Abstract
Cryptococcus gattii is the main pathogen of cryptococcosis in healthy patients and is treated mainly with fluconazole and amphotericin B. The combination of these drugs has been questioned because the mechanisms of action could lead to a theoretical antagonistic interaction. We evaluated distinct parameters involved in the in vitro combination of fluconazole and amphotericin B against Cryptococcus gattii. Fourteen strains of C. gattii were used for the determination of MIC, fractional inhibitory concentration, time-kill curve, and postantifungal effect (PAFE). Ergosterol quantification was performed to evaluate the influence of ergosterol content on the interaction between these antifungals. Interaction between the drugs varied from synergistic to antagonistic depending on the strain and concentration tested. Increasing fluconazole levels were correlated with an antagonistic interaction. A total of 48 h was necessary for reducing the fungal viability in the presence of fluconazole, while 12 h were required for amphotericin B. When these antifungals were tested in combination, fluconazole impaired the amphotericin B activity. The ergosterol content decreased with the increase of fluconazole levels and it was correlated with the lower activity of amphotericin B. The PAFE found varied from 1 to 4 h for fluconazole and from 1 to 3 h for amphotericin B. The interaction of fluconazole and amphotericin B was concentration-dependent and special attention should be directed when these drugs are used in combination against C. gattii.
INTRODUCTION
Cryptococcus gattii, an emergent fungal primary human pathogen, is the main agent of cryptococcosis in healthy individuals. The lesions caused by this organism affect mainly the lungs and central nervous system with meningitis, cranial hypertension and papilledema signs. The infection caused by C. gattii is frequently lethal, and some of the lesions and brain damage are not possible to correct surgically, requiring more intensive and prolonged treatment than other cryptococcal infections, such as those caused by C. neoformans (3, 5, 7, 27).
Amphotericin B and fluconazole are the most used polyenes and azoles medicines, respectively, which are the classes of the most commonly used antifungal agents for the treatment of cryptococcosis. Amphotericin B (AMB) is highly effective; however, its nephrotoxic and hepatotoxic effects limit its usage (21). Fluconazole (FLC) presents lower toxicity and shows adequate penetration into various tissues, including the central nervous system (4, 10). However, a large number of cases of fungal resistance development have been described (24), jeopardizing the therapy.
Considering these difficulties in treating cryptococcosis caused by C. gattii, the antifungal combination represents an important alternative to the conventional therapy. The combination of drugs usually requires lower doses of the antimicrobials. This reduction might lead to a toxicity decrease, which results in a higher tolerance to the antimicrobial by the patient. These two facts altogether increase the efficacy and speed of action, enhancing the spectrum of activity of the agents. On the other hand, the use of each drug alone would require higher doses, which would be more toxic to the patient (2). In addition, the better therapy coupled with the reduction of the probability of developing resistance to one or both antifungals during the treatment (21) are important benefits provided by this combination.
It is possible to classify the interactions between drugs in three distinct types: synergism, indifferent, and antagonism as described previously (22). The synergism will occur when the effects are greater in the treatment using combined drugs than treatments using a single-drug regimen. If the resulting effects observed in the combined treatment are smaller than what would be expected by single-drug treatments, the interaction is defined as antagonism. However, when the resulting effects have no distinct differentiation between the one-drug treatment and the combined treatment, the interaction is classified as indifferent (16, 22).
The antagonism between two or more drugs may occur because of competition to reach the same target, while the indifferent interaction indicate that drugs in combination do not have adverse effect on the therapeutic response. Amphotericin B acts by binding to the ergosterol of the fungal cell membrane causing changes in permeability and loss of intracellular content, while fluconazole inhibits the ergosterol synthesis. These action mechanisms would involve distinct interactions; however, there is an intriguing controversy regarding the combination of amphotericin B and fluconazole for fungal infections, since the existing data have failed to prove the theory of synergism or antagonism of these antifungals (12, 13, 20).
The postantifungal effect (PAFE) is defined as suppression of fungal growth that persists after limited exposure to an antifungal agent. This situation occurs mainly in vivo due to the variations of the drug concentration in the site of infection (9).
We evaluated here distinct parameters involved in the in vitro combination of fluconazole and amphotericin B against C. gattii. The influence of different concentrations of these drugs on the type of interaction and on the ergosterol contents of the plasma membrane was also evaluated. In addition, the PAFEs were determined.
