Abstract
Nineteen carbapenem-nonsusceptible Proteus mirabilis isolates were recovered from intensive care units in the Second Affiliated Hospital of Zhejiang University during a 3-month period. The isolates showed a high level of resistance against ciprofloxacin, in addition to their resistance against the carbapenems. Pulsed-field gel electrophoresis (PFGE) analysis showed that these isolates belonged to three clonal strains. PCRs and DNA sequence analysis of the carbapenemase and other β-lactamase genes indicated that all the isolates harbored the blaKPC-2 gene. Twelve of 19 isolates harbored the plasmid-mediated quinolone resistance (PMQR) genes, both the qnrD and aac(6′)-Ib-cr genes. Eight representative isolates with high levels of quinolone resistance carried the similar mutation profiles of S83I in gyrA, E466D in gyrB, and S80I in parC. Reduced carbapenem susceptibility was transferred to Escherichia coli (EC600) in a conjugation experiment, while the quinolone resistance was not. DNA hybridization showed that qnrD was located on a plasmid of approximately 4.5 kb. In summary, large clonally related isolates of KPC-2-producing P. mirabilis emerged in a Chinese hospital, and qnrD was detected in KPC-producing P. mirabilis for the first time.
INTRODUCTION
Proteus mirabilis is an opportunistic pathogen that can cause diarrhea, septicemia, meningitis, and urinary tract and respiratory system infections. P. mirabilis has now become one of the most important nosocomial infection pathogens in China. According to antimicrobial resistance surveillance of bacteria from China in 2009 (CHINET) (16), P. mirabilis was ranked fourth among the Enterobacteriaceae. However, infections caused by P. mirabilis, especially isolates recovered from sputum, might be neglected when they are recovered together with multiresistant Gram-negative bacilli such as Acinetobacter baumannii, Klebsiella pneumoniae, and Pseudomonas aeruginosa.
Since the first report of Klebsiella pneumoniae carbapenemase (KPC) in North Carolina in 2001, blaKPC has spread worldwide (10). In our hospital, blaKPC was found in most Enterobacteriaceae, including K. pneumoniae (2), Escherichia coli (2), Serratia marcescens (22), Enterobacter cloacae (1), and Citrobacter freundii (21). However, there are only a few reports regarding KPC-2 in P. mirabilis (13, 15). Quinolone resistance in the Enterobacteriaceae is usually mediated by point mutations of the gyrase and topoisomerase IV genes, leading to a target modification, and other resistance mechanisms include plasmid-mediated quinolone resistance (PMQR) determinants, which have also been found to contribute to quinolone resistance. The PMQR determinant was first described for a ciprofloxacin-resistant strain of K. pneumoniae in 1998 (7), and the latest PMQR determinant, named qnrD, was found in Salmonella enterica in 2009 (3) and has not been reported in other bacteria, until now.
From August to October 2010, a total of 19 P. mirabilis isolates were recovered from two intensive care units (ICUs) in our hospital. The isolates showed reduced carbapenem susceptibility and a much higher rate of resistance against the quinolones. To understand the mechanisms of carbapenem and quinolone resistance, carbapenem resistance genes and the quinolone resistance-associated factors, both on the chromosome and on plasmids, were investigated.
MATERIALS AND METHODS
Bacterial strains.
Nineteen nonduplicate clinical isolates of P. mirabilis were collected from two ICUs in the Second Affiliated Hospital of Zhejiang University from August to October 2010 (16 were from the neurology ICU and the other 3 were from the central ICU). Nineteen isolates were recovered from various kinds of specimens, including sputum (n = 15), central vein pipe (n = 1), urine (n = 1), drainage fluid (n = 1), and feces (1). Six P. mirabilis isolates from outpatients were used as carbapenem-susceptible control strains in pulsed-field gel electrophoresis (PFGE) analysis and antimicrobial susceptibility testing.
Antimicrobial susceptibility testing.
The MICs of ampicillin, cefoperazone-sulbactam, piperacillin-tazobactam, ceftazidime, cefotaxime, meropenem, and ertapenem were determined by the agar dilution method as recommended by the standards of the Clinical and Laboratory Standards Institute (CLSI; 2011) (4). The MICs of amikacin and ciprofloxacin were determined by Etest (AB Biodisk, Solna, Sweden), according to the manufacturer's instructions. The results were interpreted in accordance with CLSI 2011 guidelines (4). E. coli ATCC 25922 was used for quality control.
