Abstract
Topical blockade of the gp41 fusogenic protein of HIV-1 is one possible strategy by which microbicides could prevent HIV transmission, working early against infection, by inhibiting viral entry into host cells. In this study, we examined the potential of gp41 fusion inhibitors (FIs) as candidate anti-HIV microbicides. Preclinical evaluation of four FIs, C34, T20, T1249, and L'644, was performed using cellular and ex vivo genital and colorectal tissue explant models. Increased and sustained activity was detected for L'644, a cholesterol-derivatized version of C34, relative to the other FIs. The higher potency of L'644 was further increased with sustained exposure of cells or tissue to the compound. The activity of L'644 was not affected by biological fluids, and the compound was still active when tissue explants were treated after viral exposure. L'644 was also more active than other FIs against a viral escape mutant resistant to reverse transcriptase inhibitors (RTIs), demonstrating the potential of L'644 to be included as part of a multiactive antiretroviral (ARV) combination-based microbicide. These data support the further development of L'644 for microbicide application.
INTRODUCTION
Sexual intercourse remains the predominant route of HIV-1 transmission worldwide, emphasizing a clear and urgent need for additional prevention strategies, including the development of microbicides, i.e., mucosally applied products designed to prevent HIV infection (15, 30, 35, 52, 60, 68). The recent promising results from a phase IIb clinical trial, CAPRISA 004, of a vaginal microbicide based on the reverse transcriptase inhibitor (RTI) tenofovir, has demonstrated the potential utility of microbicides based on antiretroviral (ARV) drugs. However, the more recent VOICE trial of a once-daily dosing regimen with tenofovir gel failed to demonstrate any detectable efficacy in at-risk women. These studies reflect the need to develop additional microbicide candidates to potentially increase inhibitory activity. These comprise classes of inhibitors active against different steps of the viral replication cycle, including binding and fusion with target cells, reverse transcription, integration, and maturation of budded virions (2, 25, 43). The fusion peptide gp41 is not fully exposed until after HIV-1 envelope (gp120/gp41 trimer) has bound to CD4 and a coreceptor (CCR5 or CXRC4) has triggered the gp120/gp41 conformation change required to initiate fusion through the insertion of gp41 in the membrane of a target cell. Fusion inhibitors (FIs) are designed to prevent viral entry by blocking the formation of the 6-helix bundle between the N-terminal heptad repeat 1 (HR1) and the C-terminal HR2 in gp41 that leads to fusion of viral and cellular membranes. Blocking occurs by binding of FIs to the HR1 or HR2 domain. The FI enfuvirtide (T20) has been successfully used in therapy, and FIs as a class have broad activity across HIV-1 clades (66) and have been considered for microbicide development following protection of macaques from vaginal challenge with SHIV-162P3 after vaginal application of T1249 (66). Ingallinella et al. (27) have recently developed a new FI, L'644, based on a sequence of HR2, C34, conjugated to a cholesterol group functioning as a membrane anchor to increase its potency. Here we investigate the potential of four FIs, i.e., C34, T20, T1249, and L'644 (also known as C34-Chol or DS007) (Table 1), as candidate microbicides through preclinical studies with cellular and mucosal tissue explant models against R5 isolates, the predominant type associated with sexual transmission of HIV-1 (41).
Table 1.
Sequences of fusion inhibitor peptides
| Peptide | Sequence |
|---|---|
| C34 | WMEWDREINNYTSLIHSLIEESQNQQEKNEQELL |
| T20 | YTSLIHSLIEESQNQQEKNEQELLELDKWASLWNWF |
| T1249 | WQEWEQKITALLEQAQIQQEKNEYELQKLDKWASLWEWF |
| L'644 (C34-Chol) | C34-GSG-C(Chol) |
An important consideration in the design and formulation of microbicides and their eventual use is to understand the temporal window of activity for any drug candidate. Indeed, the efficacy of tenofovir gel in CAPRISA 004 has been linked to its long intracellular half-life (31, 58). The window of activity will determine required dosing relative to any act of intercourse. For example, postexposure activity would be an important determinant for the potential use of a microbicide in a postcoital scenario (either immediate or delayed), extending the flexibility of use and informing eventual product labeling. In preclinical studies this can be mimicked by measuring antiviral activity of the drug at different times of addition relative to viral exposure. FIs are conventionally thought to have a short window of activity, being effective only during the initial viral attachment and entry steps. Previous studies have suggested that L'644, through conjugation of C34 with a cholesterol group, increases the circulatory half-life of the peptide (27). This in turn may modify the potency of C34 through binding to and retention in the lipid raft component of target cells (22).
The dual use of ARV drugs for therapy and prevention, together with the increasing prevalence of strains resistant to some ARVs, mandates that strategies to prevent transmission of ARV-resistant strains also need to be taken into account in the design of effective microbicides. Indeed, it is estimated that in the developed world, 10 to 20% of new infections are caused by ARV-resistant isolates harboring mutations that confer resistance to at least one of the three main types of ARV drugs: entry/fusion inhibitors, RTIs, and/or protease inhibitors (4, 5, 7, 11, 38, 45, 59, 65). As the rollout of ARV treatment progresses in the developing world, rates of transmission of drug-resistant viruses will inevitably increase. The microbicide field benefits from the experience acquired with conventional highly active ARV therapy (HAART) regimens, where patients receive triple combinations containing at least two drugs with different mechanisms of action to help prevent emergence of resistant isolates (16, 29, 54). Hence, it is important to consider development of different ARV microbicide drug candidates able to inhibit transmission of resistant isolates, with different mechanisms of action and targeting different stages of the viral replication cycle, either as single candidates or as part of a drug combination.
In this study, we assessed and compared the activity of the cholesterol-derivatized FI L'644 to those of three nonderivatized FIs as candidate microbicides in cellular and tissue models, addressing the robustness of their activity against wild type HIV-1 isolates and a nonnucleoside RTI (NNRTI)-resistant virus.
MATERIALS AND METHODS
Reagents and plasmids.
T20 and C34 (19) were provided by the NIH AIDS Research and Reference Reagent Program (http://www.aidsreagent.org/); T1249 and L'644 (also known as C34-Chol, or DS007) were donated by the International Partnership for Microbicides (IPM) (Silver Spring, MD).
HIV-1 BaL (20), the full-length, replication- and infection-competent proviral HIV-1 clone pYU2 (36, 37), and an infectious strain resistant to multiple HIV-1 RTIs, clone HIV-1IIIB A17 (with mutations K103N and Y181C in the RT domain) (51), were provided by the NIH AIDS Research and Reference Reagent Program (http://www.aidsreagent.org/).
Seminal and cervicovaginal fluid samples.