MATERIALS AND METHODS
C. gattii strains.
We tested 14 strains of C. gattii (nine clinical and three environmental isolates, all from the culture collection of the Laboratório de Micologia da Universidade Federal de Minas Gerais, Minas Gerais, Brazil, and two reference strains from the culture collection of the University of Georgia, Atlanta, GA). All of the isolates were maintained on slant Sabouraud dextrose agar (SDA) at 4°C.
Inoculum preparation.
Prior to the tests, the strains were subcultured on SDA for 48 h at 35°C. The inoculum was prepared in sterile saline, and the transmittance of the suspensions was adjusted to 75 to 77% (530 nm), followed by further dilution in RPMI 1640 (Sigma-Aldrich) medium to achieve 1.0 × 103 to 5.0 × 103 CFU/ml (6). All of the tests were performed in duplicate for each strain.
Antifungal drug susceptibility testing.
The MICs for fluconazole (Sigma-Aldrich, St. Louis, MO) and amphotericin B (Sigma-Aldrich) were determined by using the antifungal microdilution test proposed by the Clinical and Laboratory Standards Institute (CLSI) M27-A3 method (6). Fluconazole was dissolved in sterile distilled water, and amphotericin B was dissolved in 100% dimethyl sulfoxide (Gibco-BRL, Grand Island, NY) according to the protocol of the CLSI at 1,000 μg/ml. These stock solutions were further diluted in RPMI 1640 test medium (buffered with morpholinepropanesulfonic acid [Sigma-Aldrich]) to yield twice (when a drug was tested individually) or four times (for combination of drugs) the final strength required for the test. The concentrations ranged from 0.125 to 64.0 μg/ml for fluconazole and from 0.03 to 16.0 μg/ml for amphotericin B (6).
A volume of 100 μl of the inoculum suspension was transferred to sterile flat-bottom 96-well plates containing 100 μl of each of the antifungals or RPMI 1640 (control growth). The plates were incubated at 35°C for 72 h (6). The MIC for fluconazole was determined visually as 80% growth inhibition, while for amphotericin B the reading was performed as 100% growth inhibition compared to the control (19). We also performed the MIC determination for fluconazole at 50% (the MIC-2 endpoint) of growth inhibition according to the CLSI method (6). The isolate Candida parapsilosis ATCC 22019 was used as a quality control (6).
In vitro interaction of FLC and AMB.
Fluconazole (FLC) and amphotericin B (AMB) were also tested in combination. A checkerboard microdilution method (25), which provides a matrix of all possible drug combinations in the required concentration range, was used to test the susceptibility of C. gattii to the drugs. The concentrations ranged from 0.125 to 64.0 μg/ml for FLC and from 0.016 to 1.0 μg/ml for AMB. One plate was used to test each strain. The MIC endpoint was 100% of growth inhibition. The interaction between these antifungals was quantitatively evaluated by determining the fractional inhibitory concentration (FIC). The formula for calculating the FIC was as follows: FIC = [MIC FLC in combination/MIC FLC] + [MIC AMB in combination/MIC AMB]. The FIC was calculated for all of the possible combinations of different concentrations for the same isolate and the final result was expressed as the mean of the FICs (12). Also, interaction curves were constructed. The interaction between these drugs was classified as synergism if FIC ≤ 0.5, indifferent if 0.5 > FIC ≤ 4.0, and antagonism for FIC > 4.0 as described previously (22).
Time-kill curves.
An assay was performed to evaluate the time-kill kinetics of the drugs against C. gattii. For AMB, the concentration tested was equivalent to the MIC for each strain. For FLC, the tested concentrations were equal to the MIC (endpoint at 80% of growth inhibition), twice the MIC (2× the MIC), and four times the MIC (4× the MIC) for each strain. In combination, AMB (MIC) and FLC at three different concentrations (1.0, 4.0, and 16.0 μg/ml) were tested. A 100-μl inoculum of C. gattii was placed on microtiter plates containing antifungal agents alone or in combination at different intervals for 72 h. In sequence, we added MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide; Sigma-Aldrich] (5.0 mg/ml) to determine the reduction on the metabolic cell activity. The plates were incubated at 35°C for 3 h, and isopropanol was added before the spectrophotometric reading at 490 nm. The percentage of metabolic activity compared to control growth was determined for each strain for each time of reading. The results were confirmed by plating an amount of sample from each well on SDA, followed by incubation at 35°C for 72 h prior to colony counting (14).
Ergosterol quantification.