PFGE analysis.
PFGE typing of P. mirabilis was performed as described previously (11). Chromosome DNAs were digested by ApaI for 2 h at 37οC, and electrophoresis was carried out in a Rotaphor System 6.0 instrument (Whatman Biometra, Goettingen, Germany) through a 1.0% agarose gel in 0.5× Tris-borate-EDTA buffer under the following conditions: temperature, 14οC; constant voltage, 200 V; and switch angle, 120°, with a linear switch ramp of 5 to 35 s for 19 h. The PFGE patterns were analyzed and interpreted according to the criteria of Tenover et al. (14).
PCR amplification.
Screening for the following genes was carried out by PCR amplification using specific primers: PMQR genes, including qepA (6), qnrA (9), qnrB (9), qnrC (17), qnrD (3), qnrS (9), and aac(6′)-Ib (5); quinolone resistance-determining region (QRDR) genes gyrA (18), gyrB (18), and parC (18); carbapenemase gene blaKPC (19); and other β-lactamase genes, including blaTEM (20), blaSHV (20), blaCTX-M (20), and AmpC (8). PCR amplification was performed by a TPersonal cycler (Biometra, Germany). The PCR products were sequenced using an ABI 3730 sequencer (Applied Biosystems, Foster City, CA), and the sequences were then compared with the reported sequences from GenBank. Primers qnrD-F (5′-CGAGATCAATTTACGGGGAATA-3′) and qnrD-R (5′-AACAAGCTGAAGCGCCTG-3′), amplifying a 565-bp internal fragment of the qnrD gene, were used to generate a probe for Southern blot analysis.
Genetic environment analysis of blaKPC gene.
PCR mapping of the blaKPC gene in P. mirabilis was performed, and the map was compared with that found in plasmid pKP048 (12). PCR primers and amplification conditions for blaKPC-surrounding sequences were as described previously (12). The PCR products were then sequenced.
Conjugal transfer experiment and analysis of plasmid.
Twelve qnrD-positive P. mirabilis isolates were all used as donors for the qnrD conjugation experiment, while the P. mirabilis zr61, zr28, zr69, zr55, and zr62 isolates, representing clone A with TEM, clone A without TEM, subclone B1, subclone B2, and clone C isolates, respectively, were chosen for the blaKPC conjugation experiment. Rifampin-resistant E. coli EC600 was used as the recipient strain. The E. coli transconjugants were selected on Mueller-Hinton agar medium containing 600 μg/ml rifampin plus 0.2 μg/ml ciprofloxacin for the qnrD conjugation experiments and 600 μg/ml rifampin plus 0.18 μg/ml meropenem for the blaKPC conjugation experiments. The detailed experimental method was as described previously (17). Colonies that grew on the selective medium were picked for identification by a Vitek system. Plasmid DNA preparations were obtained by an alkaline lysis technique using an AxyPrep plasmid miniprep kit (Axygen Scientific). Plasmids were separated by electrophoresis in a 0.8% agarose gel containing ethidium bromide at a constant voltage of 100 V for 1 h.
Southern blot analysis.
Plasmid DNAs from P. mirabilis isolates were separated by gel electrophoresis and then transferred to a nylon membrane (Hybond N+; Amersham Pharmacia Biotech, Orsay, France). Southern hybridization was performed using standard protocols with the qnrD-specific probe labeled by use of a DIG High Prime DNA labeling kit (Roche, Sant Cugat del Vallès, Spain) following the manufacturer's instructions.
RESULTS
Antimicrobial susceptibility.
Antimicrobial susceptibility results for the 19 P. mirabilis isolates are listed in Table 1. The 19 P. mirabilis isolates exhibited resistance or reduced susceptibility to meropenem and ertapenem. Cefoperazone-sulbactam showed the best activity against P. mirabilis, with a susceptibility rate of 100.0%, followed by ertapenem and meropenem (89.5%), ceftazidime (84.2%), and cefotaxime (73.4%). Remarkably, the highest rate of resistance was to ciprofloxacin (89.5%), other than that for resistance to ampicillin (94.7%). All six P. mirabilis isolates from outpatients were susceptible to all tested antibiotics.
