractions 3, 4, 11, and 13 were dialyzed using 3-kDa-cutoff Slide-A-Lyzer dialysis cassettes. The fractions were tested for their anti-A. actinomycetemcomitans activity, and the results showed that there was no significant anti-A. actinomycetemcomitans activity in any of the fractions (Fig. 3B). These results were further corroborated by the results of the proteinase K treatment performed as described above. This could suggest that proteins are not involved in the majority of the antibacterial effect.
Fig 3.
Garlic protein purification and characterization using SP Sepharose column chromatography. (A) The garlic proteins were purified using SP Sepharose column chromatography by elution with NaCl (0.2 to 1.0 M). The eluted fractions were processed on an SDS-PAGE gel and stained with Coomassie R250 to visualize the proteins. (B) To determine the anti-A. actinomycetemcomitans activity of partially purified protein, fractions (Frac.) 3, 4, 11, and 13 (indicated with arrows; 100 μg/ml each) were incubated with A. actinomycetemcomitans for 2 h at 37°C. The cells were then serially diluted and plated on an AAGM agar plate to enumerate the viable CFU of bacteria/ml.
Allicin exhibits antibacterial activity against A. actinomycetemcomitans.
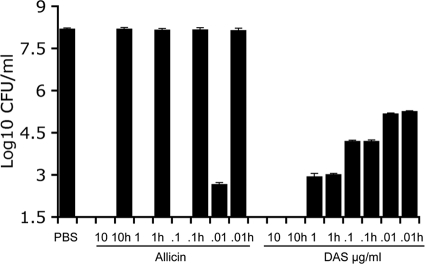
Allicin exhibited highly significantly antibacterial activity against A. actinomycetemcomitans, with concentrations of 10, 1, and 0.1 μg/ml showing complete killing. However, treatment with a concentration of allicin of 0.01 μg/ml resulted in a 5-log reduction, a result that was also highly statistically significant (P < 0.01). Allicin is, however, a thermolabile compound, and heat treatment of allicin at 100°C for 20 min resulted in complete loss of activity, even at 10 μg/ml (Fig. 4). These results suggest that allicin, although extremely effective against A. actinomycetemcomitans, is unstable at higher temperatures.
Fig 4.
Anti-A. actinomycetemcomitans activity of allicin and DAS in a 2-h cell-killing assay. To determine the anti-A. actinomycetemcomitans activity of allicin and DAS, the cells were incubated with different concentrations (10, 1, 0.1, and 0.01 μg/ml) of allicin or DAS for 2 h at 37°C. The compounds were also subjected to heat treatment (h) at 100°C for 20 min in a water bath to determine whether they retained their anti-A. actinomycetemcomitans activity. The cells were serially diluted and plated on an AAGM agar plate. The viable bacteria were enumerated after 48 h. Data represent means ± SE and significantly differ from control results (P < 0.01).
DAS exhibits antibacterial activity against A. actinomycetemcomitans.
Six different serotypes of A. actinomycetemcomitans have been previously identified (40). While the majority of bacteria had a rough-looking phenotype, some had a smooth phenotype, which may have occurred as a result of several passages in the laboratory. We tested the effects of DAS on five different serotypes of A. actinomycetemcomitans and found equal effects, with similar levels of anti-A. actinomycetemcomitans activity across all serotypes (data not shown). As no differences were observed between the different serotypes, we decided to perform all experiments using IDH781 as a representative strain. DAS exhibits significant antibacterial activity against A. actinomycetemcomitans at different concentrations. While a concentration of 10 μg/ml completely eliminated A. actinomycetemcomitans, the effect of DAS treatment diminished with decreasing concentrations. However, even at concentrations of 0.01 μg/ml, DAS treatment resulted in a 3-log reduction in bacterial levels compared to PBS treatment, a result that was statistically significant (Fig. 4; P < 0.01). To determine whether DAS is a heat-stable antimicrobial compound, DAS was subjected to heat treatment at 100°C for 20 min and then cooled, and its antibacterial effect was compared to that of untreated DAS. The results showed that heat treatment of DAS did not result in a loss of antimicrobial function even at the lowest concentration (0.01 μg/ml; Fig. 4).
Biofilm penetration assay.
To determine the effect of DAS on pregrown A. actinomycetemcomitans biofilms, A. actinomycetemcomitans was grown on coverslips and exposed to DAS at 0.01 μg/ml for 2 h. The results show that, compared to PBS treatment, treatment with DAS at 0.01 μg/ml resulted in a 2-log reduction in the number of cells, a result that was found to be statistically significant (P < 0.01; Fig. 5). However, there was a difference observed between the results of treatment of planktonic cells and treatment of biofilm; treatment of planktonic cells resulted in a 3-log reduction in cell numbers. Although the presence of a biofilm reduced the amount of killing, the reduction in A. actinomycetemcomitans numbers was still 2 logs, and the data were statistically significant in comparison to control results (Fig. 5).
Fig 5.
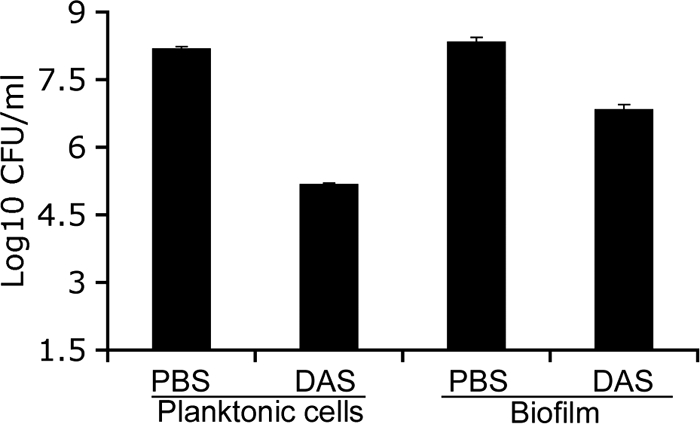
Comparative cell-killing assay in planktonic cells and biofilms of A. actinomycetemcomitans. To determine the efficacy of DAS treatment of both planktonic cells and A. actinomycetemcomitans biofilms, the cells were grown on a plastic coverslip for 48 h at 37°C. The biofilms were washed with PBS and then treated with DAS (0.01 μg/ml) for 2 h. To determine the efficacy of DAS treatment of A. actinomycetemcomitans biofilms, cells were scraped from the coverslip after treatment with DAS, serially diluted, and plated on an AAGM agar plate. For the planktonic cell-killing assay, the biofilms were scraped from the coverslip, treated with DAS for 2 h in a 1.5-ml microcentrifuge tube, serially diluted, and plated on AAGM agar for enumeration after 48 h. Data represent means ± SE and significantly differ from control results (P < 0.01).