The ergosterol quantification in the fungal cell membrane was performed as described previously (1) with modifications. The L27/01 strain was chosen from the results of drug combination and time-kill assay. The isolate was cultured (35°C, 72 h) in sterile petri dishes containing SDA. Approximately 25.0 mg of the fungal cell mass was transferred to polypropylene tubes. FLC (16.0 μg/ml), AMB (4.0 μg/ml), or a combination of AMB and FLC (4.0 μg/ml + 1.0 μg/ml, 4.0 μg/ml + 4.0 μg/ml, or 4.0 μg/ml + 16.0 μg/ml) was tested. A growth control was also performed. After incubation at 35°C for 24 h, the tubes were centrifuged (Jouan, model BR4i) at 1,643 × g for 5 min at 4°C, and the supernatant was removed. The cells were washed with sterile distilled water. The net wet weight of the cell pellet was determined. For the extraction of lipids, 3 ml of ethanolic solution of potassium hydroxide 25% was added to each cell mass, followed by agitation for 1 min. The tubes were incubated in a water bath at 85°C for 1 h and further cooled at room temperature. A mixture of 1 ml of sterile water and 3 ml of n-heptane (Sigma-Aldrich) was added, followed by agitation in a vortex for 3 min. The supernatant was removed, and the reading was performed in spectrophotometer at 282 and 230 nm. A calibration curve with standard ergosterol (Sigma-Aldrich) was constructed and used to calculate the amount of ergosterol. The results were expressed as the percentage of ergosterol compared to the growth control.
PAFE.
We determined the postantifungal effect (PAFE) of FLC and AMB for each of the 14 strains. A volume of 100 μl of the inoculum suspension (103 CFU/ml) was transferred to sterile 96-well flat-bottom plates containing 100 μl of each of the antifungals or RPMI 1640 (control growth). A growth control with 104 CFU/ml was also performed for the representative increase of 1 log10 of growth for each strain. The concentrations tested were equal to the MIC (endpoint at 80% of growth inhibition) and twice the MIC (2× the MIC) for FLC and equal to the MIC for AMB. The plates were incubated at 35°C for 1 h. After incubation, the plates were centrifuged (Jouan, model BR4i) at 1,643 × g at 4°C for 30 min. The supernatant was removed from each well, and the medium RPMI 1640 was added. The plates were further incubated at 35°C for 24 h. At different times, an MTT assay was performed. The PAFE was calculated as the time that each strain took to achieve the cell population represented by the control wells (104 CFU/ml), which is equivalent to an increase of 1 log10 in the yeast number (9).
Statistical analysis.
The results were analyzed by the nonparametric Friedman test, the Student t test, and analysis of variance, with a P value of <0.05 being considered significant.
RESULTS
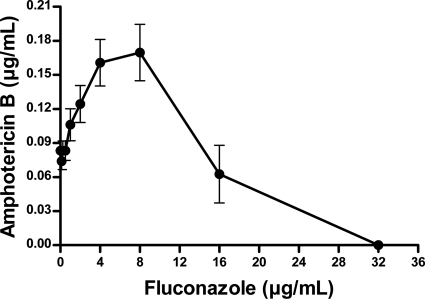
The MIC and FIC data for the 14 strains included in the present study are summarized in Table 1. The FIC data are expressed as the means of all of the FIC values obtained for each strain. MIC50 and MIC90 refer to the MIC values that inhibited 50 and 90% of the strains, respectively. In the antifungal susceptibility testing, MIC values for FLC (endpoint at 80% of growth inhibition) were lower than 16.0 μg/ml only for two strains (14.28%). For the other 12 strains (85.72%), the FLC MICs were 16.0 or 32.0 μg/ml. The MIC values for FLC, considering 50% of growth inhibition, were lower than 16.0 μg/ml for nine strains (64.28%). However, the MIC values were the same or diverge in only one dilution when comparing the two endpoints considered. A narrow range of MICs was found for AMB (0.06 to 0.125 μg/ml), and all of the isolates had low MICs for this drug. Statistical analysis showed significant difference (P < 0.001) between the antifungal agents tested. The interaction curve between the two antifungals against all of the tested C. gattii isolates is presented in Fig. 1. Regarding the mean FIC for each strain, an indifferent effect was observed in the interaction between FLC and AMB for all isolates (Table 1 and Fig. 1). Synergism and antagonism were not observed when the mean FIC was considered. However, when the FIC values were considered individually for all of the FLC concentrations tested (Table 2), we found that the FIC values increased as the concentration of the azole also increased. At lower concentrations of FLC (≤2.0 μg/ml), the FIC showed a trend predominantly toward synergism or indifferent. At FLC concentrations of ≥4.0 μg/ml, the combination between this azole and AMB resulted in indifferent or antagonistic interactions. Interestingly, the increasing FIC values occurred concomitantly with the increments of FLC and the reduction in AMB concentrations.