Seminal plasma (SP) and cervicovaginal lavage (CVL) samples were obtained under an institutional review board (IRB)-approved protocol and collected after signed informed consent was received from all donors. Samples were kindly provided by Betsy Herold (Albert Einstein College of Medicine, NY). Semen was obtained by masturbation after 48 h of sexual abstinence, and after the semen was allowed to liquefy at room temperature for 30 to 60 min, it was centrifuged at 3,000 × g for 15 min to separate spermatozoa from SP (55). Supernatants from individual samples were aliquoted and stored at −80°C. CVL samples were collected by washing the cervicovaginal area with 10 ml of sterile 0.9% saline (pH ∼5.0) (28). CVL samples were centrifuged at 1,000 × g for 15 min at 4°C. Antibiotics (500 U of penicillin/ml, 50 μg of streptomycin/ml, and 0.5 μg of amphotericin/ml) were added to the supernatants before they were aliquoted individually and stored at −80°C. For each sample, the measured pH ranged between 4 and 5. Fluids from five donors were pooled for experiments. Previous experiments with these biological fluids did not indicate any effect on viral replication capacity or on measurement of viral inhibitory activity (unpublished data).
Cell and virus culture conditions.
All cell cultures were maintained at 37°C in an atmosphere containing 5% CO2. TZM-bl cells (9, 56, 69) (NIH AIDS Research and Reference Reagent Program [http://www.aidsreagent.org/]) were grown in Dulbecco's minimal essential medium (DMEM) (Sigma-Aldrich, Inc., St. Louis, MO) containing 10% fetal calf serum (FCS), 2 mM l-glutamine, and antibiotics (100 U of penicillin/ml and 100 μg of streptomycin/ml). PM-1 cells (40) (AIDS Reagent Project, National Institute for Biological Standards and Control, United Kingdom) were maintained in RPMI 1640 medium containing 10% fetal bovine serum (FBS), 2 mM l-glutamine, and antibiotics (100 U of penicillin/ml and 100 μg of streptomycin/ml).
The molecular clones YU.2 and A17 and the laboratory-adapted isolate HIV-1 BaL were passaged through activated peripheral blood mononuclear cells (PBMCs) (21) for 11 days.
Patients and tissue explants.
Cervical tissue was obtained from patients undergoing planned therapeutic hysterectomy at St. George's Hospital or Kingston Hospital in London, United Kingdom. Penile tissue was obtained from patients undergoing gender reassignment at Charing Cross Hospital, London, United Kingdom, who had ceased hormonal therapy a minimum of 6 weeks prior to surgery. Surgically resected specimens of colorectal tissue were collected at St. George's Hospital, London, United Kingdom. All tissues were collected after signed informed consent was received from all patients and under protocols approved by the local research ethics committee. On arrival in the laboratory, resected tissue was cut into 2- to 3-mm3 explants comprising both epithelial and stromal tissue or muscularis mucosae, depending on the tissue, as described previously (12, 13, 24). Cervical and penile explants were cultured in RPMI 1640 medium supplemented with 2 mM l-glutamine, 10% FCS, and antibiotics (100 U of penicillin/ml and 100 μg of streptomycin/ml). Colorectal explants were maintained with DMEM containing 10% FCS, 2 mM l-glutamine, and antibiotics (100 U of penicillin/ml, 100 μg of streptomycin/ml, and 80 μg of gentamicin/ml). All tissues were incubated at 37°C in an atmosphere containing 5% CO2.
Infectivity and inhibition assays.
Inhibition assays were performed using a standardized amount of virus culture supernatant normalized for infectivity. TZM-bl cells were incubated with serial dilutions of compounds (between 1,000 and 0.0061 nM) for 1 h at 37°C, and then virus was added to cells and left for the time of the experiment. The extent of virus replication was determined by luciferase quantitation of cell lysates (Promega, Madison, WI) as previously described (24).
Colorectal tissue explants were incubated with compound (between 1,000 and 1 nM) for different time periods to reproduce different dosing regimens: to mimic a precoital use of microbicide, explants were treated with drug 1 h prior to virus exposure and during virus exposure (3-h pulse); to model repeated dosing, explants were exposed to drug with the 3-h pulse and during the subsequent days of culture (sustained); and to assess potency after viral exposure, explants were treated with drug for 1 h at two time points, 0 min or 1 h, after viral exposure and removal of unbound virus. Virus was added and left for 2 h, and explants were then washed 4 times with phosphate-buffered saline (PBS) to remove unbound compound and/or virus. Tissue explants were then transferred onto gel foam rafts (Welbeck Pharmaceuticals, United Kingdom) and cultured for 15 days as previously described (14, 24) in the presence or absence of compound. Approximately 50% of the supernatants were harvested every 2 to 3 days, and explants were refed with fresh medium.
Cervical explants were incubated with compound (between 1,000 and 1 nM) for different time periods: to mimic short precoital treatment, explants were incubated with drug 1 h prior to and during virus exposure (3-h pulse); to take into account the physiology of the female genital tract, where a microbicide could be retained for a longer period of time than in the other tissue models, explants were treated with drug with the 3-h pulse plus 21 h further after removal of unbound virus (24-h pulse); to mimic a repeated exposure to microbicide, explants were treated with drug with the 3-h pulse and during the subsequent days of culture (sustained); and to assess potency after viral exposure, explants were incubated with drug for 1 h either 0 min or 1 h after viral exposure and removal of unbound virus. Tissue was exposed to virus for 2 h and then washed 4 times with PBS. Explants were then transferred to a fresh tissue culture plate (13). Following overnight incubation, tissue explants were moved to a fresh tissue culture plate, and migratory cells left in the original plate were washed twice with PBS and cocultured with 4 × 104 PM-1 cells/well with or without compound in 96-well plates. Tissue explants and cellular cocultures were cultured for 15 days in the presence or absence of compound. Approximately 50% of the supernatants of explants and cellular cultures were harvested every 2 to 3 days, and both cultures were refed with fresh medium in the presence or absence of compound.
Penile tissue explants were treated with compound (between 1,000 and 1 nM) for different time periods to reproduce different dosing regimens: to mimic a precoital use of microbicide, explants were treated with drug 2 h prior to virus exposure (2-h prepulse), and to model repeated dosing, explants were expose to drug with the 2-h prepulse, during incubation with virus, and during the subsequent days of culture (sustained). Based on previous experiments, penile tissue explants required a longer virus exposure time (24 h) than other mucosal models. Penile explants were then washed 4 times with PBS and transferred to a fresh tissue culture plate (12). Following overnight incubation, tissue explants were transferred to a fresh tissue culture plate, and migratory cells left in the original plate were washed twice with PBS and cocultured with 4 × 104 PM-1 cells/well with or without compound in 96-well plates. Explants and cellular cocultures were maintained for 15 days as described above for cervical explants and migratory cell cultures.
For all tissue explant models and migratory/PM-1 cells cocultures, the extent of virus replication was determined by measuring the p24 antigen concentration in supernatants (HIV-1 p24 enzyme-linked immunosorbent assay [ELISA]; AIDS Vaccine Program, National Cancer Institute, Frederick, MD) (24).
Each experiment was performed three times with a different donor for each of the triplicates. For all compounds tested, cell and tissue viabilities were determined by measuring tetrazolium salt [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT)] cleavage into a blue product (formazan) by viable cells (61) as described previously (46). Briefly, cells or explants were incubated with 100 or 200 μl complete RPMI-MTT (0.5 mg/ml) at 37°C for 3 h, respectively. The formazan salts were solubilized by addition of 100 μl 20% sodium dodecyl sulfate in 1:1 H2O–N,N-dimethylformamide (cells) or 1 ml methanol (explants), and viability was determined by measuring the optical density at 570 nm (reference, 690 nm) in a Synergy-HT plate reader. For tissue studies, this value was corrected for explant dry weight.