Table 1.
Antimicrobial susceptibility patterns of 19 KPC-producing P. mirabilis isolates
| Antimicrobial agent | MIC (μg/ml) |
||||||
|---|---|---|---|---|---|---|---|
| Clone A (n = 14) | Clone B |
Clone C (n = 1) | 50% | 90% | Other 6 isolates | ||
| B1 (n = 2) | B2 (n = 2) | ||||||
| Meropenem | 0.25–2 | 1–2 | 0.5–1 | 0.25 | 1 | 1 | ≤0.125 |
| Ertapenem | 0.125–0.5 | 0.25 | 0.125–0.25 | 0.125 | 0.125 | 0.25 | ≤0.125 |
| Ceftazidime | 0.5–128 | 1–8 | 1–2 | 0.5 | 1 | 16 | ≤0.25 |
| Cefotaxime | 0.25–16 | 2–8 | 1–2 | 1 | 1 | 8 | ≤0.25 |
| Ciprofloxacin | 0.012–>32 | 12–24 | 8–>32 | 12 | >32 | >32 | 0.012–0.19 |
| Amikacin | 0.75–>256 | 1 | 0.75–>256 | 2 | 128 | >128 | 0.15–2 |
| Ampicillin | 8–>128 | 128 | 128 | 128 | 2 | >256 | 1–16 |
| Cefoperazone-sulbactam | 2–16 | 16 | 8–16 | 8 | 8 | 16 | ≤0.25–1 |
| Piperacillin-tazobactam | 0.5–32 | 32 | 8 | 32 | 32 | 32 | ≤0.25–1 |
PFGE typing and gene detection.
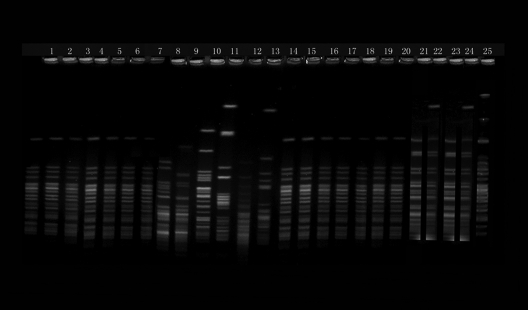
Nineteen carbapenem-nonsusceptible P. mirabilis isolates belonged to three clones, named clone A (14 isolates), clone B (2 isolates of subclone B1 and 2 isolates of subclone B2), and clone C (1 isolate). All six isolates from outpatients were distinguishable (Fig. 1). PCRs and DNA sequence analysis indicated that all 19 isolates produced KPC-2 and only 3 isolates produced TEM-1. Genetic environment analysis showed that three open reading frames (ORFs) with the order of ISKpn8, blaKPC-2, and ISKpn6-like were identified in a 3,241-bp nucleotide segment completely identical to that in previously described plasmid pKP048 (12).
Fig 1.
PFGE patterns of chromosomal DNA restriction fragments from 25 nonduplicate clinical P. mirabilis isolates. Lanes: 1 to 7 and 14 to 20, clinical isolates of P. mirabilis that belonged to clone A; 21 and 23, clinical isolates of P. mirabilis that belonged to subclone B1; 22 and 24, clinical isolates of P. mirabilis that belonged to subclone B2;; 25, clinical isolates of P. mirabilis that belonged to clone C; and 8 to 13, PFGE typing of six normal P. mirabilis isolates from intestinal tract.
The resistance profiles of the isolates showed high levels of quinolone resistance (Table 2). The DNA and derived amino acid sequences of the QRDR genes gyrA, gyrB, and parC for 8 clinical isolates with similar profiles of resistance to ciprofloxacin (MICs, 4 to >32 mg/liter), including 3 of clone A, 4 of clone B, and 1 of clone C, were compared with GenBank sequences assigned accession numbers AF397169 (gyrA), AF503506 (gyrB), and AF363611(parC). All the 8 isolates showed the same mutations that resulted in amino acid changes of S83I in gyrA, E466D in gyrB, and S80I in parC, which have been clarified to be associated with resistance to quinolones (18). Screening of PMQR determinants in 19 P. mirabilis isolates revealed that 12 isolates harbored both qnrD and aac(6′)-Ib-cr. All the PMQR determinant-positive isolates belonged to clone A, except one which belonged to subclone B2.