Table 1.
MIC, mean FIC, and interaction between FLC and AMB against 14 C. gattii strains
| C. gattii strain or parametera | MIC (μg/ml)b |
Mean FIC (μg/ml) | Interaction | ||
|---|---|---|---|---|---|
| FLC (50%) | FLC (80%) | AMB | |||
| ATCC 24065 | 2.0 | 4.0 | 0.06 | 1.19 | Indc |
| ATCC 32608 | 8.0 | 16.0 | 0.06 | 1.45 | Ind |
| L135/03 (C) | 8.0 | 16.0 | 0.06 | 1.90 | Ind |
| L28/02 (C) | 16.0 | 32.0 | 0.06 | 2.18 | Ind |
| 23/10993 (C) | 8.0 | 16.0 | 0.125 | 1.71 | Ind |
| 196L/03 (C) | 16.0 | 32.0 | 0.06 | 2.52 | Ind |
| 1913 ER (C) | 8.0 | 16.0 | 0.125 | 1.42 | Ind |
| 547/OTTI/94-PI-10 (E) | 32.0 | 32.0 | 0.06 | 1.93 | Ind |
| L27/01 (C) | 32.0 | 32.0 | 0.06 | 2.06 | Ind |
| LMM 818 (C) | 16.0 | 32.0 | 0.125 | 1.49 | Ind |
| 29/10893 (C) | 8.0 | 8.0 | 0.125 | 1.07 | Ind |
| L24/01 (C) | 8.0 | 16.0 | 0.06 | 2.66 | Ind |
| ICB 133 (E) | 8.0 | 16.0 | 0.125 | 0.77 | Ind |
| ICB 181 (E) | 8.0 | 16.0 | 0.06 | 1.45 | Ind |
| MIC50 | 8.0 | 16.0 | 0.06 | ||
| MIC90 | 32.0 | 32.0 | 0.125 | ||
| MIC range | 2.0–32.0 | 4.0–32.0 | 0.06–0.125 | ||
| Geometric mean | 10.25 | 18.22 | 0.079 | 1.61 | Ind |
Fig 1.
Combination curve of fluconazole and amphotericin B against 14 C. gattii strains.
Table 2.
FIC data for 14 C. gattii strains for each FLC concentration tested
| Strain | FIC (μg/ml)a at an FLC concn (μg/ml) of: |
|||||||
|---|---|---|---|---|---|---|---|---|
| 0.125 | 0.25 | 0.5 | 1.0 | 2.0 | 4.0 | 8.0 | 16.0 | |
| ATCC 24065 | 1.03 | 1.06 | 1.13 | 1.25 | 1.50 | |||
| ATCC 32608 | 1.01 | 1.02 | 1.03 | 1.06 | 1.13 | 2.33 | 2.58 | |
| L135/03 | 1.01 | 1.02 | 1.03 | 1.06 | 2.21 | 2.33 | 4.67* | |
| L28/02 | 1.00 | 1.01 | 1.02 | 1.03 | 2.15 | 2.21 | 4.42* | 4.67* |
| 23/10993 | 1.01 | 1.02 | 1.03 | 2.06 | 2.13 | 2.25 | 2.50 | |
| 196L/03 | 1.00 | 2.09 | 1.02 | 2.11 | 2.15 | 2.21 | 4.42* | 5.16* |
| 1913 ER | 1.01 | 1.02 | 1.03 | 1.06 | 1.13 | 2.25 | 2.50 | |
| 547/OTTI/94-PI-10 | 1.00 | 1.01 | 2.10 | 2.11 | 2.15 | 2.21 | 2.33 | 2.58 |
| L27/01 | 1.00 | 1.01 | 1.02 | 2.11 | 2.15 | 2.21 | 2.33 | 4.67* |
| LMM 818 | 1.00 | 1.01 | 1.02 | 1.03 | 2.06 | 2.13 | 2.25 | 1.50 |
| 29/10893 | 0.50** | 0.51 | 0.54 | 1.13 | 1.25 | 2.50 | ||
| L24/01 | 1.01 | 2.10 | 2.11 | 2.15 | 2.21 | 4.42* | 4.67* | |
| ICB 133 | 0.49** | 0.50** | 0.51 | 0.54 | 0.61 | 1.25 | 1.50 | |
| ICB 181 | 1.01 | 1.02 | 1.03 | 1.06 | 1.13 | 2.33 | 2.58 | |
Boldface values: *, antagonistic interaction; **, synergistic interaction. FLC, fluconazole.