Statistical and mathematical analysis.
Fifty percent inhibitory concentrations (IC50s) were calculated from sigmoid curve fits (Prism; GraphPad). All IC50 data presented fulfill the criterion of r2 being >0.7. IC50s were statistically compared using an unpaired t test or one-way analysis of variance (ANOVA) when more than two groups were compared, and P values were calculated.
RESULTS
L'644 demonstrates sustained activity in TZM-bl cells not seen with other FIs.
To evaluate the potential of C34, T20, T1249, and L'644 as candidate microbicides, we first tested their comparative inhibitory activities against an R5-isolate, HIV-1 BaL, in TZM-bl indicator cells. L'644 is a derivatized version of C34 with a cholesterol group that allows the peptide to anchor on the cellular surface. Hence, to demonstrate the potential retention of this compound we compared the following conditions: after preincubation of TZM-bl cells with FIs for 1 h, cells were either washed (pulsed) or not (sustained) before addition of virus. L'644 was the most potent peptide under sustained-exposure conditions, with IC50s in the subnanomolar range (0.01 to 0.34 nM), as shown in Table 2. Under pulsed-exposure conditions, where unbound compound was removed by washing before virus was added, the inhibitory activities of C34 and T20 were completely lost within the range of concentrations tested, and the IC50 of T1249 increased 4 log units. This increase was not statistically significant (P = 0.0966), but there was a trend to an increase of IC50. However, the potency of L'644 was retained, with IC50s still in the nanomolar range (0.32 to 4.67 nM) (Table 2). No cytotoxicity for any of the FIs was observed by MTT viability assay at the concentrations tested (data not shown).
Table 2.
Sensitivity of HIV-1 BaL to fusion inhibitors in TZM-bl cells
| Condition | IC50 (nM)a |
|||
|---|---|---|---|---|
| C34 | T20 | T1249 | L'644 | |
| Sustained | 8.25 ± 6.15 | 43.81 ± 30.38 | 0.81 ± 0.43 | 0.19 ± 0.17 |
| Pulse | NA | NA | 290.76 ± 285.97 | 2.38 ± 2.18 |
The data are the means ± standard deviations obtained from three independent experiments performed in triplicates. NA, not applicable because 50% inhibition was not achieved.
Biological fluids do not interfere with L'644 activity.
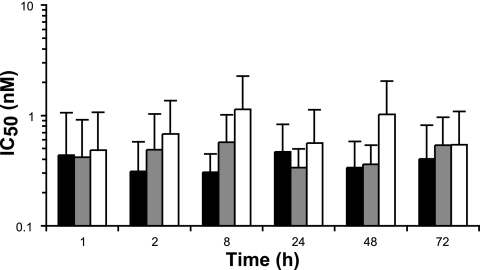
For compounds to be viable as microbicide candidates, it is important that they maintain their activity in the presence of semen and cervicovaginal fluid. Peptide inhibitors of gp41-mediated fusion have been shown to be protective against in vivo challenge when applied vaginally in nonhuman primate (NHP) studies (66, 67). However, L'644 has yet to be tested in vivo and, due to its cholesterol-derivatized nature, might be more susceptible to degradation; thus, we next assessed its activity in TZM-bl cells in the presence of biological fluids. L'644 was incubated with seminal plasma (SP) or cervicovaginal lavage (CVL) fluid (diluted 1:2) for up to 72 h before titration on TZM-bl cells against HIV-1 BaL (final dilution of SP and CVL specimens, 1:4). Comparing the IC50s of L'644 alone and in the presence of SP or CVL fluid, no statistical difference was seen at any of the time points (with P values of >0.4 even after 8 h [P = 0.4558] or 48 h [P = 0.6069] of incubation, by one-way ANOVA). Hence, neither of the biological fluids affected the IC50 (Fig. 1) or the IC90 (data not shown) values, indicating that the inhibitory activity of L'644 was not compromised by these biological fluids. No cytotoxic effect was detected during the duration of the experiment (data not shown).
Fig 1.
Activity of L'644 in the presence of biological fluids against HIV-1 BaL in TZM-bl cells. L'644 was mixed with SP (gray bars) or CVL (white bars) specimens, or not (black bars), at a 1:2 ratio in culture medium, for up to 72 h at 37°C. TZM-bl cells were treated for 1 h in the presence or absence of 100 μl of the mixture before addition of 100 μl of HIV-1 BaL. Luciferase expression (relative light units) was determined after 24 h, and the extent of inhibition by each drug was calculated. The IC50 was calculated after normalization of the percentage of inhibition relative to the relative light units obtained for cells grown in the absence of virus (0% infectivity) and for cells infected with virus in the absence of drug (100% infectivity). Data are the means (± standard deviations) from three independent experiments performed in triplicate.
Inhibitory activities of C34, T20, T1249, and L'644 in colorectal explants.
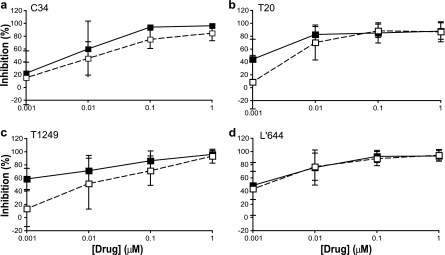
As candidate microbicides, the four FIs were then tested in mucosal tissue explants. The compounds were first tested in the colorectal explant model against HIV-1 BaL in a sustained-exposure mode (compound maintained throughout explant exposure to virus and culture). C34 tended to be the least active FI, with an IC50 of 7.29 ± 5.84 nM, compared to T20 (1.21 ± 0.74 nM), T1249 (2.87 ± 3.09 nM), and L'644 (3.82 ± 4.30 nM) (Fig. 2). When unbound compound was washed off after infection and explants cultured in the absence of compound (3-h pulse), there was a slight decrease in the activity of all FIs, reflected by an increase in IC50s for C34 (13.91 ± 6.45 nM), T20 (4.26 ± 2.65 nM), and T1249 (27.65 ± 33.04 nM) but not for L'644, where the IC50 did not change (IC50 of 3.06 ± 3.25 nM) (Fig. 2). Despite the fact that the increases in IC50s for the other three compounds were not significant, with P values of between 0.25 and 0.40, there was a trend to increase that was not seen for L'644. None of the compounds affected colorectal tissue viability as assessed by MTT assay (data not shown).
Fig 2.
Titration of C34, T20, T1249, and L'644 against HIV-1 BaL in colorectal explants. Colorectal explants were treated for 1 h in the presence or absence of C34 (a), T20 (b), T1249 (c), or L'644 (d). HIV-1 BaL was added and left for 2 h before four washes with PBS. Explants were then transferred to gel foam rafts and cultured in the presence (sustained [■]) or absence (3 h pulse [□]) of drug. The concentrations of p24 in the harvested supernatants were quantified by ELISA, and the extent of inhibition by each compound or combination was calculated. The percentage of inhibition was normalized relative to the p24 values obtained for explants not exposed to virus (0% infectivity) and for explants infected with virus in the absence of compound (100% infectivity). Data are means (± standard deviations) from three independent experiments performed in triplicate.
