Abstract
Acyclovir pharmacokinetics was evaluated in 68 HIV-seronegative, herpes simplex virus 2 (HSV-2)-seropositive African women, who received a single oral 400-mg dose of acyclovir, with plasma acyclovir concentrations measured over 8 h. Geometric mean peak concentration and area under the concentration-time curve were 0.31 μg/ml and 1.59 h · μg/ml, respectively, 54% and 52% lower than values from non-Africans. Lower acyclovir concentrations may partly explain the reduced acyclovir suppression of HSV-2 genital ulcer recurrence in HPTN 039 African women participants.
TEXT
Acyclovir (ACV) is a nucleoside analogue with activity against herpes simplex virus (HSV) and varicella-zoster virus (5, 19). HSV-2 infection, the primary cause of genital ulcers, is associated with increased risk of HIV-1 infection (7). However, two clinical trials of ACV suppressive therapy against HSV-2 proved ineffective for reducing HIV-1 acquisition (4, 21). Furthermore, in HPTN 039 (phase III, randomized clinical trial of twice-daily ACV, 400 mg orally), ACV was less effective in reducing the frequency of recurrent genital herpes and the quantity of HSV-2 DNA in ulcers among African women compared to that among men from the United States participating in the same study and to that observed in previous studies from North America (4, 9, 11, 17). Adherence to ACV in HPTN 039 was high and comparable among the women and male participants, based on pill counts of returned products; thus, a pharmacokinetic (PK) explanation was sought for the lower ACV efficacy on HSV-2 suppression in HPTN 039 African women. A PK substudy for ACV was designed to test this hypothesis.
Sixty-eight HIV-1-seronegative, HSV-2-seropositive heterosexual, healthy women were recruited from former participants of HPTN 039 in South Africa, Zambia, and Zimbabwe. Informed consent was obtained; the protocol was approved by internal review boards (IRBs) at the participating sites. Concomitant medications were not allowed in the study. ACV tablets were provided by Carlsbad Laboratories (San Diego, CA), the manufacturer of the ACV used for HPTN 039. Before ACV administration, blood was collected for measurement of serum creatinine and predose drug concentration (0 h). A single oral 400-mg acyclovir dose was given to fasting participants with 200 ml of water under direct observation. After ACV administration, blood was drawn 1, 2, 4, 6, and 8 h after dosing. The samples were centrifuged, and the plasma was stored at −70°C until analysis. Plasma concentrations of ACV were determined using a liquid chromatography-mass spectrometry method (precision and accuracy less than ±15%; Advion Biosciences, Inc., Ithaca, NY). Observed peak plasma concentration (Cmax), time to Cmax (Tmax), area under the plasma concentration-time curve from time zero to infinity (AUC0-∞) and from 0 to 8 h (AUC0-8), and half-life (t1/2) were calculated by noncompartmental methods using WinNonlin (version 5.0.1; Pharsight, Mountain View, CA). Using a t test, the PK parameters were compared with previously reported values (means and standard deviations [SD]) from 8 comparable, fasted, single-oral-400-mg-dose ACV PK studies identified through PubMED (1–3, 6, 8, 14, 15, 20).
Sixty-six black African women were included in the analysis. The participants' mean (±SD) age was 36.5 (±8.1) years, range of 21 to 54 years, and mean body weight was 71 (±16) kg, range of 40 to 129 kg. Compared to the 8 non-African studies, our subjects are older but of similar weights (Table 1).
Table 1.