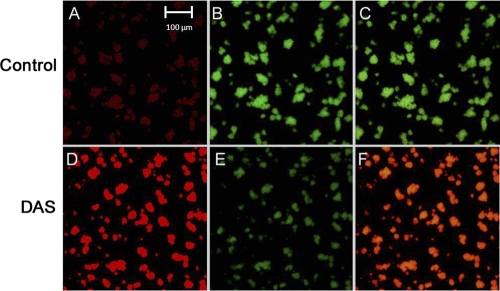
To determine whether DAS penetrated biofilms of A. actinomycetemcomitans, the cells were grown on coverslips and treated with DAS as described in Materials and Methods. The biofilms were strained (LIVE/DEAD BacLight bacterial viability assay) and observed under confocal laser scanning microscopy. The results of this experiment revealed that most of biofilm bacteria treated with DAS were dead, as demonstrated by red fluorescence (Fig. 6D). In combined channels, dead cells (red fluorescence) were predominantly present (Fig. 6F). The images from confocal microscopy of the biofilm assay corroborate the culture results, as evidenced by greater areas of cell death among DAS-treated cells (Fig. 6D to F) compared to PBS-treated controls (Fig. 6A to C).
Fig 6.
Confocal microscopy (450-μm field with ×60 magnification using Zeiss objective lens) of DAS-treated (D to E) A. actinomycetemcomitans compared to PBS-treated (A to C [Control]) A. actinomycetemcomitans IDH781. Panel D shows cells visualized using an HeNe laser focused at a wavelength of 543 nm, which is specific for propidium iodide. Panels B and E show cells visualized using an HeNe laser at a wavelength of 488 nm, which is specific for SYTO9, while panels C and F are merged images showing comparisons of live to dead cells. The PBS-treated cells are greener than the DAS-treated cells, which are yellow (panel F versus panel C; combination of red and green), indicating that the number of dead cells was higher in the DAS-treated sample than in the control sample.
Scanning electron microscopy analyses.
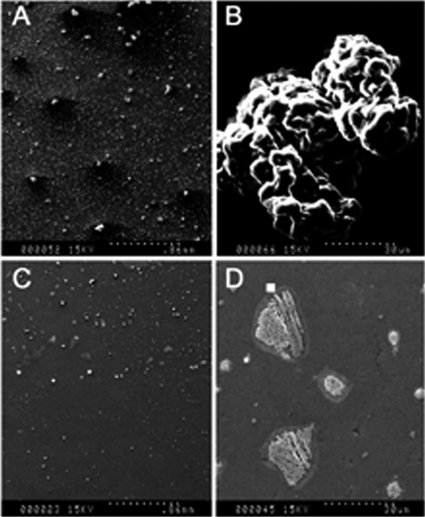
To visualize the effect of DAS on A. actinomycetemcomitans in terms of colony morphology, the cells were treated with DAS for 2 h and then serially diluted and added to plastic coverslips in a 6-well cell culture plate and incubated for 48 h. The biofilm were processed for SEM. The scanning electron microscopy analysis results shown in Fig. 7 revealed that A. actinomycetemcomitans grew as multilayered bacteria of rod-shaped, tubular morphology, a characteristic feature of well-established rough strains of A. actinomycetemcomitans biofilm (7A and B). However, when the bacteria were treated with DAS, the colony appeared flat, with complete loss of colony architecture apart from the drastic reduction in the number of colonies, indicating the presence of severe stress. The cells that survived in the DAS were not able to form a biofilm with a typical architecture. It was also difficult to locate the colonies, as they were far fewer than those seen in untreated controls (Fig. 7C and D).
Fig 7.
Scanning electron micrographs of A. actinomycetemcomitans cells treated with DAS. A. actinomycetemcomitans cells were incubated with DAS at 0.01 μg/ml for 2 h, and then the cells were serially diluted and added onto a plastic coverslip in a 6-well cell culture plate and processed for SEM. (A and B) PBS-treated cells; (C and D) DAS-treated cells (magnification in panels A and C, ×35; magnification in panels B and D, ×1,000). (A) The control (PBS) cells after 24 h of incubation. The colonies were densely attached to the coverslip. (B) A biofilm is seen as multiple layers of bacteria having a rod-shaped and tubular morphology, characteristic of well-established A. actinomycetemcomitans biofilms. (C and D) A. actinomycetemcomitans cells were treated with DAS. (C) Very few colonies were observed in DAS-treated cells, and the remaining viable cells were unable to form a complete biofilm. (D) DAS-treated A. actinomycetemcomitans cells were unable to form a viable biofilm, and the biofilms that did form were deformed and lacked the normal structure and colony morphology.
Efficacy of DAS in the presence of saliva.
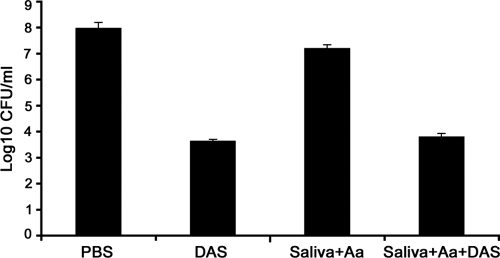
For an agent to be effective in the oral cavity, it must demonstrate activity in the presence of saliva. To test the possibility of its use as a therapeutic agent, the efficacy of DAS against A. actinomycetemcomitans in the presence of saliva was tested and compared to that of DAS without saliva treatment. DAS was dissolved in whole saliva at the lowest concentration (0.01 μg/ml) and incubated with A. actinomycetemcomitans in a 2-h cell-killing assay. The results showed that, while PBS or saliva alone does not demonstrate antimicrobial effect, saliva mixed with DAS at 0.01 μg/ml resulted in a 3-log reduction of A. actinomycetemcomitans cell numbers, indicating that DAS retains its antimicrobial activity in the presence of saliva (Fig. 8). The antimicrobial effect was comparable to that exhibited by DAS without the addition of saliva, indicating that the addition of saliva to DAS does not affect its antimicrobial activity.