L'644 is more active than other fusion inhibitors in penile tissue explants.
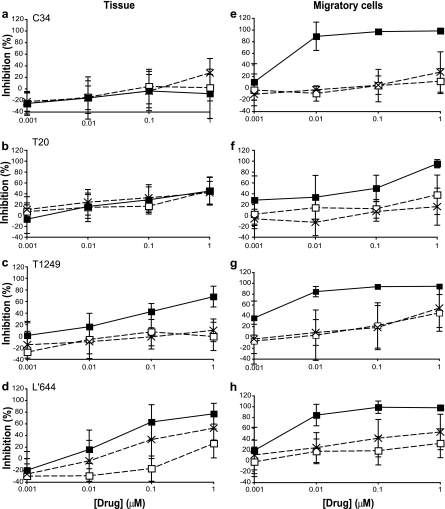
To further establish the potential of C34, T20, T1249, and L'644 as candidate microbicides, we next assessed and compared their inhibitory activities in penile tissue explants. In this system we first assessed whether pretreatment of tissue with compound (and its subsequent removal) prior to viral exposure had any impact on HIV infection, as observed for L'644 when using TZM-bl cells. Here explants were exposed to compound for 2 h, and the compound was removed (2-h pulse) by washing before addition of virus, which was incubated with tissue for 21 h before explants were washed and cultured in the absence of compound for a further 14 days. None of the FIs tested inhibited HIV-1 BaL when tissue was exposed to compound for 2 h prior to its removal and addition of virus (Fig. 3a, b, c, and d). In contrast, when FIs were maintained throughout the culture (sustained), IC50s could be determined for T1249 (214.83 ± 160.37 nM) and L'644 (68.35 ± 94.36 nM) (Fig. 3c and d), although the other inhibitors displayed no inhibitory activity.
Fig 3.
Antiviral activities of C34, T20, T1249, and L'644 in penile explants and migratory cells. Penile explants were treated for 2 h in the presence or absence of C34 (a), T20 (b), T1249 (c), and L'644 (d) and washed before treatment with HIV-1 BaL for 24 h. Explants were then washed, and after overnight culture, migratory cells (from tissue treated with C34 [e], T20 [f], T1249 [g], or L'644 [h] or without drug) were harvested and cocultured with PM-1. Levels of p24 in the harvested supernatants from tissue explants and migratory cells treated in the absence or presence of drug (2-h prepulse (□) or sustained (■)) were quantified by ELISA. The extent of inhibition by each compound was calculated. The percentage of inhibition was normalized relative to the p24 values obtained for explants not exposed to virus (0% infectivity) and for explants infected with virus in the absence of compound (100% infectivity). Data are the means (± standard deviations) from three independent experiments performed in triplicate.
Cells migrating out of male genital tissue explants, which include dendritic and CD4+ T cells, have been shown to productively disseminate HIV-1 infection (12). Hence, we also wanted to evaluate the capacities of the four FIs to inhibit dissemination by migratory cells obtained from the penile tissue explants. Similar to the case for the tissue, dissemination was not fully inhibited in cells obtained from penile explants prepulsed with FIs for 2 h prior to viral exposure (Fig. 3e, f, g, and h). However, when migratory cells were maintained in culture in the presence of compound, all FIs were active against HIV-1 BaL (Fig. 3e, f, g, and h), with IC50s in the nanomolar range for C34 (2.75 ± 1.93 nM), T20 (114.79 ± 99.43 nM), T1249 (1.21 ± 0.78 nM), and L'644 (2.76 ± 1.31 nM). No statistical difference was seen between these IC50s, with P values ranging between 0.12 and 0.99. No toxicity was detected in tissue or cells for any of the FIs as assessed by MTT viability assay (data not shown).
L'644 inhibits HIV-1 infection of cervical tissue and migratory cells.
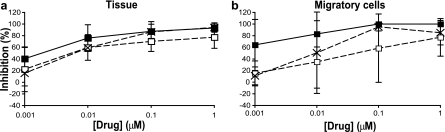
As L'644 was more potent than the other FIs tested in TZM-bl cells and penile tissue, it was selected for further analysis in the cervical explant tissue model, for which the limited availability of tissue constrained the number of conditions that could be evaluated. Cervical explants were treated with compound before (1 h) and during (2 h) exposure to virus. Explants were then washed and cultured either without compound (3-h pulse), with L'644 added back to the culture and left for a further 21 h whereupon it was removed by washing (24-h pulse), or with L'644 maintained throughout the culture period (14 days). Compound exposure time correlated with increased inhibitory activity in tissue (Fig. 4a), as shown by a slight reduction of IC50s between the 3-h pulse (9.37 ± 8.12 nM), 24-h pulse (6.37 ± 1.55 nM), and sustained exposure (3.53 ± 2.97 nM).
Fig 4.
Inhibitory activity of L'644 in cervical explants and migratory cells against HIV-1 BaL. Cervical explants were treated for 1 h in the presence or absence of L'644 (a) before treatment with BaL for 2 h. Explants were then washed four times with PBS. After overnight culture in the presence or absence of drug, migratory cells (from tissue treated with drug or without drug) were harvested and cocultured with PM-1. Levels of p24 in the harvested supernatants from tissue explants and migratory cells treated in the absence or presence of drug (3-h pulse [□], 24-h pulse [×], or sustained [■]) were quantified by ELISA. The extent of inhibition by each compound was calculated. The percentage of inhibition was normalized relative to the p24 values obtained for explants not exposed to virus (0% infectivity) and for explants infected with virus in the absence of compound (100% infectivity). Data are the means (± standard deviations) from three independent experiments performed in triplicate.
Migratory cells capable of disseminating HIV-1 infection have also been described in cervical tissue (26). After overnight culture of the explants described above, migratory cells were isolated and cultured in the absence (3- and 24-h pulse exposure) or presence (sustained exposure) of compound. Similarly to the dose-response curves obtained for L'644 in cervical explants, the inhibitory activity of the compound increased with time of exposure (Fig. 4b). The IC50s tended to decrease from the 3-h pulse condition (375.31 ± 524.82 nM) to the 24-h pulse condition (20.65 ± 27.04 nM) and again to the sustained condition (2.29 ± 2.31 nM). Due to donor-to-donor variation, this decrease was not statistically significant, with P values of >0.4 as determined by an unpaired t test.
L'644 had no impact on the viability of cervical explants or migratory cells as assessed by an MTT viability assay (data not shown).
L'644 demonstrates postexposure activity in colorectal and cervical tissue explants.