Demographics of participants in this study compared to those from 8 non-African pharmacokinetic studies of healthy volunteers or pregnant women receiving a single fasted oral dose of 400 mg acyclovir with resultant PK dataa
| Study (reference) | Location | No. of subjects | Subject status | Sex (%) | Age, yrb | Weight, kgb |
|---|---|---|---|---|---|---|
| Lu et al. (current study) | Africa | 66 | HV | F (100) | 36.5 ± 8.1 | 71 ± 16 |
| Amini et al. (1) | Iran | 12 | HV | M (100) | 27.8 ± 4.9** | 82 ± 7.6* |
| Bahrami et al. (2) | Iran | 12 | HV | – | – | – |
| Bangaru et al. (3) | India | 12 | HV | – | 25 ± 3.4** | 55 ± 6.2** |
| Farfal et al. (6) | Poland | 10 | HV | M (100) | 26 ± 4** | 81 ± 9 |
| Frenkel et al. (8) | U.S. | 8 | Pregc | F (100) | 33 ± 4.5d | 74 ± 10d |
| Landowski et al. (14) | U.S. | 11 | HV | F (36) | 29.0 ± 5.8** | 76 ± 16 |
| Palma-Aguirre et al. (15) | Mexico | 24 | HV | F (46) | 24.7 (18–49)e | 46–84f |
| Vergin et al. (20) | Germany | 24 | HV | F (50) | 24.8 ± 2.2** | – |
HV, healthy volunteers; Preg, pregnant; F, female; M, male; –, data were not available. *, P < 0.05; **, P ≤ 0.01 compared to this study using t test comparison of means and standard deviations.
Most data are presented as means ± SD.
HSV-2 seropositive.
Values were calculated from reported data of individuals.
Only mean (range), but not SD, was available from the reference.
Range but not mean ± SD was available.
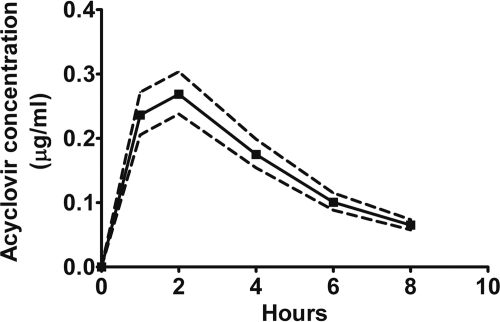
The plasma concentration-time curve is shown in Fig. 1. The geometric mean (95% confidence interval [CI]) for PK parameter estimates were Cmax, 0.31 (0.28, 0.34) μg/ml; AUC0-8, 1.30 (1.26, 1.56) h · μg/ml; AUC0-∞, 1.59 (1.43, 1.76) h · μg/ml (percent extrapolated, 17%); and t1/2, 2.8 (2.5, 3.0) h. Tmax mode was 1 h, median of 2 h, and ranged from 1 to 4 h. Mean (95% CI) serum creatinine concentration in this study was 0.78 (0.74, 0.82) mg/dl. The PK parameters did not correlate with weight, body mass index, or serum creatinine clearance (Cockcroft-Gault). In our study of African women, the median Cmax was 54% lower (range of 28% to 59%, P ≤ 0.006, Table 2) than in the 8 non-African studies. Similarly, AUC0-∞ was significantly lower than in all other studies: mean of 52% lower (range of 26% to 62%, each P ≤ 0.01). However, Tmax was 25% to 31% longer in 2 of 6 studies, and t1/2 was 24% longer in 2 of 6 studies with available comparative data (P ≤ 0.05).
Fig 1.
Acyclovir plasma concentration-time profile following a single oral dose of 400 mg acyclovir in 66 African women. Data are presented as geometric means (±95% confidence intervals).
Table 2.