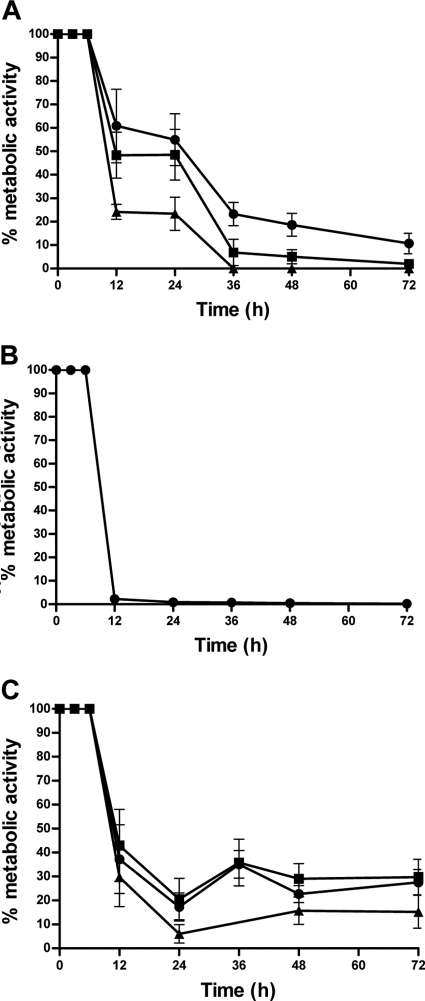
To evaluate the kinetic of the action of the antifungal agents tested, the assays of cell viability using MTT were performed (Fig. 2). For FLC, at the MIC, reduction of metabolic activity to ≥80% occurred only after 48 h. FLC concentrations at 2× the MIC and 4× the MIC reduced this period to 36 h (Fig. 2A). Statistical analysis demonstrated that increasing the FLC concentration did not have significant influence (P > 0.05) on the time to reduce the fungal viability. Within 12 h, there was a reduction of 100% of the viable cells when AMB was used at the MIC (Fig. 2B). When the tests were carried out with combinations of the drugs, the profile of reduction of the metabolic activity was different from the profiles of the drugs used individually. With all of the tested concentrations of FLC in combination with AMB at the MIC for each strain, we observed a profile of interference of one drug on the effect of the other (Fig. 2C). It was possible to observe a reduction in cell viability of ≥80% after 24 h, but the metabolic activity increased in the following measurements, with no significant difference between the concentrations of FLC tested. Intriguingly, as already mentioned, all of the strains were 100% inhibited when AMB was tested alone after 12 h at the MIC (Fig. 2B). CFU counting provided results identical to those obtained with MTT.
Fig 2.
Time-kill curves of fluconazole and amphotericin B alone or in combination against 14 C. gattii strains. (A) Time-kill curve performed with fluconazole at 1× MIC (●), 2× the MIC (■), and 4× the MIC (▲). (B) Time-kill curve performed with amphotericin B (MIC). (C) Time-kill curve performed with antifungal combinations: FLC (1.0 μg/ml) + AMB (MIC) (♦), FLC (4.0 μg/ml) + AMB (MIC) (■), and FLC (16.0 μg/ml) + AMB (MIC) (▲). AMB, amphotericin B; FLC, fluconazole.
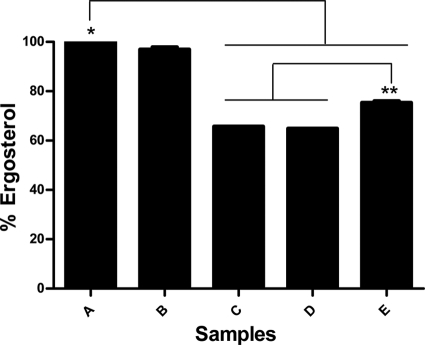
The ergosterol assays (Fig. 3) showed that treatment with FLC (16 μg/ml) caused a reduction of 36% of the ergosterol content compared to the growth control. On the other hand, AMB did not alter the ergosterol amount compared to the growth control. We observed that increasing the FLC concentration resulted in lower ergosterol content (P < 0.05). The treatment with FLC (1.0 μg/ml) and AMB (4.0 μg/ml) reduced the ergosterol contents in 25%. When the FLC concentration of 16.0 μg/ml was used in combination with AMB, the reduction in ergosterol content was similar (35%) to treatment with FLC alone in the same concentration. A statistical difference was observed between the growth control and treatment with FLC alone or in combination in all of the concentrations tested. In addition, a statistically significant difference was observed between treatment with FLC (1.0 μg/ml) and AMB (4.0 μg/ml) compared to treatment with FLC (16.0 μg/ml) alone or in combination (P < 0.05). A schematic association of the data obtained from MIC, FIC, and ergosterol quantification is presented in Fig. 4.