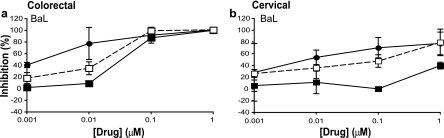
To further characterize the window of activity of L'644, we next assessed whether it was active when applied after exposure to virus. In TZM-bl cells, no inhibitory activity was detected when cells were infected, washed to remove unbound virus, and treated with L'644 1 h after virus removal (data not shown). To test if these results were also applicable in tissue, we first compared the activities of L'644 when colorectal explants were treated with L'644 either during viral exposure (2 h) or immediately following viral exposure (0 min) or when L'644 addition was delayed by 60 min following viral exposure. In these experiments, activity was assessed using two clade B R5 isolates, HIV-1 BaL and YU2. Here, a delay in compound addition correlated with a decrease in the potency of L'644 against both isolates. Compared to IC50s when tissue was treated with compound during viral exposure (7.46 ± 9.18 nM for BaL and 37.89 ± 23.93 nM for YU.2), the reduction in antiviral activity was reflected by an approximately 3-fold increase in the IC50s for both viruses when L'644 was added immediately (0 min) after virus removal (18.48 ± 5.02 nM for BaL and 110.30 ± 110.63 nM for YU.2) and a greater decrease in activity when it was added 60 min later (271.91 ± 33.35 nM for BaL and 128.30 ± 166.14 nM for YU.2), which was significant for BaL (P < 0.0001) (Fig. 5a and data not shown). Despite the increase in IC50 when L'644 was added after virus removal, the dose-response curves reached levels of inhibition at a higher concentration of compound (1 μM) that were similar to those when L'644 was added during viral exposure.
Fig 5.
Postexposure antiviral activity of L'644 in mucosal tissue explants. Colorectal (a) and cervical (b) explants infected with BaL for 2 h were either treated with L'644 (●) for 1 h before viral exposure or treated for 1 h with L'644 at 0 min (□) or 60 min (■) after removal of unbound virus. The concentrations of p24 in the harvested supernatants were quantified by ELISA, and the extent of inhibition by each compound or combination was calculated. The percentage of inhibition was normalized relative to the p24 values obtained for explants not exposed to virus (0% infectivity) and for explants infected with virus in the absence of compound (100% infectivity). Data are means (± standard deviations) from three independent experiments performed in triplicate.
We then tested the activity of L'644 against HIV-1 BaL in female genital tissue explants under the same conditions. When cervical explants were treated with L'644 during viral exposure, an IC50 of 9.37 ± 8.12 nM was calculated from the dose-response curve; however, when tissue was treated with L'644 at 0 min or 1 h after virus removal, IC50s significantly increased, to 159.57 ± 70.53 nM (P = 0.0215) and to an estimated 1275.72 ± 172.89 nM (P = 0.0002), respectively (Fig. 5b).
L'644 is active against an RTI-resistant isolate in colorectal explant cultures.
For L'644 to provide benefit in a combination microbicide containing two or more compounds, it should be active not only against wild-type virus but also against isolates resistant to other ARV drugs used in therapy, particularly resistant isolates of increasing prevalence in infected populations where treatment is available. Therefore, we assessed the activity of L'644 and the other FIs against an RTI escape mutant, A17. This isolate is highly resistant to a number of nonnucleoside RTIs (NNRTIs) through two single point mutations in the RT domain (K103N and Y181C) (24). We have previously demonstrated that this isolate replicates in colorectal tissue (23), but it replicates poorly in cervical explants; therefore, the colorectal model was used to determine the activities of FIs against this virus in mucosal tissue. The four FIs were evaluated by pretreating colorectal explants with compound for 1 h prior to addition of virus for a further 2 h. After viral exposure, explants were washed and cultured in the absence (3-h pulse) or presence (sustained) of compound. As with wild-type virus, the activities against A17 of C34, T20, and T1249 measured under sustained-exposure conditions was reduced when the compounds were used only for a 3-h pulse, with C34 not even reaching an IC50 within the range of concentrations tested. However, the difference in activity between conditions was more pronounced for the resistant isolate, as reflected by an increase of IC50s of at least 2 log units (Table 3) (with P values of < 0.001 for C34, 0.1572 for T20, and 0.0024 for T1249). In contrast, L'644 retained far more activity with shorter exposure of tissue to compound (3-h pulse instead of sustained), with a slight, nonsignificant (P = 0.2770) increase in the IC50 (from 6.11 ± 4.05 nM with sustained treatment to 62.36 ± 53.59 nM with a 3-h pulse) (Table 3).
Table 3.
Sensitivity of HIV-1 A17 to fusion inhibitors in colorectal explants
| Condition | IC50 (nM)a |
|||
|---|---|---|---|---|
| C34 | T20 | T1249 | L'644 | |
| Sustained | 38.78 ± 12.71 | 25.17 ± 13.69 | 22.49 ± 2.59 | 6.11 ± 4.05 |
| 3-h pulse | NA | 3,491.61 ± 2,213.86 | 1,616.81 ± 109.77 | 62.36 ± 53.59 |
The data are the means ± standard deviations obtained from three independent experiments performed in triplicates. NA, not applicable because 50% inhibition was not achieved.
DISCUSSION
In this study, we have performed the first comparative preclinical evaluation of four FIs, C34, T1249, T20, and L'644, as potential candidate microbicides using cellular and tissue explant models and a variety of exposure times. All four FIs are peptides derived from the fusogenic protein gp41 of the HIV-1 viral envelope. L'644 was recently developed by Ingallinella et al., using a new approach to improve C34 activity by attaching a cholesterol group as a “membrane anchor” (27). Studies by Ingallinela et al. suggested that binding of L'644 to, and retention on, the lipid raft compartment of the target cell membrane provided increased potency over the parent compound, C34. To assess the potential benefit for microbicide use of conjugating C34 to cholesterol, we compared the potencies of all four FIs under different dosing conditions across a range of culture models. L'644 was clearly the most potent FI when the TZM-bl reporter cell line was used, and activity was retained when unbound compound was removed before virus addition (Table 2), mirroring the results of Ingallinella et al. (27). However, infection of TZM-bl cells may not be representative of infection of target cells in primary tissue. TZM-bl cells express enhanced levels of the viral receptors CD4 and CCR5 (56), and as a result fusion may occur at an exaggerated speed. Furthermore, recent evidence suggests that in these cells virus is rapidly endocytosed following CD4 binding (48), and as a consequence exposure of HIV envelope fusion intermediates to extracellular FI may be temporally restricted. In addition, recent studies by Miyauchi et al. have reported that the longevity of gp41 intermediates as well as the conformation changes in the viral envelope after binding to CD4 determines the potency of FIs derived from gp41 HR2 (47, 48). These early steps of HIV-1 fusion can be modulated by factors such as density and/or affinity of coreceptors to Env and tropism. Thus, the competitive advantage of L'644 over other fusion inhibitors in this model may reflect membrane anchoring, providing more rapid interaction with transiently exposed fusion intermediates on virus and/or allowing greater access to endosomes. It remains unclear whether the viral entry mechanisms in TZM-bl cells reflect aspects of viral entry in primary mucosal target cells, which are thought to be represented predominantly by resting CD4 CCR5 T cells (22).
Importantly, none of the FIs displayed any detectable toxicity for cells or tissues used in this study. Furthermore, the activity of L'644 was not compromised following 72 h of incubation in semen or cervical lavage specimens, suggesting that it is sufficiently stable in relevant biological fluids to be compatible with microbicide use.