Table 2.
Antimicrobial susceptibility patterns of P. mirabilis isolates that belonged to various clones and their E. coli transconjugants
| Antimicrobial agent | MIC (μg/ml) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| P. mirabilis zr61 | E. coli transconjugant zr61 | P. mirabilis zr28 | E. coli transconjugant zr28 | P. mirabilis zr69 | E. coli transconjugant zr69 | P. mirabilis zr55 | E. coli transconjugant zr55 | E. coli EC600 | |
| Meropenem | 0.5 | 2 | 0.25 | 2 | 1 | 8 | 1 | 1 | 0.0625 |
| Ertapenem | 0.25 | 4 | 0.25 | 8 | 0.5 | 16 | 0.5 | 4 | 0.125 |
| Ceftazidime | 128 | 16 | 0.5 | 32 | 8 | 128 | 2 | 32 | 0.25 |
| Cefotaxime | 32 | 16 | 0.5 | 32 | 8 | 128 | 1 | 8 | 0.5 |
| Ciprofloxacin | >32 | 0.064 | >32 | 0.19 | 24 | 0.125 | >32 | 0.19 | 0.25 |
Conjugation and analysis of plasmid.
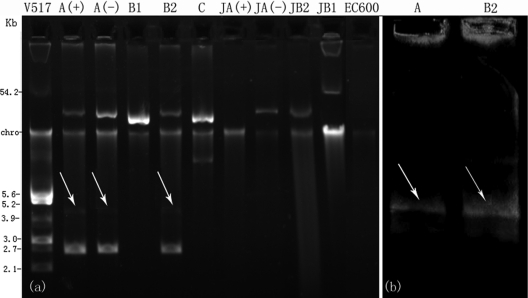
P. mirabilis isolates failed to transfer quinolone resistance to E. coli EC600 by conjugation. Transfer of reduced carbapenem susceptibility from P. mirabilis to E. coli EC600 was successful for all isolates except P. mirabilis zr62, which belonged to clone C. The bacteria that grew on medium containing rifampin and meropenem were named E. coli transconjugants zr61, zr28, zr69, and zr55, respectively. The plasmid profiles indicated that blaKPC was located on an approximately 45-kb plasmid in clone A and subclone B2 isolates, while in subclone B1, it was located on an approximately 54-kb plasmid. All qnrD-positive isolates of clone A and subclone B2 harbored two other plasmids with sizes of approximately 4.5 kb and 2.6 kb, respectively (Fig. 2a). A plasmid was not observed in E. coli transconjugant zr61, which may be due to the low plasmid copy number.
Fig 2.
(a) Plasmid profiles of P. mirabilis and E. coli transconjugants. Lanes show E. coli V517 (lane V517); P. mirabilis clone A with blaTEM-1 [lane A(+)], P. mirabilis clone A without blaTEM-1 [lane A(−)], subclone B1 (lane B1), subclone B2 (lane B2), clone C (lane C), E. coli transconjugant of TEM-producing clone A isolate [lane JA(+)], E. coli transconjugant of TEM-negative clone A isolate [lane JA(−)], E. coli transconjugant of the subclone B2 isolate (lane JB2), E. coli transconjugant of the subclone B1 isolate (lane JB1), and E. coli EC600 (lane EC600; negative control). (b) Hybridization with qnrD (lane A, P. mirabilis isolate belonging to clone A; lane B2, isolate belonging to subclone B2). Arrows indicate location of the qnrD plasmid.
DNA hybridization.
Southern blot analysis of P. mirabilis zr61 (clone A) and zr55 (subclone B2) hybridized with a qnrD-specific probe confirmed that qnrD was encoded on the 4.5-kb plasmid (Fig. 2b).