Fig 8.
Anti-A. actinomycetemcomitans activity of DAS in the presence of saliva. Saliva was collected from periodontally healthy subjects and clarified by centrifugation (10,000 × g for 20 min). A. actinomycetemcomitans (Aa) cells were incubated with DAS and either saliva or saliva with DAS for 2 h at 37°C. The cells were then serially diluted and plated on an AAGM agar plate. The viable bacterial cells were enumerated after 48 h, and the results showed a statistically significant difference with the addition of DAS. Data represent means ± SE, and error bars indicate ranges (P < 0.01).
DAS treatment reduces A. actinomycetemcomitans GST activity.
Previous reports have shown that the antimicrobial activity of DAS could be related to its inhibitory effect on arylamine transferase and/or GST (45). GSTs are members of a large supergene family of detoxifying enzymes in nature. When we searched the A. actinomycetemcomitans genome in the ORALGEN database (http://www.oralgen.lanl.gov/_index.html) for genes encoding arylamine transferase and GST, only the latter was found (accession no. AA02492). We analyzed the gst DNA sequences from different serotypes of A. actinomycetemcomitans and found no significant differences between them. The effect of DAS on GST activity in DAS-treated A. actinomycetemcomitans cells was tested at different time intervals according to a previously published method (34). The data revealed that exposure to DAS inhibits GST-specific activity (<0.001 nmol/h/mg) compared to control results (4.58 nmol/h/mg protein).
DAS does not exhibit cell cytotoxicity against oral epithelial cell lines. (i) Cell viability.
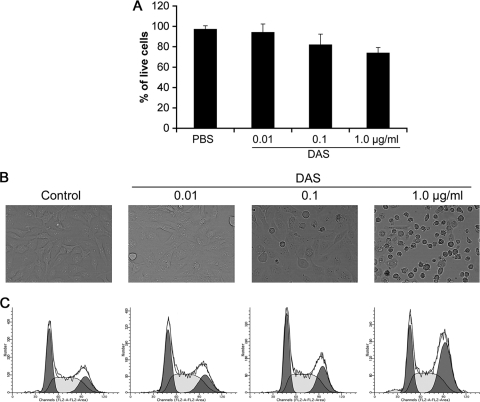
A Trypan blue exclusion assay was carried out to determine whether DAS affects the viability of OBA9. The cells were treated with different concentrations (0.01, 0.1, and 1 μg/ml) of DAS in 96-well plates for 12 h. As shown in Fig. 9A, there was no inhibition of growth of cells at a concentration of 0.01 whereas at a higher concentration (1 μg/ml) the inhibition was reduced to 30%.
Fig 9.
DAS does not exhibit cell cytotoxicity against OBA9 cells. OBA9 cells were treated for 12 h with different concentrations of DAS (1, 0.1, and 0.01 μg/ml) and analyzed using a cell viability assay. (A) Trypan blue exclusion test of OBA9 cell viability. The total cell volumes were washed two times with PBS and suspended in 1 ml of PBS, and the viable cells were counted by the trypan blue method using a cell counter. The numbers of live cells from independent triplicate experiments are expressed in percentages. (B) Light microscopy using an Olympus microscope at ×1,000 magnification to examine DAS-treated cells for changes in morphology. (C) Cells were collected in a hypotonic buffer with propidium iodide, and cell numbers were analyzed by flow cytometry. There was no evidence of cell death observed after treatment with DAS at 0.01 μg/ml, but higher concentrations of DAS (0.1 and 1 μg/ml) resulted in increasing evidence of cell death.
(ii) Cell morphology in OBA9 cells treated with DAS.
Microscopic analysis of DAS-treated gingival keratinocyte OBA9 cells revealed starkly distinct morphological features (Fig. 9B). The control (PBS-treated) cells and the cell treated with DAS at a concentration of 0.01 μg/ml revealed no evident cytopathic changes, whereas cells treated with higher concentrations (0.1 and 1.0 μg/ml) of DAS showed alterations in cell morphology. This effect was mild at a concentration of 0.1 μg/ml but was significantly greater at a concentration of 1 μg/ml, perhaps due to a cytotoxic effect. The cells appeared flattened and rounded, and most of the cells lost attachment to the substrate.
(iii) Flow cytometry.
To determine whether DAS induces cell apoptosis in the OBA9 oral cell line, cells were analyzed by flow cytometry. The results showed that there was no evidence of apoptosis at any concentration of DAS at 12 h (Fig. 9C). However, treatment with DAS at 0.1 and 1 μg/ml resulted in an increase in cell death due to cell arrest in the G2/M phase compared to the control cell treatment.
We also determined the effect of DAS treatment on levels of GST, and the results revealed that there were no changes in GST activity when cells are treated with different concentrations of DAS. The range of GST activities showed a mean of 7.07 ± 0.126 nmol/min/mg for PBS treatment, whereas treatment with DAS at 1 μg/ml (the highest concentration) showed activity at 7.69 ± 0.105 nmol/min/mg, a result that was found to be statistically insignificant (Fig. 10).
Fig 10.
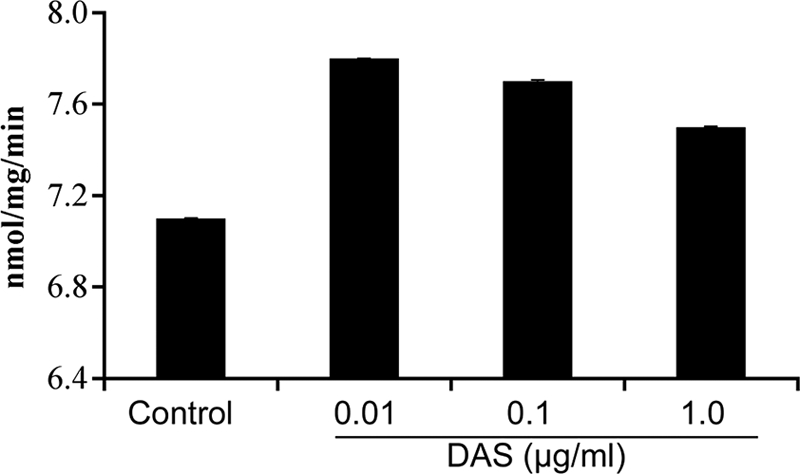
Effect of DAS on OBA9 GST activity. OBA9 cells were incubated with various concentrations of DAS for 12 h and then washed with PBS. The cells were lysed and centrifuged, and the supernatant was used as the enzyme source to measure GST-specific activity at 340 nm.