Given the potential limitations of the TZM-bl cell assays, we also ascertained the relative activities of the FIs in primary mucosal tissues. We first evaluated them using an established colorectal model of HIV-1 infection (14, 24). Here, the activities of the four FIs were relatively similar when exposure was sustained throughout the culture period (14 days); thus, the competitive advantage seen for L'644 on TZM-bl cells was not replicated in this challenge model. We and others have previously demonstrated that the primary target cells for infection in this model are activated CD4+ CCR5+ cells (1, 14, 34, 57). The likely slower kinetics of fusion, compared to those of TZM-bl cells, coupled with maintaining FI concentrations throughout the culture period may have blunted any competitive advantage attributable to the potential faster binding kinetic that might be provided by “membrane anchoring” of L'644. Indeed, little is known about the specificity of any “anchoring” effect, and thus it is anticipated that this may occur in a nonspecific manner for all cells within an explant irrespective of their susceptibility to infection. Indeed, general binding of L'644 to cells of all types within an explant could potentially act as a sink restricting the availability of compound for binding to HIV target cells. Interestingly, when exposure to FIs was restricted to the period of viral exposure, the IC50 for L'644 did not change, while it was increased for the other compounds. This suggests that when exposure to drug is limited in this model, there is some modest advantage to the cholesterol derivatization.
Activity of the FIs in the penile explant model was either poor or absent. When tissue was pretreated with FIs prior to viral exposure, there was no viral inhibition. This is perhaps not unexpected for the nonderivatized FIs; however, the lack of activity by L'644 does not replicate the advantage seen when using TZM-bl cells. There was no activity against penile glans infection with C34 and T20 when inhibitors were sustained throughout the culture, while the activities of T1249 and L'644 were greatly reduced with respect to those seen in colorectal and cervical tissue explants (see below). Nevertheless, L'644 was the most active inhibitor in this model. It is perhaps interesting to note that of the other three FIs, only T1249 displayed detectable activity in this model, likely reflecting the more lipophilic nature of this compound than of C34 and T20 (42).The reasons behind the differences in activity between the FIs in this model merit further investigation, but they likely reflect differences in drug penetration between the different tissue types. Indeed, the activities of FIs against dissemination of virus by cells that migrate out of penile tissue during the first 24 h of culture were broadly similar to those seen in colorectal tissue and cervical tissue (see below). Here also, as inhibitor concentrations were sustained throughout the culture, there was little or no competitive advantage provided by the cholesterol conjugation. Furthermore, a recent study by Dinh et al. (10) has revealed significant differences between male and female genital epithelia. Interestingly, despite the epithelium in both tissues being stratified squamous, while filaggrin (also known as filament aggregation protein), which is present in the superficial strata of epithelia (stratum corneum) creating the cornified layer of certain types of stratum corneum (44, 62), and a cornified layer were detectable in male genital epithelium, they were absent in female cervicovaginal tissue. This previous study also revealed differences between cervicovaginal and glans epithelia in expression of several proteins, including E-cadherins and desmogleins 1/2, which are involved in epithelial adhesion and stability (49), and involucrin, a structural protein to which protein and lipids bind (3, 6). These differences could affect the antiviral activity of peptidic fusion inhibitors, limiting the access to target cells or even sequestering the peptides in certain layers of the epithelium.
Experiments using cervical explants were limited by the availability of tissue; therefore, we chose to study the activity of L'644 only in this model. Here, the activity when compound was maintained throughout the culture was comparable to that seen with colorectal tissue (IC50s of 3.53 nM and 3.83 nM, respectively) and much better than that seen with penile tissue (68.35 nM). Furthermore, the activity of L'644 when maintained only during viral exposure showed a modest decrease, with an IC50 of 9.37 nM. The activity of L'644 against HIV dissemination by cells that migrate out of cervical tissue in the first 24 h of culture (dendritic cells and T cells, as previously described [26]) was similar to that observed with penile tissue (IC50s of 2.29 nM and 2.76 nM, respectively). Activity of L'644 was decreased with shorter compound exposure. Dendritic cells are known to transfer both captured (65) and de novo-produced (cis) virus to infected T cells. Fusion inhibitors, including T1249 and T20, have been shown to efficiently block DC-mediated infection in trans or cis (17, 18, 32, 33, 63). Previous studies have suggested that transfer to T cells by trans infection has a relatively short time window of a few hours following viral exposure and that by 24 h following exposure most transfer is by cis infection through de novo-produced virus from infected dendritic cells (39, 50, 64). The requirement for increased concentrations of L'644 to block dissemination by migratory cells that emigrate out of tissue in the first 24 h of culture may reflect a difference in the potency of L'644 against these two pathways.
The first clinical trial in which a microbicide was shown to be effective was CAPRISA 004 (31), in which a vaginal gel containing the RTI tenofovir was applied 12 h before intercourse and a second dose of the gel was applied within 12 h after sex. Local preexposure to tenofovir has also been successful in nonhuman primate models, using vaginal application 30 min before challenge (53) and using rectal dosing 15 min or 2 h before viral exposure (8). However, recently, once-daily dosing of the tenofovir vaginal gel in women at risk of HIV infection failed to demonstrate any detectable efficacy in the VOICE trial. FIs, including T1249, have been shown to be protective in vivo when applied vaginally to rhesus macaques (66, 67) 30 min prior to challenge; however, they have not been tested for activity outside this dosing regimen. L'644 has yet to be tested in any in vivo challenge model, and therefore it is not yet known whether the advantages seen with this compound over other FIs in the in vitro models described here also translate to greater or prolonged activity in vivo.
To further define the window of activity for L'644, we assessed the activity of the compound after viral exposure in cellular and tissue models. In the TZM-bl cell model, none of the FIs tested showed activity when added 1 h after virus removal. The lack of delayed activity in this model likely reflects the speed of fusion events and/or internalization of viral particles by these cells, as discussed above. However, when delayed-dosing experiments were performed with mucosal tissue explant models (colorectal and cervical), L'644 was still active, although less potent, when added up to an hour postinfection (Fig. 5). While the postexposure activity observed in these in vitro models is unlikely to be sufficient to propose that L'644 could be used as a postcoital microbicide, they do suggest that the window of activity for this drug maybe wider than that for other FIs. This could be further investigated in NHP challenge studies where microbicide dosing is delayed relative to viral challenge.
A further important consideration in the design of future microbicides will be the efficacy of candidate compounds against viral escape mutants resistant to compounds commonly used in therapy and/or other ARVs used in prevention (microbicides or oral preexposure prophylaxis [PrEP]). Here, we assessed the potential of all four FIs against an RTI-resistant isolate, A17, in colorectal explants. Interestingly, L'644 was the only FI that maintained its antiviral activity without substantial change in the IC50s during both sustained and pulse exposure to tissue (Table 3). Hence, L'644 would be a good candidate for use in combination-based microbicides containing NNRTIs, since it is active against both wild-type and NNRTI-resistant isolates.
This study has provided preclinical evaluation of four FIs as potential microbicide candidates. All FIs were active against infection of cervical and colorectal tissue under sustained conditions; thus, FIs are likely to be more effective as microbicides if delivered from sustained-release devices such as intravaginal rings or long-acting gels. L'644 appeared to extend the window of activity in these models and thus may provide some advantage when considering the design of a microbicide to be used in a coitus-dependent regimen (applied close to each act of intercourse). Nonhuman primate challenge studies may help determine whether such in vitro differences are matched with an increased window of protection in vivo relative to those for other FIs known to work in this model (66, 67). Any potential gains in activity will need to be matched by potential increased costs in manufacture related to the cholesterol derivatization.