DISCUSSION
KPCs have largely been detected in many genera from many countries. However, reports about KPC-producing Proteus spp. have been rare. Tibbetts et al. (15) first reported a single isolate of P. mirabilis harboring blaKPC-2 in 2008. In the current study, we first reported the clonal dissemination of KPC-2-producing P. mirabilis in ICUs. The 3,241-bp structure sequenced in this study was identified in Enterobacteriaceae isolates from our hospital, indicating probable transmission of blaKPC-2 from other common species of Enterobacteriaceae to P. mirabilis. It also indicated that interhospital clonal spread has happened because the sequence of the 3,241-bp nucleotide segment was completely identical to that of plasmid pKP048 (12), which was from a transconjugant of K. pneumoniae strain KP048 isolated from another hospital in the same city.
As is known, KPCs are capable of hydrolyzing β-lactams, including carbapenems, cephalosporins, penicillins, and aztreonam. Nevertheless, many KPC-producing P. mirabilis isolates in the current study were susceptible to carbapenems and even cephalosporins. The conjugation study indicated that plasmid-mediated KPC-2 contributed to carbapenem resistance or reduced carbapenem susceptibility in P. mirabilis. Interestingly, the MICs of carbapenems and cephalosporins for some E. coli transconjugants were higher than those for P. mirabilis (Table 2). For most of our previous studies, we found that the resistance level in the E. coli transconjugants is always lower than or similar to that in their parent isolates, as observed for S. marcescens, K. pneumoniae, E. coli, and C. freundii (2, 21, 22). However, in our recent study, we did observe higher MICs for the E. coli transconjugant than its parent isolate, the KPC-producing Morganella morganii (unpublished data). We suspect that the expression level or the stability of the KPC enzyme depends on the genetic background of the carrier of this gene rather than the KPC gene and the plasmid itself. However, further studies are required to elucidate the detailed mechanism of the phenomenon.
Antimicrobial susceptibility results showed a very high rate of resistance to ciprofloxacin (89.5%). The screening and identification of mutations in the gyrA, gyrB, and parC genes from the 8 typical isolates which had higher resistance levels (MICs, 4 to >32 mg/liter for ciprofloxacin) indicated that all the 8 isolates had the same mutation profiles of S83I in gyrA, E466D in gyrB, and S80I in parC, indicating that those mutations do contribute to the higher level of quinolone resistance. On the other hand, screening of PMQR determinants identified that the predominant genes among KPC-producing P. mirabilis isolates are qnrD (63.2%, 12/19), a novel PMQR determinant first found and only found in an S. enterica isolate from China, and aac(6′)-Ib-cr (63.2%, 12/19). This was the first detection of the qnrD gene in KPC-producing P. mirabilis isolates.
Conjugal studies proved that P. mirabilis isolates failed to transfer quinolone resistance to E. coli EC600 by conjugation, indicating that the quinolone resistance of P. mirabilis is mainly referred to the chromosomal QRDRs rather than plasmid-mediated factors. These data further supported the view that QRDRs are responsible for the higher level of quinolone resistance, while the plasmid-mediated factors play only a minor role in resistance or a lower level of resistance.
In summary, we have identified by clonal typing that the carbapenem-resistant P. mirabilis isolates from the intensive care units are very closely related and that they carried both the plasmid-mediated blaKPC gene and the qnrD gene on separate plasmids and also found that the QRDRs on the chromosomes responsible for the higher level of quinolone resistance had similar mutation profiles. It is highly possible that KPC genes are transmitted even among different species to create multiple-drug-resistant pathogens. Care has to be taken to prevent the spread of such factors conferring high-level resistance.