Fig 3.
Percent ergosterol levels of strain L27-01 after 24 h of different treatments. Bars: A, control growth; B, AMB (4.0 μg/ml); C, FLC (16.0 μg/ml); D, FLC (16.0 μg/ml) + AMB (4.0 μg/ml); E, FLC (1.0 μg/ml) + AMB (4.0 μg/ml). * and **, statistically different (P < 0.05). AMB, amphotericin B; FLC, fluconazole.
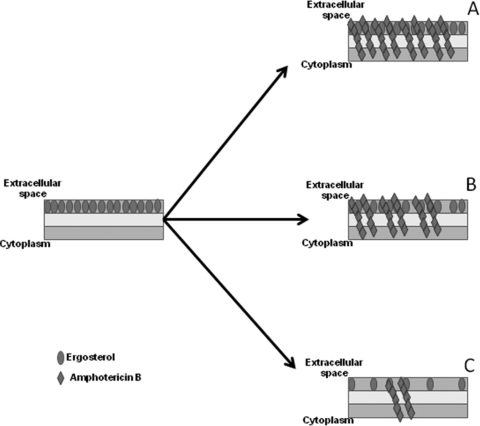
Fig 4.
Schematic representation of the reduction in ergosterol content after treatment with fluconazole and its influence on amphotericin B activity. (A) Without fluconazole, the action of amphotericin B depends on the constitutive levels of ergosterol of each strain. (B) Fluconazole at 2 to 4 μg/ml leads to an increasing loss of the ergosterol content and starts to impair the action of amphotericin B but with no visually interference on the fungus killing. (C) Fluconazole at ≥4 μg/ml leads to lower ergosterol levels, and fewer sites for amphotericin B remain, probably leading to the antagonistic interaction.
The PAFE found for FLC varied from 1 to 3 h (mean PAFE, 1.8 h) for a FLC concentration equal to the MIC and from 2 to 4 h (mean PAFE, 2.5 h) when the concentration of FLC tested was 2× the MIC. The PAFE found for AMB was from 1 to 3 h (mean PAFE, 2.1 h). The PAFE for FLC increased with higher concentrations of the azole.
DISCUSSION
The elevated MIC values found for fluconazole suggest a tendency toward less susceptibility of C. gattii to this azole. An interesting and similar observation was published by Soares et al. (26). These authors studied an isolate of C. gattii that presented an MIC ≥64.0 μg/ml for fluconazole and an MIC of ≥2.0 μg/ml for itraconazole. The in vitro MIC values were correlated with the in vivo response to the treatment with fluconazole or amphotericin B in that study. This correlation was also demonstrated previously (19) using a murine model of cerebral infection caused by C. gattii.
The mean FIC in most of the studies with triazoles and polyenics against pathogenic fungi demonstrated indifferent interaction, as we observed. Barchiesi et al. (2) observed indifference or addition interaction between fluconazole and amphotericin B, however, against another agent of cryptococcosis (C. neoformans). Cuenca-Estrella et al. (8) found an indifferent effect by combining amphotericin B and itraconazole against isolates of Aspergillus fumigatus. For Trichoderma spp., the evaluation of fluconazole and amphotericin B was also indifferent (17). Based on the Etest, Kontoyiannis et al. (15) found an antagonistic interaction between itraconazole and amphotericin B against isolates of Aspergillus fumigatus. O'Shaughnessy et al. (23) observed antagonism in the combination between amphotericin B and voriconazole against Aspergillus spp.
Our in vitro evaluation of the combination of fluconazole and amphotericin B indicated that, although the theoretical action mechanisms of both drugs show a tendency to antagonism, the interaction between these antifungals depends on the concentration tested. At low concentrations (≤2.0 μg/ml), fluconazole appears to have a small or no influence on the activity of amphotericin B. However, at concentrations of ≥4.0 μg/ml, the reduction of ergosterol content of the membrane impairs the activity of amphotericin B, which may generate an antagonism between the two drugs, as illustrated in Fig. 4.