Acyclovir pharmacokinetic parameters of this study compared to those from 8 non-African pharmacokinetic studies of healthy volunteers or pregnant women receiving a single fasted oral dose of 400 mg acyclovir with resultant PK dataa
| Study (reference) | Cmax (μg/ml) | Tmax (h) | AUC0-∞ (h · μg/ml) | t1/2 (h) |
|---|---|---|---|---|
| Lu et al. (current study) | 0.31 (0.28, 0.34) | 1.56 (1.4,1.8) | 1.59 (1.43, 1.76) | 2.8 (2.5,3.0) |
| Lu et al. (current study) | 0.34 ± 0.14 | 1.8 ± 0.9 | 1.73 ± 0.73 | 2.9 ± 1.1 |
| Amini et al. (1) | 0.76 ± 0.31** | 2.6 ± 1.2** | 4.3 ± 1.16** | 3.1 ± 0.5 |
| Bahrami et al. (2) | 0.80 ± 0.33b** | 2.4 ± 0.7b* | 4.55 ± 1.93b** | 3.8 ± 1.7b* |
| Bangaru et al. (3) | 0.83 ± 0.24** | 1.4 ± 0.6 | 3.63 ± 1.17** | 3.8 ± 0.9** |
| Farfal et al. (6) | 0.71 ± 0.19** | 1.6 ± 0.4 | 2.38 ± 0.59** | – |
| Frenkel et al. (8) | 0.54 ± 0.20c** | – | – | – |
| Landowski et al. (14) | 0.47 ± 0.14d** | – | 2.35 ± 0.67d** | – |
| Palma-Aguirre et al. (15) | 0.70 ± 0.24** | 1.4 ± 0.7 | 2.87 ± 0.97** | 2.6 ± 0.6 |
| Vergin et al. (20) | 0.80 ± 0.26** | 1.8 ± 0.6 | 3.92 ± 1.26** | 2.6 ± 0.3 |
The first row of values are geometric means (95% confidence intervals, lower and upper bounds) as reported in the text. All other values are means ± standard deviations (SD), including for our present study, to allow for comparisons to other studies. Statistical tests are based on comparisons of arithmetic means. Cmax, observed peak plasma concentration; Tmax, time to Cmax; AUC0-∞, area under the plasma concentration-time curve from time zero to infinity; t1/2, half-life; –, data were not available. *, P < 0.05; **, P ≤ 0.01 compared to this study using t test of arithmetic mean and standard deviation.
Data were calculated based on acyclovir serum concentrations.
Values were transformed from reported highest mean acyclovir plasma concentrations with 1 μM equal to 0.225 μg/ml.
Values were calculated from reported data of individuals.
In this PK study of ACV among healthy women from three sub-Saharan countries, Cmax and AUC were substantially lower than in 8 studies of similar design conducted outside sub-Saharan Africa. This may partly explain the lower efficacy of ACV observed in African participants than in U.S. participants in HPTN 039.
Bioequivalence standards limit the role of formulation to explain the PK differences between our FDA-approved generic ACV compared to the Zovirax used in 7 of 8 comparator studies (13). Other potential causes for this difference include environmental or biological differences between sub-Saharan African and U.S. study participants in HPTN 039. The PK differences may be due to reduced bioavailability of ACV in our study. Given that participants fasted in this and the other studies, diet is an unlikely explanation. PK differences may result from variations in the frequency distribution of drug transporter polymorphisms involved in ACV absorption or, less likely, elimination. ACV is a substrate of P-glycoprotein (P-gp; also known as MDR1), a drug efflux pump that limits absorption of drugs from the gastrointestinal tract and promotes excretion into bile and urine (16). ACV plasma concentrations are reduced with increased expression of MDR1 (14). Furthermore, Caucasians and Asians, in contrast to Africans, have a significantly higher frequency of silent C3435T polymorphism of the MDR1 gene, leading to much lower expression of intestinal P-gp (10, 12, 18). Future studies are needed to evaluate MDR1 expression in African women to determine whether higher MDR1 expression contributes to lower plasma levels of ACV relative to prior PK studies in other populations.
Limitations of this study include use of historical controls with potentially important differences in assay methods and drug formulations and no pharmacogenomic assessment. Theoretically, the magnitude of these differences observed in a fasting study, especially with a low bioavailability drug like ACV, may differ in clinical use.
In conclusion, we report significantly lower plasma acyclovir concentrations in African women than in non-African populations. This finding may partly explain the lower efficacy of acyclovir in suppression of genital ulcers and quantity of HSV-2 in genital ulcers among African women in HPTN 039. Whether higher doses of acyclovir or valacyclovir with greater bioavailability will improve efficacy has not been studied, and the mechanism of this pharmacokinetic difference has not been identified. More generally, our results reinforce the need for selected studies in diverse populations where pharmacogenetic polymorphisms or host environment may plausibly alter a drug's pharmacokinetics.
ACKNOWLEDGMENTS
We thank all the study participants for their time and dedication. We are grateful for the efforts of the clinical study team and the technical expertise of Tom Alexander at Advion Biosciences, Inc.