In summary, data from these studies indicate that L'644 has potential as a microbicide candidate, either alone or in combination with another ARV drug(s), since it is able to inhibit both wild-type and RTI-resistant isolates.
ACKNOWLEDGMENTS
We thank Robert Haggar, David Melville, and the Colorectal Surgery Team, St. George's Hospital, London, for their assistance in obtaining human colorectal tissue. We are grateful to Kingston Hospital and St. George's Hospital, London, for the donation of cervical tissue. We are most grateful to James Bellringer, Consultant Urologist and Gender Surgeon of Charing Cross Hospital, London, for provision of penile tissue samples. We thank the International Partnership for Microbicides (IPM) for donation of L'644.
This work was funded by IPM and NIH grant U19 AI76982-04 and supported by an equipment grant from the Fondation Dormeur.
Footnotes
Published ahead of print 13 February 2012
REFERENCES
- 1. Anton PA, et al. 2000. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS 14:1761–1765 [DOI] [PubMed] [Google Scholar]
- 2. Arien KK, Jespers V, Vanham G. 2011. HIV sexual transmission and microbicides. Rev. Med. Virol. 21:110–133 [DOI] [PubMed] [Google Scholar]
- 3. Banks-Schlegel S, Green H. 1981. Involucrin synthesis and tissue assembly by keratinocytes in natural and cultured human epithelia. J. Cell Biol. 90:732–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bennett DE, et al. 2003. Prevalence of mutations associated with antiretroviral drug resistance among men and women newly diagnosed with HIV in 10 US cities, 1997–2001. Antivir. Ther. 8:S133 [Google Scholar]
- 5. Briones C, et al. 2001. Primary genotypic and phenotypic HIV-1 drug resistance in recent seroconverters in Madrid. J. Acquir. Immune Defic. Syndr. 26:145–150 [DOI] [PubMed] [Google Scholar]
- 6. Candi E, Schmidt R, Melino G. 2005. The cornified envelope: a model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 6:328–340 [DOI] [PubMed] [Google Scholar]
- 7. Chaix M, et al. 2003. French National Sentinel Survey of antiretroviral resistance in patients with HIV-1 primary infection and in antiretroviral-naive chronically infected patients in 2001–2002. Antivir. Ther. 8:S137. [DOI] [PubMed] [Google Scholar]
- 8. Cranage M, et al. 2008. Prevention of SIV rectal transmission and priming of T cell responses in macaques after local pre-exposure application of tenofovir gel. PLoS Med. 5:e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Derdeyn CA, et al. 2000. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J. Virol. 74:8358–8367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dinh MH, Okocha EA, Koons A, Veazey RS, Hope TJ. 2012. Expression of structural proteins in human female and male genital epithelia and implications for sexually transmitted infections. Biol. Reprod. 86:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duwe S, et al. 2001. Frequency of genotypic and phenotypic drug-resistant HIV-1 among therapy-naive patients of the German Seroconverter Study. J. Acquir. Immune Defic. Syndr. 26:266–273 [DOI] [PubMed] [Google Scholar]
- 12. Fischetti L, Barry SM, Hope TJ, Shattock RJ. 2009. HIV-1 infection of human penile explant tissue and protection by candidate microbicides. AIDS 23:319–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fletcher P, et al. 2005. The nonnucleoside reverse transcriptase inhibitor UC-781 inhibits human immunodeficiency virus type 1 infection of human cervical tissue and dissemination by migratory cells. J. Virol. 79:11179–11186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fletcher PS, et al. 2006. Ex vivo culture of human colorectal tissue for the evaluation of candidate microbicides. AIDS 20:1237–1245 [DOI] [PubMed] [Google Scholar]
- 15. Foss AM, Vickerman PT, Heise L, Watts CH. 2003. Shifts in condom use following microbicide introduction: should we be concerned? AIDS 17:1227–1237 [DOI] [PubMed] [Google Scholar]
- 16. Fox R, Gourlay YJ. 2000. The impact of highly active antiretroviral combination therapy in HIV infected patients in Glasgow. Health Bull. (Edinb.) 58:309–315 [PubMed] [Google Scholar]
- 17. Frank I, Robbiani M. 2011. Attachment and fusion inhibitors potently prevent dendritic cell-driven HIV infection. J. Acquir. Immune Defic. Syndr. 56:204–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Frank I, et al. 2008. A fusion inhibitor prevents spread of immunodeficiency viruses, but not activation of virus-specific T cells, by dendritic cells. J. Virol. 82:5329–5339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gallo SA, Sackett K, Rawat SS, Shai Y, Blumenthal R. 2004. The stability of the intact envelope glycoproteins is a major determinant of sensitivity of HIV/SIV to peptidic fusion inhibitors. J. Mol. Biol. 340:9–14 [DOI] [PubMed] [Google Scholar]
- 20. Gartner S, et al. 1986. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science 233:215–219 [DOI] [PubMed] [Google Scholar]
- 21. Gordon CJ, et al. 1999. Enhancement of human immunodeficiency virus type 1 infection by the CC-chemokine RANTES is independent of the mechanism of virus-cell fusion. J. Virol. 73:684–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haase AT. 2011. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu. Rev. Med. 62:127–139 [DOI] [PubMed] [Google Scholar]
- 23. Herrera C, Cranage M, McGowan I, Anton P, Shattock RJ. 2011. Colorectal microbicide design: triple combinations of reverse transcriptase inhibitors are optimal against HIV-1 in tissue explants. AIDS 25:1971–1979 [DOI] [PubMed] [Google Scholar]
- 24. Herrera C, Cranage M, McGowan I, Anton P, Shattock RJ. 2009. Reverse transcriptase inhibitors as potential colorectal microbicides. Antimicrob. Agents Chemother. 53:1797–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hladik F, Doncel GF. 2010. Preventing mucosal HIV transmission with topical microbicides: challenges and opportunities. Antiviral Res. 88(Suppl. 1):S3–S9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hu Q, et al. 2004. Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue. J. Exp. Med. 199:1065–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ingallinella P, et al. 2009. Addition of a cholesterol group to an HIV-1 peptide fusion inhibitor dramatically increases its antiviral potency. Proc. Natl. Acad. Sci. U. S. A. 106:5801–5806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. John M, et al. 2005. Cervicovaginal secretions contribute to innate resistance to herpes simplex virus infection. J. Infect. Dis. 192:1731–1740 [DOI] [PubMed] [Google Scholar]
- 29. Jordan R, Gold L, Cummins C, Hyde C. 2002. Systematic review and meta-analysis of evidence for increasing numbers of drugs in antiretroviral combination therapy. BMJ 324:757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kalichman SC, Rompa D, Luke W, Austin J. 2002. HIV transmission risk behaviours among HIV-positive persons in serodiscordant relationships. Int. J. STD AIDS 13:677–682 [DOI] [PubMed] [Google Scholar]
- 31. Karim QA, et al. 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ketas TJ, et al. 2003. Human immunodeficiency virus type 1 attachment, coreceptor, and fusion inhibitors are active against both direct and trans infection of primary cells. J. Virol. 77:2762–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ketas TJ, et al. 2003. Entry inhibitors SCH-C, RANTES, and T-20 block HIV type 1 replication in multiple cell types. AIDS Res. Hum. Retroviruses 19:177–186 [DOI] [PubMed] [Google Scholar]