Most studies of antifungal interaction have explored only the global mean FIC or even one FIC result, which often does not correspond to the kinetics of the combination of these drugs in the range of concentrations tested, providing controversial results or even wrong conclusions. A few studies, such as that performed by Meletiadis et al. (18), reported synergism and antagonism dependent on the concentration for voriconazole and amphotericin B against Aspergillus species.
The time-kill curves (Fig. 2) reinforced the kinetics of growth and the dynamism of the action of the drugs either alone or in combination. In addition, we observed faster reduction of metabolic activity for amphotericin B alone compared to the combination of this drug with fluconazole in three different concentrations tested. Even fluconazole levels higher than the MIC were able to disturb the kinetic profile of amphotericin B. Thus, we provide here for the first time dynamic information about the in vitro combination between fluconazole and amphotericin B against C. gattii. We also think that the in vivo profiles of these drugs may be similar to our results, since different plasma levels of each drug may lead to distinct modes of interaction, varying from synergism to antagonism.
The lipid quantification demonstrated that reduction of the ergosterol content caused by fluconazole was the probable reason for the lower activity of amphotericin B in combination. Interestingly, the ergosterol contents obtained in the assay with fluconazole alone and in combination were similar, reinforcing that the presence of amphotericin B did not appear to influence the action of the azole. This fact helps to explain the demonstrated concentration-dependent interaction of the antifungals, since higher fluconazole concentrations lead to lower ergosterol contents. Consequently, few sites would be available for amphotericin B action, and that might lead to an antagonistic interaction. On the other hand, lower fluconazole levels did not appear to interfere with amphotericin B action against C. gattii.
To our knowledge, this is the first investigation about the postantifungal effect of fluconazole and amphotericin B against C. gattii. The PAFE found for fluconazole and for amphotericin is in agreement with the results of Garcia et al. (11), who observed an increase in PAFE values with higher concentrations of fluconazole and amphotericin B against Candida spp.
In conclusion, our data suggest that the interaction of the combination between fluconazole and amphotericin B against C. gattii is concentration dependent. Although it may be considered as an alternative for the treatment of infections caused by C. gattii, special attention should be provided to the plasma levels of the drugs, since low concentrations of the azole would interact with amphotericin B synergistically or additionally. With this possibility, less-toxic doses of amphotericin B could be used. Furthermore. the rates of resistance to fluconazole might be reduced. However, higher fluconazole levels may jeopardize the treatment in combination with amphotericin B. Since these drugs are the two main anticryptococcal agents used in therapy, we suggest that further studies are needed in order to better define the most appropriate treatments against cryptococcosis, particularly that caused by C. gattii.
ACKNOWLEDGMENTS
This study was supported by Fundação de Amparo a Pesquisa do Estado de Minas Gerais and Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Footnotes
Published ahead of print 30 January 2012
REFERENCES
- 1. Arthington-Skaggs BA, Jradi H, Desai T, Morrison CJ. 1999. Quantification of ergosterol content: novel method for determination of fluconazole susceptibility of Candida albicans. J. Clin. Microbiol. 37:3332–3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barchiesi F, et al. 2000. Interactions between triazoles and amphotericin B against Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:2435–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Byrnes EJ, III, Bartlett KH, Perfect JR, Heitman J. 2011. Cryptococcus gattii: an emerging fungal pathogen infecting humans and animals. Microbes Infect. 13:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Casalinuovo IA, Di Francesco P, Garaci E. 2004. Fluconazole resistance in Candida albicans: a review of mechanism. Eur. Rev. Med. Pharmacol. Sci. 8:69–77 [PubMed] [Google Scholar]
- 5. Chen A, Playford SC, Sorrell TC. 2010. Antifungal therapy in invasive fungal infections. Curr. Opin. Pharmacol. 10:522–530 [DOI] [PubMed] [Google Scholar]
- 6. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A3, 3rd ed Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 7. Corrêa MPSC, et al. 1999. Cryptococcosis in children in the State of Pará, Brazil. Rev. Soc. Bras. Med. Trop. 32:505–508 [PubMed] [Google Scholar]