Footnotes
Published ahead of print 13 February 2012
REFERENCES
- 1. Amini H, Javan M, Gazerani P, Ghaffari A, Ahmadiani A. 2008. Lack of bioequivalence between two aciclovir tablets in healthy subjects. Clin. Drug Invest. 28:47–53 [DOI] [PubMed] [Google Scholar]
- 2. Bahrami G, Mirzaeei S, Kiani A. 2005. Determination of acyclovir in human serum by high-performance liquid chromatography using liquid-liquid extraction and its application in pharmacokinetic studies. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 816:327–331 [DOI] [PubMed] [Google Scholar]
- 3. Bangaru RA, Bansal YK, Rao AR, Gandhi TP. 2000. Rapid, simple and sensitive high-performance liquid chromatographic method for detection and determination of acyclovir in human plasma and its use in bioavailability studies. J. Chromatogr. B Biomed. Sci. Appl. 739:231–237 [DOI] [PubMed] [Google Scholar]
- 4. Celum C, et al. 2008. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet 371:2109–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elion GB, et al. 1977. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc. Natl. Acad. Sci. U. S. A. 74:5716–5720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Farfal S, Klimowicz A, Bielecka-Grzela S. 2006. Acyclovir concentrations in the skin of humans after a single oral dose assessed by in vivo cutaneous microdialysis. Skin Res. Technol. 12:228–234 [DOI] [PubMed] [Google Scholar]
- 7. Freeman EE, et al. 2006. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 20:73–83 [DOI] [PubMed] [Google Scholar]
- 8. Frenkel LM, et al. 1991. Pharmacokinetics of acyclovir in the term human pregnancy and neonate. Am. J. Obstet. Gynecol. 164:569–576 [DOI] [PubMed] [Google Scholar]
- 9. Fuchs J, et al. 2010. Clinical and virologic efficacy of herpes simplex virus type 2 suppression by acyclovir in a multicontinent clinical trial. J. Infect. Dis. 201:1164–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fung KL, Gottesman MM. 2009. A synonymous polymorphism in a common MDR1 (ABCB1) haplotype shapes protein function. Biochim. Biophys. Acta 1794:860–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gupta R, et al. 2004. Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J. Infect. Dis. 190:1374–1381 [DOI] [PubMed] [Google Scholar]
- 12. Hoffmeyer S, et al. 2000. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc. Natl. Acad. Sci. U. S. A. 97:3473–3478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krauss GL, Caffo B, Chang YT, Hendrix CW, Chuang K. 2011. Assessing bioequivalence of generic antiepilepsy drugs. Ann. Neurol. 70:221–228 [DOI] [PubMed] [Google Scholar]
- 14. Landowski CP, et al. 2003. Gene expression in the human intestine and correlation with oral valacyclovir pharmacokinetic parameters. J. Pharmacol. Exp. Ther. 306:778–786 [DOI] [PubMed] [Google Scholar]
- 15. Palma-Aguirre JA, et al. 2007. Bioavailability of two oral suspension and two oral tablet formulations of acyclovir 400 mg: two single-dose, open-label, randomized, two-period crossover comparisons in healthy Mexican adult subjects. Clin. Ther. 29:1146–1152 [DOI] [PubMed] [Google Scholar]
- 16. Palmberger TF, Hombach J, Bernkop-Schnurch A. 2008. Thiolated chitosan: development and in vitro evaluation of an oral delivery system for acyclovir. Int. J. Pharm. 348:54–60 [DOI] [PubMed] [Google Scholar]
- 17. Reitano M, et al. 1998. Valaciclovir for the suppression of recurrent genital herpes simplex virus infection: a large-scale dose range-finding study. International Valaciclovir HSV Study Group. J. Infect. Dis. 178:603–610 [DOI] [PubMed] [Google Scholar]
- 18. Schaeffeler E, et al. 2001. Frequency of C3435T polymorphism of MDR1 gene in African people. Lancet 358:383–384 [DOI] [PubMed] [Google Scholar]
- 19. Schaeffer HJ, et al. 1978. 9-(2-Hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature 272:583–585 [DOI] [PubMed] [Google Scholar]
- 20. Vergin H, Kikuta C, Mascher H, Metz R. 1995. Pharmacokinetics and bioavailability of different formulations of aciclovir. Arzneimittelforschung 45:508–515 [PubMed] [Google Scholar]
- 21. Watson-Jones D, et al. 2008. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N. Engl. J. Med. 358:1560–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]