DISCUSSION
With the rise in bacterial resistance to antibiotics, there is considerable interest in the development of antimicrobial chemotherapy and other classes of antimicrobials for the control of infection. Alternative therapeutic agents such as DASs that are known for their antimicrobial activities provide a novel method of antimicrobial treatment with no known resistance or side effects. A. actinomycetemcomitans-associated LAP is a severe form of periodontal disease, affecting more than 70,000 adolescents in the United States annually, with 15- and 5-fold-higher prevalences in African-American and Hispanic populations, respectively (81). The loss of teeth in young adolescents results in significant functional, esthetic, economical, and psychological impacts on the lives of the adolescents and their families. The significance of this study was to explore the therapeutic efficacy of DAS that could make the treatment of LAP more effective and reliable. The presence of many virulence factors in A. actinomycetemcomitans and its ability to invade tissues, in addition to the emergence of different antibiotic-resistant strains, has resulted in increased recurrence of LAP.
A recent study (8) has shown that both garlic extract and allicin inhibit the growth of oral bacteria. The results of the present study also demonstrate that garlic extract significantly reduces the viability of A. actinomycetemcomitans cells, indicating that the extract has potent antimicrobial compounds. Our results suggest that the garlic extract exhibits significant anti-A. actinomycetemcomitans activity and that the activity is retained even after heat treatment.
To investigate whether proteins in garlic exhibited any antimicrobial effect, garlic proteins were purified using Sepharose column chromatography. The protein fractions were tested for their antimicrobial activity, and they did not show antimicrobial activity. However, it is possible that some proteins may need to interact with each other to demonstrate activity. Hence, the garlic extract was subjected to proteinase K treatment to completely eliminate proteins in the extract, and the results from this experiment showed that the garlic extract retains most of its activity even after proteinase K treatment. These results suggest that the ingredient in garlic extract that is active against A. actinomycetemcomitans may not be protein.
Among other compounds in garlic extract, allicin is a major compound with known antibacterial activity (8, 48, 46). We tested the effect of allicin against A. actinomycetemcomitans in a 2-h cell-killing assay. These results show that allicin is extremely effective against A. actinomycetemcomitans and results in a 5-log reduction of A. actinomycetemcomitans cell numbers. However, allicin is a thermolabile compound and is extremely unstable at higher temperatures. When allicin was subjected to heat treatment at 100°C for 20 min, the antibacterial activity was completely abolished. Previous studies have shown that allicin is formed after garlic is crushed and is immediately converted to organosulfur compounds. The results of our experiments positively correlate with those of previous studies (48).
Among the other compounds in garlic, the organosulfur compounds have gained significance with respect to treatment of infections over the last decade. The organosulfur compounds diallyl sulfide (DAS), diallyl disulfide (DADS), and diallyl trisulfide (DATS) have been shown to possess significant antibacterial properties. Unlike allicin, DASs are thermostable compounds and can be hypothesized to function effectively in the oral cavity. The inhibitory effect of DASs on several pathogens has been studied extensively (1, 7, 17, 37, 42, 51, 52, 53, 58, 62, 63), and recent reports have shown that DASs were synergistically effective against antibiotic-resistant strains when the DASs were combined with antibiotics (80). Our results also suggest that the anti-A. actinomycetemcomitans activity is from the thermostable compounds present in the garlic extracts. DAS treatment resulted in statistically significant reductions of A. actinomycetemcomitans cell numbers in the 2-h cell-killing assay. The results were similar for all the DASs, including DADS and DATS (data not shown), with no statistically significant differences. While a concentration of 1 μg/ml resulted in complete elimination of A. actinomycetemcomitans, treatment with 0.01 μg/ml resulted in a 3-log reduction of cell numbers, a result that was found to be statistically significant.
Bacteria in the oral cavity exist in a complex biofilm called dental plaque, and one of the ideal requisites of an effective antimicrobial agent is to be effective in biofilm. We decided to investigate the efficacy of DAS in pregrown A. actinomycetemcomitans biofilms, and the assay showed that, while DAS showed statistically significant killing of A. actinomycetemcomitans biofilm cells, the effect was less than that seen with planktonic cells. Confocal images show red areas on the surface of the biofilm, with the central core of the biofilm remaining yellow (representing a mixture of live and dead cells). Moreover, image analyses of the z axis also show incomplete penetration of DAS to the bottom of the biofilm (data not shown). Similarly, the SEM images also demonstrate that, while PBS-treated cells show a globular and rough morphology, treatment with DAS results in alteration of colony morphology and leads to cell destruction. Although most of the biofilm appears to have been destroyed, the bottom layer of the colony still appears to have been viable. These data suggest that, while DAS is extremely efficient at killing A. actinomycetemcomitans in the planktonic state, its effect on biofilm warrants further investigation. It is possible that DAS may not completely penetrate the biofilm or that higher concentrations may be required to achieve the desired effect.
Our results show that DAS retains its antibacterial effect after heat treatment at 100°C for over 60 min. It has been previously reported that heat treatment of garlic extract resulted in the highest antibacterial activity and that this positively correlates with increases in DAS content as evidenced by GC and mass spectrometric analyses (11, 15, 42, 65). Recently, the inhibitory mechanism of DAS against Listeria monocytogenes and E. coli O157:H7 was demonstrated by Fourier transform infrared (FT-IR) and Raman spectroscopy (54). Further, the results of that study demonstrated that DAS contributed more to the antimicrobial effect than phenolic compounds isolated from garlic extract. Another study has also shown that heat treatment of these compounds results in significant inhibition of different antibiotic-resistant strains of H. pylori and mosquito (47). The results of previous studies on heat treatment of DAS correlate positively with the results from our studies. An in vivo model showed that two DASs significantly decreased methicillin-resistant S. aureus (MRSA) viability in blood and kidney and reduced the plasma levels of fibronectin and inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) in diabetic mice (71, 72). In addition to the bactericidal effect, DASs enhance antioxidant activities by scavenging superoxide ions and enhancing glutathione peroxidase levels (24, 78, 80).