- 34. Lapenta C, et al. 1999. Human intestinal lamina propria lymphocytes are naturally permissive to HIV-1 infection. Eur. J. Immunol. 29:1202–1208 [DOI] [PubMed] [Google Scholar]
- 35. Leynaert B, Downs AM, de Vincenzi I. 1998. Heterosexual transmission of human immunodeficiency virus: variability of infectivity throughout the course of infection. European Study Group on Heterosexual Transmission of HIV. Am. J. Epidemiol. 148:88–96 [DOI] [PubMed] [Google Scholar]
- 36. Li Y, et al. 1992. Complete nucleotide sequence, genome organization, and biological properties of human immunodeficiency virus type 1 in vivo: evidence for limited defectiveness and complementation. J. Virol. 66:6587–6600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li Y, et al. 1991. Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured human brain tissue: identification of replication-competent and -defective viral genomes. J. Virol. 65:3973–3985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Little SJ, et al. 2002. Antiretroviral-drug resistance among patients recently infected with HIV. N. Engl. J. Med. 347:385–394 [DOI] [PubMed] [Google Scholar]
- 39. Lore K, Smed-Sorensen A, Vasudevan J, Mascola JR, Koup RA. 2005. Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4+ T cells. J. Exp. Med. 201:2023–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lusso P, et al. 1995. Growth of macrophage-tropic and primary human immunodeficiency virus type 1 (HIV-1) isolates in a unique CD4+ T-cell clone (PM1): failure to downregulate CD4 and to interfere with cell-line-tropic HIV-1. J. Virol. 69:3712–3720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Margolis L, Shattock R. 2006. Selective trasnmission of CCR5-utilizaing HIV-1: the ‘gatekeeper’ prooblem resolved? Nat. Rev. Microbiol. 4:312–317 [DOI] [PubMed] [Google Scholar]
- 42. Matos PM, Castanho MA, Santos NC. 2010. HIV-1 fusion inhibitor peptides enfuvirtide and T-1249 interact with erythrocyte and lymphocyte membranes. PLoS One 5:e9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mayer KH, Venkatesh KK. 2010. Chemoprophylaxis for HIV prevention: new opportunities and new questions. J. Acquir. Immune Defic. Syndr 55(Suppl. 2):S122–S127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McGrath JA, Uitto J. 2008. The filaggrin story: novel insights into skin-barrier function and disease. Trends Mol. Med. 14:20–27 [DOI] [PubMed] [Google Scholar]
- 45. Mendoza C, et al. 2003. Evidence for a different transmission efficiency of viruses with distinct drug-resistance genotypes. Antivir. Ther. 8:S144 [Google Scholar]
- 46. Mesquita PM, et al. 2008. Candidate microbicide PPCM blocks human immunodeficiency virus type 1 infection in cell and tissue cultures and prevents genital herpes in a murine model. J. Virol. 82:6576–6584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Miyauchi K, Kim Y, Latinovic O, Morozov V, Melikyan GB. 2009. HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell 137:433–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Miyauchi K, Kozlov MM, Melikyan GB. 2009. Early steps of HIV-1 fusion define the sensitivity to inhibitory peptides that block 6-helix bundle formation. PLoS Pathog. 5:e1000585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Niessen CM. 2007. Tight junctions/adherens junctions: basic structure and function. J. Investig. Dermatol. 127:2525–2532 [DOI] [PubMed] [Google Scholar]
- 50. Nobile C, et al. 2005. Covert human immunodeficiency virus replication in dendritic cells and in DC-SIGN-expressing cells promotes long-term transmission to lymphocytes. J. Virol. 79:5386–5399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nunberg JH, et al. 1991. Viral resistance to human immunodeficiency virus type 1-specific pyridinone reverse transcriptase inhibitors. J. Virol. 65:4887–4892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Padian NS, Shiboski SC, Glass SO, Vittinghoff E. 1997. Heterosexual transmission of human immunodeficiency virus (HIV) in northern California: results from a ten-year study. Am. J. Epidemiol. 146:350–357 [DOI] [PubMed] [Google Scholar]
- 53. Parikh UM, et al. 2009. Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J. Virol. 83:10358–10365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Parpia T, et al. 2001. Effect of combination therapy on immunologic progression of human immunodeficiency virus at a population level. Am. J. Epidemiol. 153:898–902 [DOI] [PubMed] [Google Scholar]
- 55. Patel S, et al. 2007. Seminal plasma reduces the effectiveness of topical polyanionic microbicides. J. Infect. Dis. 196:1394–1402 [DOI] [PubMed] [Google Scholar]
- 56. Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 72:2855–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Poles MA, Elliott J, Taing P, Anton PA, Chen IS. 2001. A preponderance of CCR5(+) CXCR4(+) mononuclear cells enhances gastrointestinal mucosal susceptibility to human immunodeficiency virus type 1 infection. J. Virol. 75:8390–8399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rohan LC, et al. 2010. In vitro and ex vivo testing of tenofovir shows it is effective as an HIV-1 microbicide. PLoS One 5:e9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Salomon H, et al. 2000. Prevalence of HIV-1 resistant to antiretroviral drugs in 81 individuals newly infected by sexual contact or injecting drug use. Investigators of the Quebec Primary Infection Study. AIDS 14:F17–23 [DOI] [PubMed] [Google Scholar]
- 60. Shattock R, Solomon S. 2004. Microbicides—aids to safer sex. Lancet 363:1002–1003 [DOI] [PubMed] [Google Scholar]
- 61. Slater TF, Sawyer B, Straeuli U. 1963. Studies on succinate-tetrazolium reductase systems. Iii. Points of coupling of four different tetrazolium salts. Biochim. Biophys. Acta 77:383–393 [DOI] [PubMed] [Google Scholar]
- 62. Steven AC, Steinert PM. 1994. Protein composition of cornified cell envelopes of epidermal keratinocytes. J. Cell Sci. 107:693–700 [PubMed] [Google Scholar]
- 63. Tardif MR, Gilbert C, Thibault S, Fortin JF, Tremblay MJ. 2009. LFA-1 antagonists as agents limiting human immunodeficiency virus type 1 infection and transmission and potentiating the effect of the fusion inhibitor T-20. Antimicrob. Agents Chemother. 53:4656–4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Turville SG, et al. 2004. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood 103:2170–2179 [DOI] [PubMed] [Google Scholar]
- 65. UK Collaborabive Group on Monitoring the Transmission of HIV Drug Resistance 2001. Analysis of prevalence of HIV-1 drug resistance in primary infections in the United Kingdom. BMJ 322:1087–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Veazey RS, et al. 2008. Tropism-independent protection of macaques against vaginal transmission of three SHIVs by the HIV-1 fusion inhibitor T-1249. Proc. Natl. Acad. Sci. U. S. A. 105:10531–10536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Veazey RS, et al. 2005. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature 438:99–102 [DOI] [PubMed] [Google Scholar]
- 68. Vittinghoff E, et al. 1999. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. Am. J. Epidemiol. 150:306–311 [DOI] [PubMed] [Google Scholar]
- 69. Wei X, et al. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46:1896–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]