Footnotes
Published ahead of print 21 February 2012
REFERENCES
- 1. Cai JC, Zhou HW, Chen GX, Zhang R. 2008. Detection of plasmid-mediated carbapenem-hydrolyzing beta-lactamase KPC-2 in a strain of carbapenem-resistant Enterobacter cloacae. Zhonghua Yi Xue Za Zhi 88:135–138 (In Chinese.) [PubMed] [Google Scholar]
- 2. Cai JC, Zhou HW, Zhang R, Chen GX. 2008. Emergence of Serratia marcescens, Klebsiella pneumoniae, and Escherichia coli isolates possessing the plasmid-mediated carbapenem-hydrolyzing beta-lactamase KPC-2 in intensive care units of a Chinese hospital. Antimicrob. Agents Chemother. 52:2014–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cavaco LM, Hasman H, Xia S, Aarestrup FM. 2009. qnrD, a novel gene conferring transferable quinolone resistance in Salmonella enterica serovar Kentucky and Bovismorbificans strains of human origin. Antimicrob. Agents Chemother. 53:603–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clinical and Laboratory Standards Institute 2011. Performance standards for antimicrobial susceptibility testing; 21th informational supplement. CLSI document M100-S21. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 5. Jiang Y, et al. 2008. Plasmid-mediated quinolone resistance determinants qnr and aac(6′)-Ib-cr in extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in China. J. Antimicrob. Chemother. 61:1003–1006 [DOI] [PubMed] [Google Scholar]
- 6. Ma J, et al. 2009. High prevalence of plasmid-mediated quinolone resistance determinants qnr, aac(6′)-Ib-cr, and qepA among ceftiofur-resistant Enterobacteriaceae isolates from companion and food-producing animals. Antimicrob. Agents Chemother. 53:519–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martinez-Martinez L, Pascual A, Jacoby GA. 1998. Quinolone resistance from a transferable plasmid. Lancet 351:797–799 [DOI] [PubMed] [Google Scholar]
- 8. Perez-Perez FJ, Hanson ND. 2002. Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 40:2153–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robicsek A, et al. 2006. Fluoroquinolone-modifying enzyme: a new adaptation of a common aminoglycoside acetyltransferase. Nat. Med. 12:83–88 [DOI] [PubMed] [Google Scholar]
- 10. Sacha P, et al. 2009. The KPC type beta-lactamases: new enzymes that confer resistance to carbapenems in Gram-negative bacilli. Folia Histochem. Cytobiol. 47:537–543 [DOI] [PubMed] [Google Scholar]
- 11. Seifert H, et al. 2005. Standardization and interlaboratory reproducibility assessment of pulsed-field gel electrophoresis-generated fingerprints of Acinetobacter baumannii. J. Clin. Microbiol. 43:4328–4335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shen P, et al. 2009. Novel genetic environment of the carbapenem-hydrolyzing beta-lactamase KPC-2 among Enterobacteriaceae in China. Antimicrob. Agents Chemother. 53:4333–4338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sheng ZK, Li JJ, Sheng GP, Sheng JF, Li LJ. 2010. Emergence of Klebsiella pneumoniae carbapenemase-producing Proteus mirabilis in Hangzhou, China. Chin. Med. J. (Engl.). 123:2568–2570 [PubMed] [Google Scholar]
- 14. Tenover FC, et al. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel-electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tibbetts R, Frye JG, Marschall J, Warren D, Dunne W. 2008. Detection of KPC-2 in a clinical isolate of Proteus mirabilis and first reported description of carbapenemase resistance caused by a KPC beta-lactamase in P. mirabilis. J. Clin. Microbiol. 46:3080–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang F, et al. 2010. CHINET 2009 surveillance of bacterial resistance in China. Chin. J. Infect. Chemother. 10:325–334 (In Chinese.) [Google Scholar]
- 17. Wang MH, et al. 2009. New plasmid-mediated quinolone resistance gene, qnrC, found in a clinical isolate of Proteus mirabilis. Antimicrob. Agents Chemother. 53:1892–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weigel LM, Anderson GJ, Tenover FC. 2002. DNA gyrase and topoisomerase IV mutations associated with fluoroquinolone resistance in Proteus mirabilis. Antimicrob. Agents Chemother. 46:2582–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yigit H, et al. 2001. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 45:1151–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu YS, et al. 2007. Resistance of strains producing extended-spectrum beta-lactamases and genotype distribution in China. J. Infect. 54:53–57 [DOI] [PubMed] [Google Scholar]
- 21. Zhang R, Yang L, Cai JC, Zhou HW, Chen GX. 2008. High-level carbapenem resistance in a Citrobacter freundii clinical isolate is due to a combination of KPC-2 production and decreased porin expression. J. Med. Microbiol. 57:332–337 [DOI] [PubMed] [Google Scholar]
- 22. Zhang R, Zhou HW, Cai JC, Chen GX. 2007. Plasmid-mediated carbapenem-hydrolysing beta-lactamase KPC-2 in carbapenem-resistant Serratia marcescens isolates from Hangzhou, China. J. Antimicrob. Chemother. 59:574–576 [DOI] [PubMed] [Google Scholar]