A critical factor for the successful use of an agent in the oral cavity is to bind and demonstrate efficacy in the presence of saliva. We decided to investigate whether DAS can work when mixed with saliva. The results confirmed that DAS retained its anti-A. actinomycetemcomitans activity even in the presence of saliva. The bactericidal activity of these sulfides correlates with the number of sulfur atoms contained within them (73). It has also been indicated that the bactericidal effects of DAS and DADS on H. pylori, K. pneumoniae, and P. aeruginosa were due to their activity with respect to inhibition of arylamine N-acetyltransferase, an enzyme found in these pathogens (18, 19, 37, 53, 73). In another study, a 30-min exposure of C. albicans to DADS (0.5 mM) induced 70% cell death within 2 h. The early intracellular events associated with DADS-induced cell death corresponded to increased oxidative stress mediated through decreased GSH levels, resulting in the drastic death of C. albicans organisms (51). In this context, we searched the whole-genome sequence database of A. actinomycetemcomitans for the genes encoding arylamine N-acetyltransferase and GST, but only the latter was found (bphH gst; accession number AA02492). Based on these studies, we hypothesized that A. actinomycetemcomitans cell death is associated with altered A. actinomycetemcomitans-GST levels and that this mediates the drastic death response induced by DAS as previously reported (51). Our kinetic enzyme assay revealed that when A. actinomycetemcomitans is treated with DAS, the activity of GST is completely inhibited compared to the activity seen with PBS-treated controls, as evidenced by the colorimetric assay. Whether the inhibition of GST leads to severe oxidative stress and subsequent cell death needs to be further investigated by analyzing DAS-GST interactions. The effects of DAS on GST activity can be different for prokaryotic and eukaryotic systems. In prokaryotic systems, GST is one of the most common mechanisms that bacteria use to deal with oxidative stresses. While this might kill the cells, it is possible to argue that a similar effect of GST can be seen in eukaryotic cell lines. A previous report has also demonstrated that diallyl disulfide (DADS), another organosulfur compound, causes cell apoptosis in MCF-7 breast cancer cell lines (50). We also decided to investigate GST activity and cell apoptosis in an oral gingival cell line (OBA9) in the presence of different concentrations of DAS. The results showed that there were no statistically significant differences in GST activity even upon exposure to a DAS concentration of 1 μg/ml. These results suggest that DAS seems to function differently in eukaryotic and prokaryotic systems.
The cytotoxic effects of DAS have been reported to occur at concentrations ranging from 150 to 500 μg/ml, and several other studies have shown that lower concentrations (5 to 100 μM) of the compounds have no cytotoxic effect (3, 10, 25, 31). The antimicrobial effects observed in our study were found at a concentration of 0.01 μg/ml. To date, however, there have been no reports on the cytotoxic effects of DASs on oral cell lines. The cells showed no evidence of apoptosis at any of the concentrations tested, as evidenced by the results of flow cytometry analysis and trypan blue analysis. However, flow cytometry analysis revealed that treatment with DAS at increasing concentrations, and especially at 1 μg/ml, resulted in increased number of cells in the G2 phase compared to the numbers seen with PBS-treated controls. It is, therefore, possible that treatment with DAS at higher concentrations causes cell death by G2/M arrest. However, the effects are seen at concentrations 100 times higher than the therapeutic concentrations. Further studies need to be carried out to understand the interactions of DAS with different cell cycle proteins.
There are several alternative products from plants that exhibit therapeutic properties, including antimicrobial effects. However, their use in conventional treatment has not well received, because certain doubts linger over the multiplicity of compounds present in them and their potential cytotoxic effects. In our experiments, DAS has shown significant antimicrobial properties against A. actinomycetemcomitans, with no cytotoxicity at therapeutic concentrations. Consequently, use of DASs seems to be safe. However, for use of DAS under in vivo conditions, there are many other aspects of the compound that need to be understood. Investigation of the bioavailability of DAS following consumption is critical to understanding the dosing of DAS for therapeutic benefit. Our in vitro antimicrobial concentrations were very low, but if the agent shows poor bioavailability, then higher concentrations of DAS may need to be administered to achieve a local therapeutic concentration. Similarly, systemic cytotoxicity following oral administration of DAS needs to be evaluated under in vivo conditions. Further studies analyzing these aspects and experiments aimed at understanding the exact mechanism behind the antibacterial action are required prior to the use of this agent for therapeutic benefit.
We conclude that DAS exhibits a significant antimicrobial effect against the periodontopathogen A. actinomycetemcomitans, with no cytotoxic effect at its therapeutic concentration in vitro. Importantly, the DASs are heat stable and their antibacterial properties are unaffected by the presence of saliva. All these properties of DAS make them attractive candidates for use as therapeutic agents for treating oral diseases. Further studies are needed to understand and define the mechanisms of action of the DASs against A. actinomycetemcomitans and other systemic pathogens in animal models to establish the safety of these agents in regular use.
ACKNOWLEDGMENTS
K.V. thanks the Department of Oral Biology of the NJDS for financial support.
We thank David Furgang for help with antibacterial assays.