- 8. Cuenca-Estrella M, et al. 2005. Combined activity in vitro of caspofungin, amphotericin B, and azole agents against itraconazole-resistant clinical isolates of Aspergillus fumigatus. Antimicrob. Agents Chemother. 49:1232–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ernst EJ, Klepser ME, Pfaller MA. 2000. Postantifungal effects of echinocandin, azole, and polyene antifungal agents against Candida albicans and Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:1108–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fica AC. 2004. Treatment of systemic fungal infections. I. Fluconazole, itraconazole, and voriconazole. Rev. Chilena Infectol. 21:26–38 [Google Scholar]
- 11. García MT, Llorente MT, Mínguez F, Prieto J. 2002. Post-antifungal effect and effects of sub-MIC concentrations on previously treated Candida spp. influence of growth phase. J. Infect. 45:263–267 [DOI] [PubMed] [Google Scholar]
- 12. Gómez-López A, Cuenca-Estrella M, Mellado E, Rodriguez-Tudella JL. 2003. In vitro evaluation of combination of terbinafine with itraconazole or amphotericin B against Zygomycota. Diagn. Microbiol. Infect. Dis. 45:199–202 [DOI] [PubMed] [Google Scholar]
- 13. Johnson MD, MacDougall C, Ostrosky-Zeichner L, Perfect JR, Rex JH. 2004. Combination antifungal therapy. Antimicrob. Agents Chemother. 48:693–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klepser ME, Wolfe EJ, Jones RN, Nightingale CH, Pfaller MA. 1997. Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B tested against Candida albicans. Antimicrob. Agents Chemother. 41:1392–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kontoyiannis DP, et al. 2000. Itraconazole-amphotericin B antagonism in Aspergillus fumigatus: an Etest-based strategy. Antimicrob. Agents Chemother. 44:2915–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kontoyiannis DP, Lewis RE. 2003. Combination chemotherapy for invasive fungal infections: what laboratory and clinical studies tell us thus far. Drug Resist. Update 6:257–269 [DOI] [PubMed] [Google Scholar]
- 17. Kratzer C, Tobudic S, Schmoll M, Graninger W, Georgopoulos A. 2006. In vitro activity and synergism of amphotericin B, azoles, and cationic antimicrobials against the emerging pathogen Trichoderma spp. J. Antimicrob. Chemother. 58:1058–1061 [DOI] [PubMed] [Google Scholar]
- 18. Meletiadis J, Stergiopoulou T, O'Shaughnessy EM, Peter J, Walsh TJ. 2007. Concentration-dependent synergy and antagonism within a triple antifungal drug combination against Aspergillus species: analysis by a new response surface model. Antimicrob. Agents Chemother. 51:2053–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mendes FES, et al. 2010. Correlation of the in vitro antifungal drug susceptibility with the in vivo activity of fluconazole in a murine model of cerebral infection caused by Cryptococcus gattii. Eur. J. Clin. Microbiol. Infect. Dis. doi:10.1007/s10096-010-1034-8 [DOI] [PubMed] [Google Scholar]
- 20. Mukherjee PK, Sheehan DJ, Hitchcock CA, Ghannoum MA. 2005. Combination treatment of invasive fungal infections. Clin. Microbiol. Rev. 18:163–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nooney L, Matthews RC, Burnie JP. 2005. Evaluation of Mycograb, amphotericin B, caspofungin, and fluconazole in combination against Cryptococcus neoformans by checkerboard and time-kill methodologies. Diagn. Microbiol. Infect. Dis. 51:19–29 [DOI] [PubMed] [Google Scholar]
- 22. Odds FC. 2003. Synergy, antagonism, and what the checkerboard puts between them. J. Antimicrob. Chemother. 52:1. [DOI] [PubMed] [Google Scholar]
- 23. O'Shaughnessy EM, Meletiadis J, Stergiopoulou TT, Demchok JP, Walsh TJ. 2006. Antifungal interactions within the triple combination of amphotericin B, caspofungin and voriconazole against Aspergillus species. J. Antimicrob. Chemother. 58:1168–1176 [DOI] [PubMed] [Google Scholar]
- 24. Revankar SG, et al. 2004. Cloning and characterization of the lanosterol 14 α-demethylase (ERG11) gene in Cryptococcus neoformans. Biochem. Biophys. Res. Commun. 324:719–728 [DOI] [PubMed] [Google Scholar]
- 25. Santos DA, Barros MES, Hamdan JS. 2006. Establishing a method of inoculum preparation for susceptibility testing of Trichophyton rubrum and Trichophyton mentagrophytes. J. Clin. Microbiol. 44:98–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Soares BM, et al. 2008. Cerebral infection caused by Cryptococcus gattii: a case report and antifungal susceptibility testing. Rev. Iberoam. Micol. 25:242–245 [PubMed] [Google Scholar]
- 27. Soares BM, et al. 2011. Cryptococcus gattii: in vitro susceptibility to photodynamic inactivation. Photochem. Photobiol. 87:357–364 [DOI] [PubMed] [Google Scholar]