Footnotes
Published ahead of print 13 February 2012
REFERENCES
- 1. Alam M, Dwivedi V, Khan AA, Mohammad O. 2009. Efficacy of niosomal formulation of diallyl sulfide against experimental candidiasis in Swiss albino mice. Nanomed 4:713–724 [DOI] [PubMed] [Google Scholar]
- 2. Ali M, Al-Qattan KK, Al-Enezi F, Khanafer RM, Mustafa T. 2000. Effect of allicin from garlic powder on serum lipids and blood pressure in rats fed with a high cholesterol diet. Prostaglandins Leukot. Essent. Fatty Acids 62:253–259 [DOI] [PubMed] [Google Scholar]
- 3. Alpers DH. 2009. Garlic and its potential for prevention of colorectal cancer and other conditions. Curr. Opin. Gastroenterol. 25:116–121 [DOI] [PubMed] [Google Scholar]
- 4. Amagase H. 2006. Clarifying the real bioactive constituents of garlic. J. Nutr. 136:716S–725S [DOI] [PubMed] [Google Scholar]
- 5. Ankri S, Mirelman D. 1999. Antimicrobial properties of allicin from garlic. Microbes Infect. 1:125–129 [DOI] [PubMed] [Google Scholar]
- 6. Ardila CM, Granada MI, Guzman IC. 2010. Antibiotic resistance of subgingival species in chronic periodontitis patients. J. Periodont. Res. 45:557–563 [DOI] [PubMed] [Google Scholar]
- 7. Avato P, Tursil E, Vitali C, Miccolis V, Candido V. 2000. Allylsulfide constituents of garlic volatile oil as antimicrobial agents. Phytomedicine 7:239–243 [DOI] [PubMed] [Google Scholar]
- 8. Bakri IM, Douglas CW. 2005. Inhibitory effect of garlic extract on oral bacteria. Arch. Oral Biol. 50:645–651 [DOI] [PubMed] [Google Scholar]
- 9. Banerjee SK, Mukherjee PK, Maulik SK. 2003. Garlic as an antioxidant: the good, the bad and the ugly. Phytother. Res. 17:97–106 [DOI] [PubMed] [Google Scholar]
- 10. Belloir C, Singh V, Daurat C, Siess MH, Le Bon AM. 2006. Protective effects of garlic sulfur compounds against DNA damage induced by direct- and indirect-acting genotoxic agents in HepG2 cells. Food Chem. Toxicol. 44:827–834 [DOI] [PubMed] [Google Scholar]
- 11. Benkeblia N, Onodera S, Yoshihira T, Kosaka S, Shiomi N. 2004. Effect of temperature on soluble invertase activity, and glucose, fructose and sucrose status of onion bulbs (Allium cepa) in store. Int. J. Food Sci. Nutr. 55:325–331 [DOI] [PubMed] [Google Scholar]
- 12. Block E. 1985. The chemistry of garlic and onions. Sci. Am. 252:114–119 [DOI] [PubMed] [Google Scholar]
- 13. Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 14. Castillo-Ruiz M, et al. 2011. Isolation of a novel Aggregatibacter actinomycetemcomitans serotype b bacteriophage capable of lysing bacteria within a biofilm. Appl. Environ. Microbiol. 77:3157–3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cellini L, Di Campli E, Masulli M, Di Bartolomeo S, Allocati N. 1996. Inhibition of Helicobacter pylori by garlic extract (Allium sativum). FEMS Immunol. Med. Microbiol. 13:273–277 [DOI] [PubMed] [Google Scholar]
- 16. Chandrashekar PM, Venkatesh YP. 2009. Identification of the protein components displaying immunomodulatory activity in aged garlic extract. J. Ethnopharmacol. 124:384–390 [DOI] [PubMed] [Google Scholar]
- 17. Chen GW, Chung JG, Ho HC, Lin JG. 1999. Effects of the garlic compounds diallyl sulphide and diallyl disulphide on arylamine N-acetyltransferase activity in Klebsiella pneumoniae. J. Appl. Toxicol. 19:75–81 [DOI] [PubMed] [Google Scholar]
- 18. Chen L, et al. 1999. Decrease of hepatic catalase level by treatment with diallyl sulfide and garlic homogenates in rats and mice. J. Biochem. Mol. Toxicol. 13:127–134 [DOI] [PubMed] [Google Scholar]
- 19. Chung JG, et al. 1998. Effects of garlic compounds diallyl sulfide and diallyl disulfide on arylamine N-acetyltransferase activity in strains of Helicobacter pylori from peptic ulcer patients. Am. J. Chin. Med. 26:353–364 [DOI] [PubMed] [Google Scholar]
- 20. Cortelli SC, et al. 2009. Diminished treatment response of periodontally diseased patients infected with the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans. J. Clin. Microbiol. 47:2018–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cutler RR, Wilson P. 2004. Antibacterial activity of a new, stable, aqueous extract of allicin against methicillin-resistant Staphylococcus aureus. Br. J. Biomed. Sci. 61:71–74 [DOI] [PubMed] [Google Scholar]
- 22. Desvarieux M, et al. 2005. Periodontal microbiota and carotid intima-media thickness: the Oral Infections and Vascular Disease Epidemiology Study (INVEST). Circulation 111:576–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dickson MA, et al. 2000. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell. Biol. 20:1436–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Durak I, Ozturk HS, Olcay E, Can B, Kavutcu M. 2002. Effects of garlic extract on oxidant/antioxidant status and atherosclerotic plaque formation in rabbit aorta. Nutr. Metab. Cardiovasc. Dis. 12:141–147 [PubMed] [Google Scholar]
- 25. Elango EM, et al. 2004. Inhibition of cyclooxygenase-2 by diallyl sulfides (DAS) in HEK 293T cells. J. Appl. Genet. 45:469–471 [PubMed] [Google Scholar]
- 26. Fine DH, Furgang D, Goldman D. 2007. Saliva from subjects harboring Actinobacillus actinomycetemcomitans kills Streptococcus mutans in vitro. J. Periodontol. 78:518–526 [DOI] [PubMed] [Google Scholar]
- 27. Fine DH, Furgang D, Kaplan J, Charlesworth J, Figurski DH. 1999. Tenacious adhesion of Actinobacillus actinomycetemcomitans strain CU1000 to salivary-coated hydroxyapatite. Arch. Oral Biol. 44:1063–1076 [DOI] [PubMed] [Google Scholar]
- 28. Fine DH, et al. 1999. Phenotypic variation in Actinobacillus actinomycetemcomitans during laboratory growth: implications for virulence. Microbiology 145(Pt. 6):1335–1347 [DOI] [PubMed] [Google Scholar]
- 29. Fives-Taylor PM, Meyer DH, Mintz KP, Brissette C. 1999. Virulence factors of Actinobacillus actinomycetemcomitans. Periodontol. 2000 20:136–167 [DOI] [PubMed] [Google Scholar]
- 30. Fukao T, Hosono T, Misawa S, Seki T, Ariga T. 2004. The effects of allyl sulfides on the induction of phase II detoxification enzymes and liver injury by carbon tetrachloride. Food Chem. Toxicol. 42:743–749 [DOI] [PubMed] [Google Scholar]
- 31. Germain E, Auger J, Ginies C, Siess MH, Teyssier C. 2002. In vivo metabolism of diallyl disulphide in the rat: identification of two new metabolites. Xenobiotica 32:1127–1138 [DOI] [PubMed] [Google Scholar]
- 32. Gillett R, Johnson NW. 1982. Bacterial invasion of the periodontium in a case of juvenile periodontitis. J. Clin. Periodontol. 9:93–100 [DOI] [PubMed] [Google Scholar]
- 33. Habig WH, Jakoby WB. 1981. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 77:398–405 [DOI] [PubMed] [Google Scholar]
- 34. Habig WH, Pabst MJ, Jakoby WB. 1974. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249:7130–7139 [PubMed] [Google Scholar]
- 35. Haubek D, Poulsen K, Asikainen S, Kilian M. 1995. Evidence for absence in northern Europe of especially virulent clonal types of Actinobacillus actinomycetemcomitans. J. Clin. Microbiol. 33:395–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Herrera D, Alonso B, Leon R, Roldan S, Sanz M. 2008. Antimicrobial therapy in periodontitis: the use of systemic antimicrobials against the subgingival biofilm. J. Clin. Periodontol. 35:45–66 [DOI] [PubMed] [Google Scholar]
- 37. Hsieh SE, Lo HH, Chung JG. 1998. The characteristics of arylamine N-acetyltransferase in Pseudomonas aeruginosa. Curr. Microbiol. 36:353–360 [DOI] [PubMed] [Google Scholar]
- 38. Jefferson KK, Goldmann DA, Pier GB. 2005. Use of confocal microscopy to analyze the rate of vancomycin penetration through Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 49:2467–2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kaplan AH, Weber DJ, Oddone EZ, Perfect JR. 1989. Infection due to Actinobacillus actinomycetemcomitans: 15 cases and review. Rev. Infect. Dis. 11:46–63 [DOI] [PubMed] [Google Scholar]
- 40. Kaplan JB, Schreiner HC, Furgang D, Fine DH. 2002. Population structure and genetic diversity of Actinobacillus actinomycetemcomitans strains isolated from localized juvenile periodontitis patients. J. Clin. Microbiol. 40:1181–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kulik EM, Lenkeit K, Chenaux S, Meyer J. 2008. Antimicrobial susceptibility of periodontopathogenic bacteria. J. Antimicrob. Chemother. 61:1087–1091 [DOI] [PubMed] [Google Scholar]
- 42. Kyung KH, Kim MH, Park MS, Kim YS. 2002. Allinase-independent inhibition of Staphylococcus aureus B33 by heated garlic. J. Food Science 67:6 [Google Scholar]
- 43. Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 44. Lai PK, Roy J. 2004. Antimicrobial and chemopreventive properties of herbs and spices. Curr. Med. Chem. 11:1451–1460 [DOI] [PubMed] [Google Scholar]
- 45. Lamm DL, Riggs DR. 2001. Enhanced immunocompetence by garlic: role in bladder cancer and other malignancies. J. Nutr. 131:1067S–1070S [DOI] [PubMed] [Google Scholar]
- 46. Lawson LD. 2007. Allyl sulfides and allyl thiosulfinates before and after heating at 54 degrees C for two weeks. Garlic Research Labs, Inc., Glendale, CA: http://www.mosquitobarrier.com/documents/MB_analysis_Silliker.pdf [Google Scholar]
- 47. Lawson LD, Ransom DK, Hughes BG. 1992. Inhibition of whole blood platelet-aggregation by compounds in garlic clove extracts and commercial garlic products. Thromb. Res. 65:141–156 [DOI] [PubMed] [Google Scholar]
- 48. Lawson LD, Wang ZJ. 2005. Allicin and allicin-derived garlic compounds increase breath acetone through allyl methyl sulfide: use in measuring allicin bioavailability. J. Agric. Food Chem. 53:1974–1983 [DOI] [PubMed] [Google Scholar]
- 49. Lawson LD, Wang ZJ, Hughes BG. 1991. Identification and HPLC quantitation of the sulfides and dialk(en)yl thiosulfinates in commercial garlic products. Planta Med. 57:363–370 [DOI] [PubMed] [Google Scholar]
- 50. Lei XY, et al. 2008. Apoptosis induced by diallyl disulfide in human breast cancer cell line MCF-7. Acta Pharmacol. Sin. 29:1233–1239 [DOI] [PubMed] [Google Scholar]
- 51. Lemar KM, et al. 2007. Diallyl disulphide depletes glutathione in Candida albicans: oxidative stress-mediated cell death studied by two-photon microscopy. Yeast 24:695–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu WH, Hsu CC, Yin MC. 2008. In vitro anti-Helicobacter pylori activity of diallyl sulphides and protocatechuic acid. Phytother. Res. 22:53–57 [DOI] [PubMed] [Google Scholar]
- 53. Lo HH, Hsieh SE, Chung JG. 1998. The effect of sulindac on arylamine N-acetyltransferase activity in Pseudomonas aeruginosa. Microbios 93:159–168 [PubMed] [Google Scholar]
- 54. Lu X, et al. 2011. Infrared and Raman spectroscopic studies of the antimicrobial effects of garlic concentrates and diallyl constituents on foodborne pathogens. Anal. Chem. 83:4137–4146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Madinier IM, Fosse TB, Hitzig C, Charbit Y, Hannoun LR. 1999. Resistance profile survey of 50 periodontal strains of Actinobacillus actinomycetemcomitans. J. Periodontol. 70:888–892 [DOI] [PubMed] [Google Scholar]
- 56. Meyer DH, Lippmann JE, Fives-Taylor PM. 1996. Invasion of epithelial cells by Actinobacillus actinomycetemcomitans: a dynamic, multistep process. Infect. Immun. 64:2988–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mosca A, Miragliotta L, Iodice MA, Abbinante A, Miragliotta G. 2007. Antimicrobial profiles of Prevotella spp. and Fusobacterium nucleatum isolated from periodontal infections in a selected area of southern Italy. Int. J. Antimicrob. Agents 30:521–524 [DOI] [PubMed] [Google Scholar]
- 58. O'Gara EA, Hill DJ, Maslin DJ. 2000. Activities of garlic oil, garlic powder, and their diallyl constituents against Helicobacter pylori. Appl. Environ. Microbiol. 66:2269–2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pai ST, Platt MW. 1995. Antifungal effects of Allium sativum (garlic) extract against the Aspergillus species involved in otomycosis. Lett. Appl. Microbiol. 20:14–18 [DOI] [PubMed] [Google Scholar]
- 60. Peleg A, et al. 2003. Effect of garlic on lipid profile and psychopathologic parameters in people with mild to moderate hypercholesterolemia. Isr. Med. Assoc. J. 5:637–640 [PubMed] [Google Scholar]
- 61. Rams TE, Dujardin S, Sautter JD, Degener JE, van Winkelhoff AJ. 2011. Spiramycin resistance in human periodontitis microbiota. Anaerobe 17:201–205 [DOI] [PubMed] [Google Scholar]
- 62. Rattanachaikunsopon P, Phumkhachorn P. 2009. Antimicrobial activity of elephant garlic oil against Vibrio cholerae in vitro and in a food model. Biosci. Biotechnol. Biochem. 73:1623–1627 [DOI] [PubMed] [Google Scholar]
- 63. Rattanachaikunsopon P, Phumkhachorn P. 2008. Diallyl sulfide content and antimicrobial activity against food-borne pathogenic bacteria of chives (Allium schoenoprasum). Biosci. Biotechnol. Biochem. 72:2987–2991 [DOI] [PubMed] [Google Scholar]
- 64. Sakurai K, et al. 2007. High incidence of actinobacillus actinomycetemcomitans infection in acute coronary syndrome. Int. Heart J. 48:663–675 [DOI] [PubMed] [Google Scholar]
- 65. Shobana S, Naidu KA. 2000. Antioxidant activity of selected Indian spices. Prostaglandins Leukot. Essent. Fatty Acids 62:107–110 [DOI] [PubMed] [Google Scholar]
- 66. Shoji S, Furuishi K, Yanase R, Miyazaka T, Kino M. 1993. Allyl compounds selectively killed human immunodeficiency virus (type 1)-infected cells. Biochem. Biophys. Res. Commun. 194:610–621 [DOI] [PubMed] [Google Scholar]
- 67. Spahr A, et al. 2006. Periodontal infections and coronary heart disease: role of periodontal bacteria and importance of total pathogen burden in the Coronary Event and Periodontal Disease (CORODONT) study. Arch. Intern. Med. 166:554–559 [DOI] [PubMed] [Google Scholar]
- 68. Szabo SE, Monroe SL, Fiorino S, Bitzan J, Loper K. 2004. Evaluation of an automated instrument for viability and concentration measurements of cryopreserved hematopoietic cells. Lab. Hematol. 10:109–111 [PubMed] [Google Scholar]
- 69. Thomson M, Al-Qattan KK, Bordia T, Ali M. 2006. Including garlic in the diet may help lower blood glucose, cholesterol, and triglycerides. J. Nutr. 136(Suppl. 3):800S–802S [DOI] [PubMed] [Google Scholar]
- 70. Thomson M, Ali M. 2003. Garlic [Allium sativum]: a review of its potential use as an anti-cancer agent. Curr. Cancer Drug Targets 3:67–81 [DOI] [PubMed] [Google Scholar]
- 71. Tsao SM, Hsu CC, Yin MC. 2003. Garlic extract and two diallyl sulphides inhibit methicillin-resistant Staphylococcus aureus infection in BALB/cA mice. J. Antimicrob. Chemother. 52:974–980 [DOI] [PubMed] [Google Scholar]
- 72. Tsao SM, Liu WH, Yin MC. 2007. Two diallyl sulphides derived from garlic inhibit meticillin-resistant Staphylococcus aureus infection in diabetic mice. J. Med. Microbiol. 56:803–808 [DOI] [PubMed] [Google Scholar]
- 73. Tsao SM, Yin MC. 2001. In-vitro antimicrobial activity of four diallyl sulphides occurring naturally in garlic and Chinese leek oils. J. Med. Microbiol. 50:646–649 [DOI] [PubMed] [Google Scholar]
- 74. Uchida Y, Takahashi T, Sato N. 1975. [The characteristics of the antibacterial activity of garlic (author's translation)]. Jpn. J. Antibiot. 28:638–642 [PubMed] [Google Scholar]
- 75. van Winkelhoff AJ, Herrera D, Oteo A, Sanz M. 2005. Antimicrobial profiles of periodontal pathogens isolated from periodontitis patients in The Netherlands and Spain. J. Clin. Periodontol. 32:893–898 [DOI] [PubMed] [Google Scholar]
- 76. Velliyagounder K, et al. 2003. One of two human lactoferrin variants exhibits increased antibacterial and transcriptional activation activities and is associated with localized juvenile periodontitis. Infect. Immun. 71:6141–6147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Weber ND, et al. 1992. In vitro virucidal effects of Allium sativum (garlic) extract and compounds. Planta Med. 58:417–423 [DOI] [PubMed] [Google Scholar]
- 78. Wu D, Cederbaum AI. 2002. Cyclosporine A protects against arachidonic acid toxicity in rat hepatocytes: role of CYP2E1 and mitochondria. Hepatology 35:1420–1430 [DOI] [PubMed] [Google Scholar]
- 79. Wu XJ, Hu Y, Lamy E, Mersch-Sundermann V. 2009. Apoptosis induction in human lung adenocarcinoma cells by oil-soluble allyl sulfides: triggers, pathways, and modulators. Environ. Mol. Mutagen. 50:266–275 [DOI] [PubMed] [Google Scholar]
- 80. Yin MC, Hwang SW, Chan KC. 2002. Nonenzymatic antioxidant activity of four organosulfur compounds derived from garlic. J. Agric. Food Chem. 50:6143–6147 [DOI] [PubMed] [Google Scholar]
- 81. Zambon JJ. 1985. Actinobacillus actinomycetemcomitans in human periodontal disease. J. Clin. Periodontol. 12:1–20 [DOI] [PubMed] [Google Scholar]
- 82. Zhang T, et al. 2010. Aggregatibacter actinomycetemcomitans accelerates atherosclerosis with an increase in atherogenic factors in spontaneously hyperlipidemic mice. FEMS Immunol. Med. Microbiol. 59:143–151 [DOI] [PubMed] [Google Scholar]
- 83. Zhang YK, Zhang XH, Li JM, Sun DS, Yang Q, Diao DM. 2009. A proteomic study on a human osteosarcoma cell line Saos-2 treated with diallyl trisulfide. Anticancer Drugs 20:702–712 [DOI] [PubMed] [Google Scholar]